Summary
The ability to remember and navigate spatial environments is critical for everyday life. A primary mechanism by which the brain represents space is through hippocampal place cells, which indicate when an animal is at a particular location [1]. An important issue is understanding how the hippocampal place-cell network represents specific properties of the environment, such as signifying that a particular position is near a doorway or that another position is near the end of a corridor. The entorhinal cortex (EC), as the main input to the hippocampus, may play a key role in coding these properties because it contains neurons that activate at multiple related positions per environment [2–6]. We examined the diversity of spatial coding across the human medial temporal lobe by recording neuronal activity during virtual navigation of an environment containing four similar paths. Neurosurgical patients performed this task as we recorded from implanted microelectrodes, allowing us to compare the human neuronal representation of space with that of animals. EC neurons activated in a repeating manner across the environment, with individual cells spiking at the same relative location across multiple paths. This finding indicates that EC cells represent non-specific information about location relative to an environment’s geometry, unlike hippocampal place cells, which activate at particular random locations. Given that spatial navigation is considered to be a model of how the brain supports non-spatial episodic memory [7–10], these findings suggest that EC neuronal activity is used by the hippocampus to represent the properties of different memory episodes [2, 11].
Results
We recorded 1,329 single neurons in various brain regions from 13 neurosurgical patients performing a virtual-navigation task. Patients were instructed to learn an environment’s layout and navigate between six destination “stores” as rapidly and accurately as possible. This environment was a square track (see Figure 1A), which limited the patients’ navigation path to particular regions of the environment. Navigation errors decreased across trials of the task (Figure 1B), indicating that patients successfully learned the environment’s layout.
Figure 1. Behavioral task and performance.
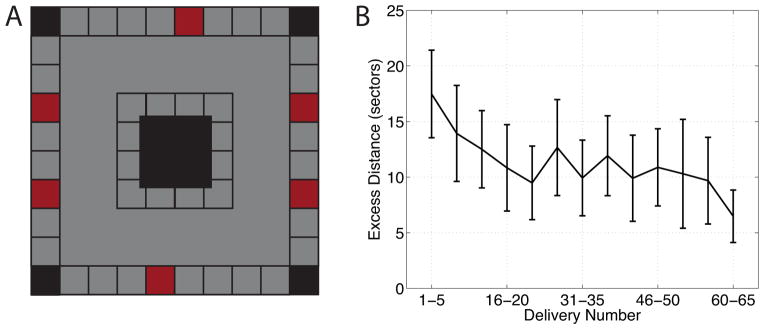
(A) An overhead schematic of the layout of the virtual environment. Red squares represent locations of the destination stores and white square are non-store buildings. Gray shading indicates regions of the environment where the patient could travel. (B) Subject average task performance as a function of delivery trial number. Performance is measured as the excess number of sectors traveled when searching for a destination store, compared to an ideal path. Error bars are 95% confidence intervals. See also Figure S1.
Our main objective was to characterize how the spiking of individual neurons varied to represent the patient’s virtual spatial location. For each cell, we computed firing rate maps corresponding to the cell’s mean rate of spiking as a function of the current virtual location. A previous analysis of this dataset [12] revealed many path cells, which coded for whether the participant was traveling clockwise or counterclockwise around the track. Thus, we computed firing rate maps separately for movements in clockwise and counterclockwise directions. We calculated these maps in a smoothed manner, as well as in a discretized manner that binned the patients’ location into one of 25 sectors on each side of the track.
Next, we identified cells that significantly varied their firing rate according to the patient’s virtual location. We used a one-way ANOVA as a screening procedure to identify individual neurons whose firing rates varied in response to the current sector of the environment. According to this measure, 313 cells (23.5%) were responsive to the location, a percentage that is in line with previous single-cell studies of human virtual navigation [12–14]. Our subsequent analyses focused on more precisely characterizing the activity of these cells.
A distinctive feature of some location-responsive cells in rodents is that they activate at multiple spatial locations that are related to each other, such as positions just before or after a curve [2], locations at particular distances from borders [4–6], or spots associated with particular landmarks [15, 16]. We sought to identify analogous types of representations in humans by searching for cells that exhibited significant path equivalence across distinct sections of the virtual environment [2]. We computed the path-equivalence coefficient for each cell, which is a measure of the similarity of the cell’s firing activity across two or more corridors (see Experimental Procedures). A cell that exhibited significant path equivalence is one that activated at the same relative position on multiple sides, such as a cell that spiked when a person was passing through the midpoint of any of the four paths. Of the 313 location-responsive cells, 30 (9.6%) exhibited significant path-equivalent firing patterns (p<0.001).
