Abstract
Rising atmospheric CO2 concentration (Ca) is expected to accelerate tree growth by enhancing photosynthesis and increasing intrinsic water-use efficiency (iWUE). However, the extent of this effect on long-term iWUE and its interactions with climate remains unclear in trees along an elevation gradient. Therefore, we investigated the variation in the radial growth and iWUE of mature Picea schrenkiana trees located in the upper tree-line (A1: 2700 m a.s.l.), middle elevation (A2: 2400 m a.s.l.), and lower forest limit (A3: 2200 m a.s.l.), in relation to the rising Ca and changing climate in the Wusun Mountains of northwestern China, based on the basal area increment (BAI) and tree-ring δ13C chronologies from 1960 to 2010. We used the CRU TS3.22 dataset to analyze the general response of tree growth to interannual variability of regional climate, and found that BAI and δ13C are less sensitive to climate at A1 than at A2 and A3. The temporal trends of iWUE were calculated under three theoretical scenarios, as a baseline for interpreting the observed gas exchange at increasing Ca. We found that iWUE increased by 12–32% from A1 to A3 over the last 50 years, and showed an elevation-dependent variation in physiological response. The significant negative relationship between BAI and iWUE at A2 and A3 showed that tree growth has been decreasing despite long-term increases in iWUE. However, BAI remained largely stable throughout the study period despite the strongest iWUE increase [at constant intercellular CO2 concentration (Ci) before 1980] at A1. Our results indicate a drought-induced limitation of tree growth response to rising CO2 at lower elevations, and no apparent change in tree growth and diminished iWUE improvement since 1980 in the upper tree-line. This study may contradict the expectation that combined effects of elevated Ca and rising temperatures have increased forest productivity, especially in high-elevation forests.
Keywords: tree rings, basal area increment, carbon isotope, SPEI, elevation gradient, physiological response, Northwestern China
Introduction
Atmospheric changes, and particularly the recent human-induced increases in carbon dioxide (CO2) concentrations and temperature, significantly affect tree growth (Martinez-Vilalta et al., 2008; Allen et al., 2010; Koutavas, 2013). Sudden, rapid changes in the atmospheric CO2 concentration (Ca) can dramatically affect short-term plant physiology and growth (Martinez-Vilalta et al., 2008), and research has shown that plants increase their intrinsic water-use efficiency (iWUE) in response to increasing CO2 (Morison, 1993; Morgan et al., 2004). Compare to these short-term experimental results, the long-term variations on the physiological responses to increased Ca, obtained from the carbon isotope discrimination (Δ13C) of tree-ring series, give insight into how trees respond to increasing atmospheric CO2 concentrations under naturally growing conditions (Peñuelas et al., 2008, 2011). Some studies found that trees may vary in their response to increasing Ca as a result of interactions with other changing environmental factors, and especially with warmer conditions (Switsur and Waterhouse, 1998; and references therein; Fischlin et al., 2007). Recently, many studies focusing on changes in tree growth in response to rising atmospheric CO2 concentrations and climate warming have been carried out in natural stands (Peñuelas et al., 2008, 2011; Linares et al., 2009; Andreu-Hayles et al., 2011; Linares and Camarero, 2012a; Granda et al., 2014; Lévesque et al., 2014; Liu et al., 2014). Whether the increased iWUE in response to rising Ca enhanced tree growth varied among sites and species. The tree growth may potentially increase in response to rising Ca in moist temperate forests (Cole et al., 2010; McMahon et al., 2010), but often decreased in tropical, dry temperate forests and at the lower-elevation forest limit (Peñuelas et al., 2008; Nock et al., 2011; Lévesque et al., 2014). However, in the Mediterranean, Juniperus thurifera showed enhanced growth at increased iWUE despite unfavorable growing climate conditions (Granda et al., 2014), whereas riparian Populus euphratica showed a significant CO2-induced growth stimulation despite the negative influences of reductions in streamflow (Liu et al., 2014). However, there have been insufficient studies on the simultaneous elevation-dependent effects on tree growth in response to increasing Ca and climate warming.
The growth rate of plant species at northern latitudes and high elevations, near their range limit, correlates well with the mean or extreme temperatures (Körner, 2012; Lenz et al., 2014). This is an important effect, since most of the world has experienced an unequivocal warming trend in the past half century, and further warming is likely (IPCC, 2013). Therefore, an elevation-dependent growth response of trees to climate is expected. At high altitudes, global warming has potentially increased radial growth (Körner, 2012), as in the case of studies in the European Alps (Hartl-Meier et al., 2014b) and in North America (Salzer et al., 2009), but growth suppression is expected to occur at lower elevations due to the predicted increase in the frequency and intensity of drought (Weltzin et al., 2003; Peñuelas et al., 2008). The synergistic effect of elevated CO2 and temperature is expected to stimulate forest productivity in temperature-limited environments (Salzer et al., 2009), but may decrease growth in water-limited environments (Linares and Camarero, 2012b). Therefore, it is necessary to find ways to detect the growth variation that occurs along an elevation gradient in response to increased Ca and warmer temperatures, since the dominant climatic stressor for trees may change with increasing elevation (Hartl-Meier et al., 2014a,b). Measurement of various tree-ring parameters allows an assessment of potential changes in tree physiology and growth over time. The tree-ring stable carbon isotope (δ13C) record provides evidence of climate effects on stomatal conductance and of tree physiological responses to increased Ca over time, whereas the basal area increment (BAI) provides a reliable indicator of tree radial growth (Linares and Camarero, 2012b).
It has been found that warming-induced prolonged drought stress significantly contributed to the marked reduction of regional BAI in recent years at mid-altitude in Tianshan Mountains (Wu et al., 2013). However, an altitudinal survey of radial growth variation and the tree physiological responses to increased Ca from lower forest limit to upper tree-line are still unclear. In the present study, we therefore combined tree-ring δ13C and BAI to examine tree responses to rising Ca and climate warming along an elevational gradient in the Wusun Mountains of northwestern China. Our first goal was to determine whether the δ13C and BAI varied between sites on the three different elevations. Second goal was to identify the climate factors influencing the δ13C and BAI and to detect the pattern of increased iWUE in response to rising Ca. Finally, we also want to detect whether the increases in iWUE could enhance growth and whether the tree growth in response to the increase in Ca and climate warming varied along an elevation gradient. Specifically, we hypothesized that (1) lower radial growth and high iWUE may be found at lower forest limit compared to upper tree-line, and (2) elevation-dependent growth variation may be due to the divergence of dominant climatic stressor and iWUE increase pattern.
Materials and methods
Study area and climate
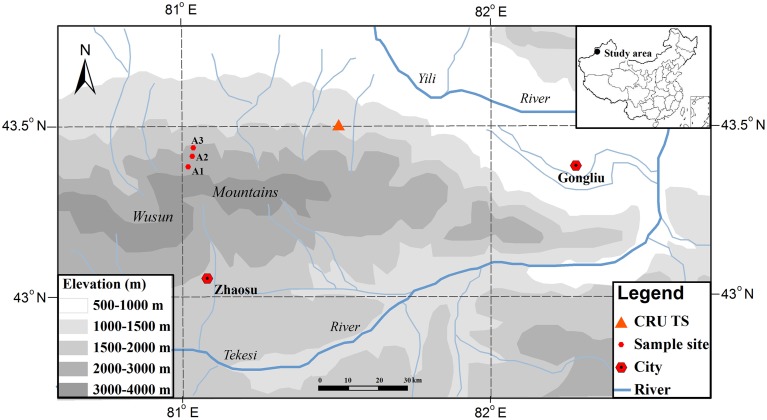
The study area is located on the northern slopes of the Wusun Mountains in the Yili valley, which is located in the western Tianshan Mountains of northwestern China (Figure 1). This area is an intermontane basin that is open toward the west and surrounded by mountains on all other sides. Under these topographic conditions, the westerly airflow directly enters the valley, bringing moisture that produces precipitation as the air rises along the mountain slope; in contrast, the topography blocks dry and warm air flowing northward from the Tarim Basin and southward from the Junggar Basin, and cold air flowing from Siberia. The Yili Valley is dominated by a temperate semiarid continental climate (Zhu, 1985).
