Abstract
Adhesive intercellular junctions between endothelial cells are formed by tight junctions and adherens junctions. In addition to promoting cell-to-cell adhesion, these structures regulate paracellular permeability, contact inhibition of endothelial cell growth, cell survival, and maintenance of cell polarity. Furthermore, adherens junctions are required for the correct organization of new vessels during embryo development or during tissue proliferation in the adult. Extensive research on cultured epithelial and endothelial cells has resulted in the identification of many molecular components of tight junctions and adherens junctions. Such studies have revealed the complexity of these structures, which are formed by membrane-associated adhesion proteins and a network of several intracellular signaling partners. This review focuses on the structural organization of junctional structures and their functional interactions in the endothelium of blood vessels and lymphatics. We emphasize the way that these structures regulate endothelial cell homeostasis by transferring specific intracellular signals and by modulating activation and signaling of growth factor receptors.
Keywords: Endothelial cells, Intercellular junctions, Adherens junctions, Tight junctions
Introduction
Cell-to-cell contacts control critical endothelial functions both in quiescent conditions and in activated situations such as inflammation and angiogenesis (Bazzoni and Dejana 2004; Wallez and Huber 2008). Junctional proteins restrain cell migration, inhibit proliferation and apoptosis, and contribute to the maintenance of apical-basal polarity. In general, therefore, junctional signals should counteract angiogenesis and should be inhibited when vessels are induced to proliferate. Indeed, rapid angiogenesis is accompanied by increased vessel permeability (Eliceiri et al. 1999), and changes in endothelial barrier function accompany most inflammatory conditions (Weis et al. 2004). An intuitive consequence of these observations is that cell-to-cell junctions need to be sufficiently dynamic to allow the vessels to grow and to return the endothelium to a quiescent state.
In the endothelium, junctional complexes comprise tight junctions (TJs), adherens junctions (AJs), and gap junctions (Simionescu 2000). Whereas the first two types of junctions establish and maintain cell-to-cell adhesion, gap junctions are specialized to allow the passage of water, ions, and other small molecules from one cell to another. These three junctional structures are formed by distinct transmembrane proteins that promote homophilic cell-to-cell interactions and transfer of intracellular signals.
Notably, the architecture of endothelial cell-to-cell junctions varies in blood vessels to meet the functional requirements of the different organs. For instance, in the brain microcirculation where strict control of endothelial permeability is exerted between the blood and the central nervous system, TJs are enriched. Conversely, in postcapillary venules where the exchange between blood and tissues is more dynamic, junctions are specialized for reversible leakage of plasma and leukocyte migration in response to inflammatory mediators. Junctions are also specialized, albeit differently, in lymphatics where the passage of solutes and leukocytes is tightly controlled (Baluk et al. 2007).
Another feature to be considered is that endothelial junctions may be dynamic structures as in other cell types. During the time that cells reach confluence, adhesive membrane proteins of AJs and TJs first form adhesive complexes at sites of cell-to-cell contacts; the complexes then organize into zipper-like structures by lateral adhesion along the cell border (Cavey et al. 2008; Chitaev and Troyanovsky 1998; Nelson and Veshnock 1987; Yap et al. 1997, 1998).
The intracellular partners of the transmembrane adhesive proteins also vary during endothelial junction maturation and stabilization (Ayalon et al. 1994; Lampugnani et al. 1997; Lampugnani and Dejana 1997). Most importantly, even after the contacts have been formed in epithelia, adhesion proteins are still in dynamic equilibrium and not only cycle continuously between the plasma membrane and intracellular compartments, but also move by diffusion within the plane of the plasma membrane (Shen et al. 2008).
Molecular organization of endothelial junctions
Although the molecular composition of the different types of junction varies, they are generally formed by both transmembrane and cytoplasmic components. At junctions, dimeric adhesive proteins bind to other identical dimers present on the adjacent cell. The result is the lateral clustering of the adhesive molecules at cell-to-cell contacts (Dejana 2004). The recognition/adhesive information is delivered inside the cell by cytoplasmic and transmembrane partners. Therefore, junctions behave as true signaling complexes (Liebner et al. 2006).
In recent years, a large effort has been made by several groups to decipher the molecular organization of intercellular junctions in endothelial cells. Several transmembrane adhesive proteins have been identified including vascular endothelial (VE−) and neural (N−) cadherin at AJs (Lampugnani and Dejana 1997; Luo and Radice 2005), occludin (Furuse et al. 1993) and members of the claudin family (Nitta et al. 2003), and the junctional adhesion molecule (JAM) family at TJs (Imhof and Aurrand-Lions 2004; Vestweber 2003). PECAM-1 (Ilan and Madri 2003; Muller 2003) or Sendo-1/Muc18/CD146 (Anfosso et al. 2001) are proteins that localize along the intercellular cleft but seem not to participate directly in AJ or TJ organization. Although the general organization of AJs and TJs in the endothelium is comparable with that of epithelial cells, there are some cell-specific features. For example, VE-cadherin, claudin-5, and PECAM-1 have been found in endothelial cells but not in epithelial cells. Furthermore, the morphology of the intercellular cleft in the endothelium differs from that of many epithelia, as TJs not only are located only on the apical side, but may also be intermingled with AJs (Fig. 3).
