Abstract
Background
Olmesartan is a type of angiotensin II receptor inhibitor that can reduce the incidence of cardiovascular events. However, its role in the function of endothelial progenitor cells in atherosclerosis patients is still unclear. Our study aimed to explore the effects and mechanism of olmesartan on endothelial progenitor cell mobilization and function in carotid atherosclerosis.
Material/Methods
Forty carotid atherosclerosis patients were enrolled. Patients were administrated olmesartan 20 mg/day for 3 months. Flow cytometry was used for counting circulating endothelial progenitor cells; colorimetric method was used to measure the serum levels of endothelial nitric oxide synthase and nitric oxide. Cell migration, adhesion, and proliferation capacity, and related signaling pathway were also analyzed. Spearman rank correlation analysis was used to investigate the influence of olmesartan on endothelial progenitor cells and clinical characteristics (e.g., sex, age, blood pressure).
Results
Compared with the control group, the number of circulating endothelial progenitor cells was significantly decreased. Olmesartan can increase circulating endothelial progenitor cells number and the serum levels of eNOS and NO. Furthermore, it can improve cell migration, adhesion, and proliferation capacities. Spearman rank correlation analysis showed there is no relationship between olmesartan promotion effects on endothelial progenitor cell mobilization and the clinical characteristics (P>0.05). P-eNOS and P-Akt expression can be unregulated by RNH-6270 treatment and blocked by LY294002.
Conclusions
Olmesartan can effectively promote the endothelial progenitor cells mobilization and improve their function in patients with carotid atherosclerosis, independent of basic characteristics. This process relies on the PI3K/Akt/eNOS signaling pathway.
Keywords: Atherosclerosis, Endothelial Growth Factors, Granulocyte-Macrophage Progenitor Cells
Background
Atherosclerosis (AS) is one of the major causes of cardiovascular disease. How to effectively inhibit the progress of atherosclerosis is currently a popular research focus [1]. In recent years, several studies have revealed that endothelial progenitor cells in the peripheral circulation, which represent endogenous endothelial regeneration ability, participated in the arterial endothelial damage repair, and is associated with the development of AS [2]. It was found that the number of peripheral blood circulating endothelial progenitor cells in AS patients decreased significantly [3], and it was correlated with the degree of cardiovascular risk events in AS patients [4]. Numerous studies showed that endothelial progenitor cells play a critical role in the process of endothelial repair. Injecting endothelial progenitor cells into an injured artery in a mouse model can induce endothelial progenitor cells to home to the ischemic damage area to participate in the repair of damaged blood vessels [5]. Endothelial progenitor cell depletion will lead to endothelial disability. The endothelial process mainly includes the endothelial progenitor cells mobilized in bone marrow, migrating to the peripheral blood circulation, and directional homing to the injured endothelial area. Then, they repair and integrate to the vascular endothelium, resulting in the formation of new blood vessels [6]. The number and function of endothelial progenitor cells can both influence the AS process. Thus, some scholars proposed treating AS by increasing the number and function of endothelial progenitor cells. Currently, drugs that can promote endothelial progenitor cell mobilization included estrogen, statins, vascular endothelial growth factor (VEGF), angiotensin-converting enzyme inhibitors (ACEI), and angiotensin receptor blockers (ARB) [7–10]. Olmesartan medoxomil, an angiotensin II (Ang II) receptor inhibitor, can specifically block the ATI receptors of Ang II. Its main active form is olmesartan. Olmesartan was found to reduce inflammatory reaction after myocardial infarction and myocardial infarction area, while increasing microvascular neogenesis [11–13]. Therefore, this study aimed to explore the role of olmesartan treatment on endothelial progenitor cell mobilization in peripheral blood in patients with carotid artery atherosclerosis, and to clarify its effect on regulating and improving vascular endothelial function.