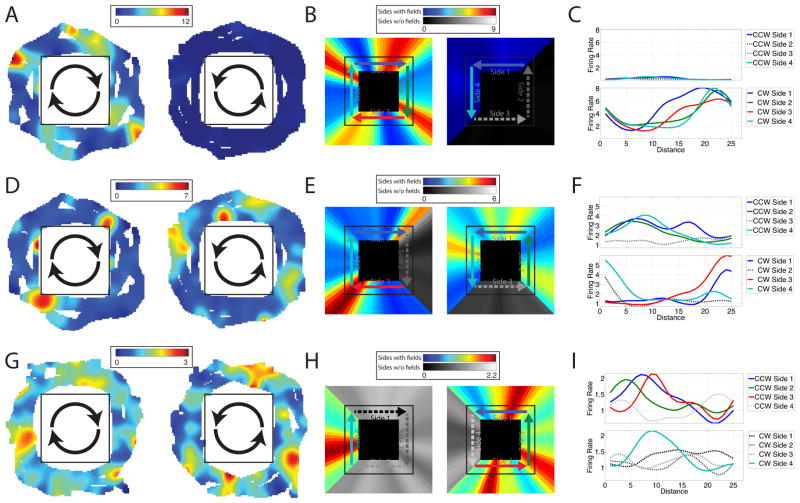
Three example path equivalent cells are shown in Figure 2. Figure 2A–C highlights one cell in the EC that activated consistently as the patient approached the end of each corridor during clockwise movement. Figure 2D–F shows a different cell in the EC that activated at similar locations across multiple corridors, with the locations of activations shifting between clockwise and counterclockwise movements. Figure 2G–I illustrates a cell from cingulate cortex that activated near the beginning of multiple corridors during counterclockwise movement. Additional example cells are shown in Figure 3.
Figure 2. Path equivalent cell firing rate maps.
Top Row: Activity of a cell in patient 2’s entorhinal cortex. (A) Two dimensional firing rate map for epochs of clockwise (left) and counterclockwise (right) movement. (B) Linearized firing rate maps (smoothed with a 12-pt window) for epochs of clockwise (left) and counterclockwise (right) movement. Sides with regions of significantly elevated firing are shown in color, and sides without significant activations are in grayscale. (C) Firing rate as a function of distance from the beginning of the side, plotted separately for each side of the environment and for clockwise (bottom) and counterclockwise (top) directions. Middle Row: Activity of a cell in patient 2’s entorhinal cortex. Bottom Row: Activity of a cell in patient 5’s cingulate cortex. See also Figure S2.
Figure 3. Examples of path equivalent cells.
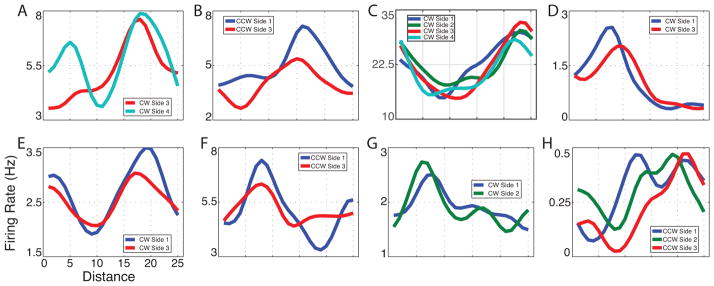
(A) A cell from patient 1’s cingulate cortex during clockwise movement. (B) A cell from patient 2’s entorhinal cortex during counterclockwise movement. (C) A cell from patient 2s entorhinal cortex during clockwise movement. (D) A cell from patient 5’s entorhinal cortex during clockwise movement. (E) A cell from patient 10’s parahippocampal gyrus during clockwise movement. (F) A cell from patient 12’s entorhinal cortex during counterclockwise movement. (G) A cell from patient 13’s hippocampus during clockwise movement. (H) A cell from patient 13’s hippocampus during counterclockwise movement. See figure S3 for additional examples.
