Abstract
Calcium and phosphate are the principle ions involved in the deposition of mineral in the human body. Inhibitors of mineralisation are essential for the prevention of ectopic mineral precipitation and deposition. In the past decade, through in vitro, in vivo and clinical observation studies, we have come to appreciate the importance of fetuin-A (Fet-A), a circulating glycoprotein, in preventing ectopic calcium phosphate mineralisation. Moreover, the detection of Fet-A-containing mineral complex, termed calciprotein particles (CPPs), has provided new ways to assess an individual's calcific risk. The pathophysiological significance of CPPs in disease states is yet to be defined, but it provides an exciting avenue to further our understanding of the development of ectopic mineralisation.
Introduction
The ability of the body to form calcified tissues is crucial to the maintenance of our structural integrity, but with mineralisation (or calcification) restricted to tissues such as bone, cartilage, dentin, cementum, enamel and otoconia. In bone and teeth, collagen I, the predominant protein in the extracellular matrix, serves as a scaffold onto which calcium (Ca), phosphate (Pi) and other ions crystalise as mineral apatite. This mineral–protein polymer gives rise to a unique material with excellent tensile and compressive strength.1
The concentrations of Ca and Pi in serum are supersaturated with respect to apatite. Therefore, one might expect that mineral nucleation and growth would occur in all extracellular fluid compartments, and thus the question with respect to biomineralisation is how the body regulates the process of crystal growth in bone and prevents crystal formation in extraosseous tissues. Failure to prevent ectopic mineralisation is common in conditions such as chronic kidney disease (CKD),2,3 chronic inflammatory disease (CID)4 and diabetes,5 and it is also seen as part of the ageing process.6
The body utilises a variety of mechanisms to control mineralisation. Inorganic molecules such as magnesium and pyrophosphate (PPi), proteins such as albumin, matrix GLA protein (MGP), osteopontin and fetuin-A (Fet-A), and pH modulate Ca Pi precipitation both at a tissue level and systemically.7,8,9,10,11,12,13,14,15,16 This review focuses on the liver-derived glycoprotein Fet-A (in humans known as α2-Heremans Schmid glycoprotein) and its role as an important systemic inhibitor of extraosseous mineralisation.17
Mineralisation from a Physicochemical Perspective
It has been a long-held view that the crystal precipitation from a solution containing Ca and Pi follows that of the classical nucleation theory (CNT). This theory proposes that in a supersaturated solution random fluctuations in particle density result in the formation of tiny crystal nucleus of the same molecular structure as the final macroscopic crystal.18 Crystal growth from the nucleus occurs via the addition of individual ions.
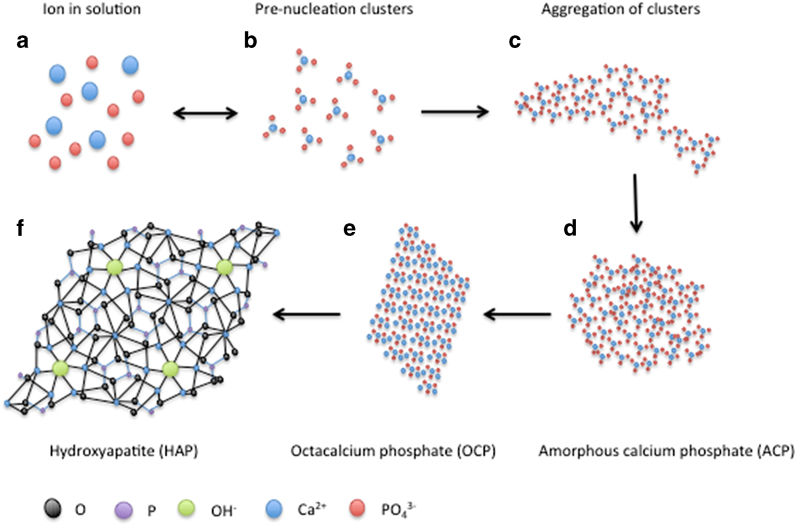
Recent studies using advanced imaging techniques such high-resolution cryo-transmission electron microscopy and atomic force microscopy have provided visual evidence of an alternative pathway to crystal nucleation, termed non-CNT (NCNT).19,20,21,22 In contrast to CNT, NCNT suggests that stable pre-nucleation complexes (PNC) exist in supersaturated solutions. In the case of Ca and Pi crystallisation, the PNC is Ca triphosphate (Ca(HPO4)3)4− (Figure 1), and its concentration in solution is in an equilibrium with the concentration of Ca and Pi ions. PNC tends to aggregate to form larger aggregates, and, over time, these aggregates coalesce into amorphous Ca Pi (ACP), an unstable phase without crystalline structure.22 The transformation (or ripening) from ACP to apatite (with a Ca:Pi ratio of 10:6) occurs with the further uptake of Ca ions and release of hydrogen ions. The final product, apatite (Ca10(PO4)6·(OH)2), is a thermodynamically stable crystal structure (Figure 1).
Figure 1.
The pathway of hydroxyapatite formation. Adapted from Habraken et al.22 with permission. A model of hydroxyapatite formation from PNC in solution. Free ions in solution (a) are in equilibrium with Ca triphosphate PNC (b). PNC aggregates (c), which then coalesce to become an ACP nucleus (d). With the addition of Ca ions and the loss of hydrogen ions, ACP transforms into OCP (e) and subsequently HAP (f), the most thermodynamically favourable state.
The speed in which crystals form in a supersaturated fluid such as the extracellular fluid depends on many factors. The degree of supersaturation has an obvious role, but inhibitors of the crystal ripening process are equally important to control physiological mineralisation and to prevent ectopic mineralisation.
Fet-A Inhibits Ca Pi Crystal Growth
Proteins modulate crystal formation in at least two ways. Albumin for example, reduces the supersaturation of serum by binding free Ca ions to its acidic amino acid residues and EF-hand-like motifs. However, this process is relatively inefficient to prevent crystal precipitation, as evidenced by the fact that despite its abundance, albumin accounts for only ∼50% of the mineralisation inhibitory activity of serum.23 Rather than binding to individual ions, Fet-A inhibits Ca Pi crystal growth by binding to clusters of Ca Pi ions, acting as a barrier to further cluster aggregation.24 The extensive computational modelling and mutant analysis work by the Jahnen–Dechent group has demonstrated that the β-sheet on the exposed surface of Fet-A is crucial to its ability to bind mineral.24 This β-sheet contains regularly spaced negatively charged acidic amino acids in a lattice-like conformation.24 Each acidic residue on the protein interacts with a Ca ion on the surface of a Ca Pi cluster, which reduces the available surface area for further cluster aggregation. In contrast to albumin, Fet-A circulates at around one-hundredth the concentration of albumin in adult human serum,25 but it is at least 10-fold more efficient in its capacity to inhibit mineral precipitation in a supersaturated solution24 (Figure 2).
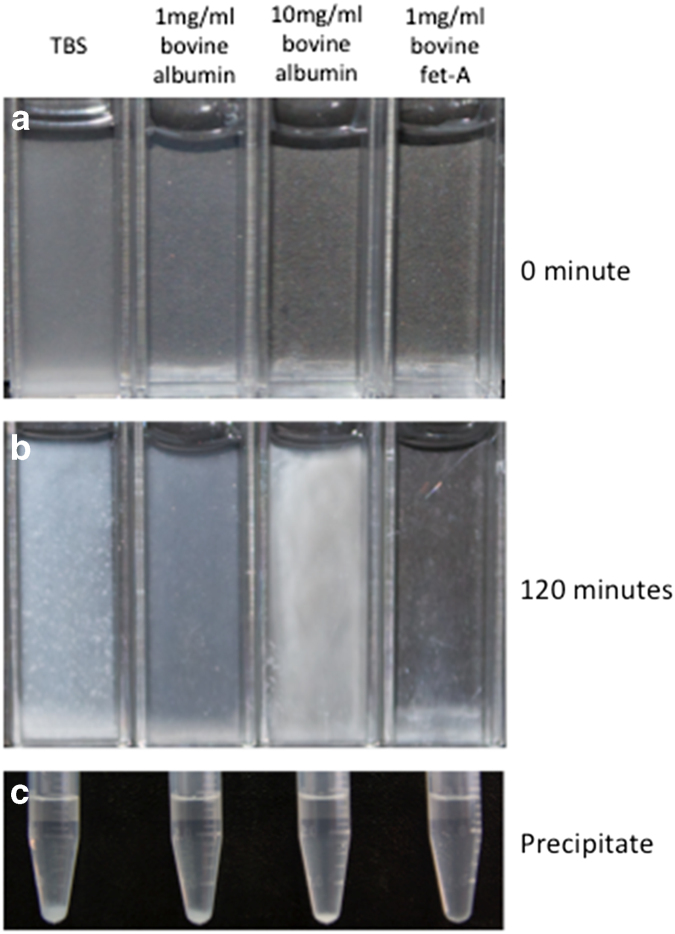
Figure 2.