Fig. 3.
Representation of three types of endothelial junctions (left) and the corresponding electron micrographs (right). In small arterioles (a, b), endothelial cell junctions are tight, probably being formed by TJs intermingled with AJs to limit exchange between blood and tissues. In venules (c, d), junctions are formed by AJs, and small areas of TJs are frequently concentrated at the apical side of the intercellular cleft. In initial lymphatics, (e, f) in which intercellular junctions control entry (intravasation) and drainage of fluid and cells from tissues, junctions are permeable, and endothelial borders have discontinuous button-like junctions with intermingled flaps resembling valve-like structures (the junctional structures are presented en face; from Baluk et al. 2007). AJ and TJ proteins are concentrated at the buttons and allow the flaps to open freely without disrupting overall vascular organization. The more distal collecting lymphatic vessels have continuous zipper-like junctions similar to those of blood vessels (not shown). In addition to AJ and TJ proteins, other junctional adhesive proteins and intracellular partners are present at endothelial junctions but, for simplicity, are not reported here. Note the different scales in the electron micrographs as well as the corresponding drawings. Bars 1 μm
The molecular organization of endothelial junctions has been described in detail in other reviews (see, for instance, Bazzoni and Dejana 2004; Ben-Ze’ev and Geiger 1998; Dejana 2004; Gonzalez-Mariscal et al. 2008; Gumbiner 2005; Ilan and Madri 2003; Imhof and Aurrand-Lions 2004; Matter and Balda 2003; Muller 2003; Vestweber 2003). Here, therefore, we briefly mention only the most important apects of their structure.
AJs in the endothelium are formed by the transmembrane adhesive protein VE-cadherin, which is, directly or indirectly, bound inside the cells to multiple intracellular partners, which include β-catenin, p120, plakoglobin, the phosphatases VE-PTP and DEP-1, and others (Bazzoni and Dejana 2004). N-cadherin is also expressed in large amounts in endothelial cells but is mostly diffuse in the cell membrane, whereas VE-cadherin is clustered at AJs both in vitro and in vivo (Navarro et al. 1998). At TJs, adhesion is instead promoted by other adhesive proteins including members of the claudin family, occludin, JAMs, and ESAM. The TJ intracellular components are members of the zonula occludens protein (ZO) family (ZO-1, -2, and -3), AF6/Afadin, PAR-3/ASIP, MUPP-1, and others (Bazzoni and Dejana 2004; Gonzalez-Mariscal et al. 2008).
Many AJ and TJ proteins are ubiquitous along the vascular tree. VE-cadherin and claudin-5 are present in most blood vessels and in confluent endothelial cells in culture. Other junctional markers such as claudin-3 (Wolburg et al. 2002), T-cadherin (Ivanov et al. 2001), and desmoplakin (Gallicano et al. 2001; Schmelz et al. 1994) have a more limited expression in particular vascular regions. Notably, junction organization and marker distribution is comparable in cultured cells and in various types of vessels in vivo (see Figs. 1, 2).
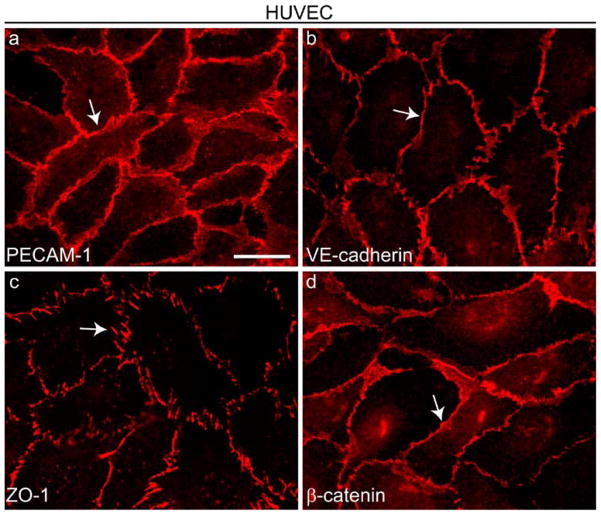
Fig. 1.