Figure 1.
Locations of the study area and of the three sampling sites and the nearest CRU TS location in the Wusun Mountains of northwestern China.
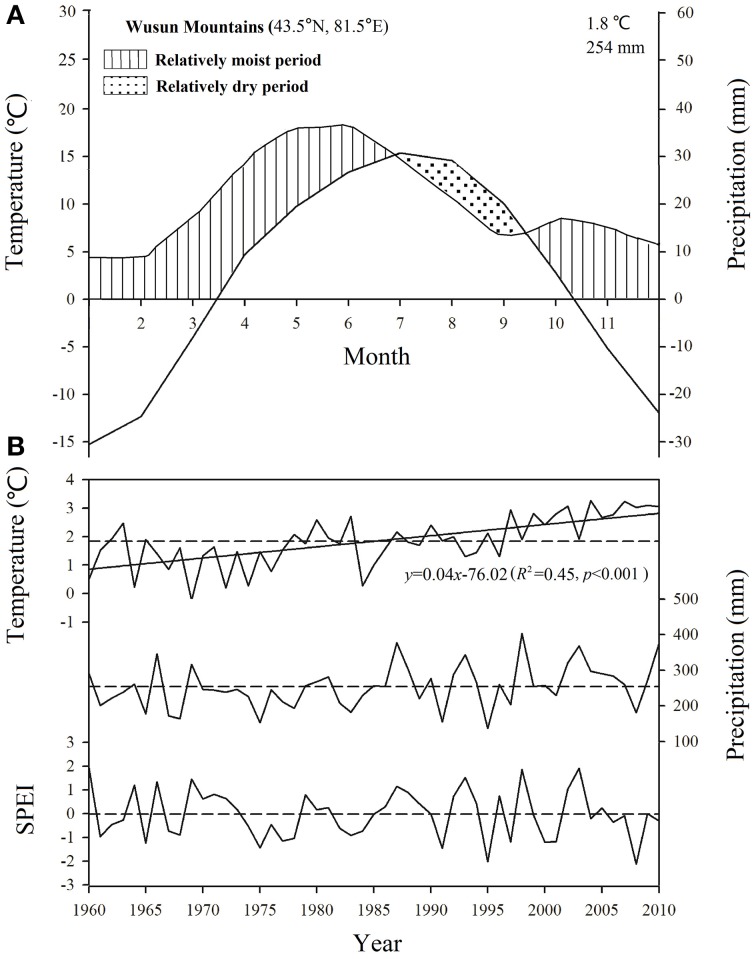
No site-specific climate data were available, therefore we used gridded climatic data from the CRU TS3.22 dataset, with a spatial resolution of 0.5 × 0.5° (Mitchell and Jones, 2005; http://www.cru.uea.ac.uk/). These data were derived from the nearest point in the CRU grid (43.5°N, 81.5°E). Hence, we only analyzed the general responses of tree growth to the inter-annual variability of regional climate in this study and our subsequent correlation analysis depended on changes in the values of each climate parameter (which should be similar along the altitudinal gradient) rather than on accurate values for each parameter. It is considered that even though the actual climate values (e.g., temperature, precipitation) will change along the altitudinal gradient, the patterns of change should be comparable along the altitudinal gradient (i.e., values should increase or decrease simultaneously at all altitudes) because they are mainly controlled by the broad-scale regional climate system. This view is supported by the results of two previous studies in the Tianshan Mountains (Guo et al., 2007; Wu et al., 2014). We used the monthly temperatures (mean, minimum, and maximum) and the monthly total precipitation from 1960 to 2010 in our analysis. The mean annual precipitation averaged 254 mm, with 62% of the precipitation falling from April to August, and the mean annual air temperature averaged 1.8°C, with the monthly mean ranging from -15.25°C in January to 15.4°C in July. The climate was dominated by a relatively dry period from July to September (Figure 2A).
Figure 2.
(A) Climate diagram based on data during 1960–2010 from the CRU TS3.22 dataset for the nearest point in the grid in the Wusun Mountains. (B) Trends in the mean annual temperature, total annual precipitation, and standardized precipitation evapotranspiration index (SPEI) in August based on a 5-month time scale for the study area from 1960 to 2010. Horizontal dashed lines represent the mean value for this period.
To quantify the intensity of the water stress, we calculated the standardized precipitation evapotranspiration index (SPEI) using the SPEI Calculator software (http://digital.csic.es/handle/10261/10002) based on the CRU TS3.22 precipitation and temperature data. This index combines the ability to characterize evaporative demand at multiple scales with the ability to account for temperature variability (Vicente-Serrano et al., 2010). We calculated the SPEI value with a timescale of 5 months based on our subsequent correlation analysis, which showed that the climatic values during the 5-month period from April to August were most significant (based on data from 1960 to 2010) and considered the value in August to represent the maximum intensity of water stress during the growing season. The mean temperature showed a significant long-term increasing trend since 1960, whereas precipitation and SPEI showed no significant long-term trend (Figure 2B).
Field sampling and stem radial growth at different elevations
Schrenk spruce (Picea schrenkiana) is one of the dominant species in the study area, and plays an important role in preventing soil erosion and soil water loss and in regulating climate, as well as in protecting the ecological stability of this inland river drainage area. Zhu et al. (2004) found that there were different relationships between the tree growth and climate at different aspects of slope and altitude of the valley. Schrenk spruce is an evergreen species that grows preferentially on shady (e.g., northwest-facing) slopes (Yuan and Li, 1995). It forms pure stands with a canopy cover ranging from 30 to 50%. The trees at our study sites grew on a northwest-facing slope (with an inclination of 30°) and the well-drained soil was a chestnut soil, which is equivalent to a Chernozems soil in the WRB soil taxonomy. We sampled this species near the upper (A1), middle (A2), and lower (A3) elevation limits of its distribution range (Figure 1; Table 1).
Table 1.
Site descriptions for the three sites used to obtain the three tree-ring chronologies.
| Site | |||
|---|---|---|---|
| A1-2700 m a.s.l. | A2-2400 m a.s.l. | A3-2200 m a.s.l. | |
| Dominant species | Schrenk spruce | Schrenk spruce | Schrenk spruce |
| Latitude | 43°25′23.4″N | 43°26′25.9″N | 43°26′16.9″N |
| Longitude | 81°02′36.7″E | 81°05′02.6″E | 81°05′32.7″E |
| Slope aspect | NW | NW | NW |
| Inclination (°) | 30 | 30 | 30 |
| Stand age (years) | 123 | 99 | 95 |
| Soil type | Chernozems | Chernozems | Chernozems |
| Soil depth (cm) | 40 | 80–100 | 80–100 |
| Sample size (no. of trees) | 20 | 22 | 24 |
The number of sampled dominant healthy trees for dendroecological analysis were 20, 22, and 24 from A1 to A3. We collected two cores per tree at breast height, with the cores obtained at right angles to each other, from even-aged mature trees growing at the upper tree line, at a middle-elevation site, and at the lower limit of the forest. After air-drying and polishing the cores, we measured the ring width using the LINTAB 6.0 software (Rinn, 2003; http://www.rinntech.de) to a precision of 0.01 mm, and cross-dated the rings using the COFECHA software (Holmes, 1983). To account for the decrease in ring width that occurs with increasing tree size, we converted the radial increment into a BAI using the following formula (Phipps and Whiton, 1988; Biondi and Qeadan, 2008):
| (1) |
where R is the tree radius and n is the year of tree-ring formation. The long-term variation of BAI was presented in the Supplementary Material (Supplementary Figure S1). In this study, we set the study period from 1960 to 2010 on account of the valid climate data acquisition and excluding the juvenile effects in the carbon analysis (McCarroll and Loader, 2004).