Fet-A is a potent inhibitor of Ca Pi precipitation in solution. Fet-A, fetuin-A. In a precipitation experiment, precipitation was instantly visible in Tris-buffered saline containing 10 mM CaCl2 and 6 mM NaH2PO4 (a). After 120 minutes, the same solution spiked with 1 mgml−1 bovine fet-A remains transparent where as precipitates are visible in albumin solutions at 1mgml−1 and 10 mgml−1 (b). Aliquots of the solutions are then centrifuged at 1500g for 5 minutes at 4°C, pellets are visible in all solutions except for the fet-A containing solution (c).
Formation of Colloidal Calciprotein Particles
Fet-A inhibits Ca Pi precipitation in two steps. First, Fet-A binds to subnanometer-sized complexes of Ca and Pi, forming an entity termed calciprotein monomers (CPM).26,27 If the solution remains supersaturated despite CPM formation, the thermodynamic drive for crystal nucleation remains and the ACP (see earlier) mineral phase emerges. At this point, it has been suggested that multiple Fet-A molecules, presumably through their binding with Ca ions, ‘coat' the exterior surfaces of ACP to form primary calciprotein particles (CPP1).26 This Fet-A ‘shield' appears to stabilise ACP and retards its progression to more crystalline mineral phases. Over time, however, the particles aggregate and develop crystalline structures to form secondary CPPs (CPP2),28 which have the mineral signatures of octacalcium phosphate or apatite27,29 (Figure 3).
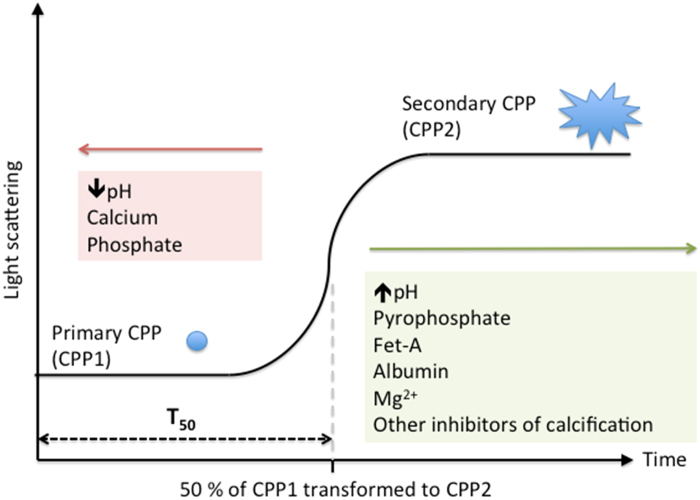
Figure 3.
Formation and transformation of CPPs in a supersaturated solution. CPP, calciprotein particle; Fet-A, fetuin-A. Adapted from Pasch et al.101 with permission. In the T50 test, a fixed amount of Ca and Pi stock solution is added to serum to induce supersaturation (10 mM Ca2+ and 6 mM PO43-). Small (~60 nm), amorphous and spherical particles (CPP1) appear immediately but are only transiently stable. After a lag period, CPP1 transforms to larger and more crystalline particles (CPP2). T50 measures the time taken for light scattering intensity to reach its half-maximal read out. Calcification inhibitors delay the transformation from CPP1 to CPP2 (green box), substrates of apatite formation and acidosis accelerates this process (red box).
The ability of proteins to inhibit mineral growth by binding to Ca Pi clusters is not unique to Fet-A. Phosphorylated proteins such as osteopontin30 and dentin matrix protein-131 are two examples of other proteins with apatite growth inhibitory activities in vitro, possibly via similar Ca Pi cluster binding mechanisms. Fet-A differs from these proteins in that protein phosphorylation is not required for its mineralisation inhibitory activity.15,24,25 Furthermore, Fet-A appears to be the only such protein that accumulates in calcified tissue but which is not synthesised locally.32,33
Modulation of Skeletal Mineralisation by Fet-A
Given that at a molecular level Fet-A inhibits apatite formation and growth, it is somewhat counterintuitive that Fet-A has also been shown to promote collagen I matrix mineralisation in cell-free conditions. A series of experiments by Price et al.34,35,36 have shown that serum alone can mineralise collagen I matrix, and such ability to induce matrix mineralisation is dependent on the presence of Fet-A in serum.37,38 It is thought that Fet-A limits mineral formation outside collagen I matrix, constraining mineralisation to the small intrafibrillar space (0.3–0.6 nm in width) where only small molecules such as Ca and Pi can readily access. The fluid within the intrafibrillar space remains supersaturated, but in the absence of a potent inhibitor mineral precipitation is strongly favoured.34
In cell culture, Fet-A has been shown to inhibit osteoblast apoptosis by preventing apatite formation. These experiments used a serum-free ‘osteogenic' medium containing supraphysiological Pi concentration.15 The high Pi concentration in culture media without the presence of inhibitors of mineralisation results in the formation of apatite crystals that are cytotoxic,29,39,40 possibly by activation of inflammasome pathways.41 Therefore, in an in vitro ‘osteogenic environment', Fet-A appears to promote cell survival by inhibiting apatite formation in the extracellular fluid. Although Fet-A may prolong survival of osteoblasts, it may also reduce osteoblast recruitment by inhibiting key signalling pathways of osteoblast differentiation from mesenchymal stem cells. Binkert et al. have shown that an Fet-A can bind to transforming growth factor-β and bone morphogenetic proteins (BMPs), cytokines crucial for osteoblastic differentiation.42 At supraphysiological doses (30 μM, ∼1.5 g l−1), Fet-A completely inhibited osteoblast maturation.42 In summary, these studies suggest that Fet-A may have different effects on in vitro mineralisation depending on the experimental conditions.
It would be hoped that an animal knockout model would help clarify some of the discrepancies from in vitro studies. However, Fet-A knockout models add further confusion to the interpretation of the cell-free and in vitro work. Fet-A knockout mice (Ahsg−/−) have a normal skeletal phenotype at birth. Ahsg−/− adult mice, however, have shortened long bones with increased mineralisation of growth plates and cortical thickness.43,44 Bone strength and mineral content are increased in Ahsg−/− in one study, but not in a subsequent experiment using animals from a different genetic background.43,44 To further complicate the picture, Fet-A heterozygotes (Ahsg+/−) did not exhibit an intermediate phenotype. In fact, reduction in serum Fet-A by two- to threefold reduced the mineralisation surface, the mineral formation rate and increased mineralisation lag time compared with wild-type or Ahsg−/− mice.43 A possible explanation for the discrepancy between the cell-free experiments and in vivo findings is that the production of local mineralisation inhibitors such as osteopontin (OPN) is upregulated in the absence of Fet-A, which can substitute for Fet-A as a modulator of local mineralisation, allowing preferential crystallisation within collagen I matrix.45 Taken together, the in vivo data suggest that bone mineralisation can occur in the absence of Fet-A with no significant compromise to its mechanical properties.
In clinical studies, the association of serum Fet-A concentration and bone mineral density (BMD) is also conflicting. As summarised in Table 1, higher serum Fet-A has been found to have either positive or no association with BMD, but no inverse associations have been reported thus far. The positive association between Fet-A and BMD is more convincingly demonstrated in women, and recent evidence suggests that oestrogen supplementation is associated with higher serum Fet-A levels,46 but the direction of causality, if any, of this relationship has yet to be demonstrated. Furthermore, in a large prospective observation cohort of subjects >65 years, baseline plasma Fet-A was not predictive of fracture risk.47
Table 1. Studies with reported associations between serum Fet-A and BMD.
| Source | Cohort | N | Association between BMD and serum fet-A |
|---|---|---|---|
| Fink et al.47 | Individuals aged >65 years | 4714 | No association with BMD after full multivariate adjustment. |
| Chailurkit et al.103 | Healthy elderly women | 82 | High fet-A was associated with increased L2–4 BMD, but not associated with femoral neck BMD |
| Ix et al.104 | Healthy individuals aged 70–79 years | 508 | High fet-A was associated with increased BMD across different sites in women. No significant association was detected in men. |
| Kirkpantur et al.105 | Haemodialysis patients | 72 | High fet-A was associated with increased BMD across different sites except for lumbar vertebrae. |
| Fiore et al.106 | Patients with established atherosclerosis | 90 | No association with BMD |
| Wilund et al.107 | Sedentary older adults | 12 | No association with BMD |
| Avila et al.108 | Prevalent female dialysis patients | 197 | Patients with a T score >−1.0 had higher fet-A compared with those with a T score ⩽−1.0. |
| Sari and Uslu109 | Postmenopausal women | 90 | Positive association with lumbar and femoral BMD |
| Sritara et al.110 | Healthy volunteers | 1741 | No association with BMD |
Abbreviations: BMD, bone mineral density; fet-A, fetuin-A.
