Abstract
Two distant Antioquian cattle farms where systemic and topical acaricides had previously failed to control infestations by Rhipicephalus (Boophilus) microplus were studied. An initial in vivo study was conducted using single subcutaneous injections with a long-acting formulation of ivermectin (630 μg/kg). Injections were made at 3-month intervals on animals at each farm to evaluate the therapeutic and persistent efficacy of ivermectin against R. microplus. Body tick counts and reproductive parameters of semi- or fully engorged females (≥5 mm) were assessed at 10-day intervals, and since no negative control group could be included, values were compared against those for day 0. Although there was an overall reduction of 50%–75% in tick numbers that persisted for 30–40 days, it was not significantly different at one of the farms and not enough to afford protection from severe infestations. The engorgement weight and egg mass weight of ticks from treated animals were significantly lower throughout the 50-day posttreatment period. Egg hatch was not significantly reduced posttreatment and remained at levels of 80%–90%. A random selection of 9 out of 28 commercial formulations of ivermectin sold in Colombia were analyzed by High Performance Liquid Chromatography (HPLC). All were within the expected labeled concentration (±15% deviation) of 1% and 3.15% ivermectin except for one. A popular unregistered injectable widely used in both farms and labeled as “natural pyrethrin”, was found to contain 10.5% ivermectin. An adult immersion test was conducted to evaluate the efficacy of topical acaricides to recommended concentrations of five commercial products and/or their combinations. Efficacy was determined by comparing the reproductive index of each treated group to that of the control group. Cypermethrin (150 ppm) was completely ineffective at both farms. Amitraz (208 ppm) exhibited low and intermediate efficacies of 14% and 56%. The combination of amitraz (100 ppm) and cypermethrin (150 ppm) was less efficacious than the amitraz alone. A generic product based on amitraz + citronella (208 ppm + 10 ppm, respectively) was shown to be less efficacious than the name-brand amitraz product. Products containing the organophosphate chlorpyrifos or trichlorfon exhibited intermediate efficacies of approximately 60% at the Tarso farm. We conclude that at these two locations, there is a high degree of resistance to many of the acaricides available in Colombia and confirm suspicions that ivermectin is no longer able to eliminate tick infestations.
Keywords: southern cattle fever tick, multi-resistance, acaricide
Introduction
Circumstantial evidence in numerous municipalities of Antioquia now indicates that resistance of Riphicephalus (Boophilus) microplus (Canestrini) to almost every class of commercially available chemical acaricide is likely widespread in this part of Colombia. Surveys of multiple farms claiming treatment failures reveal common practices known to accelerate the development of parasite resistance. Of particular concern has been the misconception by farmers and veterinarians that animals should be free of all parasites, resulting in decades of frequent and indiscriminate use of any antiparasitic on a prophylactic basis to every animal in the herd, neglecting the basic concept of preserving the population in refugia. Although treating the entire herd with acaricide has been a successful strategy in the R. microplus and Rhipicephalus (Boophilus) annulatus eradication programs that eliminated these ticks from the USA, parts of Argentina, and Australia, it is unconceivable to strive for eradication in tropical countries like Colombia with an ideal climate for tick survival and no geographical barriers to prevent tick migration using the current tick control technologies available. Therefore, the concept of preserving a portion of the population in refugia is pivotal to delay the evolution of resistance in Colombia. In addition to the lack of strategic control plans, label recommendations have been poorly observed, particularly with the preparation and application of topical acaricides with backpack sprayers, which has been the most common method for tick control in Antioquia. In small-scale dairy farms, it is common to find all family members handling backpack sprayers and making overly diluted formulations that result in under-dosing. Acaricides have been rarely rotated and are only changed when control was unsatisfactory or completely failed. Lack of government regulation or an education policy to restrict cattle movements within tick-infested areas, has likely contributed to the spread of resistance to remote areas of the country.
Although the perception that control failure seems to be widespread, it may be unfounded and partly caused by improper dosing of acaricides. Consequently, confirmation of resistance must be based on dose-response bioassays and/or assessment of the field efficacy of acaricides, for which various methods have been widely adopted and approved by the Food And Agriculture Organization (FAO)1 and World Association for the Advancement of Veterinary Parasitology (WAAVP).2 The standard in vitro bioassays recommended through the FAO guidelines for testing resistance to acaricides in R. microplus are the larval packet test (LPT) and the adult immersion test (AIT). Although, it is still difficult to predict field acaricide efficacy based on these in vitro resistance bioassays, they can certainly diagnose resistance before control failures are obvious. The LPT is laborious, and modifications like the larval immersion test (LIT) have proved better at discriminating between resistant and susceptible strains against macrocyclic lactones.3,4 In fact, numerous reports using the LIT have been recently published that demonstrate R. microplus resistance to ivermectin in other Latin American countries, including Mexico,5,6 Uruguay,7 and Brazil.3,8 To complement the above in vitro assays, field studies using the WAAVP guidelines were used simultaneously to confirm a reduction in therapeutic efficacy and protective period.2
In Colombia, at least 20 pharmaceutical companies are selling different formulations of ivermectin. Oversight of formulation practices falls under the INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) jurisdiction, and final approval for registration of veterinary drugs is under the ICA (Instituto Colombiano Agropecuario) authority. Unfortunately, scarce resources to implement quality monitoring systems of manufacturing practices and surveillance on sales of veterinary drugs has created a market prone to fraud and the presence of unregistered products. The use of products with suspect ingredients and/or concentrations is an added problem to be considered when diagnosing and confronting the appearance of resistance to antiparasitic drugs. In addition, most producers simply rely on information available at agrochemical shops to choose the most convenient product and method of application. The drive to sell products in search of perceived benefits despite real needs has undoubtedly led to poor decisions by the producers and overuse of most acaricides, consequently shortening their lifespan.
The main objective of this study was to corroborate far mers´ claims of lost efficacy to ivermectin and other common topical commercial products by using in vivo trials and AIT. Additionally, several Colombian-registered ivermectin formulations were analyzed to validate labeled concentrations.