Distribution of cell-to-cell junction proteins in cultured endothelial cells. Cultured human umbilical vein endothelial cells (HUVEC) were grown to confluence and stained by immunofluorescence with antibodies directed against four junctional proteins (arrows): PECAM-1 (a) outside adherens junctions (AJs) and tight junctions (TJs), VE-cadherin (b) at AJs, ZO-1 (c) at TJs and AJs, and β-catenin (d) at AJs. Bar 20 μm
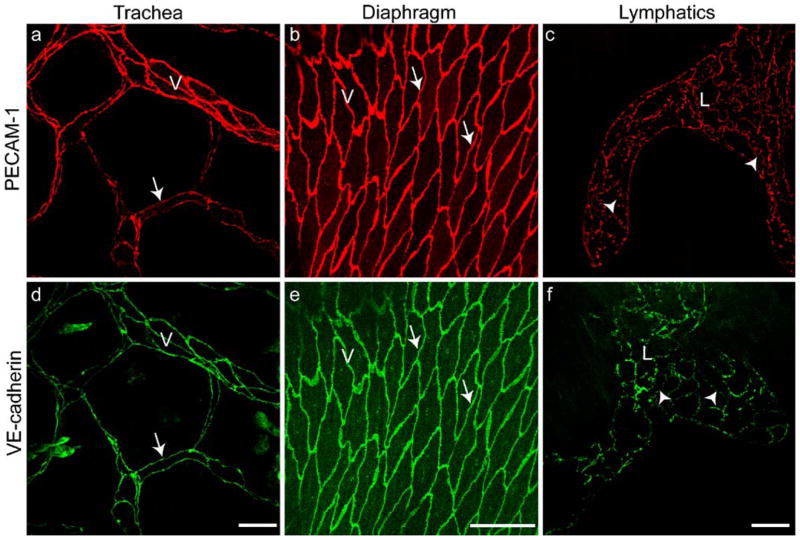
Fig. 2.
Endothelial junction organization in vivo comparable with that observed in cultured endothelial cells (Fig. 1). Staining with antibodies to PECAM-1 (a–c) and VE-cadherin (d–f). The venules (V) of mouse trachea (a, d) and diaphragm (b, e) and the lymphatics (L) of diaphragm (c, f) are immunostained (arrows zipper-like junctions of blood vessels, arrowheads button-like junctions in lymphatics; see also Fig. 3). Bars 50 μm
Junctions in the endothelium of the initial part of lymphatics differ from those in the endothelium of blood vessels (Fig. 3). A recent paper reports that junctions between endothelial cells of initial lymphatics are unique. Endothelial borders have discontinuous button-like junctions with intermingled flaps resembling valve-like structures (see Fig. 3; Baluk et al. 2007). At a molecular level, AJ and TJ proteins are concentrated at the buttons, leaving the flaps free to open without disrupting the overall junctional organization. PECAM, which is known to promote leukocyte traffic through junctions, is instead concentrated between buttons. The more distal collecting lymphatic vessels have continuous zipper-like junctions resembling those in the endothelium of blood vessels.
These data underline the specialization of endothelial junctions in lymphatics. In initial lymphatics, where intercellular junctions control entry of fluid and cells that drain from tissues, the endothelium remains permeable at valve-like regions. In collecting lymphatics, which collect lymph and cells from the initial lymphatics, dynamic junctions are not present; instead, the endothelium has only zipper-like structures of AJ and TJ similar to those in the blood vasculature.
In the brain microvasculature, as described above, endothelial junctions are rich in TJ structures that form a complex network along the rim of the endothelial cells (for reviews, see Gonzalez-Mariscal et al. 2008; Wolburg and Lippoldt 2002). Most of the components of TJs (claudins, occludin, ZO-1, 2 and 3, cingulin, 7H6, etc.) are expressed by the brain microvasculature. The integrity of TJ structures is fundamental to the maintenance of the normal functions of the central nervous system. On the one hand, conditions that alter the structure or molecular composition of TJs may lead to brain edema with harmful consequences for brain functional integrity. On the other hand, the tight cell-to-cell adhesion promoted by these structures prevents the passage of some drugs from the blood to the nervous tissue. Therefore, manipulation of TJ adhesion properties in brain microvasculature would have therapeutic consequences.
Cross talk between AJs and TJs
AJs form early during the development of the vascular system in the embryo and earlier than TJs. Inhibition of AJ organization causes major defects at early stages of development, as found in mice deficient for VE-cadherin, N-cadherin, β-catenin, VE-PTP, or DEP-1 (for a review, see Nyqvist et al. 2008).
In cultured cells, AJs organize prior to TJs. Considerable evidence indicates that TJs cannot form in the absence of AJs. Although the molecular basis of AJ and TJ reciprocal interaction is largely unknown, recent evidence highlights the role of VE-cadherin in this process. Taddei et al. (2008) have found that endothelial VE-cadherin at AJs up-regulates the TJ adhesive protein claudin-5. This effect requires the release of the inhibitory activity of the forkhead box factor FoxO1 and Tcf-4-β-catenin transcriptional repressor complex. VE-cadherin acts by inducing FoxO1 phosphorylation and inhibition through AKT activation and by limiting β-catenin nuclear translocation.
VE-cadherin and N-cadherin may have different functions in endothelial cells. VE-cadherin, similar to E-cadherin in epithelial cells, resembles an epithelial cadherin in that it limits cell migration and growth. In contrast, N-cadherin is a mesenchymal cadherin, promotes epithelial-mesenchymal transition, and increases cell motility (for a review, see Wheelock et al. 2008). Therefore, VE-cadherin is probably more effective than N-cadherin in promoting and sustaining TJs organization, further stabilizing intercellular contacts.
Another pathway involving AJ and TJ interaction is the interchange of cytoskeletal components between these two structures. A typical example is ZO-1, which has been described in epithelial cells to be concentrated at AJs at early steps of their organization (Itoh et al. 1997). ZO-1 localization at AJs is attributable to its binding to α-catenin and is transient since the protein subsequently moves away and concentrates at TJs.