Material and Methods
Research object
Forty carotid atherosclerosis patients were included between January 2014 and September 2014 from Yantai Yuhuangding Hospital. All patients were diagnosed by carotid ultrasound. Patients with diabetes or recent infection history were excluded. There were a total of 26 males and 14 females with an average age of 57±14 years old. The systolic pressure and diastolic pressure of the patients were 156.5±12.6 and 99.3±10.8 mmHg, respectively. The common carotid artery intima-media thickness (IMT) was 1.37±0.24 mm, and the plaque area was 27.5±8.77 mm2. Olmesartan was given 20 mg daily for 3 consecutive months. Another 30 healthy adults for who presented for hospital physical examination were enrolled as the control group, with 20 males and 10 females and the average age was 56±13 years. The systolic pressure and diastolic pressure of healthy control were 125.8±10.4 and 86.3±8.76 mmHg, respectively, with 0.62±0.19 mm IMT and 9.64±4.34 mm2 plaque area. There were no statistical significance in age and sex between the 2 groups (P>0.05). All included subjects voluntarily signed informed consent and the study was approved by the ethics committee.
Flow cytometry
To obtain mononuclear cells, 10 ml peripheral blood anticoagulated by heparin was density gradient centrifuged. FITC-labelled CD34 and PE-labeled CD133 antibody were added to 100 μl mononuclear cells and reacted for 10 min. Then 0.5% fetal bovine serum was added and centrifuged at 1500 rpm for 10 min. After discarding supernatant, the cells were resuspended in 400 μl 0.5% fetal bovine serum and detected on a BD flow cytometry instrument.
Serum eNOS and NO level detection
Four ml of venous blood was taken and centrifuged after 30 min standing. Colorimetry method was used to detect the eNOS and NO level according to the manufacturer’s instructions. eNOS and NO detection kits were purchased from the Nanjing Detection Biological Engineering Institute.
Endothelial progenitor cell separation and function analysis
Peripheral blood was density-gradient centrifuged to obtain mononuclear cells. The mononuclear cells were incubated with 20% M199 medium for 4 days. After being washed with PBS, the cells continued incubating 7 days for cell adhesion, migration, and proliferation ability detection. The adherent cells were digested and cultivated in a 24-well plate. After incubating at 5% CO2 for 1 h, the adhesive cells were counted. Adherent cells in 15 random ×200 vision fields were counted. We added 2×104 endothelial progenitor cells in 50 μl of medium to the upper chamber of a Boyden chamber device, and 25 μl medium with VEGF (50 ng/mL) was added to the lower chamber. After cultivation for 24 h, the cells on the lower side of the membrane were fixed with methanol and stained with Giemsa. Three randomly selected fields (400×) were counted. MTT method was used to determine cell proliferation capacity. The endothelial progenitor cells were seeded into a 96-well plate coated with fibronectin and cultured for 48 h. Then, MTT was added for 6 h and the absorbance value was detected at 562 nm wavelength.
Endothelial progenitor cell culture and Western blot
Three concentrations (0, 0.5, and 1 μM) of olmesartan activator RNH-6270 or combined 40 μmol/L PI3K inhibitor LY294002 were used to treat cells for 24 h. The cells were harvested and homogenized with lysis buffer. Total protein was separated by denaturing 10% SDS – polyacrylamide gel electrophoresis. Detection was performed with ECL luminous fluid. The primary antibodies for P-eNOS and P-Akt were purchased from Cell Signaling Technology. Antibody dilutions were 1:1000 for P-eNOS and P-Akt. Protein levels were normalized to β-actin and changes were determined.
Statistical analysis
Each experiment was repeated at least 3 times. Numerical data are presented as means and standard deviation (±SD). Differences between means were analyzed using Student’s t test, paired t test, or 1-way ANOVA. SNK test was used for comparison between groups. Spearman rank correlation analysis was used for correlation comparison. All statistical analyses were performed using SPSS 13.0 software (Chicago, IL) with P<0.05 as statistical significance.