Materials and Methods
Ivermectin analysis
A random selection of nine commercial ivermectin formulations was purchased at a local agrochemical shop from Colanta, which is the largest retailing company throughout Antioquia. They were sent, together with a popular unregistered injectable product known as Ivercyt, to the Veterinary Diagnostic Laboratory at Iowa State University to validate labeled concentrations. Samples were diluted in methanol and analyzed by High Performance Liquid Chromatography (HPLC) against standards according to a method approved by the Association of Official Agricultural Chemist.9
Selection of farms
Two farms (Tarso and San Jeronimo) where producers experienced treatment failure after using one or several products containing ivermectin were visited on separate occasions within days of dosing to substantiate further investigation. On each occasion, ticks were taxonomically identified as R. microplus, and a clear lack of efficacy was observed. The Tarso farm raises a highly selected Brangus breed imported from Texas, and comprising about 250 head. The San Jeronimo farm is a small family-run mixed dairy operation that is representative of common management practices in the area. The farm comprises 30 lactating cows along with their respective calves and includes mix crosses of Simmental, Normandy, and Holstein breeds.
Field studies
Field studies were initiated in June of 2013. The criterion for inclusion of an animal in the study was the presence of ≥40 ticks of standard 5- to 8-mm adult female ticks prior to treatment, as recommended by the WAAVP for natural field conditions.2 Animal identification was by ear tattoos at Tarso and by individual names at San Jeronimo. A long-acting formulation of 3.15% ivermectin (Ivomec GOLD®, Merial Saude Animal Ltda, Paulinia, Brazil) was administered at the recommended dosage of 630 μg/kg subcutaneously. Commercial lot numbers were as follows – Tarso: batch number BC165/11, expiration 04/2014; San Jeronimo: batch number BJ253/11, expiration 07/2014. Prior to this study, the animals were treated on an ad hoc basis with a popular unregistered product containing 10.5% ivermectin (Table 1) at Tarso, and with topical motor oil at San Jeronimo. Unfortunately, farmers did not agree to have a negative control group; therefore, efficacy could not be determined. Between 6 and 8 AM, cattle were immobilized in individual chutes, and all ticks were counted on day 0 (prior to treatment) and days 10, 20, 30, 40, and 50 posttreatment. Ticks were counted by removing them from the animals when they were ≥5 mm in length, at which point they were considered to be semi- or fully engorged and should complete engorgement within the following 24 hours (“Standard Females”10). The animals were managed similarly throughout the study period and removed from the study when tick burden was clearly affecting their well-being. In October of 2013, 3 months following the initial treatment in June, a second injection of ivermectin with commercial Ivomec GOLD®, belonging to the same commercial lot numbers as stated above for each farm, was administered to the same animals.
Table 1.
Trade names and active ingredients of topical acaricides used to expose Riphicephalus microplus using an AIT.
| TRADE NAME (MANUfACTURER) | ACTIVE INGREDIENT | BATCH NO. (EXPIRATION) | FINAL CONCENTRATION (PPM) |
|---|---|---|---|
| Ganabaño (Novartis) | Cypermethrin 15% | 003–11/E (08/2014) | 150 ppm |
| Triatox (Schering-Plough) | Amitraz 12.5% | 31112b (11/2016) | 208 ppm |
| Ganabaño + triatox | Cypermethrin + Amitraz | above products | 150 ppm Cypermethrin + 100 ppm Amitraz |
| Impacto (Aurofino) | Chlorpyrifos 25% + Cypermethrin 15% | 031/13 (05/2016) | 312 ppm Clorpirifos + 187 ppm Cypermethrin |
| Citraz (Kirovet) | Amitraz 20.8% + Citronella 1% | CIT040913 (09/2016) | 208 ppm Amitraz + 10 ppm Citronella |
| Neguvon Powder (Bayer) | Trichlorfon 97% | H130214r (02/2015) | 10000 ppm (=1%) |
Preparation of ticks and assessment of reproductive parameters
For each tick collection, the largest six engorged female ticks collected from every treated animal were pooled together and transported in cardboard boxes with fresh grass to the Veterinary Parasitology Laboratory of the University of Antioquia for processing within 24 h. Upon arrival to the laboratory, ticks were washed with distilled water, dried on paper towel, weighed, and fixed dorsally with double-side sticky tape to Petri dishes. They were then incubated at 28 °C and 95% relative humidity to allow for oviposition. The egg mass deposited after 20 days was gently removed and weighed. Twelve milligrams of eggs that were laid within the first 3–4 days from every tick were transferred into 5-mL sterile vacutainer tubes. By using a stereoscope, it had been previously determined that 300 eggs weighed 12 mg, so hatchability was based on the number of larvae arising from 12 mg of eggs. The tubes were plugged with cotton caps to allow air and moisture exchange and maintained in the incubator. Eclosion of larvae occurred after approximately 27 days after the initial oviposition, and the tubes were checked daily to determine the first day of larvae hatch. A constant number of 3–5 ticks were found in every group of 30 that would not lay eggs or would have less than 50% eclosion rates. These were removed for the calculation of mean egg weights and hatchability as they would confound the reproductive parameters of the large majority of ticks. In order to calculate percent hatch, all larvae were removed from the tubes with distilled water, transferred with a paint brush to a white sheet of paper, and then counted manually by immobilizing them to a sticky tape.
Adult immersion test
Engorged ticks were collected directly from animals in March 2014 and transported to the laboratory in cardboard boxes with fresh grass to provide humidity. Within 24 hours of their collection, they were washed three times with distilled water, weighed, and allocated to groups of ≥30 ticks that were equally distributed by weight, discarding any damaged ticks and those below 100 mg. Unlike the FAO1 protocol, which recommends 30 minutes of immersion to labeled dilutions of the commercial products, the modifications suggested by several authors of using a 5-minute immersion time in a final volume of 50 mL was followed to facilitate comparison with their studies.11–13 The control group was exposed to water. Table 1 provides a list of the topical commercial products used for the AIT.