Tree-ring δ13C and calculation of iWUE at different elevations
We selected five cores from different trees at each elevation that had the strongest correlation with the elevation-specific tree-ring chronology, few missing rings, and regular ring boundaries. We pooled the annual rings from the five samples by mixing all tree rings from a given year to produce a single composite sample, which we used to conduct the stable carbon isotope study. We extracted the α-cellulose using a modified version of the method of Green (1963) and Loader et al. (1997, 2008). To obtain highly homogenized α-cellulose, we used an ultrasound machine (JY92-2D, Scientz Industry, Ningbo, China) to break the cellulose fibers based on the method of Laumer et al. (2009).
The δ13C values were determined using a Flash EA1112 Elemental Analyzer coupled with a Finnigan Delta Plus mass spectrometer (Thermo Electron Corporation, Bremen, Germany) at the Key Laboratory of Western China's Environmental Systems, Lanzhou University. By convention, the carbon isotope ratio (δ13C) is expressed in delta (δ) notation with reference to the Vienna Pee Dee Belemnite (VPDB) standard, in parts per thousand (‰):
| (2) |
where Rsample and Rstandard are the 13C/12C ratios in the sample and in the VPDB standard, respectively. During the experiment, laboratory reference standards were periodically used to calibrate the analytical results. The analytical error (standard deviation) of the isotope measurements was less than 0.07‰ for repeated samples.
The iWUE (μmol CO2 mol−1 H2O) compares the photosynthetic uptake of CO2 through the leaf stomata with the simultaneous transpirational loss of water through the stomata using the following formula (Farquhar et al., 1982):
| (3) |
where A is the rate of CO2 assimilation by the leaves, gs is the rate of leaf stomatal conductance, and Ci is the leaf intercellular CO2 concentration. To determine Ci/Ca, we used the equation proposed by Francey and Farquhar (1982):
| (4) |
where δ13Cplant and δ13Catm refer to the δ13C composition of α-cellulose and of atmospheric CO2, respectively. The parameter a (4.4‰) represents the discrimination that occurs during diffusion of CO2 from the atmosphere into the intercellular space of cells, and b (27‰) is the assumed discrimination that occurs during carboxylation.
However, tree-ring δ13C records are widely considered to show a prominent downward trend that can be attributed to the isotopically depleted CO2 in the atmosphere (δ13Catm) that has resulted from industrialization, and should be corrected for this effect by adding published annual values for δ13Catm derived from ice cores as well as direct measurements during the modern era (McCarroll and Loader, 2004). The atmospheric δ13Catm values from 2004 to 2010 were provided by Professor Danny McCarroll (Swansea University). Hereafter, we refer to this corrected data as the δ13Ccor data (δ13Ccor in Supplementary Figure S2). As a downward trend often remains even after correcting for changes in the atmospheric CO2 concentration as a result of changes in plant physiological responses to the increased Ca (Treydte et al., 2001; Gagen et al., 2007, 2011), we further corrected this data using the method proposed by McCarroll et al. (2009). Hereafter, we refer to this corrected data as the δ13Cpin data (δ13Cpin in Supplementary Figure S2). Due to autocorrelation in the δ13Cpin trend, we further standardized the δ13Cpinseries (we scaled the series to provide a mean of 0 and a variance of 1) and fitted an autoregressive model of order 1 to each series based on the method of Lévesque et al. (2013). The resulting δ13C residuals chronology was used in the following analysis (Figure 3A).
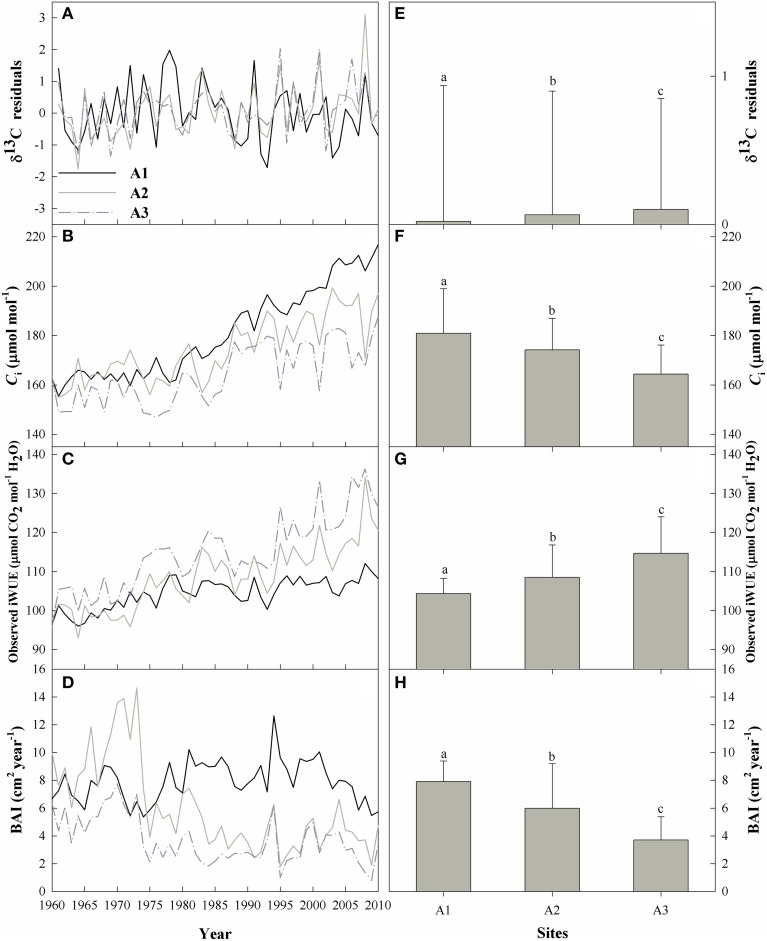
Figure 3.
Left: the temporal trends of (A) the δ13C residuals chronology, (B) the intercellular CO2 concentration (Ci), (C) the intrinsic water-use efficiency (iWUE), and (D) the basal area increment (BAI) for the three sites from 1960 to 2010. Right: the means and standard errors of (E) the δ13C residuals chronology, (F) Ci, (G) iWUE, and (H) BAI for the three sites during the period from 1960 to 2010. Bars for a parameter that are labeled with different letters differ significantly between sites (p < 0.05, ANOVA).
The tree-ring δ13C values reflect the plant's physiological response to climatic variables. Saurer et al. (2004) proposed three kinds of plant response to rising CO2: (1) Ca − Ci remains constant, Ci/Ca increases, and iWUE remains constant (a passive response); (2) Ci/Ca remains constant, but iWUE increases (an active response); and (3) Ci remains constant while iWUE increases strongly (strongest response). In this study, we used these three scenarios as a baseline for interpreting the observed the iWUE trends, based on the analysis described by Linares and Camarero (2012a) and Liu et al. (2014).
Data treatment and statistical analysis
We used One-Way ANOVA to detect significant differences in the mean value of tree growth data among the three elevations using version 16.0 of the SPSS software (SPSS Inc., Chicago, IL, USA). We also performed correlation analysis to examine the relationships between the tree-ring data and the mean monthly and seasonal climate data. We calculated Pearson's correlation coefficient (r) between the δ13C residuals chronology, BAI, and the climate variables using SPSS. The relationships between BAI and iWUE were assessed using linear regression analysis via SPSS.
Results
Temporal and elevational trends
Overall, the means of the δ13C residuals chronology, Ci, iWUE, and BAI differed significantly among the three elevations (Figure 3; Supplementary Table S1). The δ13C residuals chronology at the upper tree-line (A1) showed high δ13C enrichment before 1980 compared with the two lower-elevation sites (Figure 3A), but the chronology at the lower forest limit (A3) showed the highest δ13C for the study period as a whole (Supplementary Table S1). The δ13C enrichment was therefore significantly elevation-dependent (Figure 3E).