We found significant levels of path-equivalent cells in only two regions: the entorhinal cortex and the cingulate cortex (Figure 4A). The magnitude of individual cells’ path-equivalent firing was greater in EC compared to cingulate cortex, as indicated by the fact that the mean path-equivalence coefficient for EC path-equivalent cells (0.92) was greater than for path-equivalent cells in cingulate cortex (0.49; p<.05, rank-sum test). We specifically compared the level of path-equivalent activity between the hippocampus and its main input, the entorhinal cortex, and found that the entorhinal cortex contained more path-equivalent cells than the hippocampus (p<0.05, post-hoc test). This difference in the prevalence of path-equivalent cells cannot be attributed to a difference in the stability of the spatial coding between EC and hippocampus, as these two regions did not differ in the percentage of location sensitive cells that were stable over time (45% EC vs 53% hippocampus, n.s.). Prior research suggested functional differences across regions within the entorhinal cortex [3, 15, 17]. However, we did not find any difference in the proportion of path-equivalent cells between neurons located in the posterior vs. anterior EC, lateral vs. medial, or superior vs. inferior positions (χ2 tests, all p’s>0.1).
Figure 4. Population measurements.
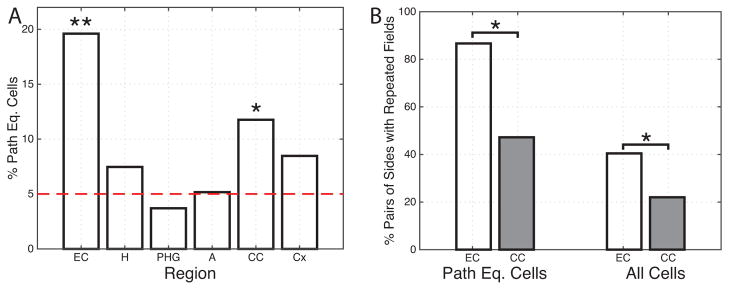
(A) Regional distribution of path-equivalent cells, which are location-responsive cells that have correlated responses across multiple corridors in the environment. EC: entorhinal cortex; H: hippocampus; PHG: parahippocampal gyrus; A: amygdala; CC: cingulate cortex; Cx: frontal/lateral-temporal cortex. (B) The percent of corridor pairs with place fields at the same relative locations. This measure is computed by identifying each cell with place fields on at least two corridors and measuring, across all pairs of corridors, how often place fields occur at the same relative location. ** denotes p<.0001, * denotes p<.05. See also Figure S4 and Table S1.
The path-equivalence measure we employed is sensitive to the overall shape of a cell’s firing pattern. Thus, this measure could be influenced by cells with diffuse firing patterns [18] rather than the spatially precise activations of conventional place or grid cells. To verify that the pattern of path-equivalent cells we observed was driven by the locations of peak spatial activations, we directly tested whether the relative locations of peak firing (place fields) were maintained across the sides of the environment. We identified each cell’s place fields and then computed, for each cell, the percent of pairs of corridors of the environment where the relative locations of the place fields overlapped by at least 50% (Figure 4B). This analysis supports the finding that the EC plays a particular role in path equivalence because cells in EC had the greatest percent of corridors where place fields were located at the same relative location. Across all cells with place fields on two or more corridors in the EC, 40% of the possible corridor pairs had fields in overlapping locations. This is significantly more than the 22% of corridor pairs for cells in cingulate cortex (p<.05, ranksum test). If we restrict this analysis to only the previously identified path-equivalent cells, the difference is more pronounced (87% compared to 47%).
One possibility is that individual neurons do not represent particular locations but rather that these signals actually encode distance traveled. We compared the location- or distance-encoding hypotheses by comparing the firing patterns of neurons that exhibited place fields during both clockwise and counterclockwise directions. For the 25 path-equivalent cells that met this criterion, we computed the correlation between the mean clockwise and counterclockwise firing patterns. We distinguished distance and location-based firing by computing this correlation two ways: with the firing rate vectors aligned by absolute location, and with the vectors ordered by distance along the direction of movement. A positive correlation in the first case indicates location coding, whereas a positive correlation in the second indicates distance coding. Of the path-equivalent cells analyzed, 11 (44%) showed significant correlations. Of these 11, 8 showed distance coding (e.g., Fig. 2I), 1 showed location coding, and 2 were ambiguous. This result supports the hypothesis that some path-equivalent cells play a role in representing relative distance (p<.05, χ2 test).