Results
Olmesartan increased circulating endothelial progenitor cell mobilization in carotid atherosclerosis patients
Compared with the control group, the number of peripheral circulating endothelial progenitor cells in patients with carotid atherosclerotic clearly decreased (P<0.05). After being treated with olmesartan for 3 months, the peripheral circulating endothelial progenitor cell number increased significantly (P<0.05), suggesting olmesartan can promote circulating endothelial progenitor cell mobilization in carotid atherosclerosis patients (Table 1). Spearman rank correlation analysis showed there is no relationship between the olmesartan promotion effects on endothelial progenitor cell mobilization and the clinical characteristics including sex, age, systolic pressure, diastolic pressure, IMT, and plaque area (P>0.05).
Table 1.
Endothelial progenitor cell number ((x±s)/2×105 cells).
| Group | Endothelial progenitor cell number |
|---|---|
| Control | 78.35±5.6 |
| Carotid atherosclerosis group (pretreatment) | 48.56±3.67# |
| Carotid atherosclerosis group (posttreatment) | 65.89±3.56* |
Compared with the control;
compared with pretreatment; P<0.05.
Olmesartan affected serum eNOS and NO level in carotid atherosclerosis patients
Compared with the control group, serum eNOS and NO level in patients with carotid atherosclerotic decreased significantly (P<0.05). After being treated with olmesartan for 3 months, eNOS and NO levels increased markedly (P<0.05), suggesting olmesartan can improve endothelial function in carotid atherosclerosis patients (Table 2).
Table 2.
Serum eNOS and NO level (x±s).
| Group | eNOS (U/L) | NO (μmol/L) |
|---|---|---|
| Control | 90.52±16.54 | 98.35±15.62 |
| Carotid atherosclerosis group (pretreatment) | 53.63±13.82# | 48.56±3.67# |
| Carotid atherosclerosis group (posttreatment) | 75.81±10.62* | 75.89±10.56* |
Compared with the control;
compared with pretreatment; P<0.05.
The effect of olmesartan on endothelial progenitor cell function in carotid atherosclerosis patients
Compared with the control group, the endothelial progenitor cell adhesion, migration, and proliferation abilities in patients with carotid atherosclerotic decreased significantly (P<0.05). After treatment with olmesartan for 3 months, these abilities gradually increased (P<0.05), suggesting olmesartan can improve endothelial progenitor cell function in carotid atherosclerosis patients (Table 3). Spearman rank correlation analysis revealed there is no relationship between olmesartan improvement on endothelial progenitor cell adhesion, migration, and proliferation abilities and the clinical characteristics including sex, age, systolic pressure, diastolic pressure, IMT, and plaque area (P>0.05).
Table 3.
Endothelial progenitor cell adhesion, migration and proliferation abilities (x±s).
| Group | Adhesion | Migration | Proliferation (OD value) |
|---|---|---|---|
| Control | 45.34±7.54 | 43.35±11.22 | 0.67±0.12 |
| Carotid atherosclerosis group (pretreatment) | 21.65±11.61# | 15.51±8.92# | 0.34±0.06# |
| Carotid atherosclerosis group (posttreatment) | 36.11±10.12* | 35.35±8.38* | 0.58±0.08* |
Compared with the control;
compared with pretreatment; P<0.05.
The mechanism of olmesartan in improving endothelial progenitor cell function
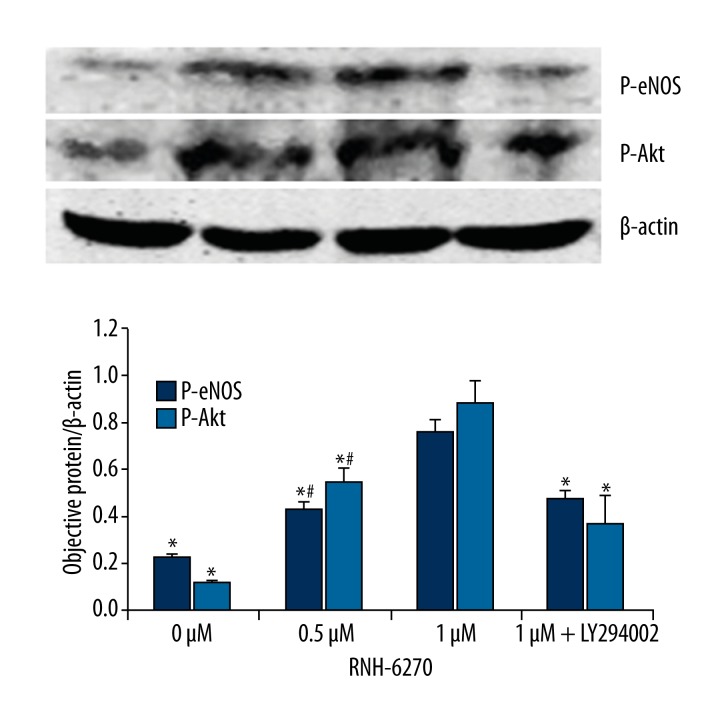
The result of Western blot analysis showed that 0.5 μM and 1 μM RNH-6270 can both increase expression level of P-eNOS and P-Akt. After being treated with PI3K inhibitor LY294002, P-eNOS and P-Akt level decreased significantly (P<0.05) (Figure 1).
