Abstract
Solid cancers and hematologic cancers frequently colonize bone and induce skeletal-related complications. Bone pain is one of the most common complications associated with cancer colonization in bone and a major cause of increased morbidity and diminished quality of life, leading to poor survival in cancer patients. Although the mechanisms responsible for cancer-associated bone pain (CABP) are poorly understood, it is likely that complex interactions among cancer cells, bone cells and peripheral nerve cells contribute to the pathophysiology of CABP. Clinical observations that specific inhibitors of osteoclasts reduce CABP indicate a critical role of osteoclasts. Osteoclasts are proton-secreting cells and acidify extracellular bone microenvironment. Cancer cell-colonized bone also releases proton/lactate to avoid intracellular acidification resulting from increased aerobic glycolysis known as the Warburg effect. Thus, extracellular microenvironment of cancer-colonized bone is acidic. Acidosis is algogenic for nociceptive sensory neurons. The bone is densely innervated by the sensory neurons that express acid-sensing nociceptors. Collectively, CABP is evoked by the activation of these nociceptors on the sensory neurons innervating bone by the acidic extracellular microenvironment created by bone-resorbing osteoclasts and bone-colonizing cancer cells. As current treatments do not satisfactorily control CABP and can elicit serious side effects, new therapeutic interventions are needed to manage CABP. Understanding of the cellular and molecular mechanism by which the acidic extracellular microenvironment is created in cancer-colonized bone and by which the expression and function of the acid-sensing nociceptors on the sensory neurons are regulated would facilitate to develop novel therapeutic approaches for the management of CABP.
Introduction
Solid cancers such as breast, prostate and lung cancer and hematologic cancers such as multiple myeloma (MM) preferentially or exclusively spread to the bone.1,2 These bone-colonizing cancers develop osteolytic, osteosclerotic or mixed bone lesions by disrupting the homeostasis of bone microenvironment.3,4,5,6 and are eventually associated with skeletal-related events (SRE) including pathologic fractures, hypercalcemia, spinal cord compressions, palliative radiotherapy to bone and surgery to bone to treat or prevent a fracture during the clinical course of the disease.7 However, the most common and detrimental SRE associated with cancer colonization in bone is the bone pain.8
Although not all patients with bone metastases experience cancer-associated bone pain (CABP), >70% of cancer patients with metastatic bone disease suffer from severe CABP in advanced stages.1 CABP most frequently occurs in bones including lumbar bones, pelvis and femoral bones that bear mechanical forces or physical loadings.9 However, anatomical location, size and number of cancer and the extent of bone destruction are not necessarily correlated with the severity of CABP. Some patients have widespread bone metastases but minimal bone pain, whereas others have minimal bone metastases but severe bone pain. Further, among cancers of the same histologic type, some cancers are associated with CABP and others are not. The reasons for these complexities are unknown.
CABP represents a combination of background, spontaneous and incident pain (pain on movement).9 Background pain is dull and continuous and exacerbates as cancer progresses. In contrast, spontaneous pain and incident pain are rapid in onset and intermittent and transient in nature. Spontaneous and incident pain are often called as breakthrough pain, as they represent an extreme pain breaking through the therapeutic regimen for background pain. Background pain can be controlled by conventional analgesics. In contrast, alleviation of breakthrough pain by currently available analgesic agents is unsatisfactory, insufficient and inadequate and occasionally associated with unwanted adverse effects.8 CABP is a mixed type chronic pain involving inflammatory and neuropathic pain. Inflammatory pain is caused by inflammatory mediators released from the tissues damaged by cancer expansion, and neuropathic pain is evoked due to the compression and invasion of the sensory nerves by cancer.8 In addition, the observation that CABP requires >10-fold greater doses of morphine than those required for relieving equivalent intensity of inflammatory pain in experimental animals10 suggests that the pathophysiology of bone pain is unique. Because of this complexity, understanding of the molecular basis of CABP is still limited.
In this review, we describe (1) the characteristics of sensory neurons innervating bone with respect to CABP, (2) the role of bone-resorbing osteoclasts and bone-colonizing cancer cells in the creation of acidic extracellular bone microenvironment and (3) the mechanism by which the sensory neurons innervating bone are activated by the acidic extracellular microenvironment and elicit CABP.
Nociceptors on sensory neurons
Sensory neurons can be divided into two general types––namely, A-fiber and C-fiber. A-fiber is subdivided into thickly myelinated A-β fibers that are positive for neurofilament 200 (NF200+) and negative for the receptor tyrosine kinase, tropomyosin receptor kinase A (TrkA−), and thinly myelinated A-δ fibers that are NF200+, TrkA− and NF200+, calcitonin gene-related peptide (CGRP)+, TrkA+. C-fiber is subdivided into CGRP+, TrkA+ unmyelinated peptidergic C fibers and unmyelinated non-peptidergic C fibers that are isolectin B4 (IB4)+, Mas-related G protein-coupled receptor member D+ (Mrgprd+), TrkA−.11 Thinly myelinated A-δ fibers (NF-200+, TrkA− and NF200+, CGRP+, TrkA+) and peptidergic C fibers (CGRP+ and TrkA+) are predominant sensory neurons innervating the bone, >80% of which are TrkA+.12,13 Although functional distinction of these neurons is not fully understood, CGRP+ peptidergic C-fiber afferent sensory neurons detect noxious stimuli and are classified as nociceptors.14 Generally, local nociceptive stimuli released from cancer cells, inflammatory cells, bone-resorbing osteoclasts or injured tissues are recognized and transduced into electrochemical signals by these nociceptors.14 The nociceptors can sense noxious thermal, mechanical and chemical stimuli and transmit these signals to the dorsal root ganglia (DRG, primary afferent neuron), then the spinal cord (secondary afferent neuron) or the central nervous system (CNS) and finally the brain.14 DRG is the cell body of sensory fibers innervating peripheral tissues and has a role as the gateway of peripheral noxious signals.15 The sensory neurons innervating bone are distal nerve fibers from a number of subpopulations of primary afferent sensory neurons associated with lumbar DRG (L3-L5).
Innervation of sensory neurons in the bone
Cancers expanding in the bone marrow can induce CABP by stretching of periosteum, which in turn excites the nociceptive sensory neurons densely innervating periosteal surfaces. Further, cancers breaking out cortical bone and proliferating along the periosteal surface also can evoke CABP in a similar manner. Thus, it has been proposed that the densely innervated periosteum is the primary site from which CABP arises.9 However, patients often experience CABP even if cancer localizes within bone marrow or mineralized bone in the absence of evident periosteal involvement,12 indicating the presence of sensory nerve fibers in non-periosteal sites in bone. An early study using a transmission electron microscopy described by Cooper16 demonstrated that nerve fibers are present in cortical bone. Furthermore, recent results have shown that mineralized bone and bone marrow are densely innervated.12,17,18,19 Of note, Mach et al.12 reported that the mineralized bone and bone marrow have greater total number of sensory fibers than periosteum.