Mean Ci decreased with decreasing elevation (Figure 3F), with a mean value of 164.4 μmol mol−1 at site A3, vs. 180.9 and 174.2 μmol mol−1 at sites A1 and A2, respectively. During the study period, iWUE increased significantly with decreasing elevation (Figures 3C,G), and increased throughout the study period, with increases of 12.0, 23.0, and 32.0% at sites A1, A2, and A3, respectively (Figure 3C). iWUE remained relatively constant at site A1 after 1980, but increased continuously at sites A2 and A3 (Figure 3C).
BAI decreased during the past 50 years at sites A2 and A3, and the decrease was significant (R2 = 0.47 and 0.36, respectively; p < 0.0001), whereas BAI increased slightly but not significantly at A1 (Figure 3D). Ci and BAI were significantly lower and iWUE was significantly higher at the lower elevations (ANOVA, p < 0.01; Figures 3F–H).
Climate–growth relationships
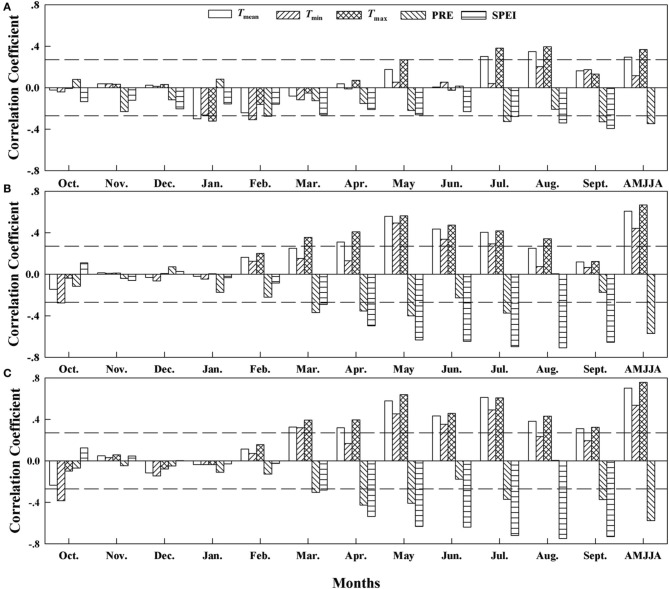
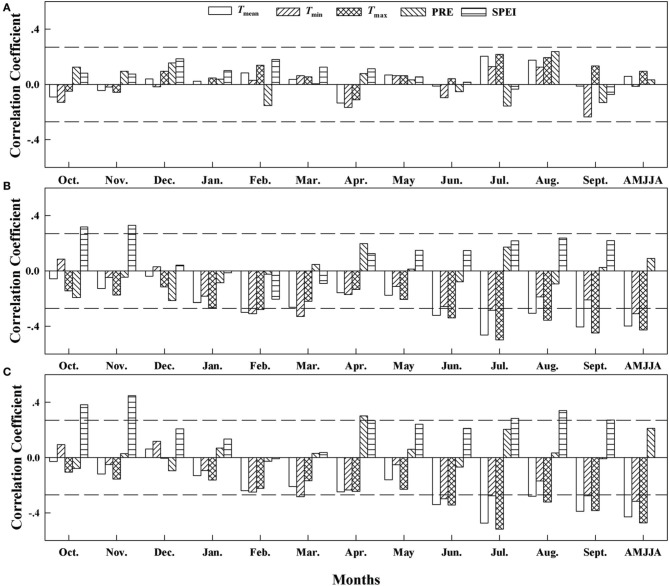
The correlation analysis based on the δ13C residuals chronologies (Figure 4) revealed significant correlations between the residuals and the mean temperature (Tmean; r = 0.29, p < 0.05), maximum temperature (Tmax, r = 0.37, p < 0.01), and precipitation (PRE; r = −0.34, p < 0.01) from April to August at site A1. At sites A2 and A3, the correlations with SPEI (at A2, r = −0.71, p < 0.001; at A3, r = −0.74, p < 0.001) in August were stronger than those with temperature during the months from April to August. The strength of the correlation and the dominant climate variables that controlled carbon isotope discrimination therefore differed among the elevations.
Figure 4.
Correlation coefficients (Pearson's r) between the δ13C residuals chronology and the climate variables from 1960 to 2010 at (A) Site A1 (2700 m a.s.l.), (B) Site A2 (2400 m a.s.l.), and (C) Site A3 (2200 m a.s.l.). Horizontal dashed lines represent the 95% confidence interval. Tmean, mean monthly temperature; Tmin, minimum monthly temperature; Tmax, maximum monthly temperature; PRE, total precipitation; SPEI, standardized precipitation evapotranspiration index. AMJJA represents the mean value from April to August.
The climate-response patterns of BAI were also elevation-dependent, and the climate signal recorded in the BAI series was much stronger (r values were much higher) at lower elevations (sites A2 and A3) than at site A1 (Figure 5). BAI was not significantly correlated with any climate variable at the highest-elevation site. Thus, the temperature parameters during the growing season had a stronger and significantly negative relationship with BAI at the two lower elevations, and the strength of this correlation increased with decreasing elevation. The correlation between the temperature series from April to August and BAI was strongest at 2200 m (site A3: for Tmean, r = −0.43, p < 0.01; for Tmin, r = −0.32, p < 0.01; and for Tmax, r = −0.47, p < 0.01). The correlations between BAI and precipitation were weak, with a significant correlation in April at site A3 (r = 0.30, p < 0.01), but we found an increasing correlation for SPEI with decreasing elevation; the correlation reached to r = 0.34 (p < 0.01) at site A3 in August with a timescale of 5.
Figure 5.
Correlation coefficients (Pearson's r) between the response of the basal area increment (BAI) to the climatic variables based on data from 1960 to 2010 at (A) Site A1 (2700 m a.s.l.), (B) Site A2 (2400 m a.s.l.), and (C) Site A3 (2200 m a.s.l.). Horizontal dashed lines represent the 95% confidence interval. Tmean, mean monthly temperature; Tmin, minimum monthly temperature; Tmax, maximum monthly temperature; PRE, total precipitation; SPEI, standardized precipitation evapotranspiration index
The climate-growth relationship revealed significant climate signals in the tree-ring δ13C series but not in the BAI series at the highest-elevation site, but the impacts of temperature and SPEI on tree growth increased with decreasing elevation in both the δ13C analysis and the BAI analysis.