More recently, the formation of AJs in epithelial cells has been found to be significantly delayed in ZO-1 knock out/ZO-2 knock down cells (Ikenouchi et al. 2007). Data show that ZO-1 plays crucial roles not only in TJ formation, but also in the conversion from “fibroblastic” AJs to belt-like “polarized epithelial” AJs through Rac1 activation.
In vivo, ablation of ZO-2 results in arrested development and embryo lethality before vascular development (Xu et al. 2008). Embryos deficient in ZO-1, instead, show defective yolk sac vascularization characterized by altered remodeling (Katsuno et al. 2008). The lack of ZO-1 might therefore lead to major defects in both vascular AJs and TJs.
Signaling through AJ
VE-cadherin might transfer intracellular signals in various ways. The p85 component of PI3K can associate with the VE-cadherin/catenin complex (Carmeliet et al. 1999), and PI3K and Akt phosphorylation are activated by VE-cadherin clustering. This may lead to the inhibition of endothelial cell apoptosis. Indeed, interference with VE-cadherin expression or function renders endothelial cells more susceptible to pro-apoptotic stimuli. Furthermore, activation of Akt by VE-cadherin causes FOXO-1 phosphorylation and inactivation of its transcriptional activity. This may in turn reprogram gene expression by endothelial cells as has been observed for claudin-5 (see above). We have also reported that, similar to E-cadherin (Nakagawa et al. 2001), VE-cadherin clustering activates the small GTPase Rac and inhibits Rho (Lampugnani et al. 2002). This effect is persistent, since Rac activity remains high in confluent cells expressing VE-cadherin, in contrast to VE-cadherin −/− cells. Nelson and colleagues (2004) have reported that the engagement of VE-cadherin in bovine pulmonary artery endothelial cells induces the sustained activation of RhoA and its effector ROCK. Evidence that Rac1 and RhoA may have opposing effects in endothelial cells is further supported by Wojciak-Stothard and collegues (2005); these authors showed that only a balanced amount of active Rac1 supports VE-cadherin-based junctional stability, whereas overexpression of a dominant active form of Rac1 leads to junction destabilization.
Although the signaling of VE-cadherin via the small GTPases Rac and Rho has not been deciphered completely, a balanced activation/inhibition is evidently required for the quiescent and activated/angiogenic endothelium. Rac activation and Rho inhibition are considered typical of confluent epithelioid cells (Zondag et al. 2000) and are induced by the expression and clustering of E- or VE-cadherin, which are typical epithelioid cadherins (see above), whereas N-cadherin, which is associated with a migratory phenotype, induces Rho activation and Rac inhibition (Charrasse et al. 2002). As reported for other cadherins, VE-cadherin clustering also induces short-lasting mitogen-activated protein kinase (MAPK) activation (Nelson et al. 2005), which is probably induced when cells first touch each other and then declines when confluency is reached and junctions are fully stabilized.
Several phosphatases (PTPμ, PTP-K, SHP1, SHP2, PTP-LAR, and PTP-B) and kinases such as src or csk have been found to associate with the cadherin/catenin complex, although not all of them are expressed by endothelial cells (for reviews, see Bazzoni and Dejana 2004; Ha et al. 2008). These phosphatases are likely to modulate phosphorylation of the complex and/or its intracellular partners. VE-PTP is an endothelial specific phosphatase that can associate with VE-cadherin (Nawroth et al. 2002). Similarly, DEP1/CD148, although not endothelial-specific, is up-regulated by cell confluency and contributes to VE-cadherin-mediated inhibition of cell growth (Lampugnani et al. 2003). Importantly, the inactivation of either one of these phosphatases induces early embryonic lethality because of major alterations of vascular development (Nyqvist et al. 2008).
The function of endothelial N-cadherin remains unclear. Unlike VE-cadherin, N-cadherin does not appear to be involved in endothelial cell-to-cell junctions in cultured confluent endothelial cells and in mature vessels in vivo (Navarro et al. 1998; Bazzoni and Dejana 2004). However, recent data show that endothelial-specific deletion of N-cadherin in mice leads to a decrease in VE-cadherin expression and a severe vascular phenotype that resembles that of VE-cadherin −/− embryos (Luo and Radice 2005). The mechanism through which N-cadherin regulates VE-cadherin expression is unknown. Others, using an in vitro model of endothelial development within stem-cell-derived embryoid bodies, have observed that N-cadherin-null endothelial cells are still able to sprout and form vascular structures (Vittet et al. 1997).
N-cadherin might also be important for the interaction between endothelial cells and pericytes. In the developing chick brain, antibodies that block N-cadherin disrupt endothelial-pericyte interaction and cause vascular haemorrhages (Gerhardt and Betsholtz 2003; Gerhardt et al. 2000). Furthermore, Tillet and coworkers have shown that a lack of N-cadherin prevents pericytes from covering endothelial outgrowths (Tillet et al. 2005). N-cadherin becomes targeted to heterotypic junctions between endothelial cells and pericytes through the activation of the G-protein coupled receptor Edg-1/S1P1 (endothelial differentiation gene), which is a receptor for platelet-derived sphingosine-1-phosphate (S1P), inducing Rac activation and microtubule polymerization (Paik et al. 2004).