Modulation of Ectopic Mineralisation by Fet-A
The spatial restriction of mineralisation to bone and teeth is sometimes lost in disease processes. Ca Pi deposits are commonly found within atherosclerotic plaques. In the elderly and in patients with CKD, diabetes and CID, ectopic mineralisation is often present in the tunica media of arteries. In patients with calciphylaxis, mineral deposits can be found also in the skin, fat and other soft tissues. Histological examinations have shown that Fet-A is intimately associated ectopic Ca Pi deposits. This is seen in calcified arterial wall,48,49,50,51,52 in ectopic joint mineralisation53 and in calcified breast implant capsules.54 Fet-A is notably absent however, in uncalcified areas, even in patients with end-stage kidney disease.48,50
Similar to osteoblast cell culture, vascular smooth muscle cell (VSMC) culture matrix mineralisation can be induced by supraphysiological concentrations of Ca and Pi ions.48 Under these conditions, Fet-A has been shown to inhibit such mineralisation in a dose-dependent manner.48,50 A reduction in the number of calcific nidi from apoptotic bodies and inhibition of mineral growth of matrix vesicles released from VSMC are again thought to account for the reduction in matrix mineralisation under these conditions.48
The ability of Fet-A to inhibit ectopic mineralisation in vivo was demonstrated in mouse knockout models. Ahsg−/− mice on the calcification-prone DBA-2 genetic background resulted in extensive ectopic mineralisation affecting virtually all organs.17,55 Similar extensive ectopic mineralisation involving the kidney, heart and lungs were seen in Ahsg−/− on the calcification-resistant C57BL/6 genetic background, when these animals were challenged with a high-Pi diet and either vitamin D supplementation17 or with the induction of CKD through renal ablation.56 With respect to vascular calcification, Ahsg−/− mice crossed with atherosclerotic-prone ApoE−/− mice developed increased aortic intimal mineralisation, but this was only apparent in nephrectomised mice fed a high-Pi diet.45 Despite these impressive phenotypes, two features are worth further consideration. First, Ahsg−/− mice do not display an overt calcific phenotype unless combined or challenged with other pro-calcific factors. This is seen in Ahsg−/− mice with a calcification-resistant background in which no significant ectopic mineralisation was seen in the absence of a high-Pi diet. Although the DBA/2 Ahsg−/− mice exhibited spontaneous ectopic calcification without exogenous stimuli, the DBA/2 strain itself is deficient in PPi,57,58 another potent inhibitor of mineralisation. This suggests that under physiological circumstances Fet-A is not absolutely required to inhibit extraosseous mineralisation, but its importance is evident when mineral metabolism is perturbed or when redundant mechanisms against ectopic mineralisation fail. The second important observation is that vascular medial mineralisation was not a prominent feature associated with Ahsg−/− mice, even in those with CKD fed a high-Pi diet.17,45,56 The preference for intimal mineralisation in Ahsg−/− mice differs from the exclusive medial mineralisation seen in other calcification inhibitor knockout models such as the Mgp−/− mice,59 and the PPi-deficient ENPP−/− mice.60 The reason for these phenotypic differences is unclear at this stage, but it suggests that Ahsg−/−mice may be a more suitable model to study endothelial injury and arterial intimal mineralisation.
Association Between Fet-A and Adverse Outcomes
The use of serum Fet-A as a risk marker was first studied in a haemodialysis cohort61 and subsequently replicated in patients on peritoneal dialysis and on patients with CKD, hypertension, diabetes and coronary heart disease (see Table 2 for the respective studies and references). A reduction in serum Fet-A may reflect decreased synthesis and consequent reduced capacity to inhibit extraosseous mineralisation, but it may also reflect the degree of extraosseous mineralisation owing to a ‘consumptive phenomenon' in which Fet-A is removed from the circulation and sequestered in areas of mineral deposition.58,62 As summarised in Table 2, although the majority of studies suggest an inverse association between serum Fet-A and adverse outcomes, several studies find no relationship or even positive associations. The discrepancies may stem from the fact that serum Fet-A is raised in obesity and insulin resistance,63 conditions that in themselves are associated with adverse outcomes.
Table 2. Clinical studies assessing the relationship between total serum Fet-A, vascular parameters and patient outcome (updated from Hamano et al. 64).
| Source | Cohort | N |
Association between adverse outcomes and total serum fet-A |
|
|---|---|---|---|---|
| Univariate association | Significant after MVA? | |||
| Kettler et al.61 | HD patients | 312 | Low serum fet-A was associated with inflammation, cardiovascular and all-cause mortality | No |
| Mehrotra et al.111 | Patients with type II diabetes | 88 | Low serum fet-A was associated with CACS in patients with diabetic nephropathy | Yes |
| Moe et al.50 | Patients with ESKD | 51 | Low serum fet-A was associated with CACS, but not aortic calcification | NP |
| Stenvinkel et al.112 | Incident dialysis patients | 256 | Low serum fet-A was associated with malnutrition, inflammation, atherosclerosis, cardiovascular and all-cause mortality | Yes |
| Wang et al.113 | PD patients | 238 | Low serum fet-A was associated with valvular calcification | Yes |
| Honda et al. | Patients with ESKD | 176 | Low serum fet-A was associated with mortality | Yes |
| Hermans et al.114 | Prevalent dialysis patients | 131 | Fet-A was inversely associated with increased APWV in univariate analysis | No |
| Jung et al.115 | HD patients | 40 | Fet-A was not associated with CACS | No |
| Cozzolino et al.116 | HD patients | 115 | Low fet-A was associated with increased CACS | Yes |
| Mori et al.117 | Healthy subjects | 141 | Higher fet-A was associated with increased carotid artery stiffness | Yes |
| Russo et al.118 | Pre-dialysis CKD patients | 53 | Low fet-A was associated with increased CACS | NP |
| Hermans et al.119 | HD and PD patients | 987 | Low fet-A was a predictor of overall mortality | Yes |
| Ix et al.120 | Patients with CAD | 970 | Low fet-A was associated with mitral annular calcification and with aortic stenosis in patients with diabetes | Yes |
| Ix et al.121 | Stages 3–4 CKD patients | 822 | Fet-A was not associated with all-cause or cardiovascular mortality | No |
| Mikami et al.122 | Patients with diabetic nephropathy | 85 | Fet-A was not associated with CACS | No |
| Shroff et al.123 | Children on dialysis | 61 | Low serum fet-A was associated with higher APWV | Yes |
| Metry et al.124 | HD patients | 222 | Low serum fet-A was associated with mortality | No |
| Zheng et al.125 | African-American HD patients | 17 | Serum fet-A was inversely associated with CACS | NP |
| Hamano et al.64 | Pre-dialysis CKD patients | 73 | Serum fet-A was not associated with CACS | NP |
| Lorant et al.126 | Patients with type II diabetes | 76 | Lower serum fet-A was associated with increased prevalence of PAD in patients | Yes |
| Mori et al.127 | Patients undergoing coronary angiography | 92 | Lower serum fet-A was associated with CAC | Yes |
| Lim et al.128 | Patients post acute myocardial infarction | 754 | Lower serum fet-A was associated with poorer 1 year survival | Yes |
| Marechal et al.129 | Renal transplant recipients | 277 | Lower serum fet-A was associated with aortic calcification and cardiovascular events | Yes |
| Pateinakis et al.130 | Haemodialysis patients | 81 | Lower serum fet-A was independently associated with increased APWV but not cIMT | No |
| Guarneri et al.131 | Patients with essential hypertension | 105 | Lower serum fet-A was independently associated with increased cIMT | Yes |
| Scialla et al.90 | Incident dialysis patients | 602 | The lowest serum fet-A tertile was associated with a significant increase in cardiovascular mortality risk | No |
| Jung et al.132 | PD patients | 67 | Lower serum fet-A was independently associated with increased APWV | Yes |
| Roos et al.133 | Patient post acute coronary syndrome | 1049 | Serum fet-A was not predictive of subsequent cardiovascular events | No |
| Emoto et al.134 | Type II diabetes without significant renal impairment | 416 | Patients with calcified carotid plaques had lower serum fet-A compared with those without calcified carotid plaques. | Yes |
| Rittig et al.135 | Patients at an increased risk of diabetes | 315 | Plasma fet-A was positively associated with cIMT. | Yes |
| Jensen et al.136 | Elderly (>65 years old) subjects | 3810 | Higher serum fet-A was associated with lower incident cardiovascular disease only in non-obese individuals. | Yes |
| Kaess et al.137 | Community cohort without pre-existing CVD | 1870 | Fet-A was not associated with CACS | No |
| Ford et al.138 | Non-diabetic stage 3 and stage 4 CKD patients | 73 | Lower plasma fet-A predicted increase in APWV after 1 year | Yes |
| Smith et al.65 | Stage 3 and stage 4 CKD patients | 184 | Total serum fet-A was not predictive of all-cause mortality | No |
Abbreviations: APWV, aortic pulse-wave velocity; CACS, coronary artery calcification score; CAD, coronary artery disease; cIMT, carotid intimal media thickness; CKD, chronic kidney disease; CVD, cardiovascular diseases; ESKD, end-stage kidney disease; fet-A, fetuin-A; HD, haemodialysis; MVA, multivariate analysis; NP, not performed; PAD, peripheral artery disease; PD, peritoneal dialysis.
Association Between CPPs and Adverse Outcomes
More recent data suggest that total immunoreactive serum Fet-A may not be the most clinically relevant measurement with respect to pathology.64,65 Circulating Fet-A may exist as free protein measured in the circulation of normal adults with a concentration of ∼0.3 mg l−1,66 but in pathological conditions Fet-A is also bound to circulating CPPs,25,64,66 which make up a variable percentage of the total circulating Fet-A pool.
