Abstract
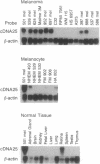
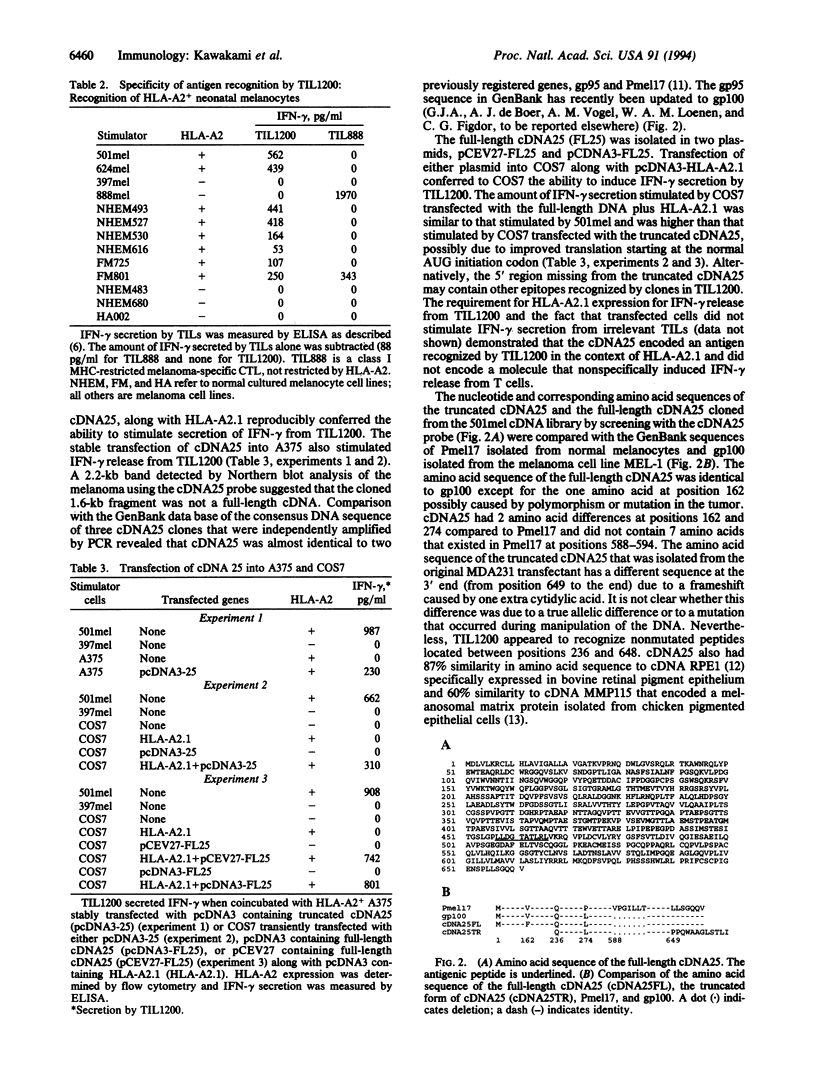
The cultured T-cell line TIL1200, established from the tumor-infiltrating lymphocytes (TILs) of a patient with advanced metastatic melanoma, recognized an antigen on most HLA-A2+ melanomas and on all HLA-A2+ cultured neonatal melanocytes in an HLA-A2 restricted manner but not on other types of tissues or cell lines tested. A cDNA encoding an antigen recognized by TIL1200 was isolated by screening an HLA-A2+ breast cancer cell line transfected with an expression cDNA library prepared from an HLA-A2+ melanoma cell line. The nucleotide and amino acid sequences of this cDNA were almost identical to the genes encoding glycoprotein gp100 or Pmel17 previously registered in the GenBank. Expression of this gene was restricted to melanoma and melanocyte cell lines and retina but was not expressed on other fresh or cultured normal tissues or other types of tumor tested. The cell line transfected with this cDNA also expressed antigen recognized by the melanoma-specific antibody HMB45 that bound to gp100. A synthetic 10-amino acid peptide derived from gp100 was recognized by TIL1200 in the context of HLA-A2.1. Since the administration of TIL1200 plus interleukin 2 resulted in regression of metastatic cancer in the autologous patient, gp100 is a possible tumor rejection antigen and may be useful for the development of immunotherapies for patients with melanoma.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adema G. J., de Boer A. J., van 't Hullenaar R., Denijn M., Ruiter D. J., Vogel A. M., Figdor C. G. Melanocyte lineage-specific antigens recognized by monoclonal antibodies NKI-beteb, HMB-50, and HMB-45 are encoded by a single cDNA. Am J Pathol. 1993 Dec;143(6):1579–1585. [PMC free article] [PubMed] [Google Scholar]
- Anichini A., Maccalli C., Mortarini R., Salvi S., Mazzocchi A., Squarcina P., Herlyn M., Parmiani G. Melanoma cells and normal melanocytes share antigens recognized by HLA-A2-restricted cytotoxic T cell clones from melanoma patients. J Exp Med. 1993 Apr 1;177(4):989–998. doi: 10.1084/jem.177.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A. B., Schreurs M. W., de Boer A. J., Kawakami Y., Rosenberg S. A., Adema G. J., Figdor C. G. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994 Mar 1;179(3):1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichard V., Van Pel A., Wölfel T., Wölfel C., De Plaen E., Lethé B., Coulie P., Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993 Aug 1;178(2):489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystryn J. C., Rigel D., Friedman R. J., Kopf A. Prognostic significance of hypopigmentation in malignant melanoma. Arch Dermatol. 1987 Aug;123(8):1053–1055. [PubMed] [Google Scholar]
- Colombari R., Bonetti F., Zamboni G., Scarpa A., Marino F., Tomezzoli A., Capelli P., Menestrina F., Chilosi M., Fiore-Donati L. Distribution of melanoma specific antibody (HMB-45) in benign and malignant melanocytic tumours. An immunohistochemical study on paraffin sections. Virchows Arch A Pathol Anat Histopathol. 1988;413(1):17–24. doi: 10.1007/BF00844277. [DOI] [PubMed] [Google Scholar]
- Crowley N. J., Darrow T. L., Quinn-Allen M. A., Seigler H. F. MHC-restricted recognition of autologous melanoma by tumor-specific cytotoxic T cells. Evidence for restriction by a dominant HLA-A allele. J Immunol. 1991 Mar 1;146(5):1692–1699. [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M., Hoak D., Gough F., McNutt M. A. Monoclonal antibodies specific for melanocytic tumors distinguish subpopulations of melanocytes. Am J Pathol. 1986 May;123(2):195–203. [PMC free article] [PubMed] [Google Scholar]
- Hom S. S., Schwartzentruber D. J., Rosenberg S. A., Topalian S. L. Specific release of cytokines by lymphocytes infiltrating human melanomas in response to shared melanoma antigens. J Immunother Emphasis Tumor Immunol. 1993 Jan;13(1):18–30. doi: 10.1097/00002371-199301000-00003. [DOI] [PubMed] [Google Scholar]
- Hunt D. F., Henderson R. A., Shabanowitz J., Sakaguchi K., Michel H., Sevilir N., Cox A. L., Appella E., Engelhard V. H. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992 Mar 6;255(5049):1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C. H., Robbins P. F., Rivoltini L., Topalian S. L., Miki T., Rosenberg S. A. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Nishimura M. I., Restifo N. P., Topalian S. L., O'Neil B. H., Shilyansky J., Yannelli J. R., Rosenberg S. A. T-cell recognition of human melanoma antigens. J Immunother Emphasis Tumor Immunol. 1993 Aug;14(2):88–93. doi: 10.1097/00002371-199308000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Rosenberg S. A., Lotze M. T. Interleukin 4 promotes the growth of tumor-infiltrating lymphocytes cytotoxic for human autologous melanoma. J Exp Med. 1988 Dec 1;168(6):2183–2191. doi: 10.1084/jem.168.6.