Cadherin association with growth factor receptors
Cadherins may associate with growth factor receptors and modulate their intracellular signaling properties (Cavallaro and Christofori 2004). The general consequence of this is that the type of cellular response to growth factors is dictated by cell density. When endothelial cells are activated by VEGF, VEGFR2 (Flk-1 or KDR) associates with the VE-cadherin/catenin complex and is de-phosphorylated by DEP-1 and other phosphatases associated with the complex (Lampugnani et al. 2003). Receptor internalization is also reduced (Lampugnani et al. 2006), and since VEGFR2 signals from intracellular compartments, the lack of internalization is accompanied by the inhibition of MAPK activation and cell proliferation.
The kinase Csk, which is an inhibitor of src, has been found to be associated with VE-cadherin. The down-regulation of Csk in endothelial cells induces a significant increase in proliferation. Csk binding to VE-cadherin requires phosphorylation of tyrosine 685, and the mutation of this tyrosine inhibits Csk association with VE-cadherin, partially blocking its inhibitory activity on cell growth (Baumeister et al. 2005).
Recent evidence suggests that VE-cadherin associates with transforming growth factor-β (TGF-β) receptor (Rudini et al. 2008). VE-cadherin expression and junctional clustering are required for optimal TGF-β signaling in endothelial cells, and the anti-proliferative and anti-migratory responses of this growth factor are increased in the presence of VE-cadherin. Endothelial cells lacking VE-cadherin are less responsive to TGF-β/ALK1- and TGF-β/ALK5-induced Smad phosphorylation and target gene transcription. VE-cadherin co-immunoprecipitates with all the components of the TGF-β receptor complex (TβRII, ALK1, ALK5, endoglin) and promotes TGF-β signaling by enhancing TβRII/ALK1 or ALK5 assembly into an active receptor complex.
Overall, these data suggest that VE-cadherin plays an important role in endothelial cell stabilization by inhibiting VEGFR2 on one hand and activating TGF-β signaling on the other. These two effects contribute to the inhibition of endothelial cell growth and motility.
The interaction of N-cadherin and the fibroblast growth factor receptor (FGFR) has been documented in various cell types (Cavallaro et al. 2001). N-cadherin might interact with growth factor receptors in vascular cells. Moreover, N-cadherin controls the level of β1 integrin in endothelial cells (Luo and Radice 2005), and integrated N-cadherin/FGFR signaling has been implicated in β1-mediated endothelial cell adhesion. Interestingly, N-cadherin supports the motility and the metastatic potential of breast cancer cells by binding to FGFR and preventing its internalization. Recent observations support the hypothesis that N-cadherin-mediated modulation of FGFR activity is involved in endothelial cell survival. Indeed, a peptide interfering with the adhesive function of N-cadherin in endothelial cells inhibits FGFR signaling, thus resulting in apoptosis (Erez et al. 2004). It is attractive to speculate that different cadherins associate with different growth factor receptors and modulate downstream effects in a specific way.
Signaling through TJs
TJ components interact with several signal transduction molecules such as G-proteins, protein kinases (Bazzoni and Dejana 2004; Gonzalez-Mariscal et al. 2008; Traweger et al. 2008), and, in general, molecules that regulate cell growth and survival. ZO-1 together with the homologous proteins ZO-2/-3, are members of the membrane-associated guanylate kinase homologs (MAGUKs; Bazzoni and Dejana 2004; Katsuno et al. 2008), which exhibit a PDZ-binding domain in the C-terminus and a src homology region 3 (SH3). PDZ domains are known to mediate the anchorage of transmembrane proteins to the cortical actin cytoskeleton. ZO-1 exists in two splicing variants characterized by the presence of the 80-amino-acid α-domain. The α+ isoform is present in epithelial cells, whereas the α− isoform is restricted to endothelial cells and Sertoli cells (Balda and Anderson 1993). ZO-1 localizes to the nucleus in sparse or migrating cells when TJs are not or only poorly developed (Gottardi et al. 1996). Similarly, ZO-2 is able to shuttle from the cytoplasm to the nucleus where it can influence gene transcription and cell behavior (Traweger et al. 2008).
In other systems, the loss-of-function mutation of the Drosophila MAGUK family member discs-large-1 leads to an overgrowth phenotype, suggesting that these proteins are involved in the downstream signaling from cell-to-cell junctions to regulate contact inhibition of growth (Woods and Bryant 1991). Furthermore, ZO-1 interacts with the Y-box transcription factor ZONAB, which inhibits the expression of ErbB2 and cell growth. ZO-1 localization at TJ is altered in tumor cells, and the expression of deletion mutants of this protein in epithelial cells causes epithelial-mesenchymal transition. Recently, ZONAB has been shown to interact directly with the Ras family member RalA when cells become confluent, leading to de-repression of target gene promoters normally repressed by ZONAB in non-confluent cells (Frankel et al. 2005). The interaction of ZONAB with ZO-1 has also been suggested to decrease the transcriptional repression by reducing the nuclear localization of ZONAB. Interestingly, oncogenic Ras alleviates transcriptional repression by ZONAB (Frankel et al. 2005), underlining the concept of multilateral cross talk of TJ proteins with other signaling pathways.