After 5 minutes of immersion, the ticks were dried with adsorbent paper, weighed, and fixed dorsally with double-side sticky tape to sterile Petri dishes (10 ticks per plate). They were then incubated at a temperature of 28 °C and 95% relative humidity for a period of 20 days. After 20 days of incubation, the egg mass of each individual tick was weighed, and approximately 12 mg of the eggs oviposited during the first 3–4 days of oviposition were collected and placed into sterile vacutainer tubes plugged with cotton. The tubes were incubated for another 2 weeks, and the fertility of each tick was determined as described in “Preparation of ticks and assessment of reproductive parameters.”
To estimate the efficacy of each acaricide, the index of reproduction (IR) was calculated according to the following formula, as recommended by FAO1 guidelines. The IR estimates the number of larvae produced per female exposed in the bioassay:
Percent control was calculated according to Drummond et al.14 as follows:
Treatments were considered effective with no indication of resistance when the efficacy (% control) was ≥90%. To assess the modified AIT, also recommended in the FAO guidelines, ticks were examined at 7 days posttreatment. Treated females that did not oviposit or produced a small infertile egg mass were considered dead.
Data analysis
Statistical analyses were performed using SPSS v-10 software. For the in vivo studies, data on female reproductive parameters were analyzed using a Student’s t-test and differences considered significant when P < 0.05. Tick counts were log transformed prior to analysis in order to achieve normality and equality of variances. Percent egg hatch was subjected to arcsine transformation and comparisons performed upon the transformed data. For the in vitro studies, the differences between the reproductive parameters of each acaricide tested and the control group were subjected to one-way analysis of variance (ANOVA) with subsequent application of the Tukey test for post hoc comparisons. Homoscedasticity (Levene’s test) was checked for each data group in order to verify the assumption required by ANOVA. When variances between groups were not similar, a nonparametric Kruskal–Wallis test was used to compare medians between groups.
Results
Ivermectin analysis of commercial formulations
The analysis of ivermectin in 9 of 28 randomly selected commercial preparations sold at the largest agrochemical retailing company throughout Antioquia is shown in Table 2. The results show that, except for one brand, all products contained ivermectin that matched labeled concentrations (±15%) of 1% and 3.15%. An unregistered injectable product known as Ivercyt and marketed as a natural pyrethrin (with allegedly no withdrawal times for milk or sacrifice) was found to contain 10.5% ivermectin. This product is sold illegally at some agrochemical shops and by private veterinarians.
Table 2.
Ivermectin concentrations in some injectable formulations available in Colombia.
| COMMERCIAL NAME | MANUFACTURER | BATCH No. | EXPIRATION DATE | REGISTRATION NUMBER ICAa | CONCENTRATION (%) | |
|---|---|---|---|---|---|---|
| LAbEL | MEASUREd (±S.D) | |||||
| IVOMEC | Merial Saúde Animal Ltd. Paulinia, Brazil | BE314/11 | 11/2016 | 1727-DB | 1.0 | 0.85 (0.05) |
| Exend | Genfar S.A. Villa Rica, Cauca, Co | 041012 | 10/2015 | 3800-DB | 1.0 | 0.95 (0.04) |
| Res-Vet | Merca Vet Ltd., Medellin, CO | 26–06–12 | 06/2015 | 8264 | 3.15 | 3.47 (0.18) |
| Provimec | Biovet S.A., Funza, CO | PVMO30511 | 05/2015 | 4496-DB | 1.0 | 1.07 (0.08) |
| Vimec L.A. | Vicar S.A, Bogota, CO | VL3K5121 | 11/2015 | 4778-DB | 1.0 | 0.84 (0.08) |
| Ivegan | Erma S.A., Cali, CO | 0024637 | 11/2017 | 3939-DB | 1.0 | 1.02 (0.10) |
| Kaput L.A. | Lab-vet, Bogota, CO | 23–04 | 04/2015 | 6187 | 1.0 | 1.17 (0.07) |
| Ivervem | Laboratorios V.M. Ltd., Bogota, CO | 3485 | 08/2013 | 3854-DB | 1.0 | 0.37 (0.08) |
| Iverbest | Callbest Ltd., Bogota, CO | 10120902 | 09/2015 | 6297-mv | 3.15 | 3.84 (0.45) |
| Ivercyta | Unknown | Unregistered | Label claima | 10.5 (0.2) | ||
Notes:
The product label falsely claims this is a “natural pyrethrin” that is effective as an endectocide and leaves no chemical residues after its parenteral administration. The recommended treatment is 1 mL/50 kg body weight subcutaneously (=dosage of 2.1 mg/kg body weight).
Field studies with ivermectin
The reproductive parameters and total body counts of ticks collected from animals treated with ivermectin on the first trial are shown in Table 3. Prior to treatment, there was a large variation in the numbers of ticks observed on treated animals (40–300 mature ticks per animal). In spite of the limitation of not having a control group to determine efficacy, ivermectin was unable to eliminate the initial infestation in any of the treated animals, and although it seemed to reduce the initial infestation at both sampled farms, the trend was not significantly different at the San Jeronimo farm (Table 3).
Table 3.
