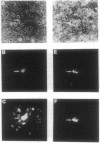
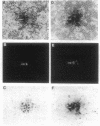
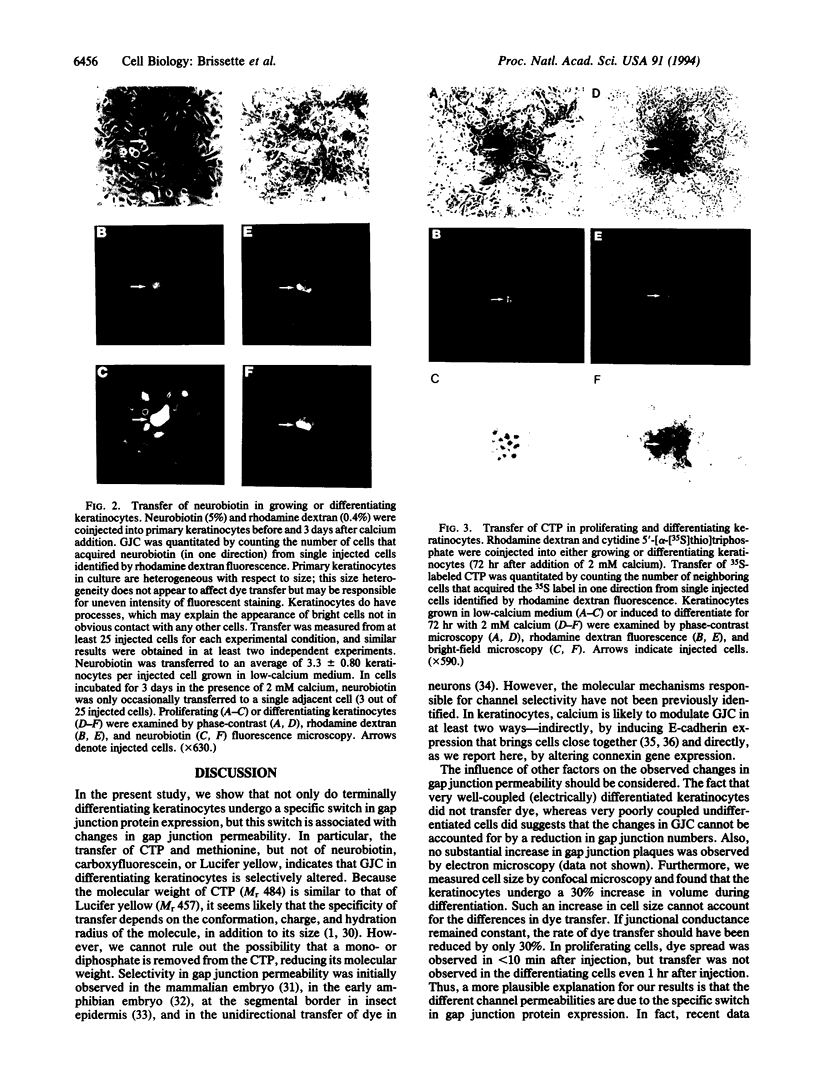
Abstract
Gap junctional communication provides a mechanism for regulating multicellular activities by allowing the exchange of small diffusible molecules between neighboring cells. The diversity of gap junction proteins may exist to form channels that have different permeability properties. We report here that induction of terminal differentiation in mouse primary keratinocytes by calcium results in a specific switch in gap junction protein expression. Expression of alpha 1 (connexin 43) and beta 2 (connexin 26) gap junction proteins is down-modulated, whereas that of beta 3 (connexin 31) and beta 4 (connexin 31.1) proteins is induced. Although both proliferating and differentiating keratinocytes are electrically coupled, there are significant changes in the permeability properties of the junctions to small molecules. In parallel with the changes in gap junction protein expression during differentiation, the intercellular transfer of the small dyes neurobiotin, carboxyfluorescein, and Lucifer yellow is significantly reduced, whereas that of small metabolites, such as nucleotides and amino acids, proceeds unimpeded. Thus, a switch in gap junction protein expression in differentiating keratinocytes is accompanied by selective changes in junctional permeability that may play an important role in the coordinate control of the differentiation process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brink P. R., Dewey M. M. Evidence for fixed charge in the nexus. Nature. 1980 May 8;285(5760):101–102. doi: 10.1038/285101a0. [DOI] [PubMed] [Google Scholar]
- Brissette J. L., Kumar N. M., Gilula N. B., Dotto G. P. The tumor promoter 12-O-tetradecanoylphorbol-13-acetate and the ras oncogene modulate expression and phosphorylation of gap junction proteins. Mol Cell Biol. 1991 Oct;11(10):5364–5371. doi: 10.1128/mcb.11.10.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cox R. P., Krauss M. R., Balis M. E., Dancis J. Evidence for transfer of enzyme product as the basis of metabolic cooperation between tissue culture fibroblasts of Lesch-Nyhan disease and normal cells. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1573–1579. doi: 10.1073/pnas.67.3.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. P., Krauss M. R., Balis M. E., Dancis J. Studies on cell communication with enucleated human fibroblasts. J Cell Biol. 1976 Dec;71(3):693–703. doi: 10.1083/jcb.71.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto G. P., el-Fouly M. H., Nelson C., Trosko J. E. Similar and synergistic inhibition of gap-junctional communication by ras transformation and tumor promoter treatment of mouse primary keratinocytes. Oncogene. 1989 May;4(5):637–641. [PubMed] [Google Scholar]