Some members of the JAM family have been found at TJs in the endothelium. The family is formed by at least five members: JAM-A (also called F11R or JAM-1), JAMB, JAM-C, JAM-4, and JAM-L. Another protein that is closely related is named ESAM and presents many similarities with the JAM group (Bazzoni and Dejana 2004; Ebnet et al. 2004; Imhof and Aurrand-Lions 2004). JAM-A, -B, and ESAM have been found to be concentrated at endothelial TJs, although they might also occur along the intercellular cleft. JAM-A (but also JAM-B and JAM-C) presents, at its C-terminus, a consensus binding sequence for type-II PDZ domains. Moreover, JAM-A is rather promiscuous and interacts with several PDZ-containing partners such as ZO-1, AF6/afadin, partitioning defective (PAR) 3/6/atypical PKC (aPTC) complex, CASK/lin 2, and MUPP-1 (Bazzoni and Dejana 2004; Ebnet et al. 2004). Some of these interactions are likely to be important for the anchorage of the protein to the actin cytoskeleton.
Several observations of various cell types suggest that JAMs are important in the establishment and maintenance of cell polarity. JAM-A (but also JAM-C and possibly JAM-B) binds the PAR3/PAR6 complex, which can associate with the λ and ζ isoforms of aPKC and the small GTPase cdc42. This complex plays a central role in establishing cell polarity in Caenorhabditis elegans and in TJ organization in mammalian cells (Ebnet et al. 2001; Itoh et al. 2001). Angiogenesis also involves the modulation of cell polarity. JAM-C has been shown to be required for tumor angiogenesis and hypoxia-induced retinal neovascularization (Lamagna et al. 2005).
Concluding remarks
Endothelial cell-to-cell junctions present a remarkably complex organization that includes not only homophilic cell-to-cell adhesion proteins, but also signaling partners. Ablation of various components of either AJs or TJs leads to major problems in vascular development in the embryo. In the adult, the lack of a correct structural and functional organization of junctions induces vascular fragility and increase in permeability. Phosphorylation of tyrosine or serine residues may also cause major changes in the functional properties of junctions (for a review, see Dejana et al. 2008). Such modifications may increase junctional protein turnover and/or the strength of their association with cytoskeletal or signaling partners. These changes cannot be easily appreciated in vivo by simple morphological analysis; more specific tools such as antibodies directed against the phosphorylated/activated forms of junctional proteins are required.
Many pathological conditions associated with inflammation, tumor vascularization, or cerebral edema are characterized by fragile or leaky blood vessels. Defects in endothelial permeability and fluid clearance also occur in lymphatics. The characterization of the structure and function of endothelial junctions opens new therapeutic possibilities for manipulating endothelial permeability and limiting tissue damage.
Acknowledgments
This work was supported by the Associazione Italiana per la Ricerca sul Cancro, Association for International Cancer Research, European Community (Integrated Project Contract no. LSHG-CT-2004–503573; NoE MAIN 502935; NoE EVGN 503254; EUSTROKE consortium; Angioscaff consortium; Optistem consortium), Istituto Superiore di Sanità, Italian Ministry of Health, MIUR (COFIN prot: 2006058482_002), and Fondation Leducq Transatlantic Network of Excellence (E.D.). Additional support came from US National Institutes of Health grants HL24136 and HL59157 from the National Heart, Lung, and Blood Institute and CA82923 from the National Cancer Institute and AngelWorks Foundation (D.McD.).
As a reflection of the broad interest in this field, many studies been performed, and we apologize to authors whose work could not be cited because of space constraints. We thank Amy Haskell and Hiroya Hashizume for electron microscopy.
Contributor Information
Elisabetta Dejana, Email: elisabetta.dejana@ifom-ieo-campus.it, IFOM, FIRC Institute of Molecular Oncology, Via Adamello 16, 20139 Milan, Italy. Department of Biomolecular Sciences and Biotechnologies, School of Sciences, University of Milan, Milan, Italy.
Fabrizio Orsenigo, IFOM, FIRC Institute of Molecular Oncology, Via Adamello 16, 20139 Milan, Italy.
Cinzia Molendini, IFOM, FIRC Institute of Molecular Oncology, Via Adamello 16, 20139 Milan, Italy.
Peter Baluk, Department of Anatomy, Comprehensive Cancer Center, and Cardiovascular Research Institute, University of California, San Francisco, USA.