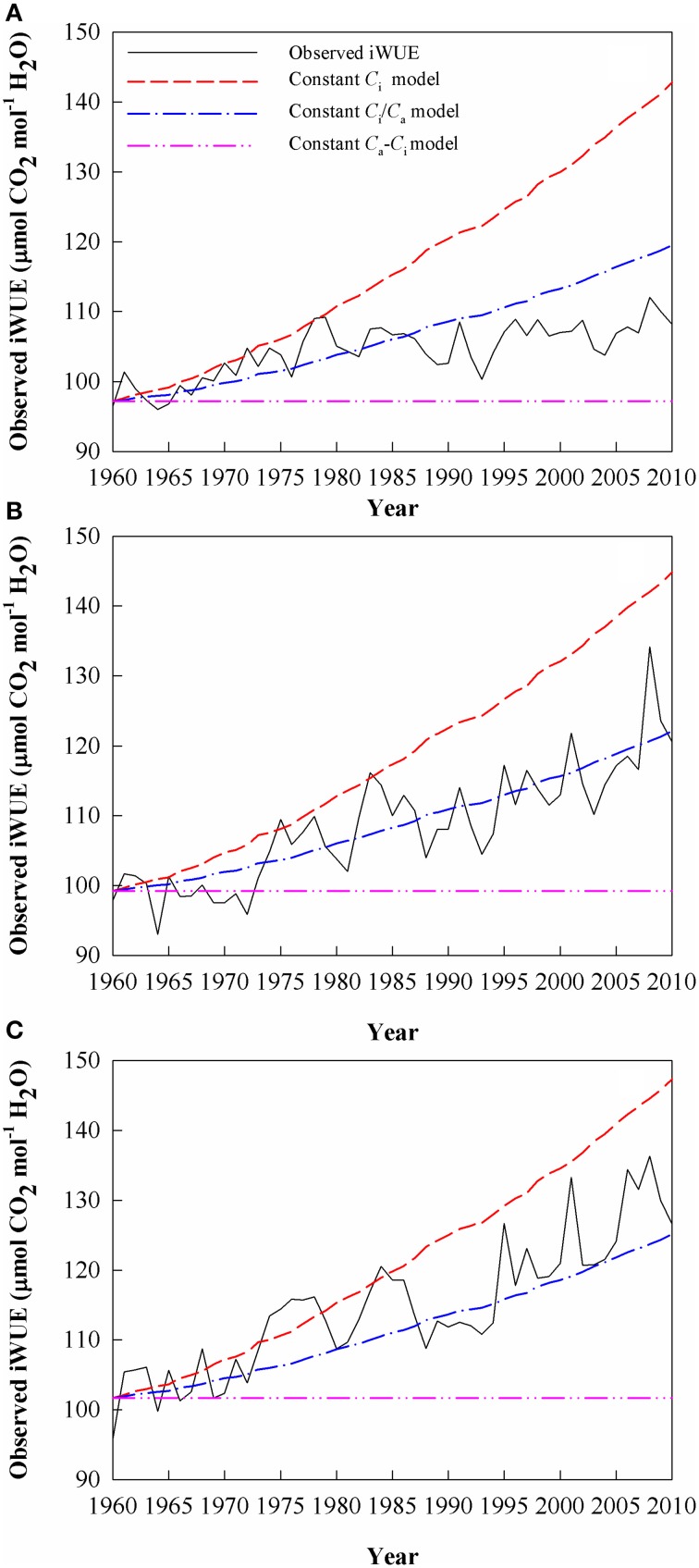
Physiological responses of trees to increasing Ca at different elevations
Since 1960, iWUE at the highest-elevation site increased by 12.0% (by 0.23 μmol CO2 mol−1 H2O year−1), vs. increases of 24 and 32% (0.44 and 0.60 μmol CO2 mol−1 H2O year−1), respectively, at the middle-elevation and low-elevation sites (Figure 6). The dominant scenario that explains the iWUE trends differed among the sites and between two subsets of the study period (Figure 6). At the highest-elevation site, iWUE was dominated by constant Ci until 1980 (which assumes that is the strongest response to Ca), with a 13.0% increase during this period, after which iWUE followed a pattern with constant Ca − Ci, with no overall increase or decrease for iWUE from 1980 to 2010 (Figure 6A). However, the iWUE values at the mid-elevation site and at the lower forest limit were mainly dominated by a constant Ca − Ci scenario during the 1960s. Then, the iWUE increase is fallen within the range of expected iWUE values represented by the constant Ci and the constant Ci/Ca scenarios at the mid-elevation and lower forest limit since 1973 (Figures 6B,C). After 1980, the iWUE trends at the mid-elevation site remained close to those in the constant Ci/Ca scenario, but the contemporaneous iWUE values at the lower forest limit were higher than those predicted under a constant Ci/Ca scenario.
Figure 6.
The observed and theoretical changes in intrinsic water-use efficiency (iWUE) calculated using Equation (3). The three scenarios for the theoretical regulation of leaf gas exchange in response to an increasing atmospheric CO2mole fraction (Ca) are: (i) a constant intercellular CO2 mole fraction (Ci); (ii) a constant Ci/Ca ratio, and (iii) a constant Ca - Ci value. Values are for (A) Site A1 (2700 m a.s.l.), (B) Site A2 (2400 m a.s.l.), and (C) Site A3 (2200 m a.s.l.).
Relationships between radial growth and iWUE
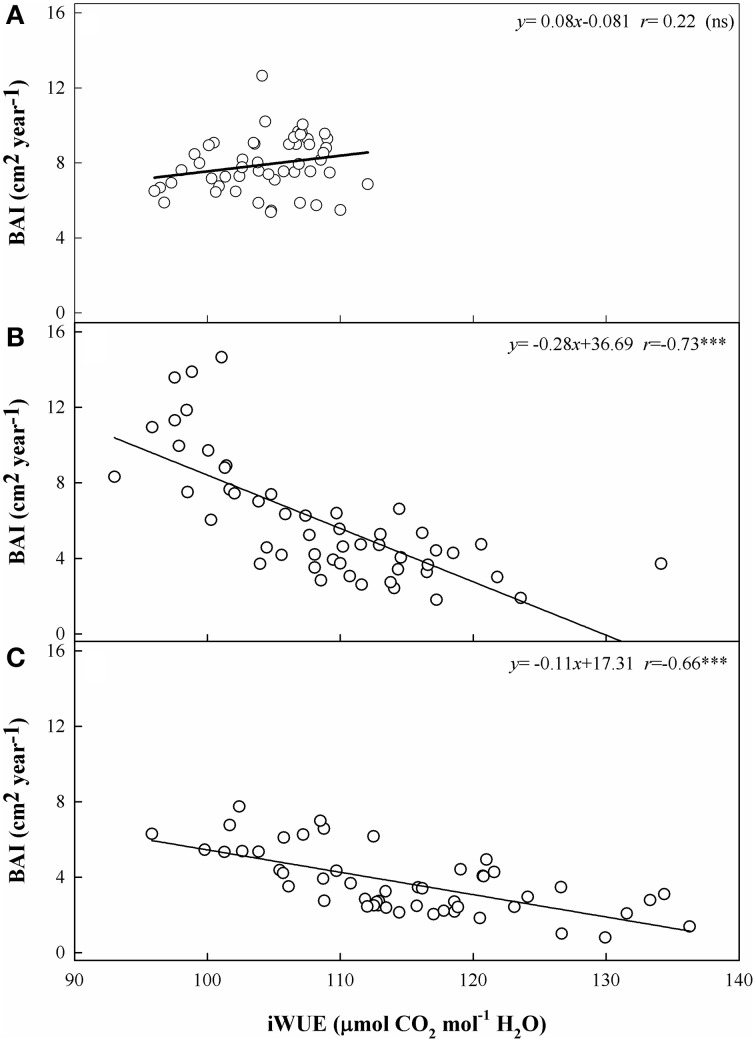
We detected the effects of long-term gas exchange on tree growth via the relationship between iWUE and BAI. BAI decreased significantly as iWUE increased at the low and middle elevation sites (r = −0.66 and r = −0.73, p < 0.001) (Figures 7B,C). In contrast, BAI at the upper tree-line remained stable (i.e., there was no statistically significant trend) despite increased iWUE (r = 0.22; Figure 7A).
Figure 7.
Relationships between the basal area increment (BAI) and the intrinsic water use efficiency (iWUE) at the three sites from 1960 to 2010. (A) Site A1 (2700 m a.s.l.), (B) Site A2 (2400 m a.s.l.), and (C) Site A3 (2200 m a.s.l.). Relationships between the two variables are calculated using Pearson's correlation coefficient (r). Significance levels: ***, p < 0.001; ns, not significant.
Discussion
Temporal and elevation effects on the climate constraints
The weather conditions during the growing season (April to August) played a dominant role in determining the variation in tree-ring δ13C. Tree-ring δ13C is expected to be strongly affected by photosynthetic rates, which are governed both by temperature and by the photon flux at the upper tree line (McCarroll and Pawellek, 2001). The stronger moisture signal reflected in δ13C (Figure 4) at the low-elevation site (A3) than that at the highest-elevation site (A1) further indicates that trees suffered from enhanced water stress at A3. Moisture conditions, and specifically the vapor-pressure difference between the atmosphere and the intercellular air spaces of the leaves, directly determine the stomatal aperture, which in turn controls the leaf intercellular CO2 concentration (Ci) and thereby governs δ13C (Farquhar et al., 1982).