- Guthrie S., Turin L., Warner A. Patterns of junctional communication during development of the early amphibian embryo. Development. 1988 Aug;103(4):769–783. doi: 10.1242/dev.103.4.769. [DOI] [PubMed] [Google Scholar]
- Haefliger J. A., Bruzzone R., Jenkins N. A., Gilbert D. J., Copeland N. G., Paul D. L. Four novel members of the connexin family of gap junction proteins. Molecular cloning, expression, and chromosome mapping. J Biol Chem. 1992 Jan 25;267(3):2057–2064. [PubMed] [Google Scholar]
- Hennemann H., Dahl E., White J. B., Schwarz H. J., Lalley P. A., Chang S., Nicholson B. J., Willecke K. Two gap junction genes, connexin 31.1 and 30.3, are closely linked on mouse chromosome 4 and preferentially expressed in skin. J Biol Chem. 1992 Aug 25;267(24):17225–17233. [PubMed] [Google Scholar]
- Hennemann H., Schwarz H. J., Willecke K. Characterization of gap junction genes expressed in F9 embryonic carcinoma cells: molecular cloning of mouse connexin31 and -45 cDNAs. Eur J Cell Biol. 1992 Feb;57(1):51–58. [PubMed] [Google Scholar]
- Hennings H., Holbrook K. A. Calcium regulation of cell-cell contact and differentiation of epidermal cells in culture. An ultrastructural study. Exp Cell Res. 1983 Jan;143(1):127–142. doi: 10.1016/0014-4827(83)90115-5. [DOI] [PubMed] [Google Scholar]
- Hennings H., Michael D., Cheng C., Steinert P., Holbrook K., Yuspa S. H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980 Jan;19(1):245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Hoh J. H., John S. A., Revel J. P. Molecular cloning and characterization of a new member of the gap junction gene family, connexin-31. J Biol Chem. 1991 Apr 5;266(10):6524–6531. [PubMed] [Google Scholar]
- Jongen W. M., Fitzgerald D. J., Asamoto M., Piccoli C., Slaga T. J., Gros D., Takeichi M., Yamasaki H. Regulation of connexin 43-mediated gap junctional intercellular communication by Ca2+ in mouse epidermal cells is controlled by E-cadherin. J Cell Biol. 1991 Aug;114(3):545–555. doi: 10.1083/jcb.114.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam E., Melville L., Pitts J. D. Patterns of junctional communication in skin. J Invest Dermatol. 1986 Dec;87(6):748–753. doi: 10.1111/1523-1747.ep12456937. [DOI] [PubMed] [Google Scholar]
- Kam E., Watt F. M., Pitts J. D. Patterns of junctional communication in skin: studies on cultured keratinocytes. Exp Cell Res. 1987 Dec;173(2):431–438. doi: 10.1016/0014-4827(87)90283-7. [DOI] [PubMed] [Google Scholar]
- Kamibayashi Y., Oyamada M., Oyamada Y., Mori M. Expression of gap junction proteins connexin 26 and 43 is modulated during differentiation of keratinocytes in newborn mouse epidermis. J Invest Dermatol. 1993 Dec;101(6):773–778. doi: 10.1111/1523-1747.ep12371693. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kumar N. M., Gilula N. B. Molecular biology and genetics of gap junction channels. Semin Cell Biol. 1992 Feb;3(1):3–16. doi: 10.1016/s1043-4682(10)80003-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lo C. W., Gilula N. B. Gap junctional communication in the post-implantation mouse embryo. Cell. 1979 Oct;18(2):411–422. doi: 10.1016/0092-8674(79)90060-6. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Mege R. M., Matsuzaki F., Gallin W. J., Goldberg J. I., Cunningham B. A., Edelman G. M. Construction of epithelioid sheets by transfection of mouse sarcoma cells with cDNAs for chicken cell adhesion molecules. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7274–7278. doi: 10.1073/pnas.85.19.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Zampighi G. A., Hall J. E. Single-membrane and cell-to-cell permeability properties of dissociated embryonic chick lens cells. J Membr Biol. 1992 Jun;128(2):91–102. doi: 10.1007/BF00231882. [DOI] [PubMed] [Google Scholar]
- Missero C., Serra C., Stenn K., Dotto G. P. Skin-specific expression of a truncated E1a oncoprotein binding to p105-Rb leads to abnormal hair follicle maturation without increased epidermal proliferation. J Cell Biol. 1993 Jun;121(5):1109–1120. doi: 10.1083/jcb.121.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado A., Yuste R., Katz L. C. Extensive dye coupling between rat neocortical neurons during the period of circuit formation. Neuron. 1993 Jan;10(1):103–114. doi: 10.1016/0896-6273(93)90246-n. [DOI] [PubMed] [Google Scholar]
- Risek B., Klier F. G., Gilula N. B. Multiple gap junction genes are utilized during rat skin and hair development. Development. 1992 Nov;116(3):639–651. doi: 10.1242/dev.116.3.639. [DOI] [PubMed] [Google Scholar]
- Robinson S. R., Hampson E. C., Munro M. N., Vaney D. I. Unidirectional coupling of gap junctions between neuroglia. Science. 1993 Nov 12;262(5136):1072–1074. doi: 10.1126/science.8093125. [DOI] [PubMed] [Google Scholar]
- Salomon D., Saurat J. H., Meda P. Cell-to-cell communication within intact human skin. J Clin Invest. 1988 Jul;82(1):248–254. doi: 10.1172/JCI113578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf S. J., Horn G. T., Erlich H. A. Direct cloning and sequence analysis of enzymatically amplified genomic sequences. Science. 1986 Sep 5;233(4768):1076–1078. doi: 10.1126/science.3461561. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D., Wang H. Z., Westphale E. M., Beyer E. C. Multiple connexins confer distinct regulatory and conductance properties of gap junctions in developing heart. Circ Res. 1992 Nov;71(5):1277–1283. doi: 10.1161/01.res.71.5.1277. [DOI] [PubMed] [Google Scholar]
- Warner A. E., Lawrence P. A. Permeability of gap junctions at the segmental border in insect epidermis. Cell. 1982 Feb;28(2):243–252. doi: 10.1016/0092-8674(82)90342-7. [DOI] [PubMed] [Google Scholar]
- Willecke K., Heynkes R., Dahl E., Stutenkemper R., Hennemann H., Jungbluth S., Suchyna T., Nicholson B. J. Mouse connexin37: cloning and functional expression of a gap junction gene highly expressed in lung. J Cell Biol. 1991 Sep;114(5):1049–1057. doi: 10.1083/jcb.114.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]