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Zakut R., Topalian S. L., Stötter H., Rosenberg S. A. Shared human melanoma antigens. Recognition by tumor-infiltrating lymphocytes in HLA-A2.1-transfected melanomas. J Immunol. 1992 Jan 15;148(2):638–643. [PubMed] [Google Scholar]
- Kim R. Y., Wistow G. J. The cDNA RPE1 and monoclonal antibody HMB-50 define gene products preferentially expressed in retinal pigment epithelium. Exp Eye Res. 1992 Nov;55(5):657–662. doi: 10.1016/0014-4835(92)90170-w. [DOI] [PubMed] [Google Scholar]
- Kwon B. S., Chintamaneni C., Kozak C. A., Copeland N. G., Gilbert D. J., Jenkins N., Barton D., Francke U., Kobayashi Y., Kim K. K. A melanocyte-specific gene, Pmel 17, maps near the silver coat color locus on mouse chromosome 10 and is in a syntenic region on human chromosome 12. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9228–9232. doi: 10.1073/pnas.88.20.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Fleming T. P., Crescenzi M., Molloy C. J., Blam S. B., Reynolds S. H., Aaronson S. A. Development of a highly efficient expression cDNA cloning system: application to oncogene isolation. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5167–5171. doi: 10.1073/pnas.88.12.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochii M., Agata K., Eguchi G. Complete sequence and expression of a cDNA encoding a chicken 115-kDa melanosomal matrix protein. Pigment Cell Res. 1991 Feb;4(1):41–47. doi: 10.1111/j.1600-0749.1991.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Nordlund J. J., Kirkwood J. M., Forget B. M., Milton G., Albert D. M., Lerner A. B. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983 Nov;9(5):689–696. doi: 10.1016/s0190-9622(83)70182-9. [DOI] [PubMed] [Google Scholar]
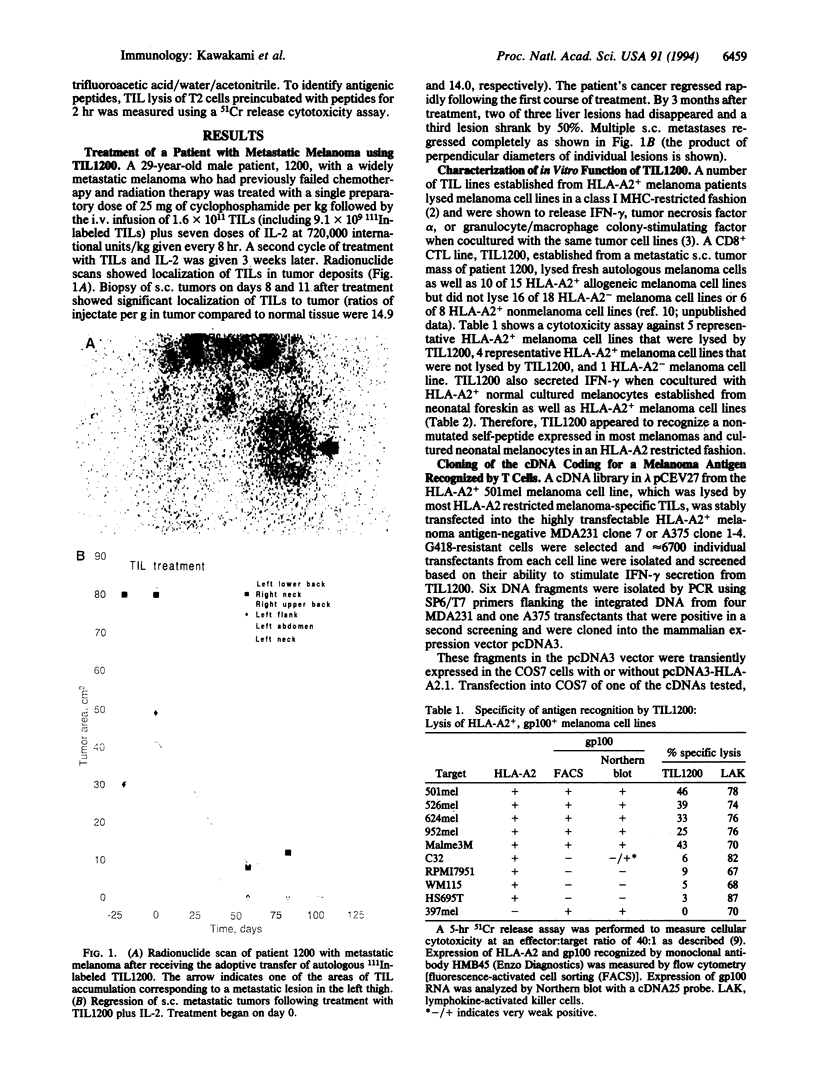
- Pockaj B. A., Sherry R. M., Wei J. P., Yannelli J. R., Carter C. S., Leitman S. F., Carasquillo J. A., Steinberg S. M., Rosenberg S. A., Yang J. C. Localization of 111indium-labeled tumor infiltrating lymphocytes to tumor in patients receiving adoptive immunotherapy. Augmentation with cyclophosphamide and correlation with response. Cancer. 