Tick counts and reproductive parameters of Riphicephalus microplus collected from cattle treated with Ivomec GOLD® at two Antioquian farms with suspected treatment failures.
| DAYS POST-TREATMENT | PARAMETER EVALUATED | TARSO (N = 6 ANIMALS) | SAN JERONIMO (N = 5 ANIMALS) |
|---|---|---|---|
| 0 | Tick counts | 105 ± 76 | 120 ± 102 |
| 10 | 26 ± 21* | 68 ± 36ns | |
| 20 | 27 ± 21* | 41 ± 34ns (1 out)a | |
| 30 | 66 ± 44* | 36 ± 12ns | |
| 40 | 82 ± 46* (1 out)a | 82 ± 65ns (2 out)a | |
| 50 | 35 ± 19* | 108 ± 52ns | |
| 60 | 12 ± 7* | ND | |
|
| |||
| 0b | Female weight (mg) | 133.4 ± 25.4 | 200 ± 53 |
| 10 | 77.1 ± 19.4* | 114 ± 32* | |
| 20 | 78.2 ± 17.7* | 88 ± 31* | |
| 30 | 80.5 ± 18.0* | 102 ± 29* | |
| 40 | 93.9 ± 35.0* | 102 ± 24* | |
| 50 | 60.9 ± 37.4* | 164 ± 35* | |
|
| |||
| 0 | Egg mass weight/tick (mg) | 64.0 ± 15.7 | 104.5 ± 31.4 |
| 10 | 24.4 ± 19.4* | 45.5 ± 18.8* | |
| 20 | 27.4 ± 12.4* | 36.5 ± 16.3* | |
| 30 | 32.8 ± 11.0* | 48.2 ± 16.1* | |
| 40 | 40.5 ± 20.0* | 45.9 ± 14.3* | |
| 50 | 24.4 ± 20.8* | 78.5 ± 22.2* | |
|
| |||
| 0 | Hatchability (%) | 82.7 ± 14.0 | 82.1 ± 9.6 |
| 10 | 68.2 ± 20.0* | 91.6 ± 9.2* | |
| 20 | 84.2 ± 16.4ns | 89.3 ± 10.7* | |
| 30 | 75.2 ± 18.3ns | 77.9 ± 11.8ns | |
| 40 | 79.6 ± 15.6ns | 87.7 ± 13.2ns | |
| 50 | 64.3 ± 22.4* | 84.3 ± 13.9ns | |
Notes: At each time point, the six largest ticks collected from each treated animal were pooled. Reproductive parameters represent the arithmetic mean (±SD) of 30 ticks.
Number of animals removed from study due to poor health related to high tick burden.
Initial female weights were different between farms: Tarso had applied Ivercyt 19 days before; San Jeronimo used motor oil (mixed with amitraz at a 1% final concentration) topically with a brush to manage Riphicephalus microplus.
Means differ significantly (P > 0.05) from day 0 by Student’s t-test.
Abbreviations: ns, nonsignificantly different from day 0; ND, not determined.
At the Tarso farm, there was a significant reduction in all posttreatment counts, but the greatest reduction was observed at day 60 when the effect of ivermectin would be greatly diminished due low concentration in the blood. Therefore, it is likely that factors determining the natural variation in tick infestations played a major role in lowering body burdens.
Treatment of test animals with ivermectin significantly reduced female body weights throughout the study period (Table 3). In fact, the loss in engorgement weight at 10 days posttreatment was almost 45% from the initial weights at both farms. The decrease in female weights resulted in reduced oviposition (Fig. 1). Hatch rates were between 80% and 90% at most time intervals, except for days 10 and 50 at the Tarso farm. This coincided with the lowest observed body and egg mass weights. Regardless of the time interval and farm, it was observed that low-weight females were associated with a higher number of nonlaying females and generally a lower hatch rate.
Figure 1.
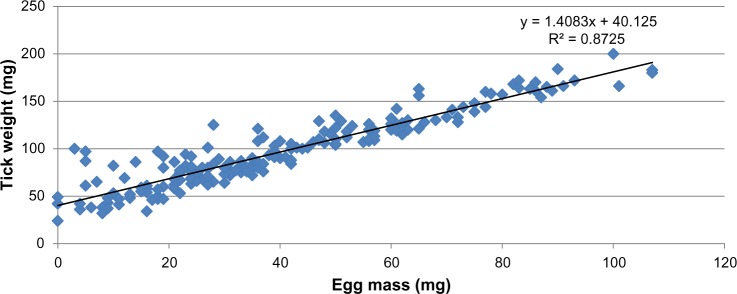
Scattergram showing the correlation (Pearson r = 0.93) between female tick weight and egg mass produced from ticks collected at Tarso (n = 188).
The observed tick burdens, particularly at Tarso, varied throughout the year with periods of relatively few ticks present on the animals when no treatments were necessary. Inversely, there were time periods when very high numbers of fully engorged females were observed on animals at both farms. Therefore, the authors acknowledge the need for a large negative control group if the efficacy of ivermectin, or any other acaricide, is going to be determined in the future under field conditions. During the second field trial, the tick numbers were very high at Tarso during the month of October (Fig. 2A). At this time, the administration of ivermectin to six animals with >300 ticks per animal was followed by total tick body counts of 79 ± 19 at 10 days postadministration (Fig. 2B). In the 10-day posttreatment interval, the severe infestation found within this group of 27 head resulted in four animals with rectal temperatures ≥41 °C, severe subcutaneous abscesses and associated myiasis in five animals (Fig. 2D–E), and extensive areas of ulcerated, bleeding, and purulent skins in three animals (Fig. 2F). The mean (±standard deviation [SD]) weights (mg) of 30 ticks collected from these animals at day 0 and 10 were significantly different (P > 0.05) with values of 131 ± 37 and 81 ± 20, respectively. The mean (±SD) egg mass weight (mg) was also significantly different between day 0 (60 ± 23) and day 10 (30 ± 12). However, no difference was observed in egg hatch rate at day 0 (87% ± 20%) and day 10 (86% ± 12%).
Figure 2.
