Abstract
Osteosarcoma is the most common form of primary bone tumors with high prevalence in children. Survival rates of osteosarcoma are low, especially in the case of metastases. Mouse models of this disease have been very valuable in investigation of mechanisms of tumorigenesis, metastasis, as well as testing possible therapeutic options. In this chapter, we summarize currently available mouse models for osteosarcoma and provide detailed methodology for the isolation of cell lines from genetically engineered mouse models (GEMMs), gene modification and tumor cell injection methods, as well as imaging techniques.
Introduction
Osteosarcoma (OS), the most common form of primary bone cancer, is most often found near the metaphyseal growth plates in long bones of the extremities, such as the distal femur, proximal tibia and proximal humerus. It is a rare malignancy, on average occurring in about 4–5 patients per million per year, but the incidence is increased in children and adolescents, as well as in the elderly.1 Despite the differences in the origin and aggressiveness of the disease between children and the elderly, in both cases this is a devastating disease with poor outcome.
Current therapeutic strategies for OS consist of a multi-modal regimen using preoperative chemotherapy followed by surgery and then post-operative chemotherapy.2 OS cells are highly resistant to radiotherapy; thus, this treatment has limited benefits and is not part of the standard treatment.3 However, it can be used in combination with surgery and chemotherapy in patients with unresectable tumors.3 Ongoing clinical trials are focused on testing different immunomodulatory strategies, including interferons and viral delivery of granulocyte–macrophage colony-stimulating factor.3 In addition, small molecules that specifically target intracellular signaling pathways such as the mammalian target of rapamycin (mTOR) and src, as well as bisphosphonates, have been considered as new strategies and novel therapeutic approaches.3
However, despite improvements in the treatment regimen and an increased number of clinical trials, many of which are nowadays internationally oriented (such as the European and American OS Group, EURAMOS), not much further progress has been made to increase patient survival over the last three decades. As a result, the 5-year overall survival rate has remained stable at ∼65% in case of local disease, and did not influence survival in case of metastases, present in about one-fifth to one quarter of patients at diagnosis, which continue to have a poor 5-year survival rate of about 20%.4,5 These facts highlight an urgent need for development of new therapeutic strategies for the treatment of bone sarcomas. Preclinical studies with fast and reliable animal models that closely mimic the human disease would be valuable in achieving this goal.
OS is a disease of mesenchymal cell origin, and malignant cells produce osteoid matrix. Therefore, the cells most implicated in its origin are of the osteoblast lineage.6 However, there has been recent interest in the cell type(s) along the differentiation pathway from mesenchymal stem cells to differentiated osteoblasts that may also be capable of serving as the cell of origin in OS. Experimental evidence exists to support the possibility that OS may originate from preosteoblasts that have differentiated to a point of commitment toward the osteoblast lineage.6 However, evidence also exists to support the potential for less differentiated cells, such as mesenchymal stem cells (MSCs), to transform into OS, as well as other sarcoma types.
Currently available mouse models
Genetically engineered mouse models
Historically, the first genetically engineered mouse model (GEMM) to display OS is the H2K-fos-tg mouse model, where AP-1 transcription factor c-fos is overexpressed. In this model, the osteoblasts were shown to be the target cells for transformation.7 Although the tumor histopathology is similar to human osteoblastic OSs, this GEMM does not reproduce the metastatic disease that frequently occurs in humans.
Recently, new GEMMs of OS have been developed based on the knowledge of Li-Fraumeni and retinoblastoma familial predisposition syndromes and the role of p53 and Rb pathways in this disease. Mice with germline mutations of p53 develop OS but also other tumors. Mice with homozygous Rb deletions are embryonic lethal and Rb heterozygotes do not develop OS. The ability to use conditional lineage-restricted alleles has further increased our understanding of the specificity of cell types that generate OS.8 Mouse models based on deletion of Prx-1-Cre that is active in early limb bud mesenchymal tissue lead to OS but also poorly differentiated soft tissue sarcomas.9 Use of osterix (Osx-cre), a gene that is actively expressed in more differentiated preosteoblasts, leads to OS with high penetrance that is dependent on p53 mutation and potentiated by loss of Rb.10
Recent advances in GEMMs have seen the in vivo application of shRNA-based transgenic approaches to suppress, rather than knockout, genes of relevance to osteosarcomagenesis. A theoretical advantage to this approach is the potential for reversibility of gene suppression upon removal of the shRNA, with the ability to study biologic consequences and model pharmacologic intervention. A recent report of this approach in OS demonstrated that p53-directed shRNA driven by Osx-cre produced an osteoblastic subtype of OS in mice with high reproducibility.11 Interestingly, these tumors lost p53 pathway function during progression and metastasis and were no longer reliant on shRNA suppression of p53.