Owing to the higher density and size of CPPs, Fet-A bound to CPPs is readily detectable by differential centrifugation, or by ultrafiltration of body fluids.25,64 Circulating CPPs in serum were initially discovered in etidronate-treated rats with profound hypercalcaemia (4.3 mM) and hyperphosphataemia (5.1 mM).67 A high-molecular-weight species in serum containing Fet-A, MGP, Ca and Pi was detected by the above-mentioned methods.67 Subsequent studies have demonstrated that a similar high-molecular-weight species containing Fet-A (without MGP) was present in the serum of rats with adenine-induced renal failure68 and in the sera of patients with CKD and CID,25,66 but it is notably undetectable in the serum of normal adults.25,64,66 Interestingly, especially high levels of CPPs were recorded in dialysis patients with calciphylaxis.66,69 We characterised the morphology of these particles in serum of dialysis patients as CPP2.29 Although it is conceivable that in the aforementioned animal models CPPs may form in the intravascular space as a result of very high Ca and Pi concentrations, patients with moderate CKD and CID often have Ca and Pi concentrations within their respective reference intervals where spontaneous in situ formation in serum seems improbable. The origin of circulating CPPs remains unknown, but the fact that bisphosphonates, parathyroid hormone, vitamin D and osteoprotegerin are associated with serum CPPs64,70 suggests that bone is the most likely candidate. CPP-like species have also been detected in the spent dialysate of some patients undergoing peritoneal dialysis.28,71
In CKD, serum CPPs are associated with coronary artery calcification,64 increased aortic stiffness25 and is predictive of all-cause mortality.65 The well-known cardiovascular risk factor of inflammation becomes important here, as the independent predictive power of CPPs is attenuated, when high-sensitivity C-reactive protein is entered into the multivariate model.65 This is interesting, as CPPs are also detected in patients with inflammation but normal renal function,72 implying that inflammation per se may be important in the formation of CPPs.
Are CPPs Pathogenic?
Although the association between circulating CPPs and diseases is interesting, whether these particles are themselves pathogenic is unclear. Existing publications suggest that the core component of CPPs, Ca Pi nanocrystals, can be toxic when applied to cultured cell lines.10,40,73,74,75,76,77,78,79,80,81,82,83,84,85 Furthermore, the nanocrystal shape and size modulated their cytotoxic effects.40,76,77,78 It is important to point out that the particles used in these studies were synthesised in a protein-free environment, but the CPPs, as detected in serum, are synthesised in a protein-rich environment in vivo.25,64 Theoretically, proteins can modulate the cytotoxicity of Ca Pi nanocrystals in at least two ways. Proteins such as Fet-A reduce aggregation and ripening of nanocrystals, thereby reducing their cytotoxicity. In addition to a given protein's physicochemical effects on crystal formation, the intracellular uptake of proteins, compared with unbound protein, is enhanced when proteins are bound to these nanocrystals.86 The intracellular fate and effects of these proteins bound to CPPs is uncertain, but it is possible that they may exert additional effects on cells.
Murine studies have shown that cells of the reticuloendothelial system remove CPPs from the circulation partly via the cell surface class A scavenger receptor.86 Macrophage uptake studies suggest that CPP-associated Fet-A is taken up much more readily than free Fet-A, suggesting that Fet-A is not the ligand responsible for macrophage endocytosis.29,86 It is possible that other proteins associated with CPPs, such as OPN, may facilitate macrophage uptake of these particles.87,88 Regardless of the exact mechanism of cell entry, CPP-induced proinflammatory cytokines release at high doses,29,39 although the ‘Fet-A shell' appears to dampen the inflammatory response to otherwise naked Ca Pi nanocrystals. Similar results have been replicated in VSMC culture.10 It is therefore an oversimplication to categorise CPPs as ‘good' or ‘bad' particles. In vitro and clinical data suggest that the presence of CPPs (compared with its absence) is associated with cytotoxicity and adverse clinical outcomes, but this is likely an effect mediated by its mineral core. The protein components, on the other hand, actually protect cells from harmful effects of the mineral core.
Are CPPs the Mediators of in vitro ‘Pi Toxicity'?
There is strong epidemiological evidence to suggest that high serum Pi is associated with adverse outcomes in patients with CKD.89,90,91 The importance of Pi in mediating adverse outcomes is backed by observations that increased Pi intake induces vascular calcification in animal models.17,56,92,93 In vitro experiments have also suggested that increased extracellular Pi may induce VSMC to express markers associated with osteoblastic/chrondrocytic-like cells, possibly via the Type III sodium-dependent Pi co-transporter (Pit-1).94 It is worth considering that the effect ascribed to a high extracellular Pi in in vitro studies may be partly or entirely owing to the effect of CPPs that form in this environment, rather than free Pi ions per se. Increased Pi concentration in cell culture medium is often achieved by adding a small volume of a concentrated stock Pi solution. The high Pi concentration at the point of contact between stock Pi solution and medium may result in the nucleation of Ca Pi mineral phases and subsequent formation of CPPs. Indeed, CPPs can spontaneously form in cell culture medium after prolonged incubation,95 and the addition of Pi (and Ca) would only serve to hasten the formation of CPPs in cell culture environments.96 This phenomenon has been experimentally verified by Sage et al., reporting that the addition of Pi resulted in nanocrystal formation with morphology similar to CPP1. The cellular effects of ‘high Pi' such as increased BMP-2 and OPN synthesis is primarily mediated by the effect of nanocrystals on cells.97 Furthermore, experiments often use a Pit-1 antagonist, phosphonoformic acid (PFA), to demonstrate that reducing intracellular Pi uptake in response to high extracellular Pi can reverse the cellular effects of high extracellular Pi. However, PFA is also a potent inhibitor of Ca Pi crystal formation.98 Therefore, the effect of PFA on cells could be also mediated via its inhibition of crystal formation, rather than via its blockade of Pit-1. In other in vitro work, increased Pi has been shown to induce VSMC autophagy99 and endothelial apoptosis;100 it is possible that these effects are also meditated via the formation of CPP in vitro. Future studies are needed to distinguish the cellular effect Pi ions from those of CPPs generated in vitro.
Serum Calcification Propensity
In parallel to the work on the biological effects of CPPs, understanding the mechanics of CPP formation has also paved the way to the development of a novel test, serum calcification propensity or the T50 test.101 Biomarkers in routine clinical practice frequently rely on the presence and concentration of a particular substance. Functional assays, however, are more appropriate in some instances, and an example of this is the prothrombin time (PT). PT assesses the time taken for a plasma sample to clot after the addition of thromboplastin, an activator of the extrinsic coagulation cascade. PT is therefore a functional study assessing the cumulative effect of the various pro- and anti-coagulants in plasma. A similar concept, based on CPP biophysics, has been developed to determine the ability of the serum to resist crystal apatite formation. When large amounts of Ca and Pi are added to serum, CPP1 are formed. The lag time between the formation of CPP1 and its transformation to CPP2 is dependent on a number of parameters (Figure 3): pH, temperature and the concentration of Fet-A, Ca and Pi ions.28,65,101 In a complex environment such as the extracellular compartment, the duration is also prolonged by the presence of other inhibitors of Ca Pi crystal growth such as PPi and magnesium ions.65,101
The lag time (T50) is the time taken for 50% of CPP1 to transform into CPP2, as determined by their difference in size and ability to scatter light. Thus, T50 is analogous to PT in that it does not give information about any single factor, but rather reflects on the overall balance between pro- and anti-calcific factors in serum; that is, longer lag time suggests that the serum contains lower levels of pro-calcific factors or more anti-calcific factors.65 The difference in light scattering from CPP1 to CPP2 can be detected via either three-dimensional dynamic light scattering or by nephelometry.28,101 A photographic representation of the concept is shown in Figure 4.
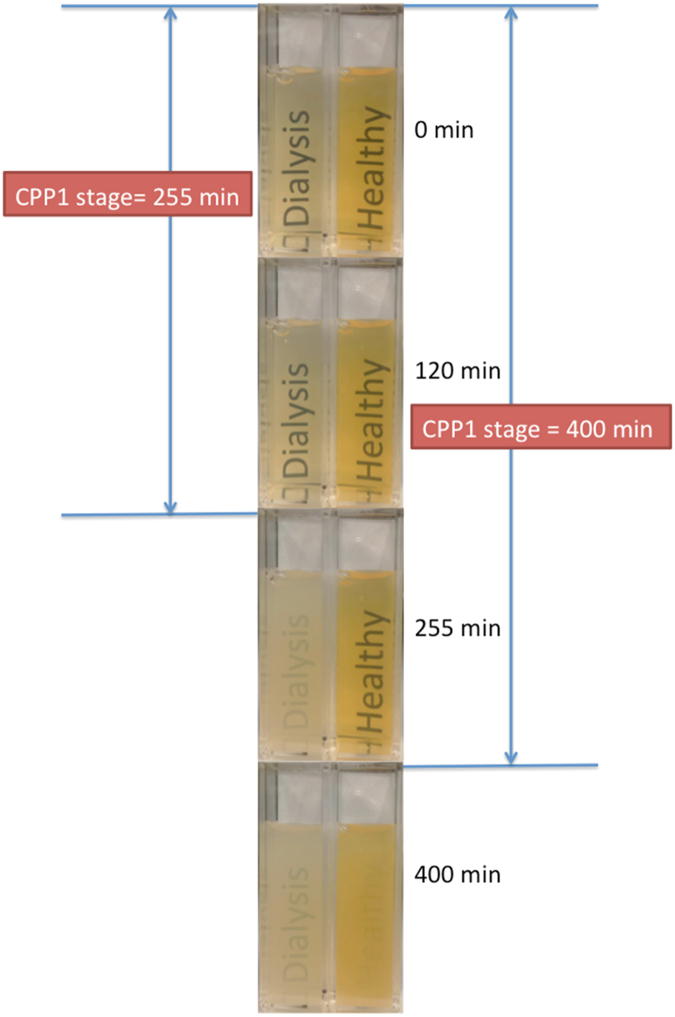
Figure 4.