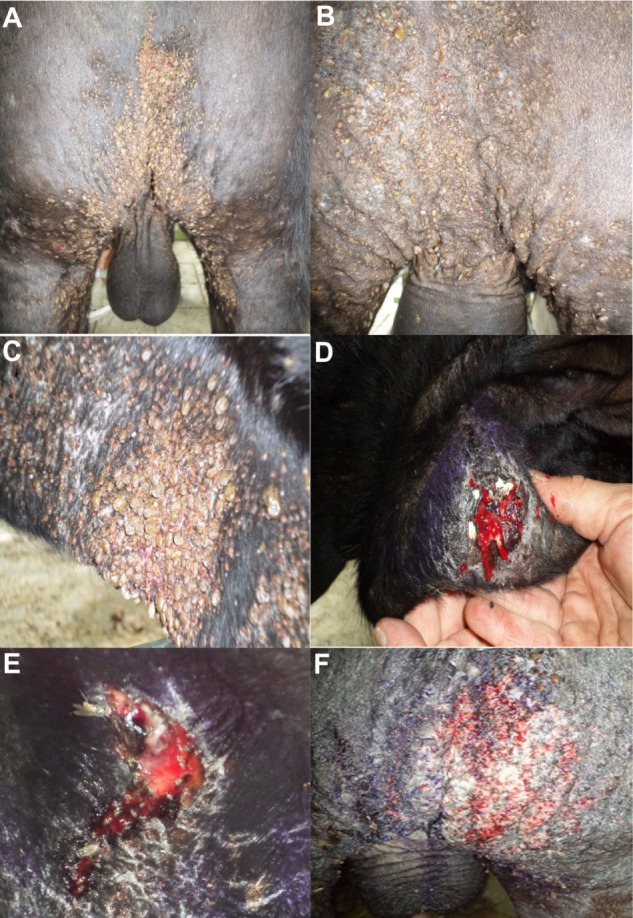
Massive infestation by R. microplus in the perineal and groin area of a Brangus bullock at the Tarso farm observed on the day (A) of application with Ivomec GOLD and 10 days later (B). Infestation by R. microplus on the brisket of a bullock (C). Secondary cutaneous lesions due to R. microplus infestation showing: subcutaneous abscess in the brisket (D), myiasis (E), and extensive areas in the perineal region with purulent, exudative and ulcerated skin (F).
At San Jeronimo, the second ivermectin administration was administered when animals had a mean (±SD) tick body count of 86 ± 42. At 10 days postinjection, the mean (±SD) body tick count was 52 ± 36 and did not differ statistically (P = 0.16) from day 0. The mean (±SD) weights (mg) of 30 ticks collected from these animals were significantly different (P < 0.01) between day 0 (193 ± 37 mg) and day 10 (97 ± 34 mg) posttreatment. The mean (±SD) egg mass weight was significantly different between day 0 and 10 (91 ± 19 versus 36 ± 22 mg). Percent hatch was unchanged by ivermectin from day 0 (93 ± 7) to day 10 postinjection (91 ± 10).
Adult immersion test
The reproductive parameters and mortality of engorged ticks collected from both farms in March 2014 and exposed to topical commercial acaricides is shown in Table 4. Parameters for the control ticks (exposed to water) were similar to values expected for healthy ticks, with an egg mass production corresponding to 40%–50% of their body weights, and fertility of eggs laid in the first days of incubation being approximately 90%. When comparing the IR of ticks exposed to different acaricides to the control group, neither product produced an efficacy above 90%, which is the threshold considered by FAO for no resistance. Cypermethrin (150 ppm) was totally ineffective at lowering egg production or percent hatch, which resulted in complete lack of efficacy on ticks from either farm. The lack of efficacy, as measured by the IR, was also reflected at 7 days postexposure with only four dead of 69 exposed ticks, a value similar to those of the control group. Amitraz at the recommended label concentration (208 ppm) had low (14%) and intermediate (56%) efficacies on ticks collected from the San Jeronimo and Tarso farms, respectively. The combination of cipermethrin (150 ppm) and half the usual concentration of amitraz (100 ppm) was not as efficacious to ticks collected from both farms than amitraz alone at 208 ppm. A generic product containing amitraz (208 ppm) and citronella (10 ppm) had an even lower efficacy of 44% on the Tarso ticks. The two compounds with the highest efficacy were the organophosphates chlorpyrifos and trichlorfon. Both produced approximately 60% mortality. Of the two parameters that determine the IR, fecundity was the most affected by most products. The fertility of eggs laid by each tick observed was typically normal or essentially zero with very few intermediate observations. However, for trichlorfon and to a lesser extent chlorpyrifos, a large number of ticks laid eggs in which half hatched and the other half did not. With respect to mortality observed at 7 days posttreatment, a tendency for a greater number of dead ticks increased in direct correlation with efficacy of the compound. However, a larger study is needed to establish whether there is a good correlation of aca-ricide efficacy as measured by IR and mortality at day 7.
Table 4.
Reproductive parameters and mortality of engorged female Rhipicephalus microplus collected from Tarso farm immersed for 5 minutes in recommended concentrations of formulated acaride containing one or two active ingredients.