Discussion
We examined human single-neuron recordings during virtual navigation and found a set of location-responsive cells that exhibited repeated firing patterns across multiple related areas of an environment. The key feature of these path-equivalent cells is that they consistently activated at the same relative position across separate corridors. This is the first evidence in humans that individual cells generalize features across multiple settings. By activating at multiple locations, these cells behave very differently from place cells, which activate at only one location per environment. Because path-equivalent cells are input to the hippocampus, it indicates that a critical function of the human hippocampus is to build distinctive neuronal representations from non-specific entorhinal input. An additional contribution of our work is showing that humans exhibit clear spatially modulated neuronal firing in virtual navigation, supporting the view that virtual and physical navigation are supported by some similar mechanisms, as previously demonstrated in rodents in various brain structures [19–21].
Our demonstration of EC path-equivalent cells complements previous studies describing rodent neurons with repeating spatial firing patterns. One example is a study by Derdikman et al. [4], which measured the activity of entorhinal grid cells as rodents navigated a constrained track. During movement in one direction of a hairpin maze, grid cells activated to represent equally spaced groups of locations that were consistently positioned across multiple corridors. As that paper demonstrates, grid cells generally reset their grids at entrances to individual corridors, giving rise to the appearance of a repeating pattern across different sections of the environment. Some of the cells in our study appear to exhibit a similar pattern, in which they reset their representation upon entering each corridor. This supports the view that the neural representation of space can be segmented by entrances to different compartments [2, 22].
Our findings are also related to data from Frank et al. [2], who reported path-equivalent cells in rodent EC. The path-equivalent cells described in that study activated at analogous locations both within and across environments. Although several aspects of our findings are similar to the cells from that study, one critical difference is that when path-equivalent cells activate, the rat always has the same compass-like absolute heading. In contrast, for the path equivalent cells that we report, each activation corresponds to a circular heading and location within the environment. In a previous study from this dataset, we reported path cells that encoded direction in a circular manner such that they activated during either clockwise or counterclockwise movement [12]. Thus, one possibility is that the entorhinal representation of direction in humans can be transformed according to an environment’s layout so that it may depart from a fixed compass-like orientation scheme. Although human EC path-equivalent share features with grid cells, it is premature to conclude that the data reported here are from grid cells. As we demonstrate in Figure S2, owing to the four-way symmetry of our square environment, our data are not consistent with a grid cell that encoded the patient’s position using a triangular coordinate system in two-dimensional space. We could not test whether the cells in our dataset exhibit grids aligned individual corridors [4] because the length of each corridor was too short to observe a possible grid repetition.
As studies of rodent spatial navigation characterize the functional relationship between different brain regions, theories of hippocampal function are converging on the idea that rodent spatial navigation is a model for studying other aspects of cognition, including episodic memory [10, 23–25]. These theories share the idea that the representation of specific episodic memories can be considered analogous to the representation of locations by place cells. The role of the EC in this system may be to represent non-specific features of the behavioral setting [3, 5, 12, 14, 26] for encoding into specific memories (or locations) by the hippocampus [27]. During navigation, EC neurons may represent the attributes of a setting, with each cell activating at related locations, as in our findings and in some earlier animal work [2]. To our knowledge, our findings are the first demonstration of this type of featural neuronal coding in the human EC (cf. Mormann et al., 2008 [28]). By demonstrating a key difference between hippocampal and entorhinal representations during navigation, our results support theoretical models regarding the diversity of information processing throughout the medial temporal lobe [27, 29, 30].
Experimental Procedures
Participants and Task Design
The task design and methods for data acquisition are described in a previous study that examined this same dataset [12]. All data analyses and results reported here are novel, although the prior study did qualitatively describe the activity of one cell we examined here. Thirteen patients undergoing surgical treatment for medication-resistant epilepsy participated in the study. All surgeries were performed by I.F. and the research protocol was approved by the University of California, Los Angeles Institutional Review Board. Patients played a 3D virtual navigation game on a laptop computer in their hospital room [12–14, 31]. The virtual environment consisted of six destination stores surrounding the perimeter of a square track, with the center of the environment obstructed by buildings (Figure 1A). On each delivery trial the patient transported a passenger to their requested store destination as accurately as possible. After arrival at the destination, on-screen text displayed the name of the next randomly selected destination store.