A conceptual representation of the T50 test. CPP, calciprotein particle; T50, lag time. Protocol adapted from Pasch et al.101. To form a precipitation mix, Ca and Pi stock solution was added to serum of a healthy volunteer (right) and a dialysis patient (left) and incubated at 37°C. A standard flash enabled camera was used to capture the images obtained at the given time intervals. CPP1 stage appears translucent due to a relatively small degree of light scattering. After 255 minutes, the dialysis patient sample becomes opaque, indicating that CPP1 has transformed into CPP2, but the sample from the healthy volunteer remained translucent until 400 min, when it underwent a similar change in opacity.
The advantage of this assay is that it captures various known (and possibly unknown) factors that modulate mineralisation. We have shown that in a pre-dialysis CKD cohort T50 is inversely associated with known promoters of mineralisation such as ionised Ca and Pi, but it is positively associated with inhibitors of mineralisation such as PPi and serum Fet-A.65 In the same cohort, a lower baseline T50 is associated with increased aortic pulse-wave velocity and increased inflammatory markers. Furthermore, a lower T50 was found to predict all-cause mortality, even after adjustment for routine clinical and biochemical parameters.65 Similar results have recently been replicated in renal transplant recipients.102 The T50 assay has the potential to revolutionise the assessment of ‘calcific risk', but its availability is limited and not completely physiological, as it relies on adding large amounts Ca and Pi ions to reliably induce a highly supersaturated environment.101 It is also important to note that unlike the serum CPPs detected in patients with CKD and CID, the CPPs generated in the T50 assay represent a ‘test tube' phenomenon.
Summary and Conclusions
Through evolution, vertebrates have developed sophisticated mechanisms to restrict physiological mineralisation to bone and teeth. Fet-A is one of a number of overlapping factors that limit Ca Pi mineralisation in extraosseous tissues, while permitting physiological ossification. The study of Fet-A and its interaction with mineral has revealed a new paradigm to understand and assess the effect of disturbed Ca and Pi metabolism. Ectopic mineralisation is found in many disease states, but it is especially prevalent in those with CKD. Although the mainstay of therapy of ectopic mineralisation is currently focused on controlling serum Ca and Pi levels, finding ways to support inhibitors of mineralisation may be of equal importance in the treatment of unwanted mineralisation.
Acknowledgments
MMXC is a recipient of a joint National Health and Medical Research Council (NHMRC) and Jacquot Foundation Postgraduate Research Scholarship. ERS has received research funding from Monash University, The Royal Melbourne Hospital Foundation, Amgen and Baxter, honoraria from Shire, and served as a consultant for Vifor Pharma. SGH has received research funding from the Jacquot Foundation, the Kinkaid-Smith fund and research funding or honoraria from Amgen, Baxter, Gilead, Novartis and Shire. The opinions expressed by this article are solely those of the individual authors and do not reflect the views of NHMRC or the Jacquot Foundation.
Footnotes
The authors declare no conflict of interest.
References
- Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int 2006; 17: 319–336. [DOI] [PubMed] [Google Scholar]
- Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC. Electron beam computed tomography in the evaluation of cardiac calcifications in chronic dialysis patients. Am J Kidney Dis 1996; 27: 394–401. [DOI] [PubMed] [Google Scholar]
- Raggi P, Boulay A, Chasan-Taber S, Amin N, Dillon M, Burke SK et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol 2002; 39: 695–701. [DOI] [PubMed] [Google Scholar]
- Wang S, Yiu K-H, Mok M-Y, Ooi GC, Khong P-L, Mak K-FH et al. Prevalence and extent of calcification over aorta, coronary and carotid arteries in patients with rheumatoid arthritis. J Intern Med 2009; 266: 445–452. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J et al. Effect of type 1 diabetes on the gender difference in coronary artery calcification: a role for insulin resistance? The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes 2003; 52: 2833–2839. [DOI] [PubMed] [Google Scholar]
- Mönckeberg JG. Über die reine Mediaverkalkung der Extremitätenarterien und ihr Verhalten zur Arteriosklerose. Virchow's Arch für Pathol Anat und Physiol und für Klin Medizin 1903; 171: 141–167. [Google Scholar]
- Kircelli F, Peter ME, Sevinc Ok E, Celenk FG, Yilmaz M, Steppan S et al. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol Dial Transplant 2012; 27: 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill WC, Lomashvili KA, Malluche HH, Faugere M-C, Riser BL. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int 2011; 79: 512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addison WN, Azari F, Sørensen ES, Kaartinen MT, McKee MD. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem 2007; 282: 15872–15883. [DOI] [PubMed] [Google Scholar]
- Dautova Y, Kozlova D, Skepper JN, Epple M, Bootman MD, Proudfoot D. Fetuin-A and albumin alter cytotoxic effects of calcium phosphate nanoparticles on human vascular smooth muscle cells. PLoS ONE 2014; 9: e97565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gericke A, Qin C, Spevak L, Fujimoto Y, Butler WT, Sorensen ES et al. Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif Tissue Int 2005; 77: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmey D, Johnson KA, Zelken J, Camacho NP, Hoylaerts MF, Noda M et al. Elevated skeletal osteopontin levels contribute to the hypophosphatasia phenotype in Akp2(−/−) mice. J Bone Miner Res 2006; 21: 1377–1386. [DOI] [PubMed] [Google Scholar]
- Yagami K, Suh JY, Enomoto-Iwamoto M, Koyama E, Abrams WR, Shapiro IM et al. Matrix GLA protein is a developmental regulator of chondrocyte mineralization and, when constitutively expressed, blocks endochondral and intramembranous ossification in the limb. J Cell Biol 1999; 147: 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two GLA-containing proteins. J Cell Biol 2004; 165: 625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinke T, Amendt C, Trindl A, Pöschke O, Müller-Esterl W, Jahnen-Dechent W. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem 1996; 271: 20789–20796. [DOI] [PubMed] [Google Scholar]
- Takeuchi S, Hirukawa K, Togari A. Acidosis inhibits mineralization in human osteoblasts. Calcif Tissue Int 2013; 93: 233–240. [DOI] [PubMed] [Google Scholar]
- Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest 2003; 112: 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside J. Nucleation. In: Nancollas GH (ed).Biological Mineralization and Demineralization Springer Berlin Heidelberg: Berlin, Heidelberg, Germany, 1982; pp 23–35. [Google Scholar]
- Dey A, Bomans PHH, Müller FA, Will J, Frederik PM, de With G et al. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat Mater 2010; 9: 1010–1014. [DOI] [PubMed] [Google Scholar]
- Pouget EM, Bomans PHH, Goos JACM, Frederik PM, de With G. Sommerdijk NAJM. The initial stages of template-controlled CaCO3 formation revealed by cryo-TEM. Science 2009; 323: 1455–1458. [DOI] [PubMed] [Google Scholar]
- Baumgartner J, Dey A, Bomans PHH, Le Coadou C, Fratzl P. Sommerdijk NAJM et al. Nucleation and growth of magnetite from solution. Nat Mater 2013; 12: 310–314. [DOI] [PubMed] [Google Scholar]
- Habraken WJEM, Tao J, Brylka LJ, Friedrich H, Bertinetti L, Schenk AS et al. Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate. Nat Commun 2013; 4: 1507. [DOI] [PubMed] [Google Scholar]
- Garnett J, Dieppe P. The effects of serum and human albumin on calcium hydroxyapatite crystal growth. Biochem J 1990; 266: 863–868. [PMC free article] [PubMed] [Google Scholar]
- Heiss A, DuChesne A, Denecke B, Grötzinger J, Yamamoto K, Renné T et al. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J Biol Chem 2003; 278: 13333–13341. [DOI] [PubMed] [Google Scholar]
- Smith ER, Ford ML, Tomlinson LA, Rajkumar C, McMahon LP, Holt SG. Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol Dial Transplant 2012; 27: 1957–1966. [DOI] [PubMed] [Google Scholar]
- Rochette CN, Rosenfeldt S, Heiss A, Narayanan T, Ballauff M, Jahnen-Dechent W. A shielding topology stabilizes the early stage protein-mineral complexes of fetuin-A and calcium phosphate: a time-resolved small-angle X-ray study. Chembiochem 2009; 10: 735–740. [DOI] [PubMed] [Google Scholar]