Consistent with these earlier studies, we also observed that CGRP+ sensory neurons innervate the mineralized bone and bone marrow by immunohistochemical examination (Figure 1). CGRP is a widely used marker for sensory neurons and has been implicated in human migraine.20 Mice lacking CGRP showed attenuated responses to nociceptive chemicals and inflammation.21 Of interest, tumor-associated angiogenesis and tumor growth were significantly reduced in CGRP−/− mice,22 suggesting that tumor colonization is under the influence of CGRP+ sensory neurons. These results suggest that CGRP is not only a phenotypic marker for sensory neurons but also an algesic substance and angiogenetic factor.
Figure 1.
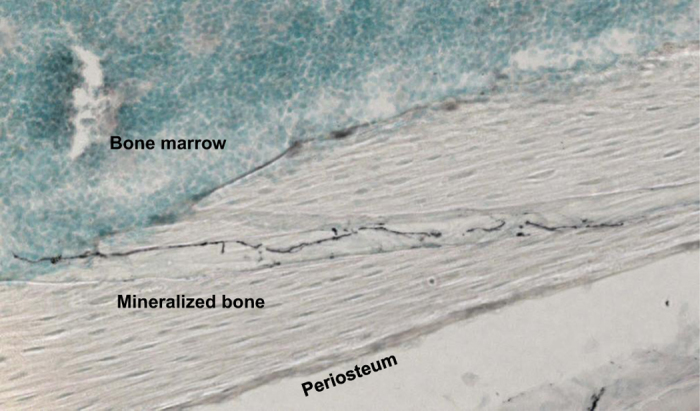
Innervation of CGRP-positive sensory neurons in the bone. Bones were fixed, decalcified and cut on a cryostat (30 μm). Sections were then incubated with a guinea pig anti-CGRP (1:2000), followed by an incubation with goat anti-guinea pig IgG (1:500). The immunostained sections were examined under a confocal laser-scanning microscope (LSM510, Ver 3.2, Carl Zeiss). CGRP-positive sensory neurons (black) run through mineralized bone into the bone marrow. CGRP, calcitonin gene-related peptide.
CGRP+ sensory neurons and sympathetic fibers in the periosteum exhibited the pathological sprouting and reorganization in the presence of cancer cells in bone, resulting in the formation of neuroma-like structures.23 This neuroma formation is speculated to contribute to the occurrence of breakthrough or incident pain and exacerbation of CABP, making control of CABP difficult. Nerve growth factor (NGF) derived from cancer cells and bone marrow stromal cells is likely responsible for the sprouting of sensory neurons, as anti-NGF-neutralizing antibodies blocked the sprouting and reduced CABP.23 We observed similar sprouting of CGRP+ sensory neurons in bone colonized by breast cancer cells and MM cells (unpublished data). These results suggest that soluble mediators produced in cancer-colonized bone microenvironment promote the remodeling of the sensory neurons innervating bone and regulate the progression of CABP.
Proposed mechanisms of CABP
Micro-fractures resulting from decreased bone density and/or disrupted bone architecture due to increased osteoclastic bone resorption in the presence of cancer cells undoubtedly cause CABP (Table 1). However, the occurrence of CABP in patients with prostate cancers that predominantly develop osteosclerotic bone metastases24 suggests a mechanism(s) other than cancer-associated osteolysis. Other mechanisms proposed are stretching of periosteum by tumor expansion in the bone marrow cavity, which in turn excites the nociceptive sensory neurons innervating periosteal surfaces, and direct nerve injury by cancer invasion.9 In addition to these mechanisms, increased neuron sprouting and neuroma formation, upregulation of the expression of nociceptors and excitement and sensitization of nociceptive sensory neurons by increased release of noxious substances from cancer cells, bone cells and nerve cells have been also implicated in CABP.25 However, the observations that the severity of CABP is not correlated with clinical and pathological features of cancer and that not all bone cancers cause CABP indicate heterogeneity, diversity and complexity of underlying mechanisms of CABP.
Table 1. Mechanism of bone pain.
| Microfractures |
| Stretching of periosteum by tumor expansion in bone marrow |
| Sensory nerve injury by tumor invasion |
| Increased neuron remodeling and neuroma formation |
| Elevated expression of nociceptors |
| Sensitization or excitation of nociceptors |
| Increased production of noxious substances |
| Neurotrophins - NGF, BDNF, NT-3 |
| Inflammatory mediators - IL-1β, IL-6, TNFα, TGFβ |
| Chemokines – MIP-1α, MCP-1 |
| Protons |
| Lysophosphatidic acid |
| Bradykinin |
| Substance P |
| Calcitonin gene-related protein |
| Endothelin |
| Vascular endothelial growth factor |
| Prostaglandins |
| Glutamate |
| Histamine |
| ATP |
Accumulating clinical data have shown that the specific inhibitors of osteoclasts, bisphosphonate and denosumab significantly reduce CABP,26,27 suggesting that a variety of algogenic factors released at the tumor-bone interface during osteoclastic bone resorption may excite and sensitize peripheral nociceptive sensory neurons and evoke CABP by binding to their cognitive receptors present on the sensory neurons.25,28 In addition, it has been well-recognized that aggressive cancer cells secrete substantial amounts of protons/lactate into the extracellular environments to avoid intracellular acidification due to elevated aerobic glycolysis known as the Warburg effect.29 Protons are one of these algogenic mediators.25,28 Thus, protons released from osteoclasts and cancer cells co-operatively create an acidic extracellular microenvironment in cancer-colonized bone.
Proton release by osteoclasts in CABP
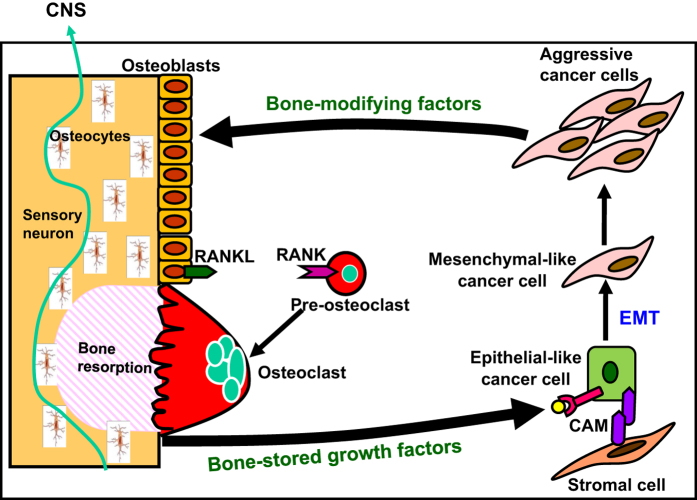
Osteoclasts are multinucleated giant cells formed by the fusion of mononuclear progenitors of the monocyte/macrophage lineage.30 They are the principal bone resorbing cells and have a central role in the formation of the skeleton and regulation of its mass. For osteoclast formation from the osteoclast precursors, macrophage colony-stimulating factor (M-CSF) and receptor for activation of nuclear factor kappa B (NF-κB) (RANK) ligand (RANKL)31 produced in neighboring osteoblasts or stromal cells are essential. In cancer-colonized bone and bone metastasis, osteoclasts are increased and activated to destroy bone by factors produced by cancers.3,4,5,6 Bone destruction, in turn, further stimulates the colonization of cancer cells in bone via the release of bone-stored growth factors including transforming growth factor-β (TGF-β) and insulin-like growth factors (IGFs). This interactive process between bone-colonizing cancer cells and bone-resorbing osteoclasts is called ‘the vicious cycle' (Figure 2). Thus, osteoclasts are a central regulatory factor in the development and progression of cancer colonization in bone and bone metastasis.