In addition to these p53-based models, other GEMMs of OS have been reported. Examples include the use of targeted apc:twist double-mutant mice,12 overexpression of SV40 T antigen in mature osteoblasts using the osteocalcin promoter,13 upregulation of Hedgehog signaling in osteoblasts crossed to mice with a p53 heterozygous background14 and conditional expression of the intracellular domain of Notch1 in immature osteoblasts.15 Collectively, these and future GEMM OS models provide futher insight into human OS genetics and biology.
Models based on cell/tumor grafts
Another approach to model OS in mice is mainly based on the injection of murine (allograft) or human (xenograft) OS cells (Figure 1). The advantage of these models is that they are easy to set up, affordable, have a quick onset (tumor development between 1 and 2 months) and are reproducible. Tumor cells can be inoculated into immunocompetent (syngeneic models) or immunocompromised mice (xenogeneic models). The different cell lines used for paratibial and intraosseous models are described in Table 1. Some studies use subcutaneous injections of OS cells; however, cell grafts or tumor fragment transplantation in orthotopic sites are considered more relevant preclinical models.
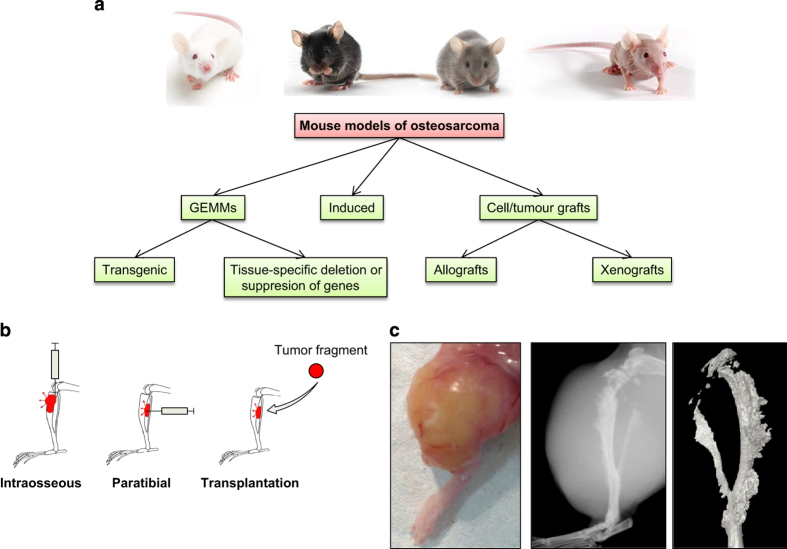
Figure 1.
Summary of models of osteosarcoma. (a) A summary of the available mouse models for OS. (b) Illustration of the different injection modes. (c) Picture taken after the skin removal of a tumor obtained 1 month after injection of KHOS cells. Radiography of the tibia carrying the tumor. Three-dimensional reconstruction of the tumoral tibia after microCT analysis.
Table 1. The main cell lines inoculated in in vivo mouse models, as well as their origin, tumorigenic capacity and the mouse strains best suited for their use.