| PARAMETER AI (BRAND NAME) FARM | NUMBER | TICK WEIGHT (MEAN +SD) (MG) | EGG MASS (MEAN +SD) (MG) | FECUNDITY (EGG+FEMALES) | FERTILITY (% HATCH) | REPRODUCTIVE INDEX (FECUNDITY X FERTILITY) | EFFICACYa | MORTALITY OF ADULTS AT 7 DAYS” |
|---|---|---|---|---|---|---|---|---|
| Control (dH2O) | ||||||||
| Tarso | 38 | 205.5 ±49.1 | 83.8 ±40.0 | 0.40 ±0.16 | 87.8 ± 25.9 | 37.2 ± 18.8 | – | 3/38 |
| San Jeronimo | 28 | 171.9 ±50.0 | 77.6 ±39.2 | 0.44 ±0.14 | 92.0 ± 17.9 | 42.1 ± 15.1 | – | 1/28 |
|
| ||||||||
| Cypermethrin (Ganabaño®) | ||||||||
| Tarso | 38 | 203.4 ± 50.0 | 86.7 ± 31.2 | 0.44 ± 0.19 | 82.6 ± 22.9 | 38.7 ± 22.9 | 0% | 3/38 |
| San Jeronimo | 31 | 175.1 ±47.8 | 83.3 ±37.8 | 0.47 ±0.16 | 90.0± 16.4 | 43.9 ±16.6 | 0% | 1/31 |
|
| ||||||||
| Amitraz (Triatox®) | ||||||||
| Tarso | 38 | 206.6 ± 58.0 | 41.8 ± 47.7*** | 0.21 ±0.23*** | 53.9 ±46.6** | 16.25 ±22.8*** | 56.3% | 17/38 |
| San Jeronimo | 31 | 177.0 ± 50.6 | 74.4 ± 42.3 | 0.41 ± 0.19 | 77.9 ± 37.1 | 36.1 ± 23.6 | 14.2% | 7/31 |
|
| ||||||||
| Cypermethrin + Amitraz (Ganabano® + Triatox®) | ||||||||
| Tarso | 38 | 205.1 ± 54.0 | 55.7 ± 43.5* | 0.26 ± 0.19* | 60.0 ± 40.1* | 18.6 ± 19.8*** | 50.0% | 13/38 |
| San Jeronimo | 30 | 183.5 ± 63.6 | 74.7 ± 44.4 | 0.41 ± 0.24 | 76.3 ± 36.9 | 36.9 ± 26.9 | 12.3% | 6/30 |
|
| ||||||||
| Amitraz + citronella (Citraz®) | ||||||||
| Tarso | 38 | 207.1 ± 53.3 | 52.5 ±46.3* | 0.25 ±0.21* | 65.1 ±43.4 | 20.9 ±21.8** | 43.8% | 17/38 |
| San Jeronimo | – | – | – | – | – | – | – | – |
|
| ||||||||
| Cypermethrin + chlorpyrifos (Impacto®) | ||||||||
| Tarso | 38 | 204.4 ± 50.9 | 52.8 ± 42.9* | 0.24 ± 0.17** | 54.9 ± 40.0** | 14.7 ± 18.1*** | 60.4% | 12/38 |
| San Jeronimo | – | – | – | – | – | – | – | – |
|
| ||||||||
| Trichlorfon (Neguvon®) | ||||||||
| Tarso | 38 | 209.7 ± 43.1 | 52.0 ± 36.1* | 0.25 ± 0.18* | 48.9 ± 38.4*** | 14.6 ± 18.25*** | 60.7% | 12/38 |
| San Jeronimo | – | – | – | – | – | – | – | – |
Notes: Efficacy is expressed as percentage (%) of IR compared to the control group.
Efficacy:% control = [(Σ IR control − Σ IR treated)/Σ IR control] × 100.
Female ticks that did not oviposit by day 7 or produced only nonviable eggs were considered dead. Significance:
P < 0.05;
P < 0.01;
P < 0.001 compared to control. “–” indicates data was not recorded for this comparison.
Discussion
The objective of this study was to investigate producer concerns at two locations in Antioquia, Colombia, where a long-acting systemic acaricide containing ivermectin and several topical acaricides of other chemical classes were no longer able to eliminate R. microplus infestations from cattle. This perception was confirmed in this study through the administration of Ivomec GOLD® to R. microplus–infested cattle on two separate occasions 3 months apart. The observed reduction in standard females 10 days postinjection was approximately 45% and 75% at San Jeronimo and Tarso farms, respectively. In a study conducted at the USDA Agricultural Research Service (ARS) Cattle Fever Tick Research Laboratory in Edinburg, Texas, USA, the therapeutic efficacy of Ivomec GOLD® was shown to be 99.9% against all stages of ivermectin-susceptible R. microplus at the time of treatment.15 Furthermore, the protective period against larval reinfestation was shown to be 14 days, if a level of control at ≥99% was desired, and dropped to 70.4% 28 days posttreatment. Considering that R. microplus require at least 18 days on a host to complete development, Davey et al.15 concluded that cattle could be treated with Ivomec GOLD® at intervals of 31 days, without risk of having viable ticks detach from infested animals. In the present field study, fecund ticks were present at 10 days posttreatment, and in some animals, body counts were even higher than before the administration. However, without a negative control group, it was impossible to calculate the degree of efficacy and dynamic fluctuations in tick populations under natural conditions could have played a role in lowering total body counts. In any case, these results are most worrisome when compared to those of a field study conducted in the Mexican tropics that included a negative control group.16 In their study, the therapeutic and persistent efficacy of Ivomec GOLD® was 95% at 56 days post-treatment and consistent with the prolonged effect claimed on the pro duct label of approximately 75 days.
Ivermectin treatment significantly reduced female engorgement weights and egg production. In fact, the engorgement weights were approximately 60% of those recorded before treatment at both farms. This result was similar to that reported by Lopes et al.17 using experimentally controlled conditions. Therefore, even though ticks are surviving ivermectin treatment that is expected to kill 100%, there remains a distinct deleterious effect not overcome by resistance mechanism(s). The initial mean engorgement weights observed were very different between Tarso (133 mg) and San Jeronimo (200 mg). This difference could be attributed to previous treatments of the unregistered Ivercyt product containing 10.5% ivermectin at the Tarso farm (19 days before the onset of the study) and topically applied engine oil at the San Jeronimo farm (used on an as-needed basis). The application of motor oil has been reported to be the most common method used for tick control by resource-poor farmers in South Africa.18 A study that compared total tick burdens of cattle treated with engine oil with an untreated control group showed it to be 15%–65% efficacious and an inexpensive method of tick control.19 However, the effect was shown to be transient and almost certainly required direct contact between the oil and the tick to achieve good results.