Figure 2.
Vicious cycle between osteoclasts and cancer cells in the bone. Bone-derived growth factors, (GFs) such as insulin-like growth factors (IGF) and transforming growth factor-β (TGF-β), promote proliferation and inhibit apoptosis and stimulate epithelial–mesenchymal transition (EMT) and production of bone-modifying cytokines such as parathyroid hormone-related protein (PTH-rP), prostaglandin E2 (PGE2) and interleukin-11 (IL-11) in bone-colonizing cancer cells, supporting the ‘Seed and Soil' theory proposed by Paget.91 These bone-modifying factors further stimulate osteoclastic bone resorption via activation of receptor activator of the nuclear factor-κB (RANKL)/RANK pathway in osteoblasts and osteoclasts, thereby further increasing the release of bone-stored growth factors, thus establishing ‘vicious cycle' between bone-resorbing osteoclasts and bone-colonizing cancer cells.3,4,5,6 Some bone-colonizing cancer cells reside in stromal cell niche via cell–cell contact mediated by cell adhesion molecules (CAMs) and stay dormant or undergo EMT and acquire further aggressiveness. Sensory neurons innervate bone. Role of osteocytes in bone metastasis needs to be elucidated. CAM, cell adhesion molecule, CNS, central nervous system, EMT, epithelial–mesenchymal transition, RANK, receptor activation of NF-κB, RANKL, receptor activation of NF-κB ligand.
Significant reduction in bone pain by the specific inhibitors of osteoclastic bone resorption, bisphosphonates and denosumab, in patients with multiple myeloma and solid cancers26,27,32,33 indicates a critical role of osteoclasts in the pathophysiology of CABP. Consistent with these clinical observations, Honore et al.34 reported that osteoprotegerin (OPG), which is a natural soluble decoy receptor that competes with RANK for RANKL and thus inhibits RANKL-induced osteoclast formation and bone resorption,30,31 suppressed CABP using an experimental animal model. We also showed that the most potent bisphosphonate zoledronic acid significantly reduced CABP in a rat mammary tumor model.35 It is therefore important to understand how osteoclasts resorb bone to gain better insights into the mechanism underlying CABP.
Bone resorption by mature osteoclasts is a dynamic multistep process.30 First, osteoclasts migrate and attach tightly to the bone surfaces via the αvβ3 integrin, thereby forming sealing zones, followed by plasma membrane ruffled border formation. To dissolve bone minerals, protons (H+) and chloride ions (Cl−) are released via the plasma membrane (a3 isoform) vacuolar H+-ATPase proton pump36 and chloride ion-proton anti-porter ClC-737 clustered in the ruffled border into the resorption lacunae, respectively, acidifying the resorptive lacunae to a pH of ∼4.5.36 Concomitantly, the cysteine peptidase cathepsin K38 degrades bone matrix. The degraded bone matrix is trans-endocytosed from the resorption lacunae to the functional secretory domain and released into the extracellular environment.39 Finally, osteoclasts gone through bone resorption detach from the bone surface and undergo apoptosis.30
Expression of a3 isoform vacuolar H+-ATPase on bone-resorbing osteoclasts was shown by many studies.40,41,42 Using pH-activatable chemical probes, Kowada et al.43 reported that the pH in the resorption pit in vivo is between 4 and 6. We reported that the non-selective vacuolar-H+-ATPase inhibitor, bafilomycin A1, reduces inflammatory bone pain.44 Moreover, we recently found that bafilomycin A1 blocked the development of acidic environment in cancer-colonized bone determined by acridine orange staining41 and significantly reduced CABP in an animal model of MM (unpublished data). These data suggest that alogenic protons released from bone-resorbing osteoclasts via a3 vacuolar-H+-ATPase into the extracellular microenvironment likely impact pH-sensitive peripheral nerve fibers and directly contribute to CABP. Of interest, we observed that the p-type-H+-ATPase inhibitor, rabeprazole,45 that is widely used for the treatment of gastric pain failed to suppress CABP (unpublished data). The pathophysiology of CABP may be thus unique compared with that of pain in non-bone organs because of the contribution of osteoclast-specific a3 vacuolar-H+-ATPase.
Proton release by cancer cells in CABP
The observations that inhibitors of osteoclastic bone resorption reduce CABP but fail to block the progression of CABP in cancer patients27,33 and tumor-bearing animals34,35 indicate that osteoclasts are not the only cells responsible for CABP. There is no doubt that cancer cells and inflammatory cells and immune cells within and around the tumor that secrete varieties of noxious substances (Table 1) are also responsible for evoking CABP. Recent studies revealed that cancer cells, in addition to osteoclasts, secrete substantial amounts of proton/lactate and create acidic extracellular environments.29
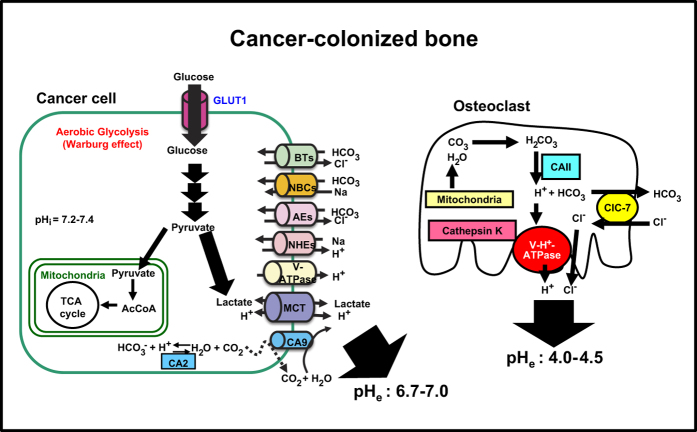
Normal cells are primarily dependent on mitochondrial oxidative phosphorylation to produce ATP. In contrast, most cancer cells rely on aerobic glycolysis known as the ‘Warburg effect', which is much less energy efficient but independent of oxgen.29 Although the mechanism of cancer dependence on the aerobic glycolysis remains unclear, it has been proposed that cancer cells possess the capacity to shift their energy metabolism to glycolysis to meet increased requirements for ATP and metabolic intermediates and precursors to maintain their aggressiveness under hypoxic conditions. The elevated aerobic glycolysis consumes high glucose and produces substantial amounts of lactate that lowers intracellular pH.29 To avoid intracellular acidification, cancers actively extrude lactate and protons via plasma membrane pH regulators such as monocarboxylate transporter 1 and 4 (MCT1 and MCT4), Na+/H+ exchangers, anion exchangers, carbonic anhydrases, V-H+-ATPase, Na+/HCO3− co-transporters and HCO3−transporter (Figure 3), creating acidic extracellular cancer microenvironment.46 Disruption of these pH regulators thus can arrest cancer cell growth and kill cancer cells by an elevation of intracellular levels of H+ and/or lactate, thereby inhibiting tumor progression. Consistent with this notion, we showed that high-metastatic B16 mouse melanoma cells expressed increased levels of the a3 isoform V-H□-ATPase compared with the low-metastatic B16 parental cells.47 Knockdown of a3 V-ATPase suppressed invasiveness and migration and significantly decreased lung and bone metastases, suggesting that a3 V-ATPase promotes distant metastasis of B16 melanoma by creating acidic extracellular environments via proton secretion. These results collectively suggest that the cancer-associated pH regulators are novel therapeutic targets for the treatment of cancer progression, invasion and metastasis. Further, it is expected that inhibitors of these pH regulators would be analgesic agents for acid-induced CABP.
