Abstract
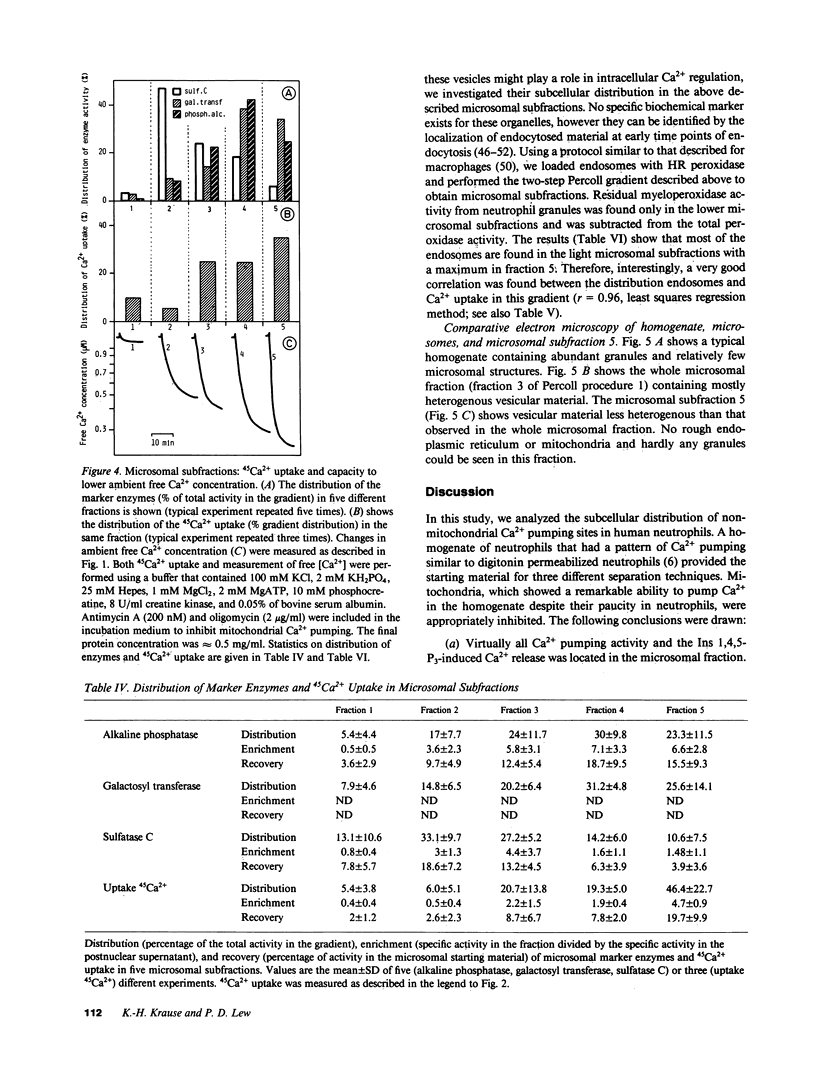
The distribution of nonmitochondrial Ca2+ pumping sites and the site of action of inositol 1,4,5-trisphosphate (Ins 1,4,5-P3) were studied in subcellular fractions of human neutrophils. In homogenates, two different Ca2+ pools could be observed: a mitochondrial Ca2+ pool and a nonmitochondrial, ATP-dependent, Ins 1,4,5-P3-responsive Ca2+ pool. When the homogenate was separated into microsomes, primary granules, and secondary granules, the nonmitochondrial Ca2+ pumping and the Ins 1,4,5-P3-induced Ca2+ release occurred only in the microsomal fraction. In a gradient developed to separate different microsomal organelles, maximal Ca2+ pumping activity occurred in fractions of low densities. Correlations between Ca2+ uptake and organelle markers were negative for the endoplasmic reticulum (r = -0.49) and positive for plasma membrane (r = 0.47), Golgi (r = 0.62), and endosomes (r = 0.96). Because the Ca2+ pumping organelles in these fractions were insensitive to micromolar vanadate and digitonin treatment, they are unlikely to be plasma membrane vesicles. We conclude first that microsomal fractions of human neutrophils contain organelles that lower the ambient free Ca2+ concentration and respond to Ins 1,4,5-P3. Second, granules are not involved in intracellular Ca2+ regulation in neutrophils. Third, nonendoplasmic reticulum organelles, such as endosomes, Golgi elements, or yet undefined specialized structures, play a major role in intracellular Ca2+ homeostasis in human neutrophils.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrein P. C., Stossel T. P. Prevention of degradation of human polymorphonuclear leukocyte proteins by diisopropylfluorophosphate. Blood. 1980 Sep;56(3):442–447. [PubMed] [Google Scholar]
- Bainton D. F., Ullyot J. L., Farquhar M. G. The development of neutrophilic polymorphonuclear leukocytes in human bone marrow. J Exp Med. 1971 Oct 1;134(4):907–934. doi: 10.1084/jem.134.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros F., Kaczorowski G. J. Mechanisms of Ca2+ transport in plasma membrane vesicles prepared from cultured pituitary cells. II. (Ca2+ + Mg2+)-ATPase-dependent Ca2+ transport activity. J Biol Chem. 1984 Aug 10;259(15):9404–9410. [PubMed] [Google Scholar]
- Bayerdörffer E., Streb H., Eckhardt L., Haase W., Schulz I. Characterization of calcium uptake into rough endoplasmic reticulum of rat pancreas. J Membr Biol. 1984;81(1):69–82. doi: 10.1007/BF01868811. [DOI] [PubMed] [Google Scholar]
- Becker G. L., Fiskum G., Lehninger A. L. Regulation of free Ca2+ by liver mitochondria and endoplasmic reticulum. J Biol Chem. 1980 Oct 10;255(19):9009–9012. [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Prentki M., Irvine R. F., Berridge M. J., Wollheim C. B. Inositol 1,4,5-trisphosphate mobilizes intracellular Ca2+ from permeabilized insulin-secreting cells. Biochem J. 1984 Oct 15;223(2):467–473. doi: 10.1042/bj2230467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B., Schlegel W. Inositol 1,4,5-trisphosphate and intracellular Ca2+ homeostasis in clonal pituitary cells (GH3). Translocation of Ca2+ into mitochondria from a functionally discrete portion of the nonmitochondrial store. J Biol Chem. 1986 Jun 5;261(16):7223–7229. [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bretz R., Stäubli W. Detergent influence on rat-liver galactosyltransferase activities towards different acceptors. Eur J Biochem. 1977 Jul 1;77(1):181–192. doi: 10.1111/j.1432-1033.1977.tb11656.x. [DOI] [PubMed] [Google Scholar]