The low and non-significant correlation (r < 0.276, p > 0.05) between the climate controls and BAI at site A1 suggests lower water stress and more adequate growth conditions for trees at the upper tree-line. In contrast with site A1, we found similar responses of tree growth to climate in trees growing at the A2 and A3 sites. Trees at both sites showed a similarly strong negative correlation with temperature throughout the growing season, but revealed increased sensitivity to moisture conditions (e.g., the strong negative correlations with SPEI; Figure 4) at the lower-elevation sites. High temperatures decrease soil water content and increase evaporation, hence reducing stomatal conductance, photosynthesis, and growth of trees (Peñuelas et al., 2008; Yin et al., 2008; Lévesque et al., 2013).
Elevation-dependent physiological responses to the Ca increases
iWUE of Schrenk spruce has increased at all three sites since 1960 (by 12.0, 24.0, and 32.0% at sites A1, A2, and A3, respectively; Figure 6). These values fall within the range of iWUE increases (up to 36.2%) that has been reported in other forests (Bert et al., 1997; Duquesnay et al., 1998; Feng, 1998; Peñuelas et al., 2011; Linares and Camarero, 2012a). Nevertheless, some studies have found that the responses of trees to increased Ca differed between stands, even though the most pronounced changes in iWUE were observed during the second half of the twentieth century from tree-ring chronologies (Waterhouse et al., 2004; Andreu-Hayles et al., 2011; Gagen et al., 2011; Linares and Camarero, 2012a). We also found this change at the three sites in our study.
The highest-elevation site (A1) showed a significant decrease in Ci/Ca before 1980, resulting in a greater iWUE improvement than at the other sites during this period, which agrees with the constant-Ci scenario (Saurer et al., 2004). Under this scenario, iWUE exceeded the expected values that would result from an active tree response to increased Ca, suggesting that stomatal closure of trees at the highest-elevation site may be reinforced by other factors (Andreu-Hayles et al., 2011). The high elevation site has a lower atmospheric pressure, which may increase the effect of any increase in the partial pressure of CO2 on the rate of photosynthesis more than at lower elevations (Hultine and Marshall, 2000). In contrast, the A2 and A3 sites followed the constant Ca − Ci scenario during the 1960s, leading to the lowest iWUE increase during this period in response to increasing Ca.
After 1980, we found a progressively smaller response to increasing Ca at the highest-elevation site. This lower sensitivity to Ca indicates that trees at the highest-elevation site may have become saturated with respect to increased Ca, as the trees exhibited their greatest iWUE improvement from 1960 to 1980 at site A1 (Waterhouse et al., 2004; Figure 6A). However, the responses of the trees at the two lower-elevation sites were within the range of expected iWUE values under the constant Ci scenario and the constant Ci/Ca scenario after 1973, a period when the greatest iWUE increases occurred at these sites. As the regional climate changes toward more aridification, generated by a significant temperature increase that was not accompanied by increased precipitation, hence increasing evaporative demand (Figure 2B), the greater iWUE improvement at lower elevations appears to have been induced by drought stress. We found that the limiting effects of drought for tree growth in lower elevation sites were strongly enhanced (Figures 4, 5). It is reasonable to expect a much lower water availability and enhanced drought stress at lower elevations, since the precipitation generally increases with altitudes in the Tianshan Mountains under the effect of westerly system (Guo et al., 2007; Wu et al., 2014).
Tree growth at different elevations
The low-elevation site had consistently higher (less negative) δ13Cpin, higher iWUE, and lower BAI than the A1 and A2 sites during the study period. Thus, the higher iWUE values since the 1970s that developed in response to the increased CO2 concentrations were consistently above those predicted under the constant Ci/Ca scenario, but this was not sufficient to reverse the decrease in BAI at site A3 (Figure 7C), as was the case for the active-response scenario (constant Ci/Ca) at site A2 (Figures 6B, 7B). The significant negative relationship between tree growth (BAI) and iWUE at the A2 and A3 sites (Figures 7B,C), accompanied by the consistent increase in iWUE over time (Figures 6B,C), indicated that tree growth has been decreasing despite long-term increases in iWUE, especially at the lower-elevation sites. A trend of decreasing BAI is a valid indicator of a decline in tree growth (Phipps and Whiton, 1988), and does not appear to be a consequence of maturation of the trees (Leblanc et al., 1992). The decreasing BAI found at the lower-elevation sites is in line with recent studies that have demonstrated a warming-induced growth decline despite increasing iWUE for forest systems at dry sites (Peñuelas et al., 2008; Andreu-Hayles et al., 2011; Linares and Camarero, 2012a; Lévesque et al., 2014). Wu et al. (2013) have demonstrated that prolonged warming-induced drought stress contributed greatly to a marked reduction of the regional BAI in recent years for Schrenk spruce at middle altitudes on the northern slopes of the Tianshan Mountains. In the present study, the close relationship between BAI and SPEI at sites A2 and A3 further indicated a link between the growth decline and warming-induced drought at the lower-elevation sites (Figure 5).
However, at the upper limit of the current distribution of Schrenk spruce in the western Tianshan Mountains, BAI remained largely stable throughout the study period. Simultaneously, the changes in iWUE of Schrenk spruce at the A1 site agreed with the constant Ci scenario before 1980, with a particularly strong improvement in iWUE before 1980 (Saurer et al., 2004; Figure 6A). However, this iWUE improvement did not induce a significant overall increase in BAI (Figure 7A). After 1980, the lack of change in iWUE during a period with a constant Ca − Ci scenario suggests that the trees reached a limit in their ability to respond to the increased Ca and subsequently showed a diminishing sensitivity to rising Ca. This diminishing sensitivity of iWUE and growth to increasing Ca at the tree line may be due to more adequate growth conditions at this site.
Conclusions
In this study, we found a warmth-induced drought limitation on tree growth accompanied by increasing iWUE at the lower-elevation sites in the spruce forest. Moreover, the continuous increases in iWUE may not have been sufficient to counteract the decreases in tree growth that occurred at lower elevations, as the negative effects of other factors on tree growth, such as drought, may have outweighed the CO2 fertilization effect. In contrast, the strongest iWUE increase, which occurred before 1980, did not significantly increase tree growth at the upper tree line. After 1980, the sensitivity of iWUE to increasing Ca diminished, suggesting the existence of a saturation effect. Therefore, the synergistic effect of elevated CO2 and higher temperatures may have had different effects in temperature-limited environments (e.g., at the upper limit of the tree's distribution) than in water-limited environments (e.g., at the lower limit of the tree's distribution). However, this study showed no apparent change of tree growth and a diminishing of iWUE improvement since 1980 onward at upper tree-line, which may contradict expectations that combined effects of elevated Ca and rising temperatures have increased forest productivity, especially for the high elevation forest (Linares et al., 2009; Salzer et al., 2009). Our results may provide insights into the tradeoffs between the responses to increased Ca and climate change, which were revealed by changes in tree physiology (δ13C) and growth (BAI) along an elevation gradient in this semi-arid region of northwestern China.
Author contributions
GW was responsible for the laboratory experiments and writing a draft of the manuscript. XL and TC contributed substantially to the conception or design of the work. GX, WW, XZ, and XZ contributed to the isotopic analyses. XL provided helpful suggestions and edits that improved the manuscript. TC, GX, WW, XZ, and XZ assisted in the statistical analysis and development of the chronologies. All the authors gave their final approval of the version to be published and agreed to be accountable for all aspects of the work by ensuring that questions related to the accuracy or integrity of all parts of the work will be appropriately investigated and resolved.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by the Major State Basic Research Development Program of China (973 Program) (2013CBA01808), by the self-determination project of the State Key Laboratory of Cryospheric Sciences (SKLCS-ZZ-2015-01-12), and by the Chinese Academy of Sciences Interdisciplinary Innovation Team Project (29Y329B91).