Figure 3.
Acidic extracellular bone microenvironment created by bone-resorbing osteoclasts and bone-colonizing cancer cells. To dissolve bone minerals, mature osteoclasts release protons (H+) and chloride ions (Cl−) into the resorption lacunae via the plasma membrane (a3 isoform) vacuolar H+-ATPase proton pump36 and chloride ion-proton anti-porter ClC-7,37 acidifying the resorption lacunae to a pH of ∼4.5.30,36,43 Concomitantly, the lysosomal cysteine peptidase cathepsin K38 degrades bone matrix including type I collagen. RANKL stimulates osteoclastogenesis and bone resorption and prolongs survival by inhibiting apoptosis. Under hypoxic environment, cancer cells shift their energy metabolism to glycolysis that is independent of oxygen. The elevated aerobic glycolysis, known as the ‘Warburg effect', consumes high glucose via glucose transporter 1 (Glut1) and produces substantial amounts of lactate that lowers intracellular pH.29 To avoid intracellular acidification, cancers actively extrude lactate and protons via plasma membrane pH regulators such as monocarboxylate transporter 1 and 4, Na+/H+ exchangers, anion exchangers, carbonic anhydrases (CAs, CA2, CA9 and/or CA12), the plasma membrane proton pump V-H+-ATPase, Na+/HCO3− co-transporters and HCO3-transporter, creating acidic extracellular cancer microenvironment.46 CAII, carbonic anhydrase II; ClC7, plasma membrane chloride ion-proton anti-porter; RANK, receptor activation of NF-κB; RANKL, receptor activation of NF-κB ligand; V-H+-ATPase, plasma membrane (a3 isoform) vacuolar H+-ATPase proton pump; MCT1 and MCT4, monocarboxylate transporter 1 and 4; NHEs, Na+/H+ exchangers; AEs: anion exchangers; CA: carbonic anhydrases, V-H+-ATPase; NBC, Na+/HCO3− co-transporters; BT, HCO3−transporter; pHi, intracellular pH; pHe, extracellular pH.
MCT1 and MCT4 are two of the major proton-coupled lactate symporters mediating bidirectional lactate transport across the plasma membrane.48 MCT expression is increased in cancers including triple-negative breast cancer,49 renal cancer50 and head and neck cancer51 and correlated with the outcome of these cancer patients. MCT4 exports intracellular lactate resulting from aerobic glycolysis (Warburg effect), elevating lactate levels in extracellular cancer environments and exposing host cells adjacent to cancers to lactate.52 In contrast, MCT1 mainly imports extracellular lactate into cancer cells to utilize for oxidative metabolism.52 Suppression of the export of lactic acid from cancer cells by disrupting MCTs reduces glycolysis and cell growth.29 Meanwhile, recent studies reported that lactate released via MCTs from astrocytes serves as an energy source for neurons,53,54 suggesting that lactate is a critical regulator of neuronal function. Therefore, nociceptive sensory neurons can be also activated by lactate released via MCTs from neighboring cancer cells and evoke CABP. We recently found that CD138+ human primary multiple myeloma cells and several human myeloma cell lines strongly express MCT1 and/or MCT4. These cells release lactate into the culture medium and lower the pH in the culture medium (unpublished observation). These results may provide us with the molecular basis that accounts for lactate acidosis associated with cancers.55 The effects of lactate on CABP are currently unknown. Studies on the expression and function of MCT in cancers and the effects of secreted lactate on sensory neurons will lead us to better understanding of the mechanism of CABP and to facilitate to design alternative therapeutic approaches for CABP in addition to cancer.
Acid-sensing by sensory neurons in bone
The pathological acidic microenvironments created by protons/lactate secreted from bone-resorbing osteoclasts and bone-colonizing cancer cells upregulate and activate acid-sensing nociceptors expressed on sensory neurons and evoke CABP. It is known that acid-sensing is represented by two types of proton-activated cationic currents in DRG neurons.56 The first type, which is seen in most DRG, is characterized by a fast and rapidly inactivating inward current carried by Na+ and by a high sensitivity to protons. It is activated at pH 7 and maximally at pH around 6. The currents of this type exhibit similar patterns to those associated with the acid-sensing ion channels (ASICs). The second type is observed only in capsaicin-sensitive DRG neurons. The current of this type is sustained, slowly inactivating, less sensitive to protons and activated only at pH <6.2, sharing many similarities to the acid-induced current associated with the transient receptor potential channel-vanilloid subfamily member 1 (TRPV1). Here the contribution of two of the specific and representative pH-sensitive acid-sensing nociceptors, TRPV1 and ASIC3, to acid-evoked CABP will be discussed.
Transient receptor potential channel-vanilloid subfamily member 1 (TRPV1)
The TRPV1/vanilloid receptor 1 (VR1)/capsaicin receptor was cloned by expression cloning by Caterina et al.57 TRPV1 has 838 amino acids with a molecular size of 95kDa and is predominantly expressed on small unmyelinated c-fiber nociceptive afferent sensory neurons. In bone, osteoblasts and osteoclasts were found to express TRPV1.58 TRPV1, which is a highly Ca2+-permeable non-selective cation channel, is activated not only by acid but also by noxious heat (<43C) and is the only channel that is excited by the vanilloid capsaicin.57 Of note, an acidic environment allows protons to enter the cells via TRPV1. TRPV1 is only activated by acid below pH 6.0, generating a sustained channel current, whereas mild acidosis between pH 6 and 7 sensitizes TRPV1 to other noxious stimuli including capsaicin, heat and inflammatory mediators.57 In addition to mild acidosis, inflammatory mediators such as prostaglandins, bradykinin, adenosine triphosphate (ATP), 5-hydroxytryptamine and NGF also can sensitize TRPV1 to protons.
Using a mouse model of inflammatory pain, we showed that TRPV1 was co-expressed on CGRP-positive sensory neurons in the DRG.59 Mice with inflammation in their hind-paw exhibited a nociceptive behavior (thermal hyperalgesia) with elevated CGRP mRNA expression in the DRG. Treatment by acid (pH 5.5) excited primary DRG sensory neuron cells and increased CGRP mRNA expression and protein production in these cells. The specific antagonist of TRPV1, 5′-iodoresiniferatoxin (I-RTX), blocked the excitation and the acid-increased CGRP expression and production. Further, primary DRG cells isolated from TRPV1−/− mice failed to show an increase in CGRP expression on treatment with pH 5.5. Of importance, inflammatory pain was markedly diminished in TRPV1−/− mice. These results collectively suggest that activation of TRPV1 in inflammatory acidic environment leads to an upregulation of CGRP expression in sensory neurons, which in turn further increases inflammation and pain. Thus, blockade of TRPV1, CGRP or both could be an effective pharmacological intervention for acid-associated inflammatory pain.