Electrophysiology
We recorded spiking activity at 28–32 kHz using 40-μm platinum-iridium microwire electrodes [32] connected to a Neuralynx recording system. Nine microwires extended from the tip of each clinical depth electrode. Action potentials were manually isolated using spike shape, clustering of wavelet coefficients, and interspike intervals [33]. We localized the locations of individual electrodes by co-registering post-operative CT scans with pre-implant MRI images and standardizing to a normalized brain [34].
Data Preprocessing
We binned the firing rate of each cell into 100-ms epochs. We labeled each epoch with the patient’s location and direction of travel (either clockwise or counterclockwise around the square path). With the exception of the firing-rate maps presented in Figure 2A,D,G, all data analyses were conducted after linearizing patients’ location into 100 discrete sectors (25 per side) along the square path.
Data Analysis
For each cell, we computed a one-way ANOVA as a screening procedure to identify cells whose firing rate varied significantly according to environment sector, assessing significance with a shuffling procedure [13]. To determine whether a cell displayed a similar firing patterns across multiple sides of the square track, we used a modified version of the path equivalence coefficient from Frank et al. [2]. The path equivalence coefficient is a measure of the degree to which a cell fires in similar relative locations on multiple trajectories. Only sides of the track that contained at least one region of three or greater contiguous sectors of elevated firing were included. We define the path-equivalence coefficient as the median correlation between the firing rates of all pairs of included sides minus the median correlation between the firing rates of all pairs of included sides and shuffled sides:
where side is the firing rate of the corresponding 25 sectors, shuf is the firing rate of the corresponding 25 sectors shuffled as described below, and i and j range from 1 to the number of included sides. To determine the firing rate values of a shuffled side, the corresponding firing rate values for the first half of that side were reversed and then the values of the two halves were swapped (e.g., a path of locations “A..BC..D” become “C..DA..B”). Statistical significance was determined using a permutation procedure (see Supplemental Experimental Procedures).
Supplementary Material
Acknowledgments
This work was supported by the Brain and Behavior Research Foundation and US National Institutes of Health grant MH061975. We thank Michael Kahana for help with task design, and Sang Ah Lee for insightful comments on the manuscript.
Footnotes
Author Contributions
Surgeries were performed by I.F. Data were collected by J.J. and N.S., and data analyses were performed by J.F.M. The paper was written by J.F.M. and J.J.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map: Preliminary evidence from unit activity in the freely-moving rat. Brain Research. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 2.Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27(1):169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- 3.Hafting Torkel, Fyhn Marianne, Molden Sturla, Moser May-Britt, Moser Edvard I. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 4.Derdikman D, Whitlock JR, Tsao A, Fyhn M, Hafting T, Moser MB, Moser EI. Fragmentation of grid cell maps in a multicompartment environment. Nature Neuroscience. 2009;12(10):1325–1332. doi: 10.1038/nn.2396. [DOI] [PubMed] [Google Scholar]
- 5.Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of Geometric Borders in the Entorhinal Cortex. Science. 2008;322(5909):1865. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- 6.Bjerknes Tale L, Moser Edvard I, Moser May-Britt. Representation of geometric borders in the developing rat. Neuron. 2014;82(1):71–78. doi: 10.1016/j.neuron.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Eichenbaum Howard, Lipton Paul A. Towards a functional organization of the medial temporal lobe memory system: role of the parahippocampal and medial entorhinal cortical areas. Hippocampus. 2008;18(12):1314–1324. doi: 10.1002/hipo.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nature Reviews Neuroscience. 2008;9(3):182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 9.Hasselmo ME. How We Remember: Brain Mechanisms of Episodic Memory. MIT Press; Cambridge, MA: 2012. [Google Scholar]