Donald M. McDonald, Department of Anatomy, Comprehensive Cancer Center, and Cardiovascular Research Institute, University of California, San Francisco, USA
References
- Anfosso F, Bardin N, Vivier E, Sabatier F, Sampol J, Dignat-George F. Outside-in signaling pathway linked to CD146 engagement in human endothelial cells. J Biol Chem. 2001;276:1564–1569. doi: 10.1074/jbc.M007065200. [DOI] [PubMed] [Google Scholar]
- Ayalon O, Sabanai H, Lampugnani MG, Dejana E, Geiger B. Spatial and temporal relationships between cadherins and PECAM-1 in cell-cell junctions of human endothelial cells. J Cell Biol. 1994;126:247–258. doi: 10.1083/jcb.126.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Anderson JM. Two classes of tight junctions are revealed by ZO-1 isoforms. Am J Physiol. 1993;264:C918–C924. doi: 10.1152/ajpcell.1993.264.4.C918. [DOI] [PubMed] [Google Scholar]
- Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister U, Funke R, Ebnet K, Vorschmitt H, Koch S, Vestweber D. Association of Csk to VE-cadherin and inhibition of cell proliferation. EMBO J. 2005;24:1686–1695. doi: 10.1038/sj.emboj.7600647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Ben-Ze’ev A, Geiger B. Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signaling and cancer. Curr Opin Cell Biol. 1998;10:629–639. doi: 10.1016/s0955-0674(98)80039-2. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogen-esis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–132. doi: 10.1038/nrc1276. [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Niedermeyer J, Fuxa M, Christofori G. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol. 2001;3:650–657. doi: 10.1038/35083041. [DOI] [PubMed] [Google Scholar]
- Cavey M, Rauzi M, Lenne PF, Lecuit T. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature. 2008;453:751–756. doi: 10.1038/nature06953. [DOI] [PubMed] [Google Scholar]
- Charrasse S, Meriane M, Comunale F, Blangy A, Gauthier-Rouviere C. N-cadherin-dependent cell-cell contact regulates Rho GTPases and beta-catenin localization in mouse C2C12 myoblasts. J Cell Biol. 2002;158:953–965. doi: 10.1083/jcb.200202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitaev NA, Troyanovsky SM. Adhesive but not lateral E-cadherin complexes require calcium and catenins for their formation. J Cell Biol. 1998;142:837–846. doi: 10.1083/jcb.142.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci. 2008;121:2115–2122. doi: 10.1242/jcs.017897. [DOI] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, Vestweber D. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) EMBO J. 2001;20:3738–3748. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Ohno S, Vestweber D. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- Erez N, Zamir E, Gour BJ, Blaschuk OW, Geiger B. Induction of apoptosis in cultured endothelial cells by a cadherin antagonist peptide: involvement of fibroblast growth factor receptor-mediated signalling. Exp Cell Res. 2004;294:366–378. doi: 10.1016/j.yexcr.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Frankel P, Aronheim A, Kavanagh E, Balda MS, Matter K, Bunney TD, Marshall CJ. RalA interacts with ZONAB in a cell density-dependent manner and regulates its transcriptional activity. EMBO J. 2005;24:54–62. doi: 10.1038/sj.emboj.7600497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicano GI, Bauer C, Fuchs E. Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development. 2001;128:929–941. doi: 10.1242/dev.128.6.929. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Wolburg H, Redies C. N-cadherin mediates pericytic-endothelial interaction during brain angiogenesis in the chicken. Dev Dyn. 2000;218:472–479. doi: 10.1002/1097-0177(200007)218:3<472::AID-DVDY1008>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Arpin M, Fanning AS, Louvard D. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci USA. 1996;93:10779–10784. doi: 10.1073/pnas.93.20.10779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Ha CH, Bennett AM, Jin ZG. A novel role of vascular endothelial cadherin in modulating c-Src activation and downstream signaling of vascular endothelial growth factor. J Biol Chem. 2008;283:7261–7270. doi: 10.1074/jbc.M702881200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J, Umeda K, Tsukita S, Furuse M. Requirement of ZO-1 for the formation of belt-like adherens junctions during epithelial cell polarization. J Cell Biol. 2007;176:779–786. doi: 10.1083/jcb.200612080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan N, Madri JA. PECAM-1: old friend, new partners. Curr Opin Cell Biol. 2003;15:515–524. doi: 10.1016/s0955-0674(03)00100-5. [DOI] [PubMed] [Google Scholar]
- Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491–497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov D, Philippova M, Antropova J, Gubaeva F, Iljinskaya O, Tararak E, Bochkov V, Erne P, Resink T, Tkachuk V. Expression of cell adhesion molecule T-cadherin in the human vasculature. Histochem Cell Biol. 2001;115:231–242. doi: 10.1007/s004180100252. [DOI] [PubMed] [Google Scholar]
- Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimori T, Nabeshima YI, Noda T, Tsukita S. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol Biol Cell. 2008;19:2465–2475. doi: 10.1091/mbc.E07-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamagna C, Hodivala-Dilke KM, Imhof BA, Aurrand-Lions M. Antibody against junctional adhesion molecule-C inhibits angiogenesis and tumor growth. Cancer Res. 2005;65:5703–5710. doi: 10.1158/0008-5472.CAN-04-4012. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Dejana E. Interendothelial junctions: structure, signalling and functional roles. Curr Opin Cell Biol. 1997;9:674–682. doi: 10.1016/s0955-0674(97)80121-4. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Andriopoulou P, Esser S, Risau W, Dejana E. Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J Cell Sci. 1997;110:2065–2077. doi: 10.1242/jcs.110.17.2065. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell. 2002;13:1175–1189. doi: 10.1091/mbc.01-07-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Cavallaro U, Dejana E. The multiple languages of endothelial cell-to-cell communication. Arterioscler Thromb Vasc Biol. 2006;26:1431–1438. doi: 10.1161/01.ATV.0000218510.04541.5e. [DOI] [PubMed] [Google Scholar]