The results of the AIT showed that the maximum efficiency that could be achieved with any product or combination was only intermediate (50%–60%) at reducing the reproductive capacity of exposed engorged females. Similar studies in Brazil reported that farmers normally start reporting acaricide resistance when the in vitro efficacy, as determined by the AIT, has fallen by 50% or more.13 In another similar study conducted in dairy farms in Venezuela, it was found that increasing the treatment concentrations above the manufacturer’s recommendations for cypermethrin or amitraz did not increase efficacy of these acaricides when treatment at the labeled rate produced 17% and 50% control, respectively.20 Therefore, although the practice is common, producers should realize that increasing treatment concentrations of products will not necessarily get better results. In the present study, cypermethrin (150 ppm) was completely devoid of efficacy, and when combined in the mixture with half the usual concentration of amitraz (100 ppm), the efficacy of the combination was lower than that of amitraz at its recommended 208 ppm concentration. This is an interesting finding as the combination of cypermethrin and amitraz has been shown to have greater efficacy against R. microplus resistant to these compounds.21 Furthermore, their study showed that when piperonyl butoxide was incorporated in the cypermethrin–amitraz mixture, a synergistic effect was attained reaching a field control efficacy against ticks in the Mexican tropics of 95% that persisted for 28 days. Acaricide resistance is so common in Brazil that most newly marketed products now contain a combination of active compounds. Often up to three active ingredients of different chemical families are used to control acaricide-resistant ticks in Brazil.11,12 More research into the potential benefits of mixtures on the control of R. microplus in Antioquia, Colombia, is needed.
The generic amitraz product (Citraz®) used at recommended concentration by the manufacturer should have been as efficacious as the name-brand amitraz product (Triatox®) since they both contained concentrations of 208 ppm amitraz, plus 10 ppm exposure citronella on the generic. The results showed a much lower efficacy for this generic product despite having the citronella, and results with ticks from other farms (data not shown) suggest even worse performance than expected. Several Brazilian studies showed that dilutions of citronella oil with concentrations above 25,000 ppm are necessary to lower the reproductive parameters of ticks.22,23 Therefore, it is unlikely that a citronella concentration of 10 ppm would have any adverse impact. In this study, no chemical analysis was conducted on any of the topical products tested. Therefore, the possibility that the concentration of the active ingredients were inadequate cannot be ruled out. This type of analysis is essential for making comparisons on products carrying the same active ingredients especially when generic products are used.
A combination of factors at the farms sampled has driven the evolution of resistance to ivermectin and other acaricides. At Tarso, the unregistered product containing 10.5% ivermectin has been applied for years at a dosage of 2100 μg/kg body weight, creating selection pressure on the tick population. Interestingly, the Tarso herd contained 20 Zebu along with the main herd of Brangus animals. The Zebu breed is known to be highly resistant to R. microplus. Ideally, these Zebu animals should be spared from any treatments to preserve part of the susceptible population in refugia. Clearly, the use of registered and unregistered acaricides, together with a lack of proper guidance on best management practices in this farm, as well as many others in Colombia, are key factors that have contributed to the appearance of resistance in R. microplus. Regulatory authorities should implement programs to control the sale of veterinary drugs and discourage the use of unregistered products. In spite of the large number of registered generic ivermectin drugs available in Colombia, HPLC analysis of a random selection (Table 1) showed that ivermectin concentrations met label claims. Although this does not necessarily imply bioequivalence of the available formulations to the patented Ivomec®, it is clear that failure to control tick infestation at these Colombian farms can be attributed to genuine ivermectin resistance and not to a poor quality of the drugs available. Government should prioritize the establishment of diagnostic laboratories with adequate staff and finances to provide science-based knowledge to assist producers to make informed rational decisions regarding the management of R. microplus. Finally, residues of chemical acaricides in animal products are a concern that is yet to be considered by regulatory authorities in Colombia. At present, the only tests performed are for the detection of antimicrobial residues in milk, which is left to the discretion of the dairy companies.24
In conclusion, the present study has confirmed popular claims that ivermectin is no longer capable of eliminating tick infestation from some Antioquian cattle farms. Integrated approaches to parasite control are needed as multiresistant strains of R. microplus are now present in the region. These programs may include changes in current farming practices and could include the use of resistant cattle breeds, pasture vacation periods, adjustments to the plant composition of pastures, and introducing biodiversity back into these tropical, but mostly monoculture paddocks.
Acknowledgments
The authors would like to thank Dr Steve Ensley and Dwayne Schrunk for performing the ivermectin concentration analysis of selected commercial products at Iowa State University. Additionally, the authors appreciate the review undertaken by Dr Don Thomas.
Footnotes
ACADEMIC EDITOR: Timothy Kelley, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Disclaimer
In conducting the research described in this report, the investigators adhered to the “Guide for the Care and Use of Laboratory Animals,” as promulgated by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council. The facilities are fully accredited by the American Association of Laboratory Animal Care. This article reports the results of research only. Mention of a proprietary product does not constitute an endorsement or a recommendation by the USDA for its use.