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2015.00309/abstract
References
- Allen C. D., Alison K. M., Haroun C., Dominique B., Nate M., Michel V., et al. (2010). A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 259, 660–684 10.1016/j.foreco.2009.09.001 [DOI] [Google Scholar]
- Andreu-Hayles L., Planells O., Gutierrez E., Muntan E., Helle G., Anchukaitis K. J., et al. (2011). Long tree-ring chronologies reveal 20th century increases in water-use efficiency but no enhancement of tree growth at five Iberian pine forests. Glob. Change Biol. 17, 2095–2112 10.1111/j.1365-2486.2010.02373.x [DOI] [Google Scholar]
- Bert D., Leavitt S. W., Dupouey J. L. (1997). Variations of wood δ13C and water-use efficiency of Abies alba during the last century. Ecology 78, 1588–1596. [Google Scholar]
- Biondi F., Qeadan F. (2008). A theory-driven approach to tree-ring standardization: defining the biological trend from expected basal area increment. Tree-Ring Res. 64, 81–96 10.3959/2008-6.1 [DOI] [Google Scholar]
- Cole C. T., Anderson J. E., Lindroth R. L., Waller D. M. (2010). Rising concentrations of atmospheric CO2 have increased growth in natural stands of quaking aspen (Populus tremuloides). Glob. Change Biol. 16, 2186–2197 10.1111/j.1365-2486.2009.02103.x [DOI] [Google Scholar]
- Duquesnay A., Breda N., Stievenard M., Dupouey J. L. (1998). Changes of tree-ring delta δ13C and water-use efficiency of beech (Fagus sylvatica L.) in north-eastern France during the past century. Plant Cell Environ. 21, 565–572 10.1046/j.1365-3040.1998.00304.x [DOI] [Google Scholar]
- Farquhar G. D., O'Leary M., Berry J. (1982). On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Funct. Plant Biol. 9, 121–137. 10.1071/PP982012118540975 [DOI] [Google Scholar]
- Feng X. (1998). Long-term Ci/Ca response of trees in western North America to atmospheric CO2 concentration derived from carbon isotope chronologies. Oecologia 117, 19–25 10.1007/s004420050626 [DOI] [PubMed] [Google Scholar]
- Fischlin A., Midgley G. F., Price J. T., Leemans R., Gopal B., Turley C., et al. (2007). Ecosystems, their properties, goods, and services, in Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, eds Parry M. L., Canziani O. F., Palutikof J. P., Der P. J. V., And L., Hanson C. E. (Cambridge: Cambridge University Press; ), 211–272. [Google Scholar]
- Francey R. J., Farquhar G. D. (1982). An explanation of 13C/12C variations in tree rings. Nature 297, 28–31 10.1038/297028a0 [DOI] [Google Scholar]
- Gagen M., Finsinger W., Wagner-Cremer F., McCarroll D., Loader N. J., Robertson I., et al. (2011). Evidence of changing intrinsic water-use efficiency under rising atmospheric CO2 concentrations in Boreal Fennoscandia from subfossil leaves and tree ring δ13C ratios. Glob. Change Biol. 17, 1064–1072 10.1111/j.1365-2486.2010.02273.x [DOI] [Google Scholar]
- Gagen M., McCarroll D., Loader N. J., Robertson L., Jalkanen R., Anchukaitis K. J. (2007). Exorcising the ‘segment length curse’: summer temperature reconstruction since AD 1640 using non-detrended stable carbon isotope ratios from pine trees in northern Finland. Holocene 17, 435–446 10.1177/0959683607077012 [DOI] [Google Scholar]
- Granda E., Rossatto D., Camarero J. J., Voltas J., Valladares F. (2014). Growth and carbon isotopes of Mediterranean trees reveal contrasting responses to increased carbon dioxide and drought. Oecologia 174, 307–317. 10.1007/s00442-013-2742-4 [DOI] [PubMed] [Google Scholar]
- Green J. W. (1963). Wood cellulose,. in Methods in Carbohydrate Chemistry, Vol. 3: Cellulose, ed Whistler R. L. (New York, NY: Academic Press; ), 9–20. [Google Scholar]
- Guo Y. Y., Liu H. Y., Ren J., Zhan X. F., Cao S. P. (2007). Responses of tree growth to vertical climate gradient in the middle section of the Tianshan Mountains. Q. Sci. 27, 322–331. (in Chinese). [Google Scholar]
- Hartl-Meier C., Dittmar C., Zang C., Rothe A. (2014a). Mountain forest growth response to climate change in the Northern Limestone Alps. Trees 28, 819–829 10.1007/s00468-014-0994-1 [DOI] [Google Scholar]
- Hartl-Meier C., Zang C., Dittmar C., Esper J., Goettlein A., Rothe A. (2014b). Vulnerability of Norway spruce to climate change in mountain forests of the European Alps. Climate Res. 60, 119–132 10.3354/cr01226 [DOI] [Google Scholar]
- Holmes R. L. (1983). Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull. 43, 51–67. [Google Scholar]
- Hultine K. R., Marshall J. D. (2000). Altitude trends in conifer leaf morphology and stable carbon isotope composition. Oecologia 123, 32–40 10.1007/s004420050986 [DOI] [PubMed] [Google Scholar]
- IPCC (2013). Climate Change 2013: The Physical Science Basis. Summary for Policymakers. London: Cambridge University Press. [Google Scholar]
- Körner C. (2012). Alpine Treelines. Functional Ecology of the Global High Elevation Tree Limits. Basel: Springer; 10.1007/978-3-0348-0396-0 [DOI] [Google Scholar]
- Koutavas A. (2013). CO2 fertilization and enhanced drought resistance in Greek firs from Cephalonia Island, Greece. Glob. Change Biol. 19, 529–539. 10.1111/gcb.12053 [DOI] [PubMed] [Google Scholar]
- Laumer W., Andreu L., Helle G., Schleser G. H., Wieloch T., Wissel H. (2009). A novel approach for the homogenization of cellulose to use micro-amounts for stable isotope analyses. Rapid Commun. Mass Spectrom. 23, 1934–1940. 10.1002/rcm.4105 [DOI] [PubMed] [Google Scholar]
- Leblanc D. C., Nicholas N. S., Zedaker S. M. (1992). Prevalence of individual-tree growth decline in red spruce populations of the Southern Appalachian Mountains. Can. J. For. Res. 22, 905–914 10.1139/x92-120 [DOI] [Google Scholar]
- Lenz A., Vitasse Y., Hoch G., Körner C. (2014). Growth and carbon relations of temperate deciduous tree species at their upper elevation range limit. J. Ecol. 102, 1537–1548 10.1111/1365-2745.12307 [DOI] [Google Scholar]
- Lévesque M., Saurer M., Siegwolf R., Eilmann B., Brang P., Bugmann H., et al. (2013). Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob. Change Biol. 19, 3184–3199 10.1111/gcb.12268 [DOI] [PubMed] [Google Scholar]
- Lévesque M., Siegwolf R., Saurer M., Eilmann B., Rigling A. (2014). Increased water-use efficiency does not lead to enhanced tree growth under xeric and mesic conditions. N. Phytol. 203, 94–109 10.1111/nph.12772 [DOI] [PubMed] [Google Scholar]
- Linares J. C., Camarero J. J. (2012a). From pattern to process: linking intrinsic water-use efficiency to drought-induced forest decline. Glob. Change Biol. 18, 1000–1015 10.1111/j.1365-2486.2011.02566.x [DOI] [Google Scholar]
- Linares J. C., Camarero J. J. (2012b). Growth patterns and sensitivity to climate predict silver fir decline in the Spanish Pyrenees. Eur. J. For. Res. 131, 1001–1012 10.1007/s10342-011-0572-7 [DOI] [Google Scholar]
- Linares J. C., Delgado-Huertas A., Camarero J. J., Merino J., Carreira J. A. (2009). Competition and drought limit the response of water-use efficiency to rising atmospheric carbon dioxide in the Mediterranean fir Abies pinsapo. Oecologia 161, 611–624. 10.1007/s00442-009-1409-7 [DOI] [PubMed] [Google Scholar]