- Burgess G. M., McKinney J. S., Irvine R. F., Putney J. W., Jr Inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate formation in Ca2+-mobilizing-hormone-activated cells. Biochem J. 1985 Nov 15;232(1):237–243. doi: 10.1042/bj2320237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Canonico P. G., Beaufay H., Nyssens-Jadin M. Analytical fractionation of mouse peritoneal macrophages: physical and biochemical properties of subcellular organelles from resident (unstimulated) and cultivated cells. J Reticuloendothel Soc. 1978 Aug;24(2):115–138. [PubMed] [Google Scholar]
- Clapper D. L., Lee H. C. Inositol trisphosphate induces calcium release from nonmitochondrial stores i sea urchin egg homogenates. J Biol Chem. 1985 Nov 15;260(26):13947–13954. [PubMed] [Google Scholar]
- Dawson A. P. Kinetic properties of the Ca2+-accumulation system of a rat liver microsomal fraction. Biochem J. 1982 Jul 15;206(1):73–79. doi: 10.1042/bj2060073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Cooper M. R. A modified procedure for the determination of leukocyte alkaline phosphatase. Biochem Med. 1970 Aug;4(1):61–68. doi: 10.1016/0006-2944(70)90103-1. [DOI] [PubMed] [Google Scholar]
- Dewald B., Bretz U., Baggiolini M. Release of gelatinase from a novel secretory compartment of human neutrophils. J Clin Invest. 1982 Sep;70(3):518–525. doi: 10.1172/JCI110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Vicentini L. M., Treves S., Riz G., Pozzan T. Inositol phosphate formation in fMet-Leu-Phe-stimulated human neutrophils does not require an increase in the cytosolic free Ca2+ concentration. Biochem J. 1985 Jul 15;229(2):361–367. doi: 10.1042/bj2290361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty R. W., Godfrey P. P., Hoyle P. C., Putney J. W., Jr, Freer R. J. Secretagogue-induced phosphoinositide metabolism in human leucocytes. Biochem J. 1984 Sep 1;222(2):307–314. doi: 10.1042/bj2220307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enyedi A., Sarkadi B., Földes-Papp Z., Monostory S., Gárdos G. Demonstration of two distinct calcium pumps in human platelet membrane vesicles. J Biol Chem. 1986 Jul 15;261(20):9558–9563. [PubMed] [Google Scholar]
- Epping R. J., Bygrave F. L. A procedure for the rapid isolation from rat liver of plasma membrane vesicles exhibiting Ca2+-transport and Ca2+-ATPase activities. Biochem J. 1984 Nov 1;223(3):733–745. doi: 10.1042/bj2230733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulski K., Carafoli E. Ca2+ transporting activity of membrane fractions isolated from the post-mitochondrial supernatant of rat liver. Cell Calcium. 1982 Aug;3(3):263–281. doi: 10.1016/0143-4160(82)90005-7. [DOI] [PubMed] [Google Scholar]