- Wald J, Wiese S, Eckert T, Jahnen-Dechent W, Richtering W, Heiss A. Formation and stability kinetics of calcium phosphate–fetuin-A colloidal particles probed by time-resolved dynamic light scattering. Soft Matter 2011; 7: 2869. [Google Scholar]
- Heiss A, Eckert T, Aretz A, Richtering W, van Dorp W, Schäfer C et al. Hierarchical role of fetuin-A and acidic serum proteins in the formation and stabilization of calcium phosphate particles. J Biol Chem 2008; 283: 14815–14825. [DOI] [PubMed] [Google Scholar]
- Smith ER, Hanssen E, McMahon LP, Holt SG. Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PLoS ONE 2013; 8: e60904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter GK. Role of osteopontin in modulation of hydroxyapatite formation. Calcif Tissue Int 2013; 93: 348–354. [DOI] [PubMed] [Google Scholar]
- Deshpande AS, Fang P-A, Zhang X, Jayaraman T, Sfeir C, Beniash E. Primary structure and phosphorylation of dentin matrix protein 1 (DMP1) and dentin phosphophoryn (DPP) uniquely determine their role in biomineralization. Biomacromolecules 2011; 12: 2933–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terkelsen OB, Jahnen-Dechent W, Nielsen H, Moos T, Fink E, Nawratil P et al. Rat fetuin: distribution of protein and mRNA in embryonic and neonatal rat tissues. Anat Embryol (Berl) 1998; 197: 125–133. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Sheardown Sa, Deal a, Møllgård K, Reader M, Dziegielewska KM. Expression and distribution of fetuin in the developing sheep fetus. Histochemistry 1994; 102: 457–475. [DOI] [PubMed] [Google Scholar]
- Price PA, Toroian D, Chan WS. Tissue-nonspecific alkaline phosphatase is required for the calcification of collagen in serum: a possible mechanism for biomineralization. J Biol Chem 2009; 284: 4594–4604. [DOI] [PubMed] [Google Scholar]
- Hamlin NJ, Price PA. Mineralization of decalcified bone occurs under cell culture conditions and requires bovine serum but not cells. Calcif Tissue Int 2004; 75: 231–242. [DOI] [PubMed] [Google Scholar]
- Price PA, June HH, Hamlin NJ, Williamson MK. Evidence for a serum factor that initiates the re-calcification of demineralized bone. J Biol Chem 2004; 279: 19169–19180. [DOI] [PubMed] [Google Scholar]
- Toroian D, Price PA. The essential role of fetuin in the serum-induced calcification of collagen. Calcif Tissue Int 2008; 82: 116–126. [DOI] [PubMed] [Google Scholar]
- Price PA, Toroian D, Lim JE. Mineralization by inhibitor exclusion: the calcification of collagen with fetuin. J Biol Chem 2009; 284: 17092–17101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H-H, Wu C-Y, Young D, Martel J, Young A, Ojcius DM et al. Physicochemical and biological properties of biomimetic mineralo-protein nanoparticles formed spontaneously in biological fluids. Small 2012; 9: 2297–2307. [DOI] [PubMed] [Google Scholar]
- Xu Z, Liu C, Wei J, Sun J. Effects of four types of hydroxyapatite nanoparticles with different nanocrystal morphologies and sizes on apoptosis in rat osteoblasts. J Appl Toxicol 2012; 32: 429–435. [DOI] [PubMed] [Google Scholar]
- Pazár B, Ea H-K, Narayan S, Kolly L, Bagnoud N, Chobaz V et al. Basic calcium phosphate crystals induce monocyte/macrophage IL-1β secretion through the NLRP3 inflammasome in vitro. J Immunol 2011; 186: 2495–2502. [DOI] [PubMed] [Google Scholar]
- Binkert C, Demetriou M, Sukhu B, Szweras M, Tenenbaum HC, Dennis JW. Regulation of osteogenesis by fetuin. J Biol Chem 1999; 274: 28514–28520. [DOI] [PubMed] [Google Scholar]
- Szweras M, Liu D, Partridge EA, Pawling J, Sukhu B, Clokie C et al. Alpha 2-HS glycoprotein/fetuin, a transforming growth factor-beta/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J Biol Chem 2002; 277: 19991–19997. [DOI] [PubMed] [Google Scholar]
- Seto J, Busse B, Gupta HS, Schäfer C, Krauss S, Dunlop JWC et al. Accelerated growth plate mineralization and foreshortened proximal limb bones in fetuin-A knockout mice. PLoS ONE 2012; 7: e47338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenfeld R, Schäfer C, Krüger T, Haarmann C, Schurgers LJ, Reutelingsperger C et al. Fetuin-A protects against atherosclerotic calcification in CKD. J Am Soc Nephrol 2009; 20: 1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin GA, Cummins KM, Wassel CL, Daniels LB, Ix JH. The association of fetuin-A with cardiovascular disease mortality in older community-dwelling adults: the Rancho Bernardo study. J Am Coll Cardiol 2012; 59: 1688–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink HA, Bůžková P, Garimella PS, Mukamal KJ, Cauley JA, Kizer JR et al. Association of fetuin-A with incident fractures in community-dwelling older adults: the Cardiovascular Health Study. J Bone Miner Res 2015; e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Reynolds JL. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol 2005; 16: 2920–2930. [DOI] [PubMed] [Google Scholar]
- Schlieper G, Aretz A, Verberckmoes SC, Kruger T, Behets GJ, Ghadimi R et al. Ultrastructural analysis of vascular calcifications in uremia. J Am Soc Nephrol 2010; 21: 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe SM, Reslerova M, Ketteler M, O'neill K, Duan D, Koczman J et al. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD). Kidney Int 2005; 67: 2295–2304. [DOI] [PubMed] [Google Scholar]
- Voigt M, Fischer D-C, Rimpau M, Schareck W, Haffner D. Fibroblast growth factor (FGF)-23 and fetuin-A in calcified carotid atheroma. Histopathology 2010; 56: 775–788. [DOI] [PubMed] [Google Scholar]
- Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 2008; 118: 1748–1757. [DOI] [PubMed] [Google Scholar]
- Kazama JJ, Gejyo F, Ei I. The immunohistochemical localization of alpha2-Heremans-Schmid glycoprotein/fetuin-A (AHSG). Nephrol Dial Transplant 2005; 20: 851–852. [DOI] [PubMed] [Google Scholar]
- Hunter LW, Lieske JC, Tran NV, Miller VM. The association of matrix Gla protein isomers with calcification in capsules surrounding silicone breast implants. Biomaterials 2011; 32: 8364–8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M, Schaefer C, Kinkeldey A, Jahnen-Dechent W. Ectopic calcification in fetuin-A deficient mice starts in the microvasculature. Bone 2011; 48: S241. [Google Scholar]
- Westenfeld R, Schafer C, Smeets R, Brandenburg VM, Floege J, Ketteler M et al. Fetuin-A (AHSG) prevents extraosseous calcification induced by uraemia and phosphate challenge in mice. Nephrol Dial Transplant 2007; 22: 1537–1546. [DOI] [PubMed] [Google Scholar]
- Meng H, Vera I, Che N, Wang X, Wang SS, Ingram-Drake L et al. Identification of Abcc6 as the major causal gene for dystrophic cardiac calcification in mice through integrative genomics. Proc Natl Acad Sci USA 2007; 104: 4530–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RS, Küçükosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IEM et al. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Natl Acad Sci USA 2013; 110: 20206–20211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997; 386: 78–81. [DOI] [PubMed] [Google Scholar]
- Lomashvili KA, Narisawa S, Millán JL, O'Neill WC. Vascular calcification is dependent on plasma levels of pyrophosphate. Kidney Int 2014; 85: 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Böhm R et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet 2003; 361: 827–833. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Dibra F, Lee MD, Oldenburg R, Uitto J. Overexpression of fetuin-a counteracts ectopic mineralization in a mouse model of pseudoxanthoma elasticum (abcc6(−/−)). J Invest Dermatol 2010; 130: 1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association between human fetuin-A and the metabolic syndrome: data from the Heart and Soul Study. Circulation 2006; 113: 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano T, Matsui I, Mikami S, Tomida K, Fujii N, Imai E et al. Fetuin-mineral complex reflects extraosseous calcification stress in CKD. J Am Soc Nephrol 2010; 21: 1998–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ER, Ford ML, Tomlinson LA, Bodenham E, McMahon LP, Farese S et al. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J Am Soc Nephrol 2014; 25: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ER, Cai MM, McMahon LP, Pedagogos E, Toussaint ND, Brumby C et al. Serum fetuin-A concentration and fetuin-A-containing calciprotein particles in patients with chronic inflammatory disease and renal failure. Nephrology (Carlton) 2013; 18: 215–221. [DOI] [PubMed] [Google Scholar]