TRPV1 has been implicated in the pathophysiology of CABP.13,25,28 In preclinical models of cancer pain, TRPV1 expression was increased in the ipsi-lateral DRG in the presence of cancer in bone.35,60 In support of the important role of TRPV1 in CABP, TRPV1 gene disruption or TRPV1 antagonist reduces CABP.61 Similarly, the specific TRPV1 antagonist I-RTX also suppressed CABP.60 Of interest, the TRPV1 antagonist SB366791 was shown to potentiate analgesic effects of morphine on bone cancer pain.62 These data suggest that suppression of TRPV1 activation under the acidic cancer environment is a promising therapeutic approach to attenuate CABP. However, preclinical and clinical studies revealed that TRPV1 antagonists induce hyperthermia as an adverse effect.63 Attempts to develop or identify antagonists devoid of temperature effects have not been successful. In this context, it is noted that recent studies have shown that the expression and activity of TRPV1 on the DRG sensory neurons are upregulated by TGF-β164 and IGF-1.65 Further, these studies showed that inhibition of TGF-β or IGF receptor signaling reduced CABP through suppression of TRPV1 activation. As TGF-β and IGF-1 are abundantly stored in the bone and released as a consequence of bone resorption by cancer-activated osteoclasts, expression and activation of TRPV1 on the sensory neurons innervating bone may be modulated by these bone-derived growth factors during the development and progression of osteolytic lesions. This notion is supported by the observations that bisphosphonate and denosumab reduced CABP in cancer patients.26,27,32,33 Suppression of TRPV1 activity and expression by blocking the activity of growth factors released from the bone is an alternative approach to attenuate CABP.
Of particular interest, Riera et al.66 recently reported that mice deficient in TRPV1 live longer and exhibit more youthful metabolic profile at old age compared with wild-type mice of the same age. These authors also found that CGRP release from sensory neurons innervating pancreatic islets is decreased, which in turn promotes insulin secretion and metabolic health in TRPV1-knockout mice. These surprising findings suggest that peripheral sensory neurons contribute to the regulation of metabolic health and longevity and that TRPV1 and CGRP may be therapeutic targets in the management of not only CABP but also glucose homeostasis and aging.
Acid-sensing ion channel 3 (ASIC3)
ASIC3 is one of the six subunits (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3 and ASIC4) of the acid-sensing ion channels that belong to the voltage-insensitive, amiloride-sensitive epithelial Na+ channel/degenerin family of cation channels.67 ASIC3 has 531 amino acids with a molecular size of 59 kDa. Different from TRPV1 that is excited with pH below 6.0, ASIC3 is a pH sensor that is activated with mild extracellular acidosis (pH 6.7-7.3). Expression of ASIC3 is predominantly observed in the DRG primary afferent sensory neurons.67,68,69 ASIC3 expression in DRG was increased in inflammatory acidic conditions and co-expressed with CGRP in the sensory neurons innervating the knee joint, indicating ASIC3 contribution to acute arthritis pain.70 ASIC3 was also detected on the CGRP-positive sensory neurons innervating periosteal surface of bone.71 In the bone, ASIC3 is expressed in monocytes, osteoclasts and osteoblasts.72 However, its functional role in bone homeostasis is unclear.
A recent study that an injection of the synthetic ASIC3 agonist, 2-guanidine-4-methylquinazoline, into mouse paw induced noxious behaviors in wild-type mice but not ASIC3−/− mice73 provided the evidence that ASIC3 activation is sufficient to evoke pain.74 However, the role of ASIC3 in CABP still remains elusive. We reported that ASCI3 mRNA expression was upregulated in DRG in a model of rat mammary tumor associated with CABP.35 Similarly, Qiu et al.75 recently showed that ASIC3 expression was elevated in DRG in rats with CABP using a different mammary tumor from ours. Although these results suggest that ASIC3 is involved in the pathophysiology of CABP, it needs to be shown that suppression of increased ASIC3 expression in DRG reduces CABP and that CABP is alleviated in ASIC3-knockout cancer-bearing mice.
We have recently found that, on acid treatment (pH 6.5), primary sensory neuron cells isolated from DRG displayed Ca2+ influx (unpublished data), which is a widely used early indicator for sensory neuron excitation.76 The specific ASIC3 antagonist APETx277 inhibited Ca2+ influx, indicating a critical role of ASIC3 in sensory neuron excitation. Further, APETx2 was shown to reduce acid-induced inflammatory pain due to complete Freund's adjuvant78 and slower the progression of osteoarthritis.79 Effects of APETx2 on CABP are unknown.
The DRG sensory neurons express both TRPV1 and ASIC3 to respond to extracellular acidification. It is important to investigate whether TRPV1 and ASIC3 have different or identical function in exciting and sensitizing sensory neurons in response to acid. ASIC3 senses mild extracellular acidification (pH 6.7-7.3),67,69 whereas TRPV1 is activated at the pH lower than 6.0.57 We reported that TRPV1 was activated at pH 5.5.59 The pH of the acidic cancer environment is shown to be 6.5–7.046 and that of resorption lacunae is assumed to be 4.0–4.5.36,43 Sensory neurons innervating bone may be excited and sensitized by discriminating mild and strong acidic extracellular microenvironment through ASIC3 and TRPV1, respectively (Figure 4).
Figure 4.
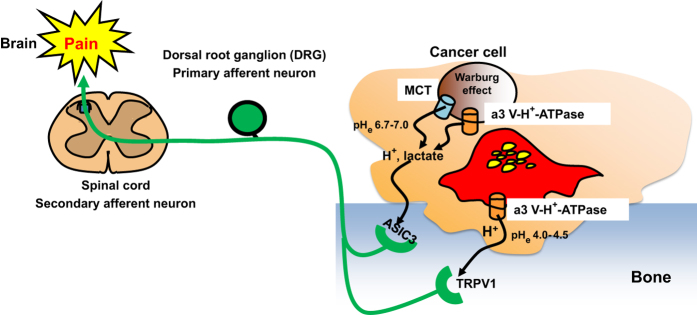
Acid-evoked cancer-associated bone pain (CABP). Bone-resorbing osteoclasts secrete protons to degrade bone minerals, and bone-colonizing cancer cells also release protons/lactate to avoid intracellular acidification due to the Warburg effect. The acidic microenvironment created by bone-colonizing cancer cells (pHe:6.7–7.0) and bone-resorbing osteoclasts (pHe:4.0–4.5) directly excites sensory neurons innervating bone via activation of the acid-sensing nociceptors, TRPV1 and ASIC3, respectively, transducing noxious signals via DRG (primary afferent neuron) and spinal cord (secondary afferent neuron) and evokes bone pain in brain. Blockade of TRPV1 and/or ASIC3 activation under the acidic cancer environments may be a promising therapeutic approach to alleviate CABP. ASIC3, acid-sensing ion channel 3; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglion; MCT, monocarboxylate transporter; TRPV1, transient receptor potential channel-vanilloid subfamily member 1.