Figure 1.
Olmesartan activates the PI3K/Akt/eNOS signaling pathway in endothelial progenitor cells from carotid atherosclerosis patients. * Compared with the 1-μM RNH-6270 group, P<0.05; # Compared with the 0-μM RNH-6270 group, P<0.05.
Discussion
Our results show that the number of peripheral circulating endothelial progenitor cells in patients with carotid atherosclerosis decreased markedly (Table 1). Further analysis on the cell function revealed that the adhesion, migration, and proliferation abilities reduced significantly (Table 3), suggesting that the number and function of endothelial progenitor cells in atherosclerosis patients were significantly damaged, impacting the affected artery intima repairmen. Serum eNOS and NO level were also confirmed to decrease (Table 2), suggesting endothelial dysfunction exists in atherosclerosis patients. Olmesartan medoxomil, a type of long-term angiotensin II receptor inhibitor with good tolerance can specifically block the ATI receptors of angiotensin II. Its main active form is olmesartan, which has been shown to promote neovascularization after myocardial ischemia. Animal studies have shown that angiotensin receptor inhibitor can have an anti-atherosclerosis effect independent of its blood pressure-lowering effect [14,15]. A 2-year multicenter study in several countries and regions (the MORE study) revealed that olmesartan can significantly reduce blood pressure of hypertension patients and improve IMT and plaque volume (PV), especially for patients with large PV [16]. It has been suggested that olmesartan can improve neovascularization in myocardial ischemia [11], improve endothelial dysfunction in patients with various cardiovascular disease, promote the endothelium-dependent relaxation in hypertension patients [17], improve circulating endothelial progenitor cell number in type 2 diabetic patients [18], and even improve the vascular morphology of hypertension patients in stage I (the VIOS study) [19].
However, the impact of olmesartan on endothelial progenitor cell mobilization and function in carotid atherosclerotic patients is still unclear. Endothelial progenitor cells can enhance endothelial cell repair in local injury and play an important role in maintaining endothelial integrity. Atherosclerosis first occurs as endothelial dysfunction, mainly characterized as endothelial cell loss and decreased eNOS activity [20]. Our study showed that 3 months of olmesartan treatment can effectively promote peripheral endothelial progenitor cell mobilization in carotid atherosclerosis patients. Further functional analysis results showed that in vitro olmesartan treatment promote the recovery of endothelial progenitor cells adhesion, migration, and proliferation abilities. Serum eNOS and NO levels also increased. The adhesion, migration, and proliferation abilities of endothelial progenitor cells can help them directionally home to the endothelial injury area, repairing endothelial tissue, and integrating to the vascular endothelium for neovascularization. An animal experiment also confirmed that the endothelial cells derived from endothelial progenitor cells can replace apoptotic endothelial cells [21]. Moreover, Spearman rank correlation analysis showed there is no relationship between olmesartan promotion effects on endothelial progenitor cell mobilization, adhesion, migration, and proliferation abilities and the clinical characteristics, including sex, age, systolic pressure, diastolic pressure, IMT, and plaque area. This indicates that olmesartan can act on endothelial progenitor cell independent of basic clinical characteristics.