Author Contributions
Conceived and designed the experiments: All authors. Analyzed the data: AL, DV, JC, RM. Wrote the first draft of the manuscript: AL, DV, JC. Contributed to the writing of the manuscript: All authors. Agree with manuscript results and conclusions: All authors. Jointly developed the structure and arguments for the paper: All authors. Made critical revisions and approved final version: All authors. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.FAO . Resistencia a los antiparasitarios. Estado actual con énfasis en America Latina. Dirección de Producción y Sanidad Animal de la FAO; Roma: 2003. p. 157. [Google Scholar]
- 2.Holdsworth PA, Kemp D, Green P, et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) guidelines for evaluating the efficacy of acaricides against ticks (Ixodidae) on ruminants. Vet Parasitol. 2006;136:29–43. doi: 10.1016/j.vetpar.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Klafke GM, Castro-Janer E, Mendes MC, Namindome A, Schumaker TTS. Applicability of in vitro bioassays for the diagnosis of ivermectin resistance in Rhipicephalus microplus (Acari: Ixodidae) Vet Parasitol. 2012;184:121–220. doi: 10.1016/j.vetpar.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Sabatini GA, Kemp DH, Hughes S, Nari A, Hansen J. Tests to determine LC50 and discriminating doses for macrocyclic lactones against the cattle tick Boophilus microplus. Vet Parasitol. 2001;95(1):53–62. doi: 10.1016/s0304-4017(00)00406-4. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, Rosado-Aguilar JA. Survey of Rhipicephalus microplus resistance to ivermectin at cattle farms with history of macrocyclic lactones use in Yucatan, Mexico. Vet Parasitol. 2010;172:109–13. doi: 10.1016/j.vetpar.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Cogollo LC, Rodriguez-Vivas RI, Ramirez-Cruz GT, Miller RJ. First report of the cattle tick Rhipicephalus microplus resistant to ivermectin in Mexico. Vet Parasitol. 2010;168:165–9. doi: 10.1016/j.vetpar.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Castro-Janer E, Rifran L, Gonzalez P, et al. Determination of the susceptibility of Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) to ivermectin and fipronil by larval immersion test (LIT) in Uruguay. Vet Parasitol. 2011;178:148–55. doi: 10.1016/j.vetpar.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Klafke GM, Albuquerque TA, Miller RJ, Schumaker TTS. Selection of an ivermectin-resistant strain of Rhipicephalus microplus (Acari: Ixodidae) in Brazil. Vet Parasitol. 2010;168:97–104. doi: 10.1016/j.vetpar.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Shurbaji M, Abu AI, Rub MH, et al. Development and validation of a new HPLC-UV method for the simultaneous determination of triclabendazole and ivermectin B1a in a pharmaceutical formulation. J AOAC Int. 2010;93:1868–73. [PubMed] [Google Scholar]
- 10.Wharton RH, Utech KBW. The relationship between engorgement and dropping of Boophilus microplus (Ixodidae) to assessment of tick numbers on cattle. Aust J Entomol. 1970;21:163–81. [Google Scholar]
- 11.Raynal JT, Silva AA, Sousa T, et al. Acaricides efficiency on Rhipicephalus (Boophilus) microplus from Bahia state North-Central región. Rev Bras Parasitol Vet. 2013;22(1):71–7. doi: 10.1590/s1984-29612013005000006. [DOI] [PubMed] [Google Scholar]
- 12.Andreotti R, Guerrero FD, Soares MA, Barros JC, Miller RJ, León AP. Acaricide resistance of Rhipicephalus (Boophilus) microplus in State of Mato Grosso do Sul, Brazil. Rev Bras Parasitol Vet. 2011;20:127–33. doi: 10.1590/s1984-29612011000200007. [DOI] [PubMed] [Google Scholar]
- 13.Veiga LP, Souza AP, Bellato V, Sartor AA, Nunes AP, Cardoso HM. Resistance to cypermethrin and amitraz in Rhipicephalus (Boophilus) microplus on the Santa Catarina Plateau, Brazil. Rev Bras Parasitol Vet. 2012;21(2):133–6. doi: 10.1590/s1984-29612012000200011. [DOI] [PubMed] [Google Scholar]
- 14.Drummond RO, Crust SF, Trevino JL, Gladney WJ, Graham OH. Boophilus annulatus and B. microplus: laboratory tests of insecticides. J Eco Entomol. 1973;66:130–3. doi: 10.1093/jee/66.1.130. [DOI] [PubMed] [Google Scholar]
- 15.Davey RB, Pound JM, Miller JA, Klavons JA. Therapeutic and persistent efficacy of a long-acting (LA) formulation of ivermectin against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) and sera concentration through time in treated cattle. Vet Parasitol. 2010;169:149–56. doi: 10.1016/j.vetpar.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 16.Arieta-Román RJ, Rodriguez-Vivas RI, Rosado-Aguilar JA, Ramirez-Cruz GT, Basto-Estrella G. Persistent efficacy of two macrocyclic lactones against natural Rhipiceplalus (Boophilus) microplus infestations in cattle in the Mexican tropics. Rev Mex Cienc Pecu. 2010;1:59–67. [Google Scholar]
- 17.Lopes WD, Teixeira WF, de Matos LV, et al. Effects of macrocyclic lactones on the reproductive parameters of engorged Rhipicephalus (Boophilus) microplus females detached from experimentally infested cattle. Exp Parasitol. 2013;135:72–8. doi: 10.1016/j.exppara.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Hlatshwayo M, Mbati PA. A survey of tick control methods used by resource-poor farmers in the Qwa-Qwa of eastern Free State Province, South Africa. Onderstepoort J Vet. 2005;72:245–9. doi: 10.4102/ojvr.v72i3.202. [DOI] [PubMed] [Google Scholar]
- 19.Dreyer K, Fourie LJ, Kok DJ. The efficacy of used engine oil against ticks on cattle. Onderstepoort J Vet. 1998;65:275–9. [PubMed] [Google Scholar]
- 20.Bravo M, Henriquez H, Coronado A. In vitro efficacy of amitraz and cypermethrin on Boophilus microplus from dairy farms in Lara State, Venezuela. Ann N Y Acad Sci. 2008;1149:246–8. doi: 10.1196/annals.1428.032. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Vivas RI, Li AY, Ojeda-Chi MM, Trinidad-Martinez I, Rosado-Aguilar JA, León AP. In vitro and in vivo evaluation of cypermethrin, ami-traz, and piperonyl butoxide mixtures for the control of resistant Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) in the Mexican tropics. Vet Parasitol. 2013;197(1–2):288–96. doi: 10.1016/j.vetpar.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 22.Carlini F, Silviera F, Buttow VF, Monteiro SG. In vitro effect of the association of citronella, Santa Maria Herb (Chenopodium ambrosioides) and Quassia tincture on cattle tick Rhipicephalus microplus. Ci Anim Bras Goiânia. 2013;14(1):113–9. [Google Scholar]
- 23.Martins RM. In vitro study of the acaricidal activity of the essential oil from Citronella of Java (Cymbopogon winterianus Jowitt) to the tick Boophilus microplus. Rev Bras Plant Med Motucatu. 2006;8(2):71–78. [Google Scholar]
- 24.Villar D, Olivera M, Ruiz JD, Chaparro J. Editorial Biogénesis. Medellin, Colombia: 2012. Aproximación al tema de residuos antimicrobianos y antiparasitarios en leche. Available from: http://editorialbio-genesis.udea.edu.co/index.php/biogenesis/article/view/153. [Google Scholar]