| Model | Cell line | Origin | Inoculation | Tumor development | Mouse strain | References |
|---|---|---|---|---|---|---|
| Allograft | K7(K8,…, K12) | Spontaneous osteosarcoma from BALB/c mice | Paratibial or intraosseous injection | Primary tumor (osteolytic)Rare lung metastasis | BALB/c | Schmidt et al. (1988)Gerstenfeld et al. (1996) |
| K7M2(ATCC CRL-2836) | Lung metastases consecutive to intraosseous injection of the K7 cell line to the tibia of a BALB/c mouse | Paratibial or intraosseous injection | Primary tumor (osteolytic) Highly metastatatic(lung metastasis in 90% of mice) | BALB/c | Khanna et al. (2000) | |
| Dunn(BFO) | Spontaneous osteosarcoma from the tail of C3H/HeN mice | Subcutaneous, intraosseous and intravenous injection | Primary tumor (osteogenic)Lung and liver micrometastasis | C3H/HeN | Dunn and Andervont (1963) | |
| LM8 | Lung metastases of Dunn osteosarcoma cells after 8 in vivo passages (Fidler's procedures) | Subcutaneous, intraosseous and intravenous injection | Primary tumor (osteogenic) Highly metastatatic(lung metastasis in 100% of mice) | C3H/HeN | Asai et al. (1998)Poste and Fidler (1980) | |
| POS-1 | Spontaneous osteosarcoma of C3H/HeN mice | Transplantation of tumoral fragments | Primary tumor (osteolytic)Lung metastasis | C3H/HeN | Nitto et al. (1998)Uesugi et al. (2000) | |
| MOS-J | Spontaneous chondroblastic osteosarcoma of C57BL/6J mice | Paratibial or intraosseous injection | Primary tumor (osteogenic)Lung metastasis | C57BL/6J | Joliat et al. (2002) | |
| Xenograft | MNNG-HOS(ATCC CRL-1547) | Transformed from HOS (ATCC CRL-1543) with MNNG, a carcinogenic nitrosamine | Paratibial or intraosseous injection | Primary tumor (mixed)Rare and big lung metastases | NUDE/SCID | McAllister et al. (1971)Rhim and Park et al. (1975) |
| KHOS(ATCC CRL-1544) | HOS cells (ATCC CRL-1543) by transformation using Kirsten murine sarcoma virus (Ki-MSV). | Paratibial or intraosseous injection | Primary tumor (mixed)Frequent and small lung metastases | NUDE/SCID | McAllister et al. (1971)Rhim, Cho et al. (1975)Rhim, Cho and Huebner (1975) | |
| KRIB | HOS cells (ATCC CRL-1543) by transformation using a v-Ki-ras oncogene. | Paratibial or intraosseous injection | Primary tumor (more an osteolytic model)Highly metastatic (lung metastasis in all mice) | NUDE/SCID | Berlin et al. (1993) | |
| 143B-HOS(ATCC CRL-8303) | HOS cells (ATCC CRL-1543) by transformation using Ki-ras oncogene. | Paratibial or intraosseous injection | Primary tumor (more an osteolytic model)Highly metastatic (lung metastasis in all mice) | NUDE/SCID | Campione-Piccardo et al. (1979)Luu et al. (2005) | |
| U-2 OS Originally 2T(ATCC HTB-96) | Moderately differentiated (epithelial) sarcoma of the tibia of a 15-year-old girl | Subcutaneous injection | Primary tumor (osteogenic)Low tumorigenicity and aggressivenessNo lung metastases | NUDE/SCID | Pontén and Saksela (1967)Manara et al. (2000) | |
| SAOS-2(ATCC HTB-85) | Primary epithelial osteosarcoma of a 11-year-old Caucasian girl | Paratibial or intraosseous injection | Primary tumor (osteogenic)Few metastasis | NUDE/SCID | Fogh, Fogh and Orfeo (1977) | |
| MG63 | Fibroblastic osteosarcoma of a 14-year-old boy | Subcutaneous | Primary tumor (osteogenic) | NUDE/SCID | Billiau et al. (1977) | |
| (ATCC CRL-1427) | Low tumorigenicity and aggressiveness | Cheon et al. (1997) |
The full references can be found in Supplementary Information.
Radiation/chemically induced OS
Exposure to carcinogens such as radioactive materials has been implicated in OS tumorigenesis. Therefore, several radiation-induced OS mouse models have been developed and utilized for the study of human OS. Murine OS models have been developed using exposure to radioactive substances such as radium, thorium and roentgen. Radioactive heavy metals have also been used for the induction of OS in mice, as these metals tend to incorporate in the ossifying matrix of the bone. In addition, alpha and beta emitters have been shown to successfully induce OS and have been adopted as a model of human radiation carcinogenesis. Most of the chemically induced mice OS models have been developed by injecting different chemical carcinogens directly into the bone.16 These models are utilized as a representation of the effect of DNA damage in pathogenesis, rather than to recapitulate the etiology of OS.16
Methods
(1) Isolation and characterization of cell lines from bone tumors
Materials
Antibiotics: Penicillin–Streptomycin.
Collagenase A.
Dispase II.
TC grade 1% trypsin-EDTA.
Phosphate-buffered saline (PBS; TC grade) and PBS supplemented with antibiotics (PBS-Ab).
OS isolation medium: modified Eagle's medium type α (alphaMEM) or Dulbecco's modified Eagle's medium (DMEM) supplemented with 15% FBS and antibiotics.