Other acid-sensing machineries potentially contributing to CABP
Transient receptor potential (TRP) ion channels are the large families of cellular sensors mediating taste perception, thermo-sensation, mechano-sensing and osmolality sensing by transducing various physical stimuli into neuronal signals in predominantly C and Aδ nociceptors in primary sensory neurons.80 Further, TRP ion channels also mediate transduction of peripheral nociceptive stimuli into pain. It is thus tempting to assume that these family members of TRP channels have a role in acid-induced CABP. However, current available evidence suggests that activation of these TRPs by acid is unlikely except for TRPV1. Of interest, recent studies described that the members of TRP channels are involved in the regulation of skeletal homeostasis by affecting intestinal calcium absorption (TRPV6), renal calcium reabsorption (TRPV5), and differentiation of osteoclasts (TRPV1, TRPV2, TRPV4 and TRPV5), chondrocytes (TRPV4) and osteoblasts (TRPV1).58
TRPV4
TRPV4 is a calcium-permeable non-selective cation channel that is critical to the regulation of osmo-, thermo- and mechano-transduction.81 Although TRPV4 was shown to be activated by acid at pH 4–6,82 it is unknown whether TRPV4 is involved in the pathophysiology of acid-induced CABP at present. Recent studies revealed that mutations of TRPV4 are associated with varieties of skeletal dysplasia and osteoarthritis.83 Different TRPV4 mutations cause dominantly inherited neurologic disorders such as congenital spinal muscular atrophy and hereditary motor and sensory neuropathy. Of interest, some patients with a TRPV4 mutation display skeletal dysplasia combined with peripheral neuropathy. Consistent with these results, we reported that TRPV4 is a regulator of chondrocyte differentiation.84 Role of TRPV4 in CABP warrants further exploration.
TRPA1
TRP Ankyrin 1 (TRPA1) is a non-selective Ca2+ permeable cation channel that uniquely possesses 17 ankyrin repeat domains.80 TRPA1 is predominantly expressed in C and Aδ nerve fibers. Although still controversial, TRPA1 is proposed to be activated by noxious cold temperatures (< 18°C) and mechanical force. Some studies reported that TRPA1-deficient mice showed impaired behavioral responses to cold plate and mechanical stimuli. Expression of TRPA1 in bone cells has been unknown, but a recent study demonstrated the expression of TRPA1 in human odontoblasts.85 It is unclear whether TRPA1 is activated by acid and has a role in CABP. Notably, however, most neurons that express TRPA1 also express TRPV1, raising the possibility that TRPA1 may modulate or partially share the acid-sensing function of TRPV1. If this is proved to be the case, hyperthermia, which is a major obstacle in the development of TRPV1 antagonists, may not be a serious problem with TRPA1 antagonists.
TRPM8
TRP Melastatin 8 (TRPM8) is expressed in a subset of C and Aδ nerve fibers in DRG, trigeminal ganglion and nodose ganglion.80 In bone, TRPM8 expression was shown in osteoblasts; however, its functional role is unknown.86 TRPM8 is activated by cold temperatures (< 15 °C) and ‘cooling' compounds such as menthol and icilin and peppermint oil. It is unclear whether TRPM8 is activated by acid. TRPM8 was initially cloned as a molecule that has high homology to a TRP-like channel that was identified in prostate cancers.80 Later, it was found that increased expression of TRPM8 is correlated with the aggressiveness of a variety of cancers including prostate, lung, breast, gastric, ovarian and liver cancer and melanoma.87 As prostate, breast and lung cancers preferentially spread to the bone and are frequently associated with CABP, it is plausible to speculate that TRPM8 contributes to the pathophysiology of CABP. The role of TRPM in CABP needs to be elucidated.
Conclusion
Bone microenvironment is hypoxic.88 Hypoxia increases the expression of hypoxia-inducible transcription factor-1 (HIF-1) that upregulates the expression and function of the plasma membrane proton/lactate transporters including glucose transporter 1, MCT4, Na+/H+ exchanger 1 and carbonic anhydrase 9.46 Therefore, the bone microenvironment by its nature is likely acidic. In addition, the bone is a unique organ in which multinucleated giant osteoclasts continuously secrete protons to maintain bone homeostasis.30 The secretion of protons by osteoclasts is increased in response to the bone-modifying factors produced by bone-colonizing cancer cells.3,4,5,6 Protons and lactate are also secreted by bone-colonizing cancer cells themselves as a consequence of elevated oxygen-independent glycolysis (Warburg effect).29,46 Collectively, cancer-colonized bone microenvironment can readily develop pathological acidosis, thereby evoking acid-induced CABP, and the contribution of acid may be more specific and critical in the pathophysiology of CABP than that of pain in other tissues and organs. In addition to bone pain, the acidic extracellular microenvironments also exacerbate aggressiveness of cancer-colonizing bone.89,90 Thus, suppression of the development of acidic extracellular cancer-colonized bone microenvironment by inhibiting the proton and lactate transporters in osteoclasts and cancer cells is a logic therapeutic approach to inhibit the progression of cancer and CABP. Interference with the activation of acid-sensing nociceptors in sensory neurons may also be an effective and selective therapeutic means for CABP. Finally, development of new analgesic drugs for CABP is expected to reduce dosing of opioids that are currently the primary agent in the management of cancer pain but cause unwanted adverse effects.
Improved survival resulting from the advancement of interdisciplinary treatments increases the opportunity for cancer patients to suffer from CABP. CABP profoundly disrupts quality of life and impairs the host immune system causing increased secondary infections and delays in the recovery from the illness, resulting in increased death. Adequate control of CABP will be a challenging goal in the management of patients with cancers in the bone.
Acknowledgments
This study is supported by the Project Development Team within the ICTSI NIH/NCRR (#TR000006), start-up fund of Indiana University School of Medicine, USA, Grants-in-Aid, #23390422 [TY], 23659870 [TY], 26670806 [TY] and 26293394 [TY] by the Ministry of Education, Culture, Sports, Science and Technology, Japan, Naito Foundation Research Grant (#0808030024) [TY] and Princess Takamatsu Cancer Research Foundation Grant (#08-24020) [TY].
Footnotes
The authors declare no conflict of interest.