The PI3K/Akt/eNOS signaling pathway was thought to be associated with endothelial progenitor cell differentiation [22]. For example, it was found that high-density lipoprotein (HDL) can help endothelial progenitor cells to differentiate to endothelial cells through activating the PI3K/Akt signaling pathway [23], and HMG-CoA reductase inhibitor and VEGF can activate eNOS to promote endothelial progenitor cell differentiation by the PI3K/Akt signaling pathway [24–26]. These studies suggest that the PI3K/Akt signaling pathway plays an important role in promoting endothelial progenitor cell proliferation and differentiation. Thus, our studies further analyzed the mechanism by which olmesartan promotes endothelial progenitor cell mobilization and improves their function. After we isolated peripheral vascular endothelial progenitor cells from carotid atherosclerosis patients treated by olmesartan activator RNH-6270 or combined PI3K inhibitor, we found that the RNH-6270 can effectively activate the PI3KK/Akt/eNOS signaling pathway with increased Akt and eNOS phosphorylation levels, and they were restrained when combined with PI3K inhibitor (Figure 1). Our findings suggest that olmesartan may improve endothelial progenitor cell function by activating the PI3KK/Akt/eNOS signaling pathway.
Conclusions
This study confirmed that olmesartan treatment can effectively promote peripheral endothelial progenitor cell mobilization and improve their function in carotid atherosclerosis patients through the PI3KK/Akt/eNOS signaling pathway, providing a theoretical basis for clinical applications.
Footnotes
Source of support: This research was supported by the Natural Science Foundation of Shandong Province (ZR2010HM091)
References
- 1.Goncharov NV, Avdonin PV, Nadeev AD, et al. Reactive Oxygen Species in Pathogenesis of Atherosclerosis. Curr Pharm Des. 2015;21(9):1134–46. doi: 10.2174/1381612820666141014142557. [DOI] [PubMed] [Google Scholar]
- 2.Du F, Zhou J, Gong R, et al. Endothelial progenitor cells in atherosclerosis. Front Biosci (Landmark Ed) 2012;17:2327–49. doi: 10.2741/4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briasoulis A, Tousoulis D, Antoniades C, et al. The role of endothelial progenitor cells in vascular repair after arterial injury and atherosclerotic plaque development. Cardiovasc Ther. 2011;29:125–39. doi: 10.1111/j.1755-5922.2009.00131.x. [DOI] [PubMed] [Google Scholar]
- 4.Werner N, Kosiol S, Schiegl T, et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 5.Werner N, Junk S, Laufs U, et al. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 6.Umemura T, Higashi Y. Endothelial progenitor cells: therapeutic target for cardiovascular diseases. J Pharmacol Sci. 2008;108:1–6. doi: 10.1254/jphs.08r01cp. [DOI] [PubMed] [Google Scholar]
- 7.Matsuura K, Hagiwara N. The pleiotropic effects of ARB in vascular endothelial progenitor cells. Curr Vasc Pharmacol. 2011;9:153–57. doi: 10.2174/157016111794519345. [DOI] [PubMed] [Google Scholar]
- 8.Povsic TJ, Najjar SS, Prather K, et al. EPC mobilization after erythropoietin treatment in acute ST-elevation myocardial infarction: the REVEAL EPC substudy. J Thromb Thrombolysis. 2013;36:375–83. doi: 10.1007/s11239-013-0944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Moran E, Ding L, et al. PPARalpha regulates mobilization and homing of endothelial progenitor cells through the HIF-1alpha/SDF-1 pathway. Invest Ophthalmol Vis Sci. 2014;55:3820–32. doi: 10.1167/iovs.13-13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Cai Y, Zhang Y, et al. Local injection of deferoxamine improves neovascularization in ischemic diabetic random flap by increasing HIF-1alpha and VEGF expression. Plos One. 2014;9:e100818. doi: 10.1371/journal.pone.0100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu XM, Jin YN, Ma L. Olmesartan medoxomil reverses glomerulosclerosis in renal tissue induced by myocardial infarction without changes in renal function. Exp Ther Med. 2014;8:105–9. doi: 10.3892/etm.2014.