Tissue digestion solution: alphaMEM supplemented with 0.1% collagenase and 0.2% dispase and filtered through 0.20 μM syringe filter.
This requires the OS to be visible by eye, an ideal size is a few millimeters in diameter.
Euthanize animals and collect tumor-containing limbs.
Transfer the limb into a falcon tube containing PBS-Ab.
In a tissue-culture hood, remove muscle, stepwise, rinsing in PBS, discarding debris and muscle, each time changing dishes.
Dissect tumors under the tissue-culture hood and transfer individual tumors to 1.5 ml Eppendorf tubes.
Wash 2–3 times with PBS-Ab. Resuspend in ∼1 ml PBS-Ab.
Mince with scissors directly in 1.5 ml tube.
Spin briefly.
Collect the supernatant carefully avoiding minced tissue and filter through a 70 μM strainer into a 50 ml Falcon containing 10 ml of DMEM containing 15% FBS. Keep under the hood.
Add 1 ml digest solution to minced OS pellet. Incubate 20 min in a 37 °C water bath with gentle shaking.
Carefully collect the supernatant and filter.
Repeat steps 6–10 two to three more times.
Centrifuge filtered fraction for 5 min at 1200 rpm.
Depending on size, resuspend pellet in 5–10 ml OS isolation medium and plate in appropriate tissue culture dish or flask. Flask of 25 or 75 cm2 is ideal.
Monitor cultures daily and replace medium after 3 days. Once the cultures reach confluence, maintain by splitting 1:5–10 using trypsin-EDTA.
*NOTE: Freezing aliquots at early passages is highly recommended. OS cell lines isolated by this method usually proliferate well for the first 2–3 passages but might slow down later on. Cultures beyond 15–20 passages are considered established cell lines.
(2) Modification and injection of tumor cells
Materials
PBS, pH=7.2.
Culture medium (DMEM, RPMI) supplemented with 10% FCS, 2 mM l-glutamine.
1% Trypsin solution.
Tissue culture flasks or dishes.
Isoflurane/oxygen-based anesthesia system fitted with an induction chamber and inhalation masks for mice or xylazine–ketamine cocktail.*NOTE: Xylazine (Rompun) at a dose of 10 mg kg−1 and ketamine (Imalgene 500) at a dose of 100 mg kg−1 should be used.*NOTE: Adequate preoperative analgesia should be applied––for example, by coinjecting 0.05 mg kg−1 fentanyl.
Matrigel (for specific experiments).
Mice: 4–8-week old.
A shaver/clippers.
Sterile forceps and scalpels.
Wound stitches/suture strings.
Sterile syringes and needles (21, 26, 28 and 31 G).
Third-generation HIV-based lentiviral vectors.
HEK 293FT cells.
Transfection agents such as calcium phosphate and lipofectamine.
Cellulose acetate membrane filter, 0.45 micron (Thermo Fisher).
Ultrafiltration tubes (Amicon Ultra-15 Centrifugal Filter Units, Millipore).
OS cell lines (HOS, MOS-J etc.).
(a) Modification of OS cells using lentiviruses
Produce lentiviral particles by transfecting 6 × 106 HEK 293FT cells 24 h after plating with 3 μg of optimized packaging and envelop plasmids (pLP1, pLP2, pLP-VSV-G) and 9 μg of the pLNT plasmid containing the gene of interest.17 Collect the virus-containing supernatant 48 h after the transfection and filter it with a 0.45 μm cellulose acetate membrane filter. For virus titration, serial dilutions of supernatants are tested on HEK 293FT cells, which are then analyzed for transgene expression 3–4 days post infection.*NOTE: Supernatants can be concentrated between 50 and 100 times by ultrafiltration.
Plate OS cells at 2 × 104 cells per well in a 24-well plate and infect the cells with the filtered supernatant at a MOI of ≤10 (10 viral particles per cell).*NOTE: HOS cells are easier to transduce compared with MOS-J cells. Cell transduction depends on the cell differentiation level.
48 hours after the transduction, when cells reach confluency, start the antibiotic selection to keep a polyclonal population expressing the transgene and amplify the cells.
(b) Preparation of cells before inoculation
Grow cells to reach a confluence of 70–80% on the day of the experiment to have cells in the exponential growth phase, which is recommended for an optimal tumor onset.*NOTE: A large amount of cells are frequently lost during the serial washings, thus prepare more cells than needed (approximately half the amount needed should be prepared extra).