1994 Mar 15;73(6):1731–1737. doi: 10.1002/1097-0142(19940315)73:6<1731::aid-cncr2820730630>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Richards J. M., Mehta N., Ramming K., Skosey P. Sequential chemoimmunotherapy in the treatment of metastatic melanoma. J Clin Oncol. 1992 Aug;10(8):1338–1343. doi: 10.1200/JCO.1992.10.8.1338. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Ruppert J., Sidney J., Celis E., Kubo R. T., Grey H. M., Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993 Sep 10;74(5):929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- Schaumburg-Lever G., Metzler G., Kaiserling E. Ultrastructural localization of HMB-45 binding sites. J Cutan Pathol. 1991 Dec;18(6):432–435. doi: 10.1111/j.1600-0560.1991.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Sensi M., Salvi S., Castelli C., Maccalli C., Mazzocchi A., Mortarini R., Nicolini G., Herlyn M., Parmiani G., Anichini A. T cell receptor (TCR) structure of autologous melanoma-reactive cytotoxic T lymphocyte (CTL) clones: tumor-infiltrating lymphocytes overexpress in vivo the TCR beta chain sequence used by an HLA-A2-restricted and melanocyte-lineage-specific CTL clone. J Exp Med. 1993 Oct 1;178(4):1231–1246. doi: 10.1084/jem.178.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilyansky J., Nishimura M. I., Yannelli J. R., Kawakami Y., Jacknin L. S., Charmley P., Rosenberg S. A. T-cell receptor usage by melanoma-specific clonal and highly oligoclonal tumor-infiltrating lymphocyte lines. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2829–2833. doi: 10.1073/pnas.91.7.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennegoor C., Hageman P., Van Nouhuijs H., Ruiter D. J., Calafat J., Ringens P. J., Rümke P. A monoclonal antibody specific for cells of the melanocyte lineage. Am J Pathol. 1988 Jan;130(1):179–192. [PMC free article] [PubMed] [Google Scholar]
- Vogel A. M., Esclamado R. M. Identification of a secreted Mr 95,000 glycoprotein in human melanocytes and melanomas by a melanocyte specific monoclonal antibody. Cancer Res. 1988 Mar 1;48(5):1286–1294. [PubMed] [Google Scholar]
- Zakut R., Topalian S. L., Kawakami Y., Mancini M., Eliyahu S., Rosenberg S. A. Differential expression of MAGE-1, -2, and -3 messenger RNA in transformed and normal human cell lines. Cancer Res. 1993 Jan 1;53(1):5–8. [PubMed] [Google Scholar]
- van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991 Dec 13;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]