- Price PA, Thomas GR, Pardini AW, Figueira WF, Caputo JM, Williamson MK. Discovery of a high molecular weight complex of calcium, phosphate, fetuin, and matrix gamma-carboxyglutamic acid protein in the serum of etidronate-treated rats. J Biol Chem 2002; 277: 3926–3934. [DOI] [PubMed] [Google Scholar]
- Matsui I, Hamano T, Mikami S, Fujii N, Takabatake Y, Nagasawa Y et al. Fully phosphorylated fetuin-A forms a mineral complex in the serum of rats with adenine-induced renal failure. Kidney Int 2009; 75: 915–928. [DOI] [PubMed] [Google Scholar]
- Cai MMX, Smith ER, Brumby C, McMahon LP, Holt SG. Fetuin-A-containing calciprotein particle levels can be reduced by dialysis, sodium thiosulphate and plasma exchange. Potential therapeutic implications for calciphylaxis? Nephrology (Carlton) 2013; 18: 724–727. [DOI] [PubMed] [Google Scholar]
- Price PA, Williamson MK, Nguyen TMT, Than TN. Serum levels of the fetuin-mineral complex correlate with artery calcification in the rat. J Biol Chem 2004; 279: 1594–1600. [DOI] [PubMed] [Google Scholar]
- Cai MMX, Wigg B, Smith ER, Hewitson TD, Mcmahon LP, Holt SG. Relative abundance of fetuin- A in peritoneal dialysis effluent and its association with in situ formation of calciprotein particles: an observational pilot study. Nephrology 2015; 20: 6–10. [DOI] [PubMed] [Google Scholar]
- Gujadhur A, Smith ER, McMahon LP, Spanger M, Chuen J, Holt SG. Large vessel calcification in Takayasu arteritis. Intern Med J 2013; 43: 584–587. [DOI] [PubMed] [Google Scholar]
- Meena R, Kesari KK, Rani M, Paulraj R. Effects of hydroxyapatite nanoparticles on proliferation and apoptosis of human breast cancer cells (MCF-7). J Nanoparticle Res 2012; 14: 712. [Google Scholar]
- Xu JL, Khor KA, Sui JJ, Zhang JH, Chen WN. Protein expression profiles in osteoblasts in response to differentially shaped hydroxyapatite nanoparticles. Biomaterials 2009; 30: 5385–5391. [DOI] [PubMed] [Google Scholar]
- Shi Z, Huang X, Cai Y, Tang R, Yang D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater 2009; 5: 338–345. [DOI] [PubMed] [Google Scholar]
- Motskin M, Wright DM, Muller K, Kyle N, Gard TG, Porter AE et al. Hydroxyapatite nano and microparticles: correlation of particle properties with cytotoxicity and biostability. Biomaterials 2009; 30: 3307–3317. [DOI] [PubMed] [Google Scholar]
- Kalia P, Vizcay-Barrena G, Fan JP, Warley A, Di Silvio L, Huang J. Nanohydroxyapatite shape and its potential role in bone formation: an analytical study. J R Soc Interface 2014; 11: 20140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Das M, Balla VK. Effect of hydroxyapatite particle size, morphology and crystallinity on proliferation of colon cancer HCT116 cells. Mater Sci Eng C Mater Biol Appl 2014; 39: 336–339. [DOI] [PubMed] [Google Scholar]
- Santos C, Gomes PS, Duarte Ja, Franke RP, Almeida MM, Costa MEV et al. Relevance of the sterilization-induced effects on the properties of different hydroxyapatite nanoparticles and assessment of the osteoblastic cell response. J R Soc Interface 2012; 9: 3397–3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Liu C, Qian J, Wang J, Zhang Y. Size-mediated cytotoxicity and apoptosis of hydroxyapatite nanoparticles in human hepatoma HepG2 cells. Biomaterials 2010; 31: 730–740. [DOI] [PubMed] [Google Scholar]
- Epple M, Ganesan K, Heumann R, Klesing J, Kovtun A, Neumann S et al. Application of calcium phosphate nanoparticles in biomedicine. J Mater Chem 2010; 20: 18. [Google Scholar]
- Pele L, Haas CT, Hewitt R, Faria N, Brown A, Powell J. Artefactual nanoparticle activation of the inflammasome platform: in vitro evidence with a nano-formed calcium phosphate. Nanomedicine (Lond) 2014; 3: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hails La, Babister JC, Inglis S, Davis Sa, Oreffo ROC, Mann S. Inhibition of hydroxyapatite nanoparticle-induced osteogenic activity in skeletal cells by adsorption of serum proteins. Small 2010; 6: 1986–1991. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhou G, Liu H, Niu X, Han J, Zheng L et al. Nano-hydroxyapatite particles induce apoptosis on MC3T3-E1 cells and tissue cells in SD rats. Nanoscale 2012; 4: 2894–2899. [DOI] [PubMed] [Google Scholar]
- Ewence AE, Bootman M, Roderick HL, Skepper JN, McCarthy G, Epple M et al. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells: a potential mechanism in atherosclerotic plaque destabilization. Circ Res 2008; 103: e28–e34. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Schäfer C, Heiss A, Gräber S, Kinkeldey A, Büscher A et al. Clearance of fetuin-A–containing calciprotein particles is mediated by scavenger receptor-A. Circ Res 2012; 111: 575–584. [DOI] [PubMed] [Google Scholar]
- Pedraza CE, Nikolcheva LG, Kaartinen MT, Barralet JE, McKee MD. Osteopontin functions as an opsonin and facilitates phagocytosis by macrophages of hydroxyapatite-coated microspheres: implications for bone wound healing. Bone 2008; 43: 708–716. [DOI] [PubMed] [Google Scholar]
- Jahnen-Dechent W, Schäfer C, Ketteler M, McKee MD. Mineral chaperones: a role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med 2008; 86: 379–389. [DOI] [PubMed] [Google Scholar]
- Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease. JAMA 2011; 305: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Scialla JJ, Kao WHL, Crainiceanu C, Sozio SM, Oberai PC, Shafi T et al. Biomarkers of vascular calcification and mortality in patients with ESRD. Clin J Am Soc Nephrol 2014; 9: 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 2005; 16: 520–528. [DOI] [PubMed] [Google Scholar]
- Lomashvili KA. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol 2004; 15: 1392–1401. [DOI] [PubMed] [Google Scholar]
- El-Abbadi MM, Pai AS, Leaf EM, Yang H-Y, Bartley BA, Quan KK et al. Phosphate feeding induces arterial medial calcification in uremic mice: role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int 2009; 75: 1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yang H-Y, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res 2006; 98: 905–912. [DOI] [PubMed] [Google Scholar]
- Vali H, McKee MD, Çiftçioglu N, Sears SK, Plows FL, Chevet E et al. Nanoforms: a new type of protein-associated mineralization. Geochim Cosmochim Acta 2001; 65: 63–74. [Google Scholar]
- Wu C-Y, Martel J, Young D, Young JD. Fetuin-A/albumin-mineral complexes resembling serum calcium granules and putative nanobacteria: demonstration of a dual inhibition-seeding concept. PLoS ONE 2009; 4: e8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage AP, Lu J, Tintut Y, Demer LL. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int 2011; 79: 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Bellosta R, Sorribas V. Phosphonoformic acid prevents vascular smooth muscle cell calcification by inhibiting calcium-phosphate deposition. Arterioscler Thromb Vasc Biol 2009; 29: 761–766. [DOI] [PubMed] [Google Scholar]
- Dai X-Y, Zhao M-M, Cai Y, Guan Q-C, Zhao Y, Guan Y et al. Phosphate-induced autophagy counteracts vascular calcification by reducing matrix vesicle release. Kidney Int 2013; 83: 1042–1051. [DOI] [PubMed] [Google Scholar]
- Di Marco GS, Hausberg M, Hillebrand U, Rustemeyer P, Wittkowski W, Lang D et al. Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am J Physiol Renal Physiol 2008; 294: F1381–F1387. [DOI] [PubMed] [Google Scholar]
- Pasch A, Farese S, Gräber S, Wald J, Richtering W, Floege J et al. Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 2012; 23: 1744–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyzer CA, De Borst MH, Van Den Berg E, Jahnen-Dechent W, Navis G, Bakker SJL et al. High calcification propensity is associated with mortality and graft failure in renal transplant recipients. Nephrol Dial Transplant 2014; 29: iii541. [Google Scholar]
- Chailurkit L, Kruavit A, Rajatanavin R, Ongphiphadhanakul B. The relationship of fetuin-A and lactoferrin with bone mass in elderly women. Osteoporos Int 2011; 22: 2159–2164. [DOI] [PubMed] [Google Scholar]
- Ix JH, Wassel CL, Bauer DC, Toroian D, Tylavsky FA, Cauley JA et al. Fetuin-A and BMD in older persons: the Health Aging and Body Composition (Health ABC) study. J Bone Miner Res 2009; 24: 514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpantur A, Altun B, Hazirolan T, Akata D, Arici M, Kirazli S et al. Association among serum fetuin-A level, coronary artery calcification, and bone mineral densitometry in maintenance hemodialysis patients. Artif Organs 2009; 33: 844–854. [DOI] [PubMed] [Google Scholar]