Trypsinize cells and wash at least two times with PBS to remove serum residues before resuspending cells into the appropriate volume. Keep the cells on ice until the injection.*NOTE: The cells should be suspended into a minimal volume to limit inflammatory process. Usually 0.5–4 million cells can be resuspended in a volume of 10–50 μl. High-precision syringes (for example, Hamilton) are recommended for the actual tumor cell injection.
(b.1) Paratibial injection of OS cells
Anesthetize the animals and shave the legs, decontaminate them with alcohol and maintain the leg outstretched between the thumb and the index finger.
Apply the needle perpendicular to the tibia. Before injecting the cells, activate the periosteum briefly with the tip of the needle.*NOTE: A limited number of animals should be injected with the same needle (3 for example) to avoid the cell sedimentation. Some examples of paratibial injections (allografts and xenografts) are used in refs 18, 19.*NOTE: To limit soft tissue injuries during paratibial injection, the use of thin wall sterile needles is recommended, like BD Ultra-Fine insulin 0.3 ml syringes (31 G × 8 mm) for example.
(b.2) Intraosseous injection of OS cells
Anesthetize the animals and shave the legs and decontaminate them with alcohol.Two different approaches can be used to perform intramedullary injections:
- In the first method:
- Make a 0.5cm skin incision just below the knee joint to expose the tibia tuberosity.
- Inject cells into the intramedullary cavity of the tibia with a 26–28G syringe and suture the skin.
- In the second option:
- Pre-drill an axial hole with a 21–26 G needle in the tibial plateau or femoral condyles through the medullary cavity to allow the access for the Hamilton needle containing the cells.*NOTE: Compared with the first approach, the second one is less invasive, and the mice recover quickly after the injection.*NOTE: It is highly recommended to check correct positioning of the drilling needle inside the metaphyseal area with an X-ray monitoring system (for example, faxitron) before proceeding with tumor cell injection.*NOTE: For both models, cells can be resuspended in a Matrigel solution (around 4 mg ml−1), but the injection should be quick and the needle should be kept on ice, as it solidifies at 37 °C.*NOTE: Tumor growth is relatively slow as the tumor has first to invade medullary and cortical bone before reaching soft tissues and becoming measurable. Intraosseous injections are considered closer to the clinical reality but are frequently associated with an acute inflammation and the induction of venous emboli, with the immediate dissemination of tumor cells in the bloodstream. Venous emboli can in part be prevented by slow extraction of the injection needle directly after injection and by lowering the amount of injected cells.*NOTE: Because some (aggressive, bone lytic) OS models are often associated with the occurrence of bone-associated pain during tumor development, analgesia must be applied––for example, daily injections of temgesic 0.1 mg kg−1 i.p.*NOTE: Some examples are shown in refs 20, 21.
(b.3) Transplantation of a fragment of OS tumor
Inject OS cells subcutaneously into a mouse.
When the tumor is palpable, excise a small fragment and insert it close to the periosteum of the diaphysis (tibia) through a 0.5 mm section inside the muscle of other mice of the same strain. Then, suture the cutaneous and muscular wounds.*NOTE: One example of transplantation is described by Lamoureux et al. 22 where POS-1 cells were first inoculated in the hind footpad of mice. When tumors are developed, 2 × 2 × 2 mm3 fragments were excised and transplanted along the tibia in other mice.*NOTE: This model requires a higher number of mice compared with the other methods due its lower rate of graft success and proliferative parts of OS tissue need to be transplanted, whereas the necrotic tissue has to be discarded. Thus, this model is more difficult to set up and has high variability. The main advantage of transplantation compared with injection models is to keep the cells in their microenvironment and to limit dissemination into the bloodstream.
(3) Imaging techniques
Many imaging techniques can be used to visualize osteosarcomas. Some examples are two-dimensional simple X-ray or more sophisticated three-dimensional microcomputed tomography (microCT) techniques. In this chapter, we will be describing in vivo bioluminescent imaging, which requires tumor cells to be labeled with firefly luciferase. The appropriate technique should be chosen depending on the objective of the study.