References
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006; 12: 6243s–6249s. [DOI] [PubMed] [Google Scholar]
- Terpos E, Berenson J, Raje N, Roodman GD. Management of bone disease in multiple myeloma. Expert Rev Hematol 2014; 7: 113–125. [DOI] [PubMed] [Google Scholar]
- Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002; 2: 584–593. [DOI] [PubMed] [Google Scholar]
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004; 350: 1655–1664. [DOI] [PubMed] [Google Scholar]
- Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer 2011; 11: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T, Tanaka S, Hata K. Role of RANKL/RANK in primary and secondary breast cancer. World J Orthop 2013; 4: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Cook RJ, Lipton A, Costa L, Coleman RE. Prognostic factors for skeletal complications from metastatic bone disease in breast cancer. Breast Cancer Res Treat 2010; 123: 767–779. [DOI] [PubMed] [Google Scholar]
- Falk S, Dickenson AH. Pain and nociception: mechanisms of cancer-induced bone pain. J Clin Oncol 2014; 32: 1647–1654. [DOI] [PubMed] [Google Scholar]
- Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain 1997; 69: 1–19. [DOI] [PubMed] [Google Scholar]
- Luger NM, Sabino MA, Schwei MJ, Mach DB, Pomonis JD, Keyser CP et al. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain 2002; 99: 397–406. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 2001; 413: 203–210. [DOI] [PubMed] [Google Scholar]
- Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei ML, Pomonis JD et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 2002; 113: 155–166. [DOI] [PubMed] [Google Scholar]
- Mantyh P. Bone cancer pain: Causes, consequences, and therapeutic opportunities. Pain 2013; 154: S54–S62. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 2009; 139: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation 2015; 18: 24–32. [DOI] [PubMed] [Google Scholar]
- Cooper RR. Nerves in cortical bone. Science 1968; 160: 327–328. [DOI] [PubMed] [Google Scholar]
- Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 1999; 25: 623–629. [DOI] [PubMed] [Google Scholar]
- Irie K, Hara-Irie F, Ozawa H, Yajima T. Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc Res Tech 2002; 58: 85–90. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Takeda S, Xu R, Ochi H, Sunamura S, Sato T et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature 2013; 497: 490–493. [DOI] [PubMed] [Google Scholar]
- Benemei S, Nicoletti P, Capone JG, Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol 2009; 9: 9–14. [DOI] [PubMed] [Google Scholar]
- Salmon AM, Damaj MI, Marubio LM, Epping-Jordan MP, Merlo-Pich E, Changeux JP. Altered neuroadaptation in opiate dependence and neurogenic inflammatory nociception in alpha CGRP-deficient mice. Nat Neurosci 2001; 4: 357–358. [DOI] [PubMed] [Google Scholar]
- Toda M, Suzuki T, Hosono K, Hayashi I, Hashiba S, Onuma Y et al. Neuronal system-dependent facilitation of tumor angiogenesis and tumor growth by calcitonin gene-related peptide. Proc Natl Acad Sci USA 2008; 105: 13550–13555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience 2010; 171: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan A, Smith MT. Pathobiology and management of prostate cancer-induced bone pain: recent insights and future treatments. Inflammopharmacology 2013; 21: 339–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW. Cancer-induced bone pain: Mechanisms and models. Neurosci Lett 2013; 557: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno N, Aogi K, Minami H, Nakamura S, Asaga T, Iino Y et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol 2005; 23: 3314–3321. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Body JJ, Stopeck A, von Moos R, Fallowfield L, Mathias SD et al. Pain outcomes in patients with advanced breast cancer and bone metastases: Results from a randomized, double-blind study of denosumab and zoledronic acid. Cancer 2013; 119: 832–838. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci 2006; 7: 797–809. [DOI] [PubMed] [Google Scholar]
- Parks SK, Chiche J, Pouysségur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer 2013; 13: 611–623. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Bone resorption by osteoclasts. Science 2000; 289: 1504–1508. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Boyle WJ, Simonet WS, Kostenuik PJ, Dougall WC, Sullivan JK et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov 2012; 11: 401–419. [DOI] [PubMed] [Google Scholar]
- Terpos E, Morga G, Dimopoulos MA, Drake MT, Lentzsch S, Raje N et al. International myeloma working group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol 2013; 31: 2347–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moos R, Body JJ, Egerdie B, Stopeck A, Brown JE, Damyanov D et al. Pain and health-related quality of life in patients with advanced solid tumours and bone metastases: integrated results from three randomized, double-blind studies of denosumab and zoledronic acid. Support Care Cancer 2013; 21: 3497–3507. [DOI] [PubMed] [Google Scholar]
- Honore P, Luger NM, Sabino MA, Schwei MJ, Rogers SD, Mach DB et al. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med 2000; 6: 521–528. [DOI] [PubMed] [Google Scholar]
- Nagae M, Hiraga T, Yoneda T. Acidic microenvironment created by osteoclasts causes bone pain associated with tumor colonization. J Bone Miner Metab 2007; 25: 99–104. [DOI] [PubMed] [Google Scholar]
- Qin A, Cheng TS, Pavlos NJ, Lin Z, Dai KR, Zheng MH. V-ATPases in osteoclasts: structure, function and potential inhibitors of bone resorption. Int J Biochem Cell Biol 2012; 44: 1422–1435. [DOI] [PubMed] [Google Scholar]
- Supanchart C, Wartosch L, Schlack C, Kühnisch J, Felsenberg D, Fuhrmann JC et al. ClC-7 expression levels critically regulate bone turnover, but not gastric acid secretion. Bone 2014; 58: 92–102. [DOI] [PubMed] [Google Scholar]
- Costa AG, Cusano NE, Silva BC, Cremers S, Bilezikian JP, Cathepsin K. its skeletal actions and role as a therapeutic target in osteoporosis. Nat Rev Rheumatol 2011; 7: 447–456. [DOI] [PubMed] [Google Scholar]
- Zhao H. Membrane trafficking in osteoblasts and osteoclasts: new avenues for understanding and treating skeletal diseases. Traffic 2012; 13: 1307–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyomura T, Murata Y, Yamamoto A, Oka T, Sun-Wada GH, Wada Y et al. From lysosomes to the plasma membrane: localization of vacuolar-type H+-ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem 2003; 278: 22023–22030. [DOI] [PubMed] [Google Scholar]
- Henriksen K, Sørensen MG, Jensen VK, Dziegiel MH, Nosjean O, Karsdal MA. Ion transporters involved in acidification of the resorption lacuna in osteoclasts. Calcif Tissue Int 2008; 83: 230–242. [DOI] [PubMed] [Google Scholar]
- Kikuta J, Wada Y, Kowada T, Wang Z, Sun-Wada GH, Nishiyama I et al. Dynamic visualization of RANKL and Th17-mediated osteoclast function. J Clin Invest 2013; 123: 866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowada T, Kikuta J, Kubo A, Ishii M, Maeda H, Mizukami S et al. In vivo fluorescence imaging of bone-resorbing osteoclasts. J Am Chem Soc 2011; 133: 17772–17776. [DOI] [PubMed] [Google Scholar]
- Nagae M, Hiraga T, Wakabayashi H, Wang L, Iwata K, Yoneda T. Osteoclasts play a part in pain due to the inflammation adjacent to bone. Bone 2006; 39: 1107–1115. [DOI] [PubMed] [Google Scholar]
- Marelli S, Pace F. Rabeprazole for the treatment of acid-related disorders. Expert Rev Gastroenterol Hepatol 2012; 6: 423–435. [DOI] [PubMed] [Google Scholar]
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011; 10: 767–777. [DOI] [PubMed] [Google Scholar]