- Gennaro R., Pozzan T., Romeo D. Monitoring of cytosolic free Ca2+ in C5a-stimulated neutrophils: loss of receptor-modulated Ca2+ stores and Ca2+ uptake in granule-free cytoplasts. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1416–1420. doi: 10.1073/pnas.81.5.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A. K. Ca-pumps in smooth muscle: one in plasma membrane and another in endoplasmic reticulum. Cell Calcium. 1985 Jun;6(3):227–236. doi: 10.1016/0143-4160(85)90008-9. [DOI] [PubMed] [Google Scholar]
- Herzog V., Fahimi H. D. A new sensitive colorimetric assay for peroxidase using 3,3'-diaminobenzidine as hydrogen donor. Anal Biochem. 1973 Oct;55(2):554–562. doi: 10.1016/0003-2697(73)90144-9. [DOI] [PubMed] [Google Scholar]
- Joseph S. K., Williams R. J., Corkey B. E., Matschinsky F. M., Williamson J. R. The effect of inositol trisphosphate on Ca2+ fluxes in insulin-secreting tumor cells. J Biol Chem. 1984 Nov 10;259(21):12952–12955. [PubMed] [Google Scholar]
- Klemper M. S. An adenosine triphosphate-dependent calcium uptake pump in human neutrophil lysosomes. J Clin Invest. 1985 Jul;76(1):303–310. doi: 10.1172/JCI111961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempner M. S., Mikkelsen R. B., Corfman D. H., André-Schwartz J. Neutrophil plasma membranes. I. High-yield purification of human neutrophil plasma membrane vesicles by nitrogen cavitation and differential centrifugation. J Cell Biol. 1980 Jul;86(1):21–28. doi: 10.1083/jcb.86.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K. H., Schlegel W., Wollheim C. B., Andersson T., Waldvogel F. A., Lew P. D. Chemotactic peptide activation of human neutrophils and HL-60 cells. Pertussis toxin reveals correlation between inositol trisphosphate generation, calcium ion transients, and cellular activation. J Clin Invest. 1985 Oct;76(4):1348–1354. doi: 10.1172/JCI112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagast H., Lew P. D., Waldvogel F. A. Adenosine triphosphate-dependent calcium pump in the plasma membrane of guinea pig and human neutrophils. J Clin Invest. 1984 Jan;73(1):107–115. doi: 10.1172/JCI111180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Dayer J. M., Wollheim C. B., Pozzan T. Effect of leukotriene B4, prostaglandin E2 and arachidonic acid on cytosolic-free calcium in human neutrophils. FEBS Lett. 1984 Jan 23;166(1):44–48. doi: 10.1016/0014-5793(84)80041-1. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Pozzan T. Role of cytosolic free calcium and phospholipase C in leukotriene-B4-stimulated secretion in human neutrophils. Comparison with the chemotactic peptide formyl-methionyl-leucyl-phenylalanine. Eur J Biochem. 1987 Jan 2;162(1):161–168. doi: 10.1111/j.1432-1033.1987.tb10556.x. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Southwick F. S., Stossel T. P., Whitin J. C., Simons E., Cohen H. J. A variant of chronic granulomatous disease: deficient oxidative metabolism due to a low-affinity NADPH oxidase. N Engl J Med. 1981 Nov 26;305(22):1329–1333. doi: 10.1056/NEJM198111263052207. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Wollheim C. B., Waldvogel F. A., Pozzan T. Modulation of cytosolic-free calcium transients by changes in intracellular calcium-buffering capacity: correlation with exocytosis and O2-production in human neutrophils. J Cell Biol. 1984 Oct;99(4 Pt 1):1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. S., Steinman R. M., Unkeless J. C., Cohn Z. A. Selective iodination and polypeptide composition of pinocytic vesicles. J Cell Biol. 1980 Sep;86(3):712–722. doi: 10.1083/jcb.86.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L., Chen T., Knapp H. R., Jr, Landon E. J. Energy-dependent calcium sequestration activity in rat liver microsomes. J Biol Chem. 1975 Jun 25;250(12):4562–4568. [PubMed] [Google Scholar]
- Morcos N. C. Localization of (Ca2+ + Mg2+)-ATPase, Ca2+ pump and other ATPase activities in cardiac sarcolemma. Biochim Biophys Acta. 1982 Jun 28;688(3):747–756. doi: 10.1016/0005-2736(82)90288-7. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G., Scott I. D. The regulation of brain mitochondrial calcium-ion transport. The role of ATP in the discrimination between kinetic and membrane-potential-dependent calcium-ion efflux mechanisms. Biochem J. 1980 Mar 15;186(3):833–839. doi: 10.1042/bj1860833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D., Akerman K. Mitochondrial calcium transport. Biochim Biophys Acta. 1982 Sep 1;683(1):57–88. doi: 10.1016/0304-4173(82)90013-1. [DOI] [PubMed] [Google Scholar]
- O'Rourke F. A., Halenda S. P., Zavoico G. B., Feinstein M. B. Inositol 1,4,5-trisphosphate releases Ca2+ from a Ca2+-transporting membrane vesicle fraction derived from human platelets. J Biol Chem. 1985 Jan 25;260(2):956–962. [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Preissler M., Williams J. A. Localization of ATP-dependent calcium transport activity in mouse pancreatic microsomes. J Membr Biol. 1983;73(2):137–144. doi: 10.1007/BF01870437. [DOI] [PubMed] [Google Scholar]
- Prentki M., Biden T. J., Janjic D., Irvine R. F., Berridge M. J., Wollheim C. B. Rapid mobilization of Ca2+ from rat insulinoma microsomes by inositol-1,4,5-trisphosphate. Nature. 1984 Jun 7;309(5968):562–564. doi: 10.1038/309562a0. [DOI] [PubMed] [Google Scholar]
- Prentki M., Janjic D., Biden T. J., Blondel B., Wollheim C. B. Regulation of Ca2+ transport by isolated organelles of a rat insulinoma. Studies with endoplasmic reticulum and secretory granules. J Biol Chem. 1984 Aug 25;259(16):10118–10123. [PubMed] [Google Scholar]
- Prentki M., Janjic D., Wollheim C. B. The regulation of extramitochondrial steady state free Ca2+ concentration by rat insulinoma mitochondria. J Biol Chem. 1983 Jun 25;258(12):7597–7602. [PubMed] [Google Scholar]
- Prentki M., Wollheim C. B., Lew P. D. Ca2+ homeostasis in permeabilized human neutrophils. Characterization of Ca2+-sequestering pools and the action of inositol 1,4,5-triphosphate. J Biol Chem. 1984 Nov 25;259(22):13777–13782. [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Quintart J., Courtoy P. J., Baudhuin P. Receptor-mediated endocytosis in rat liver: purification and enzymic characterization of low density organelles involved in uptake of galactose-exposing proteins. J Cell Biol. 1984 Mar;98(3):877–884. doi: 10.1083/jcb.98.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M., Laharrague P., Fillola G., Thomas J., Ribes G., Fontan P., Chap H., Corberand J., Douste-Blazy L. A rapid isolation procedure of plasma membranes from human neutrophils using self-generating Percoll gradients. Importance of pH in avoiding contamination by intracellular membranes. Biochim Biophys Acta. 1985 Sep 25;819(1):1–9. doi: 10.1016/0005-2736(85)90188-9. [DOI] [PubMed] [Google Scholar]