In vivo imaging system
Bioluminescence is a well-established technique commonly utilized to track tumor growth and to locate and monitor the presence of metastases in living animals. In vivo imaging system (IVIS, Xenogen/Perkin-Elmer, Hopkinton, MA, USA) is a highly sensitive in vivo imaging technology utilized to quantify bioluminescence via a digital camera and advanced computer software. This system detects photons emitted from luciferase-expressing cells in living animals23 and is primarily used to monitor primary tumor growth. Although it can also be utilized to monitor established lung metastases, a more sensitive technique for imaging small/early metastases would be in vivo lung microCT imaging, which is not described here because of space limitations.24
Materials
Syringes with 25 G needle
D-Luciferin
Isoflurane/oxygen-based anesthesia system fitted with an induction chamber and inhalation masks for mice or xylazine–ketamine cocktail.*NOTE: Xylazine (Rompun) at a dose of 10 mg kg−1 and ketamine (Imalgene 500) at a dose of 100 mg kg−1 should be used.
PBS.
OS cells labeled with firefly luciferase (described above).
Procedure
Verify luciferase activity in cells before injection.
Count and re-suspend cells in PBS to a final concentration of 1.0 × 106 cells per ml.*NOTE: The number of cells has to be adjusted depending on the cell line.
Inoculate luciferase-labeled OS cells into the tibia of 4–5-week-old mice (described above).
Prepare a sterile stock solution of D-luciferin in PBS.
Using a syringe with a 25 G needle, inject 150 μl of D-luciferin (concentration of 150 mg kg−1) into the intraperitoneal cavity or subcutaneously above the neck.*NOTE: In this protocol, i.p. or subcutaneously injection of D-luciferin is suggested because of its ease to perform. However, if injection is not properly performed, variability in expression can occur. A method to validate the quality of the injection is to utilize preformulated D-luciferin fluorescent substrates such as Rediject D-luciferin (Perkin-Elmer, Waltham, MA, USA). Alternatively, i.v. injection is also possible and provides higher signal strength.25 However, in this case, the luciferase signal will develop much faster and care must be taken to image the mouse rapidly after injection.
Record D-luciferin time of injection.
Anesthetize mice with isofluorane/oxygen and place the animal in the imaging chamber.*NOTE: During bioluminescene imaging, mice are usually anesthetized with isoflurane. However, it is important to recognize that it has been reported to decrease the luciferase activity in some cases.26 In case of an expected low luciferase signal, ketamine-based anesthesia should be considered.
Initialize the ‘Living Image' software provided by the manufacturer (Xenogen/Perkin-Elmer).
Set exposure time and imaging parameters.
Take the first image ∼8–12 min after D-luciferin injection.*NOTE: It is recommended to perform an initial kinetic experiment for each animal model taking images during different time points. This will allow you to determine the D-luciferin distribution for your experiment.
Analyze and quantify the photons emitted from luciferase-labeled cells within the animal according to the manufacturer's protocol (Xenogen/Perkin-Elmer).
Conclusions
In this chapter, we have summarized the currently available GEMMs for OS and provided methods for isolation of cell lines from available models, modification, injection/transplantation of these cell lines and imaging techniques. Mouse models have immense value for the study of complex diseases; however, it should not be forgotten that these methods have their limitations. For example, phenotypic drift of cell lines frequently occurs in laboratories. In addition, as some of these cell lines are derived from already established tumors from patients, they are not suitable for the investigation of tumor initiation. Investigators should choose the models best suited for their studies according to their question of interest, always paying attention to possible concerns such as the use of proper controls (in certain cases mice carrying Cre transgenes have been observed to have phenotypes), environmental changes between different animal facilities, the use of correct breeding strategies and possible changes in phenotypes over time.
Supplementary Material
Acknowledgments
Supported by the IBMS-ECTS Young Investigators. We thank Professor Bruno Fuchs for his critical reading of the manuscript.