- Liu X., Wang W., Xu G., Zeng X., Wu G., Zhang X., et al. (2014). Tree growth and intrinsic water-use efficiency of inland riparian forests in northwestern China: evaluation via δ13C and δ18O analysis of tree rings. Tree Physiol. 34, 966–980. 10.1093/treephys/tpu067 [DOI] [PubMed] [Google Scholar]
- Loader N., Robertson I., Barker A., Switsur V., Waterhouse J. (1997). An improved technique for the batch processing of small wholewood samples to α-cellulose. Chem. Geol. 136, 313–317 10.1016/S0009-2541(96)00133-7 [DOI] [Google Scholar]
- Loader N., Santillo P., Woodman-Ralph J., Rolfe J., Hall M., Gagen M., et al. (2008). Multiple stable isotopes from oak trees in southwestern Scotland and the potential for stable isotope dendroclimatology in maritime climatic regions. Chem. Geol. 252, 62–71 10.1016/j.chemgeo.2008.01.006 [DOI] [Google Scholar]
- Martinez-Vilalta J., Lopez B. C., Adell N., Badiella L., Ninyerola M. (2008). Twentieth century increase of Scots pine radial growth in NE Spain shows strong climate interactions. Glob. Change Biol. 14, 2868–2881 10.1111/j.1365-2486.2008.01685.x [DOI] [Google Scholar]
- McCarroll D., Gagen M. H., Loader N. J., Robertson I., Anchukaitis K. J., Los S., et al. (2009). Correction of tree ring stable carbon isotope chronologies for changes in the carbon dioxide content of the atmosphere. Geochim. Cosmochim. Acta 73, 1539–1547 10.1016/j.gca.2008.11.041 [DOI] [Google Scholar]
- McCarroll D., Loader N. J. (2004). Stable isotopes in tree rings. Q. Sci. Rev. 23, 771–801 10.1016/j.quascirev.2003.06.017 [DOI] [Google Scholar]
- McCarroll D., Pawellek F. (2001). Stable carbon isotope ratios of Pinus sylvestris from northern Finland and the potential for extracting a climate signal from long Fennoscandian chronologies. Holocene 11, 517–526 10.1191/095968301680223477 [DOI] [Google Scholar]
- McMahon S. M., Parker G. G., Miller D. R. (2010). Evidence for a recent increase in forest growth. Proc. Natl. Acad. Sci. U.S.A. 107, 3611–3615. 10.1073/pnas.0912376107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T. D., Jones P. D. (2005). An improved method of constructing a database of monthly climate observations and associated high-resolution grids. Int. J. Climatol. 25, 693–712 10.1002/joc.1181 [DOI] [Google Scholar]
- Morgan J., Pataki D., Körner C., Clark H., Del Grosso S., Grünzweig J., et al. (2004). Water relations in grassland and desert ecosystems exposed to elevated atmospheric CO2. Oecologia 140, 11–25. 10.1007/s00442-004-1550-2 [DOI] [PubMed] [Google Scholar]
- Morison J. (1993). Response of plants to CO2 under water limited conditions. Vegetatio 104, 193–209 10.1007/BF00048153 [DOI] [Google Scholar]
- Nock C. A., Baker P. J., Wanek W., Leis A., Grabner M., Bunyavejchewin S., et al. (2011). Long-term increases in intrinsic water-use efficiency do not lead to increased stem growth in a tropical monsoon forest in western Thailand. Glob. Change Biol. 17, 1049–1063 10.1111/j.1365-2486.2010.02222.x [DOI] [Google Scholar]
- Peñuelas J., Canadell J. G., Ogaya R. (2011). Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Global Ecol. Biogeogr. 20, 597–608 10.1111/j.1466-8238.2010.00608.x [DOI] [Google Scholar]
- Peñuelas J., Hunt J. M., Ogaya R., Jump A. S. (2008). Twentieth century changes of tree-ring δ13C at the southern range-edge of Fagus sylvatica: increasing water-use efficiency does not avoid the growth decline induced by warming at low altitudes. Glob. Change Biol. 14, 1076–1088 10.1111/j.1365-2486.2008.01563.x [DOI] [Google Scholar]
- Phipps R. L., Whiton J. C. (1988). Decline in long-term growth trends of white oak. Can. J. For. Res. 18, 24–32 10.1139/x88-005 [DOI] [Google Scholar]
- Rinn F. (2003). TSAPWin: Time Series Analysis and Presentation for Dendrochronology and Related Applications. Version 0.55 User Reference: RinnTech. Heidelberg. [Google Scholar]
- Salzer M. W., Hughes M. K., Bunn A. G., Kipfmueller K. F. (2009). Recent unprecedented tree-ring growth in bristlecone pine at the highest elevations and possible causes. Proc. Natl. Acad. Sci. U.S.A. 106, 20348–20353. 10.1073/pnas.0903029106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurer M., Siegwolf R. T. W., Schweingruber F. H. (2004). Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob. Change Biol. 10, 2109–2120 10.1111/j.1365-2486.2004.00869.x [DOI] [Google Scholar]
- Switsur R., Waterhouse J. (1998). Stable isotopes in tree ring cellulose, in Stable Isotopes:Intergration of Biological, Ecological and Geochemical Processes, ed Griffths H. (Oxford: BIOS; ), 303–321. [Google Scholar]
- Treydte K., Schleser G. H., Schweingruber F. H., Winiger M. (2001). The climatic significance of δ13C in subalpine spruces (Lötschental, Swiss Alps). Tellus B 53, 593–611 10.1034/j.1600-0889.2001.530505.x [DOI] [Google Scholar]
- Vicente-Serrano S. M., Begueria S., Lopez-Moreno J. I. (2010). A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. J. Clim. 23, 1696–1718 10.1175/2009JCLI2909.1 [DOI] [Google Scholar]
- Waterhouse J. S., Switsur V., Barker A., Carter A., Hemming D., Loader N. J., et al. (2004). Northern European trees show a progressively diminishing response to increasing atmospheric carbon dioxide concentrations. Q. Sci. Rev. 23, 803–810 10.1016/j.quascirev.2003.06.011 [DOI] [Google Scholar]
- Weltzin J. F., Loik M. E., Schwinning S., Williams D. G., Fay P. A., Haddad B. M., et al. (2003). Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience 53, 941–952 10.1641/0006-3568(2003)053[0941:ATROTE]2.0.CO;2 [DOI] [Google Scholar]
- Wu X., Liu H., He L., Qi Z., Anenkhonov O. A., Korolyuk A. Y., et al. (2014). Stand-total tree-ring measurements and forest inventory documented climate-induced forest dynamics in the semi-arid Altai Mountains. Ecol. Indic. 36, 231–241 10.1016/j.ecolind.2013.07.005 [DOI] [Google Scholar]
- Wu X., Liu H., Wang Y., Deng M. (2013). Prolonged limitation of tree growth due to warmer spring in semi-arid mountain forests of Tianshan, northwest China. Environ. Res. Lett. 8:024016 10.1088/1748-9326/8/2/024016 [DOI] [Google Scholar]
- Yin Z. Y., Shao X. M., Qin N. S., Liang E. (2008). Reconstruction of a 1436-year soil moisture and vegetation water use history based on tree-ring widths from Qilian junipers in northeastern Qaidam Basin, northwestern China. Int. J. Climatol. 28, 37–53 10.1002/joc.1515 [DOI] [Google Scholar]
- Yuan Y. J., Li J. F. (1995). Response functions of tree-ring chronologies in Western Tianshan Mountains. J. Glaciol. Geocryol. 17, 170–177. (in Chinese). [Google Scholar]
- Zhu H., Wang L., Shao X., Fang X. (2004). Tree ring-width response of Picea schrenkiana to climate change. Acta Geogr. Sin. 59, 863–870. (in Chinese). [Google Scholar]
- Zhu K. Z. (1985). Chinese Physical Geography Pandect. Beijing: Science Press. (in Chinese). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.