- Nishisho T, Hata K, Nakanishi M, Morita Y, Sun-Wada GH, Wada Y et al. The a3 isoform vacuolar type H+-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res 2011; 9: 845–855. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. Monocarboxylic acid transport. Compr Physiol 2013; 3: 1611–1643. [DOI] [PubMed] [Google Scholar]
- Doyen J, Trastour C, Ettore F, Peyrottes I, Toussant N, Gal J et al. Expression of the hypoxia-inducible monocarboxylate transporter MCT4 is increased in triple negative breast cancer and correlates independently with clinical outcome. Biochem Biophys Res Commun 2014; 451: 54–61. [DOI] [PubMed] [Google Scholar]
- Fisel P, Kruck S, Winter S, Bedke J, Hennenlotter J, Nies AT et al. DNA methylation of the SLC16A3 promoter regulates expression of the human lactate transporter MCT4 in renal cancer with consequences for clinical outcome. Clin Cancer Res 2013; 19: 5170–5181. [DOI] [PubMed] [Google Scholar]
- Curry JM, Tuluc M, Whitaker-Menezes D, Ames JA, Anantharaman A, Butera A et al. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle 2013; 12: 1371–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest 2013; 123: 3685–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen LH. Is lactate food for neurons? Comparison of monocarboxylate transporter subtypes in brain and muscle. Neuroscience 2007; 145: 11–19. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011; 144: 810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut JA, Madias NE. Lactic Acidosis. N Engl J Med 2014; 371: 2309–2319. [DOI] [PubMed] [Google Scholar]
- Holzer P. Acid sensing by visceral afferent neurones. Acta Physiol (Oxf) 2011; 201: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288: 306–313. [DOI] [PubMed] [Google Scholar]
- Lieben L, Carmeliet G. The involvement of TRP channels in bone homeostasis. Front Endocrinol (Lausanne) 2012; 3: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi M, Hata K, Nagayama T, Sakurai T, Nishisho T, Wakabayashi H et al. Acid activation of Trpv1 leads to an up-regulation of calcitonin gene-related peptide expression in dorsal root ganglion neurons via the CaMK-CREB cascade: a potential mechanism of inflammatory pain. Mol Biol Cell 2010; 21: 2568–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niiyama Y, Kawamata T, Yamamoto J, Omote K, Namiki A. Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience 2007; 148: 560–572. [DOI] [PubMed] [Google Scholar]
- Ghilardi JR, Röhrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci 2005; 25: 3126–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niiyama Y, Kawamata T, Yamamoto J, Furuse S, Namiki A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br J Anaesth 2009; 102: 251–258. [DOI] [PubMed] [Google Scholar]
- Brederson JD, Kym PR, Szallasi A. Targeting TRP channels for pain relief. Eur J Pharmacol 2013; 716: 61–76. [DOI] [PubMed] [Google Scholar]
- Xu Q, Zhang XM, Duan KZ, Gu XY, Han M, Liu BL et al. Peripheral TGF-β1 signaling is a critical event in bone cancer-induced hyperalgesia in rodents. J Neurosci 2013; 33: 19099–19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cai J, Han Y, Xiao X, Meng XL, Su L et al. Enhanced function of TRPV1 via up-regulation by insulin-like growth factor-1 in a rat model of bone cancer pain. Eur J Pain 2014; 18: 774–784. [DOI] [PubMed] [Google Scholar]
- Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R et al. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell 2014; 157: 1023–1036. [DOI] [PubMed] [Google Scholar]
- Deval E, Gasull X, Noël J, Salinas M, Baron A, Diochot S et al. Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol Ther 2010; 128: 549–558. [DOI] [PubMed] [Google Scholar]
- de la Rosa AD, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci USA 2002; 99: 2326–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-G, Xu T-L. ASIC3 channels in multimodal sensory perception. ACS Chem Neurosci 2011; 2: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M, Kolker SJ, Sluka KA. Acid-sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan-induced arthritis. J Pain 2009; 10: 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TH, Riede MS, Vulchanova L, Ortiz-Gonzalez XR, Elde R. An acid sensing ion channel (ASIC) localizes to small primary afferent neurons in rats. Neuroreport 1998; 9: 1109–1113. [DOI] [PubMed] [Google Scholar]
- Jahr H, van Driel M, van Osch GJ, Weinans H, van Leeuwen JP. Identification of acid-sensing ion channels in bone. Biochem Biophys Res Commun 2005; 337: 349–354. [DOI] [PubMed] [Google Scholar]
- Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F et al. A nonproton ligand sensor in the acid-sensing ion channel. Neuron 2010; 68: 61–72. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Taugher RJ, Kreple CJ. Acid-sensing ion channels in pain and disease. Nat Rev Neurosci 2013; 14: 461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Wei X, Zhang S, Yuan W, Mi W. Increased expression of acid-sensing ion channel 3 within dorsal root ganglia in a rat model of bone cancer pain. Neuroreport 2014; 25: 887–893. [DOI] [PubMed] [Google Scholar]
- Feldman P, Due MR, Ripsch MS, Khanna R, White FA. The persistent release of HMGB1 contributes to tactile hyperalgesia in a rodent model of neuropathic pain. J Neuroinflammation 2012; 9: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S et al. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J 2004; 23: 1516–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ et al. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol 2010; 161: 950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi M, Ikeuchi M, Ji Q, Tani T. Local ASIC3 modulates pain and disease progression in a rat model of osteoarthritis. J Biomed Sci 2012; 19: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Abooj M. TRP channels and analgesia. Life Sci 2013; 92: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Elias A, Mrkonjić S, Jung C, Pardo-Pastor C, Vicente R, Valverde MA. The TRPV4 channel. Handb Exp Pharmacol 2014; 222: 293–319. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 2003; 278: 22664–22668. [DOI] [PubMed] [Google Scholar]
- Nishimura G, Lausch E, Savarirayan R, Shiba M, Spranger J, Zabel B et al. TRPV4-associated skeletal dysplasias. Am J Med Genet C Semin Med Genet 2012; 160C: 190–204. [DOI] [PubMed] [Google Scholar]
- Muramatsu S, Wakabayashi M, Ohno T, Amano K, Ooishi R, Sugahara T et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem 2007; 282: 32158–32167. [DOI] [PubMed] [Google Scholar]
- Egbuniwe O, Grover S, Duggal AK, Mavroudis A, Yazdi M, Renton T et al. TRPA1 and TRPV4 activation in human odontoblasts stimulates ATP release. J Dent Res 2014; 93: 911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abed E, Labelle D, Martineau C, Loghin A, Moreau R. Expression of transient receptor potential (TRP) channels in human and murine osteoblast-like cells. Mol Membr Biol 2009; 26: 146–158. [DOI] [PubMed] [Google Scholar]
- Chen J, Luan Y, Yu R, Zhang Z, Zhang J, Wang W. Transient receptor potential (TRP) channels, promising potential diagnostic and therapeutic tools for cancer. Biosci Trends 2014; 8: 1–10. [DOI] [PubMed] [Google Scholar]
- Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res 2009; 24: 1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies RJ, Verduzco D, Gatenby RA. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat Rev Cancer 2012; 12: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppicelli S, Bianchini F, Calorini L. Extracellular acidity, a "reappreciated" trait of tumor environment driving malignancy: perspectives in diagnosis and therapy. Cancer Metastasis Rev 2014; 33: 823–832. [DOI] [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet 1889; 133: 571–573. [PubMed] [Google Scholar]