- Luo Y, Radice GL. N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J Cell Biol. 2005;169:29–34. doi: 10.1083/jcb.200411127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 2003;24:327–334. doi: 10.1016/s1471-4906(03)00117-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci. 2001;114:1829–1838. doi: 10.1242/jcs.114.10.1829. [DOI] [PubMed] [Google Scholar]
- Navarro P, Ruco L, Dejana E. Differential localization of VE-and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol. 1998;140:1475–1484. doi: 10.1083/jcb.140.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth R, Poell G, Ranft A, Kloep S, Samulowitz U, Fachinger G, Golding M, Shima DT, Deutsch U, Vestweber D. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. EMBO J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Veshnock PJ. Ankyrin binding to (Na+ + K+) ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987;328:533–536. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Pirone DM, Tan JL, Chen CS. Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA. Mol Biol Cell. 2004;15:2943–2953. doi: 10.1091/mbc.E03-10-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM, Jean RP, Tan JL, Liu WF, Sniadecki NJ, Spector AA, Chen CS. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci USA. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyqvist D, Giampietro C, Dejana E. Deciphering the functional role of endothelial junctions by using in vivo models. EMBO Rep. 2008;9:742–747. doi: 10.1038/embor.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, Proia RL, Hla T. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 2004;18:2392–2403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudini N, Felici A, Giampietro C, Lampugnani M, Corada M, Swirsding K, Garre M, Liebner S, Letarte M, ten Dijke P, Dejana E. VE-cadherin is a critical endothelial regulator of TGF-beta signalling. EMBO J. 2008;27:993–1004. doi: 10.1038/emboj.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Moll R, Kuhn C, Franke WW. Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells. II. Different types of lymphatic vessels. Differentiation. 1994;57:97–117. doi: 10.1046/j.1432-0436.1994.5720097.x. [DOI] [PubMed] [Google Scholar]
- Shen L, Weber CR, Turner JR. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J Cell Biol. 2008;181:683–695. doi: 10.1083/jcb.200711165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu M. Structural biochemical and functional differentiation of the vascular endothelium. In: Risau W, editor. Morphogenesis of the endothelium. Harwood Academic; Amsterdam: 2000. pp. 1–21. [Google Scholar]
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- Tillet E, Vittet D, Feraud O, Moore R, Kemler R, Huber P. N-cadherin deficiency impairs pericyte recruitment, and not endothelial differentiation or sprouting, in embryonic stem cell-derived angiogenesis. Exp Cell Res. 2005;310:392–400. doi: 10.1016/j.yexcr.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Traweger A, Lehner C, Farkas A, Krizbai IA, Tempfer H, Klement E, Guenther B, Bauer HC, Bauer H. Nuclear zonula occludens-2 alters gene expression and junctional stability in epithelial and endothelial cells. Differentiation. 2008;76:99–106. doi: 10.1111/j.1432-0436.2007.00227.x. [DOI] [PubMed] [Google Scholar]
- Vestweber D. Lymphocyte trafficking through blood and lymphatic vessels: more than just selectins, chemokines and integrins. Eur J Immunol. 2003;33:1361–1364. doi: 10.1002/eji.200324011. [DOI] [PubMed] [Google Scholar]
- Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P. Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci USA. 1997;94:6273–6278. doi: 10.1073/pnas.94.12.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778:794–809. doi: 10.1016/j.bbamem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Tsang LY, Haworth SG. Rac and Rho play opposing roles in the regulation of hypoxia/reoxygenation-induced permeability changes in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2005;288:L749–L760. doi: 10.1152/ajplung.00361.2004. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–337. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Hamm S, Wolburg-Buchholz K, Dehouck B, Dehouck MP, Liebner S, Furuse M, Cecchelli R, Risau B, Engelhardt B. Loss of claudin-3 but not of claudin-5 from cerebral endothelial tight junctions directly correlates with blood-brain barrier leakyness in vivo and in vitro. Eur J Physiol Suppl. 2002;443:262. [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Xu J, Kausalya PJ, Phua DC, Ali SM, Hossain Z, Hunziker W. Early embryonic lethality of mice lacking ZO-2, but not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol Cell Biol. 2008;28:1669–1678. doi: 10.1128/MCB.00891-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Pruschy M, Gumbiner BM. Lateral clustering of the adhesive ectodomain: a fundamental determinant of cadherin function. Curr Biol. 1997;7:308–315. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]
- Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondag GC, Evers EE, ten Klooster JP, Janssen L, van der Kammen RA, Collard JG. Oncogenic Ras downregulates Rac activity, which leads to increased Rho activity and epithelial-mesenchymal transition. J Cell Biol. 2000;149:775–782. doi: 10.1083/jcb.149.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]