References
- Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer 2009; 125: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev 2014; 40: 523–532. [DOI] [PubMed] [Google Scholar]
- Ando K, Heymann MF, Stresing V, Mori K, Redini F, Heymann D. Current therapeutic strategies and novel approaches in osteosarcoma. Cancers 2013; 5: 591–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DC, Carney SC, Ahlmann ER, Hendifar A, Chawla S, Fedenko A et al. A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma 2012; 2012: 704872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment – Where do we stand? A state of the art review. Cancer Treat Rev 2014; 40: 523–532. [DOI] [PubMed] [Google Scholar]
- Mutsaers AJ, Walkley CR. Cells of origin in osteosarcoma: mesenchymal stem cells or osteoblast committed cells? Bone 2014; 62: 56–63. [DOI] [PubMed] [Google Scholar]
- Grigoriadis AE, Schellander K, Wang ZQ, Wagner EF. Osteoblasts are target cells for transformation in c-fos transgenic mice. J Cell Biol 1993; 122: 685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AJ, Mutsaers AJ, Baker EK, Walkley CR. Genetically engineered mouse models and human osteosarcoma. Clin Sarcoma Res 2012; 2: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PP, Pandey MK, Jin F, Raymond AK, Akiyama H, Lozano G. Targeted mutation of p53 and Rb in mesenchymal cells of the limb bud produces sarcomas in mice. Carcinogenesis 2009; 30: 1789–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev 2008; 22: 1662–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutsaers AJ, Ng AJ, Baker EK, Russell MR, Chalk AM, Wall M et al. Modeling distinct osteosarcoma subtypes in vivo using Cre:lox and lineage-restricted transgenic shRNA. Bone 2013; 55: 166–178. [DOI] [PubMed] [Google Scholar]
- Entz-Werle N, Choquet P, Neuville A, Kuchler-Bopp S, Clauss F, Danse JM et al. Targeted apc;twist double-mutant mice: a new model of spontaneous osteosarcoma that mimics the human disease. Transl Oncol 2010; 3: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux SD, Di Grappa MA, Beristain AG, McKee TD, Wai DH, Paderova J et al. Prkar1a is an osteosarcoma tumor suppressor that defines a molecular subclass in mice. J Clin Invest 2010; 120: 3310–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LH, Wang W, Yeung W, Deng Y, Yuan P, Mak KK. Hedgehog signaling induces osteosarcoma development through Yap1 and H19 overexpression. Oncogene 2013; 33: 4857–4866. [DOI] [PubMed] [Google Scholar]
- Tao J, Jiang MM, Jiang L, Salvo JS, Zeng HC, Dawson B et al. Notch activation as a driver of osteogenic sarcoma. Cancer Cell 2014; 26: 390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KB. Osteosarcomagenesis: modeling cancer initiation in the mouse. Sarcoma 2011; 2011: 694136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D et al. A third-generation lentivirus vector with a conditional packaging system. J Virol 1998; 72: 8463–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobin B, Huin MB, Lamoureux F, Ory B, Charrier C, Lanel R et al. BYL719, a new alpha-specific PI3K inhibitor: single administration and in combination with conventional chemotherapy for the treatment of osteosarcoma. Int J Cancer 2015; 136: 784–796. [DOI] [PubMed] [Google Scholar]
- Luu HH, Kang Q, Park JK, Si W, Luo Q, Jiang W et al. An orthotopic model of human osteosarcoma growth and spontaneous pulmonary metastasis. Clin Exp Metastasis 2005; 22: 319–329. [DOI] [PubMed] [Google Scholar]
- Comstock KE, Hall CL, Daignault S, Mandlebaum SA, Yu C, Keller ET. A bioluminescent orthotopic mouse model of human osteosarcoma that allows sensitive and rapid evaluation of new therapeutic agents In vivo. In Vivo 2009; 23: 661–668. [PubMed] [Google Scholar]
- Miwa S, Hiroshima Y, Yano S, Zhang Y, Matsumoto Y, Uehara F et al. Fluorescence-guided surgery improves outcome in an orthotopic osteosarcoma nude-mouse model. J Orthop Res 2014; 32: 1596–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoureux F, Richard P, Wittrant Y, Battaglia S, Pilet P, Trichet V et al. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res 2007; 67: 7308–7318. [DOI] [PubMed] [Google Scholar]
- Lim E, Modi KD, Kim J. In vivo bioluminescent imaging of mammary tumors using IVIS spectrum. J Vis Exp 2009; 26: e1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namati E, Thiesse J, Sieren JC, Ross A, Hoffman EA, McLennan G. Longitudinal assessment of lung cancer progression in the mouse using in vivo micro-CT imaging. Med Phys 2010; 37: 4793–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyaerts M, Caveliers V, Lahoutte T. Bioluminescence imaging: looking beyond the light. Trends Mol Med 2012; 18: 164–172. [DOI] [PubMed] [Google Scholar]
- Keyaerts M, Remory I, Caveliers V, Breckpot K, Bos TJ, Poelaert J et al. Inhibition of firefly luciferase by general anesthetics: effect on in vitro and in vivo bioluminescence imaging. PloS One 2012; 7: e30061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.