- 10.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nature Neuroscience. 2013;16(2):130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckmaster CA, Eichenbaum H, Amaral DG, Suzuki WA, Rapp PR. Entorhinal Cortex Lesions Disrupt the Relational Organization of Memory in Monkeys. Journal of Neuroscience. 2004;24(44):9811–9825. doi: 10.1523/JNEUROSCI.1532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs J, Kahana MJ, Ekstrom AD, Mollison MV, Fried I. A sense of direction in human entorhinal cortex. 2010;107(14):6487–6482. doi: 10.1073/pnas.0911213107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–187. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs J, Weidemann CT, Miller JF, Solway A, Burke JF, Wei X, Suthana N, Sperling MR, Sharan AD, Fried I, Kahana MJ. Direct recordings of grid-like neuronal activity in human spatial navigation. Nature Neuroscience. 2013;16:1188–1190. doi: 10.1038/nn.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308(5729):1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- 16.Tsao Albert, Moser May-Britt, Moser Edvard I. Traces of experience in the lateral entorhinal cortex. Current Biology. 2013 doi: 10.1016/j.cub.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 17.Brun VH, Solstad T, Kjelstrup KB, Fyhn M, Witter MP, Moser EI, Moser MB. Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus. 2008;18(12):1200–1212. doi: 10.1002/hipo.20504. [DOI] [PubMed] [Google Scholar]
- 18.Quirk Gregory J, Muller Robert U, Kubie John L, Ranck James B., Jr The positional firing properties of medial entorhinal neurons: Description and comparison with hippocampal place cells. 1992;12(5):1945–1963. doi: 10.1523/JNEUROSCI.12-05-01945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aghajan Zahra M, Acharya Lavanya, Moore Jason J, Cushman Jesse D, Vuong Cliff, Mehta Mayank R. Impaired spatial selectivity and intact phase precession in two-dimensional virtual reality. Nature Neuroscience. 2014;18:121–128. doi: 10.1038/nn.3884. [DOI] [PubMed] [Google Scholar]
- 20.Harvey CD, Collman F, Dombeck DA, Tank DW. Intracellular dynamics of hippocampal place cells during virtual navigation. Nature. 2009;461:941–946. doi: 10.1038/nature08499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravassard Pascal, Kees Ashley, Willers Bernard, Ho David, Aharoni Daniel, Cushman Jesse, Aghajan Zahra M, Mehta Mayank R. Multisensory control of hippocampal spatiotemporal selectivity. Science. 2013;340(6138):1342–1346. doi: 10.1126/science.1232655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiers Hugo J, Hayman Robin MA, Jovalekic Aleksandar, Marozzi Elizabeth, Jeffery Kathryn J. Place field repetition and purely local remapping in a multicompartment environment. Cerebral Cortex. 2013:bht198. doi: 10.1093/cercor/bht198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron. 1999;23(2):209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 24.Buzsáki G. Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- 25.Moser EI, Moser MB. Grid cells and neural coding in high-end cortices. Neuron. 2013;80:765–774. doi: 10.1016/j.neuron.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 26.Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive Representation of Position, Direction, and Velocity in Entorhinal Cortex. Science. 2006;312(5774):758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- 27.Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary learning systems approach. Psychological Review. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 28.Mormann F, Kornblith S, Quiroga RQ, Kraskov A, Cerf M, Fried I, Koch C. Latency and selectivity of single neurons indicate hierarchical processing in the human medial temporal lobe. Journal of Neuroscience. 2008;28(36):8865. doi: 10.1523/JNEUROSCI.1640-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knierim JJ, Lee I, Hargreaves EL. Hippocampal Place Cells: Parallel Input Streams, Subregional Processing, and Implications for Episodic Memory. Hippocampus. 2006;16:755–764. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- 30.Solstad T, Moser EI, Einevoll GT. From grid cells to place cells: A mathematical model. Hippocampus. 2006;16:1026–1031. doi: 10.1002/hipo.20244. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs J, Kahana MJ. Direct brain recordings fuel advances in cognitive electrophysiology. Trends in Cognitive Sciences. 2010;14(4):162–171. doi: 10.1016/j.tics.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fried I, Wilson CL, Maidment NT, Engel J, Jr, Behnke E, Fields TA, MacDonald KA, Morrow JW, Ackerson L. Cerebral microdialysis combined with single-neuron and electroencephalographic recording in neurosurgical patients. Journal of Neurosurgery. 1999;91:697–705. doi: 10.3171/jns.1999.91.4.0697. [DOI] [PubMed] [Google Scholar]
- 33.Quiroga RQ, Nadasdy Z, Ben-Shaul Y. Unsupervised spike detection and sorting with wavelets and superparamagnetic clustering. Neural Computation. 2004;16:1661–1687. doi: 10.1162/089976604774201631. [DOI] [PubMed] [Google Scholar]
- 34.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Verlag; Stuttgart: 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.