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen B, Lu D, Fu Y, et al. Olmesartan prevents cardiac rupture in mice with myocardial infarction by modulating growth differentiation factor 15 and p53. Br J Pharmacol. 2014;171:3741–53. doi: 10.1111/bph.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuda T, Muto S, Fujisawa G, et al. Heart angiotensin II-induced cardiomyocyte hypertrophy suppresses coronary angiogenesis and progresses diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2012;302:H1871–83. doi: 10.1152/ajpheart.00663.2011. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki A, Koga T. Pravastatin sodium, an inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase, decreases serum total cholesterol in Japanese White rabbits by two different mechanisms. Atherosclerosis. 2002;162:299–306. doi: 10.1016/s0021-9150(01)00726-2. [DOI] [PubMed] [Google Scholar]
- 15.Takai S, Jin D, Sakaguchi M, et al. The regressive effect of an angiotensin II receptor blocker on formed fatty streaks in monkeys fed a high-cholesterol diet. J Hypertens. 2005;23:1879–86. doi: 10.1097/01.hjh.0000182527.52063.32. [DOI] [PubMed] [Google Scholar]
- 16.Stumpe KO, Agabiti-Rosei E, Zielinski T, et al. Carotid intima-media thickness and plaque volume changes following 2-year angiotensin II-receptor blockade. The Multicentre Olmesartan atherosclerosis Regression Evaluation (MORE) study. Ther Adv Cardiovasc Dis. 2007;1:97–106. doi: 10.1177/1753944707085982. [DOI] [PubMed] [Google Scholar]
- 17.Bahlmann FH, de Groot K, Mueller O, et al. Stimulation of endothelial progenitor cells: a new putative therapeutic effect of angiotensin II receptor antagonists. Hypertension. 2005;45:526–29. doi: 10.1161/01.HYP.0000159191.98140.89. [DOI] [PubMed] [Google Scholar]
- 18.Naya M, Tsukamoto T, Morita K, et al. Olmesartan, but not amlodipine, improves endothelium-dependent coronary dilation in hypertensive patients. J Am Coll Cardiol. 2007;50:1144–49. doi: 10.1016/j.jacc.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Smith RD, Yokoyama H, Averill DB, et al. The protective effects of angiotensin II blockade with olmesartan medoxomil on resistance vessel remodeling (The VIOS study): rationale and baseline characteristics. Am J Cardiovasc Drugs. 2006;6:335–42. doi: 10.2165/00129784-200606050-00006. [DOI] [PubMed] [Google Scholar]
- 20.Bonello-Palot NSS, Robert S, Bourgeois P, et al. Prelamin A accumulation in endothelial cells induces premature senescence and functional impairment. Atherosclerosis. 2014;237:45–52. doi: 10.1016/j.atherosclerosis.2014.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Lee PS, Poh KK. Endothelial progenitor cells in cardiovascular diseases. World J Stem Cells. 2014;6:355–66. doi: 10.4252/wjsc.v6.i3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai P, Liu Y. Echinocystic acid, isolated from Gleditsia sinensis fruit, protects endothelial progenitor cells from damage caused by oxLDL via the Akt/eNOS pathway. Life Sci. 2014;114:62–69. doi: 10.1016/j.lfs.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Sumi M, Sata M, Miura S, et al. Reconstituted high-density lipoprotein stimulates differentiation of endothelial progenitor cells and enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:813–18. doi: 10.1161/01.ATV.0000259299.38843.64. [DOI] [PubMed] [Google Scholar]
- 24.Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–97. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbich C, Dimmeler S. Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of HMG-CoA reductase inhibitors. Kidney Int. 2005;67:1672–76. doi: 10.1111/j.1523-1755.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 26.Besler C, Doerries C, Giannotti G, et al. Pharmacological approaches to improve endothelial repair mechanisms. Expert Rev Cardiovasc Ther. 2008;6:1071–82. doi: 10.1586/14779072.6.8.1071. [DOI] [PubMed] [Google Scholar]