- Rossier M. F., Krause K. H., Lew P. D., Capponi A. M., Vallotton M. B. Control of cytosolic free calcium by intracellular organelles in bovine adrenal glomerulosa cells. Effects of sodium and inositol 1,4,5-trisphosphate. J Biol Chem. 1987 Mar 25;262(9):4053–4058. [PubMed] [Google Scholar]
- Rubinoff M. J., Nellans H. N. Active calcium sequestration by intestinal microsomes. Stimulation by increased calcium load. J Biol Chem. 1985 Jul 5;260(13):7824–7828. [PubMed] [Google Scholar]
- STRAUS W. OCCURRENCE OF PHAGOSOMES AND PHAGO-LYSOSOMES IN DIFFERENT SEGMENTS OF THE NEPHRON IN RELATION TO THE REABSORPTION, TRANSPORT, DIGESTION, AND EXTRUSION OF INTRAVENOUSLY INJECTED HORSERADISH PEROXIDASE. J Cell Biol. 1964 Jun;21:295–308. doi: 10.1083/jcb.21.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott R. E., Horn R. G. Ultrastructural aspects of neutrophil granulocyte development in humans. Lab Invest. 1970 Aug;23(2):202–215. [PubMed] [Google Scholar]
- Smolen J. E., Korchak H. M., Weissmann G. The roles of extracellular and intracellular calcium in lysosomal enzyme release and superoxide anion generation by human neutrophils. Biochim Biophys Acta. 1981 Nov 5;677(3-4):512–520. doi: 10.1016/0304-4165(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. The interaction of soluble horseradish peroxidase with mouse peritoneal macrophages in vitro. J Cell Biol. 1972 Oct;55(1):186–204. doi: 10.1083/jcb.55.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta E. E., de Meis L. ATP-dependent calcium accumulation in brain microsomes. Enhancement by phosphate and oxalate. Biochim Biophys Acta. 1975 Jun 25;394(2):239–247. doi: 10.1016/0005-2736(75)90262-x. [DOI] [PubMed] [Google Scholar]
- Virk S. S., Kirk C. J., Shears S. B. Ca2+ transport and Ca2+-dependent ATP hydrolysis by Golgi vesicles from lactating rat mammary glands. Biochem J. 1985 Mar 15;226(3):741–748. doi: 10.1042/bj2260741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WURTMAN R. J., AXELROD J. A SENSITIVE AND SPECIFIC ASSAY FOR THE ESTIMATION OF MONOAMINE OXIDASE. Biochem Pharmacol. 1963 Dec;12:1439–1441. doi: 10.1016/0006-2952(63)90215-6. [DOI] [PubMed] [Google Scholar]
- Wibo M., Morel N., Godfraind T. Differentiation of Ca2+ pumps linked to plasma membrane and endoplasmic reticulum in the microsomal fraction from intestinal smooth muscle. Biochim Biophys Acta. 1981 Dec 21;649(3):651–660. doi: 10.1016/0005-2736(81)90170-x. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Cooper R. H., Joseph S. K., Thomas A. P. Inositol trisphosphate and diacylglycerol as intracellular second messengers in liver. Am J Physiol. 1985 Mar;248(3 Pt 1):C203–C216. doi: 10.1152/ajpcell.1985.248.3.C203. [DOI] [PubMed] [Google Scholar]
- Wuytack F., Raeymaekers L., Casteels R. The Ca2+-transport ATPases in smooth muscle. Experientia. 1985 Jul 15;41(7):900–905. doi: 10.1007/BF01970008. [DOI] [PubMed] [Google Scholar]