- Fiore CE, Celotta G, Politi GG, Di Pino L, Castelli Z, Mangiafico RA et al. Association of high alpha2-Heremans-Schmid glycoprotein/fetuin concentration in serum and intima-media thickness in patients with atherosclerotic vascular disease and low bone mass. Atherosclerosis 2007; 195: 110–115. [DOI] [PubMed] [Google Scholar]
- Wilund KR, Tomayko EJ, Evans EM, Kim K, Ishaque MR, Fernhall B. Physical activity, coronary artery calcium, and bone mineral density in elderly men and women: a preliminary investigation. Metabolism 2008; 57: 584–591. [DOI] [PubMed] [Google Scholar]
- Avila M, Prado C, Ventura M-J, Mora C, Briones D, Valdez H et al. Vitamin D receptor gene, biochemical bone markers and bone mineral density in Mexican women on dialysis. Nephrol Dial Transplant 2010; 25: 2259–2265. [DOI] [PubMed] [Google Scholar]
- Sarı A. The relationship between fetuin-A and bone mineral density in postmenopausal osteoporosis. Turkish J Rheumatol 2013; 28: 195–201. [Google Scholar]
- Sritara C, Thakkinstian A, Ongphiphadhanakul B, Chailurkit L, Chanprasertyothin S, Ratanachaiwong W et al. Causal relationship between the AHSG gene and BMD through fetuin-A and BMI: multiple mediation analysis. Osteoporos Int 2014; 25: 1555–1562. [DOI] [PubMed] [Google Scholar]
- Mehrotra R, Westenfeld R, Christenson P, Budoff M, Ipp E, Takasu J et al. Serum fetuin-A in nondialyzed patients with diabetic nephropathy: relationship with coronary artery calcification. Kidney Int 2005; 67: 1070–1077. [DOI] [PubMed] [Google Scholar]
- Stenvinkel P, Wang K, Qureshi AR, Axelsson J, Pecoits-Filho R, Gao P et al. Low fetuin-A levels are associated with cardiovascular death: impact of variations in the gene encoding fetuin. Kidney Int 2005; 67: 2383–2392. [DOI] [PubMed] [Google Scholar]
- Wang AY-M, Woo J, Lam CW-K, Wang M, Chan IH-S, Gao P et al. Associations of serum fetuin-A with malnutrition, inflammation, atherosclerosis and valvular calcification syndrome and outcome in peritoneal dialysis patients. Nephrol Dial Transplant 2005; 20: 1676–1685. [DOI] [PubMed] [Google Scholar]
- Hermans MMH, Brandenburg V, Ketteler M, Kooman JP, van der Sande FM, Gladziwa U et al. Study on the relationship of serum fetuin-A concentration with aortic stiffness in patients on dialysis. Nephrol Dial Transplant 2006; 21: 1293–1299. [DOI] [PubMed] [Google Scholar]
- Jung HH, Kim S-W, Han H. Inflammation, mineral metabolism and progressive coronary artery calcification in patients on haemodialysis. Nephrol Dial Transplant 2006; 21: 1915–1920. [DOI] [PubMed] [Google Scholar]
- Cozzolino M, Galassi A, Biondi ML, Turri O, Papagni S, Mongelli N et al. Serum fetuin-A levels link inflammation and cardiovascular calcification in hemodialysis patients. Am J Nephrol 2006; 26: 423–429. [DOI] [PubMed] [Google Scholar]
- Mori K, Emoto M, Araki T, Yokoyama H, Teramura M, Lee E et al. Association of serum fetuin-A with carotid arterial stiffness. Clin Endocrinol (Oxf) 2007; 66: 246–250. [DOI] [PubMed] [Google Scholar]
- Russo D, Corrao S, Miranda I, Ruocco C, Manzi S, Elefante R et al. Progression of coronary artery calcification in predialysis patients. Am J Nephrol 2007; 27: 152–158. [DOI] [PubMed] [Google Scholar]
- Hermans MMH, Brandenburg V, Ketteler M, Kooman JP, van der Sande FM, Boeschoten EW et al. Association of serum fetuin-A levels with mortality in dialysis patients. Kidney Int 2007; 72: 202–207. [DOI] [PubMed] [Google Scholar]
- Ix JH, Chertow GM, Shlipak MG, Brandenburg VM, Ketteler M, Whooley Ma. Association of fetuin-A with mitral annular calcification and aortic stenosis among persons with coronary heart disease: data from the Heart and Soul Study. Circulation 2007; 115: 2533–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ix JH, Shlipak MG, Sarnak MJ, Beck GJ, Greene T, Wang X et al. Fetuin-A is not associated with mortality in chronic kidney disease. Kidney Int 2007; 72: 1394–1399. [DOI] [PubMed] [Google Scholar]
- Mikami S, Hamano T, Fujii N, Nagasawa Y, Isaka Y, Moriyama T et al. Serum osteoprotegerin as a screening tool for coronary artery calcification score in diabetic pre-dialysis patients. Hypertens Res 2008; 31: 1163–1170. [DOI] [PubMed] [Google Scholar]
- Shroff RC, Shah V, Hiorns MP, Schoppet M, Hofbauer LC, Hawa G et al. The circulating calcification inhibitors, fetuin-A and osteoprotegerin, but not matrix Gla protein, are associated with vascular stiffness and calcification in children on dialysis. Nephrol Dial Transplant 2008; 23: 3263–3271. [DOI] [PubMed] [Google Scholar]
- Metry G, Stenvinkel P, Qureshi aR, Carrero JJ, Yilmaz MI, Bárány P et al. Low serum fetuin-A concentration predicts poor outcome only in the presence of inflammation in prevalent haemodialysis patients. Eur J Clin Invest 2008; 38: 804–811. [DOI] [PubMed] [Google Scholar]
- Zheng S, de Las Fuentes L, Bierhals A, Ash-Bernal R, Spence K, Slatopolsky E et al. Relation of serum fetuin-A levels to coronary artery calcium in African-American patients on chronic hemodialysis. Am J Cardiol 2009; 103: 46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorant DP, Grujicic M, Hoebaus C, Brix J-M, Hoellerl F, Schernthaner G et al. Fetuin-A levels are increased in patients with type 2 diabetes and peripheral arterial disease. Diabetes Care 2011; 34: 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Ikari Y, Jono S, Emoto M, Shioi A, Koyama H et al. Fetuin-A is associated with calcified coronary artery disease. Coron Artery Dis 2010; 21: 281–285. [DOI] [PubMed] [Google Scholar]
- Lim P, Moutereau S, Simon T, Gallet R, Probst V, Ferrieres J et al. Usefulness of fetuin-A and C-reactive protein concentrations for prediction of outcome in acute coronary syndromes (from the French Registry of Acute ST-Elevation Non-ST-Elevation Myocardial Infarction [FAST-MI]). Am J Cardiol 2013; 111: 31–37. [DOI] [PubMed] [Google Scholar]
- Maréchal C, Schlieper G, Nguyen P, Krüger T, Coche E, Robert A et al. Serum fetuin-A levels are associated with vascular calcifications and predict cardiovascular events in renal transplant recipients. Clin J Am Soc Nephrol 2011; 6: 974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pateinakis P, Papagianni A, Douma S, Efstratiadis G, Memmos D. Associations of fetuin-A and osteoprotegerin with arterial stiffness and early atherosclerosis in chronic hemodialysis patients. BMC Nephrol 2013; 14: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneri M, Geraci C, Incalcaterra F, Arsena R, Mulè G, Vaccaro F et al. Subclinical atherosclerosis and fetuin-A plasma levels in essential hypertensive patients. Hypertens Res 2013; 36: 129–133. [DOI] [PubMed] [Google Scholar]
- Jung JY, Hwang Y-H, Lee S-W, Lee H, Kim DK, Kim S et al. Factors associated with aortic stiffness and its change over time in peritoneal dialysis patients. Nephrol Dial Transplant 2010; 25: 4041–4048. [DOI] [PubMed] [Google Scholar]
- Roos M, von Eynatten M, Heemann U, Rothenbacher D, Brenner H, Breitling LP. Serum fetuin-A, cardiovascular risk factors, and six-year follow-up outcome in patients with coronary heart disease. Am J Cardiol 2010; 105: 1666–1672. [DOI] [PubMed] [Google Scholar]
- Emoto M, Mori K, Lee E, Kawano N, Yamazaki Y, Tsuchikura S et al. Fetuin-A and atherosclerotic calcified plaque in patients with type 2 diabetes mellitus. Metabolism 2010; 59: 873–878. [DOI] [PubMed] [Google Scholar]
- Rittig K, Thamer C, Haupt A, Machann J, Peter A, Balletshofer B et al. High plasma fetuin-A is associated with increased carotid intima-media thickness in a middle-aged population. Atherosclerosis 2009; 207: 341–342. [DOI] [PubMed] [Google Scholar]
- Jensen MK, Bartz TM, Mukamal KJ, Djoussé L, Kizer JR, Tracy RP et al. Fetuin-A, type 2 diabetes, and risk of cardiovascular disease in older adults: the cardiovascular health study. Diabetes Care 2013; 36: 1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaess BM, Enserro DM, McManus DD, Xanthakis V, Chen M-H, Sullivan LM et al. Cardiometabolic correlates and heritability of fetuin-A, retinol-binding protein 4, and fatty-acid binding protein 4 in the Framingham Heart Study. J Clin Endocrinol Metab 2012; 97: E1943–E1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford ML, Tomlinson LA, Smith ER, Rajkumar C, Holt SG. Fetuin-A is an independent determinant of change of aortic stiffness over 1 year in non-diabetic patients with CKD stages 3 and 4. Nephrol Dial Transplant 2010; 25: 1853–1858. [DOI] [PubMed] [Google Scholar]