Abstract
Background
The aim of this study was to examine the relationship or differences in ocular structures of amblyopic eyes compared to fellow eyes in children and young adults with hyperopic anisometropic amblyopia.
Material/Methods
Hyperopic participants with anisometropic amblyopia, defined as the presence of best-corrected visual acuity differences of at least 2 Snellen lines and 1.5 diopters between amblyopic and fellow eyes, were studied. Using the IOL Master, Pentacam Scheimpflug imaging and Spectralis optical coherence tomography, the axial length, corneal curvature, and anterior chamber depth (ACD), as well as the thickness of the cornea, peripapillary retinal nerve fiber layer (RNFL), and macula, were compared between children and young adults and between their amblyopic and fellow eyes.
Results
In 53 participants with hyperopic anisometropic amblyopia, there were significant differences in the anterior corneal curvature, ACD and axial length between the amblyopic and fellow eyes of all the patients. The mean central macular thickness in the amblyopic eyes was significantly thicker (P=.001) in the group aged 5 to 12 years; however, this was not the case in the group aged 13 to 42 years. There was no significant difference in average RNFL thickness in either group.
Conclusions
We found significantly greater mean central macular thickness in anisometropic amblyopic eyes among participants aged 5 to 12 years, but not among those who were older. Similarly, the interocular differences in axial length parameters seemed to be related to the central macular thickness differences between the amblyopic and fellow eyes in the younger group.
Keywords: Amblyopia; Anisometropia; Hyperopia; Macula Lutea; Tomography, Optical Coherence
Background
Amblyopia is defined as a unilateral or, less commonly, bilateral reduction of the best-corrected visual acuity (BCVA) not explained directly by any structural abnormality of the eye or posterior visual pathways [1–3]. Hyperopia and anisometropia are risk factors for developing amblyopia, and relatively high degrees of hyperopic anisometropia can induce the risk for children [4]. Although amblyopia is associated with a structurally normal eye, several studies suggest that subclinical abnormalities of the eye and visual pathways may exist in amblyopic eyes when compared to the sound fellow eye [5–8]. Recent developments in the field of ocular imaging technology have led to increased number of studies on ocular structures, including those that investigate the presence of retinal involvement in amblyopia. Previous studies in this area have been rather controversial, and there is no general agreement on retinal involvement [9–13].
The aim of this study was to examine the relationship and differences in ocular parameters of the affected amblyopic eye when compared to the unaffected fellow eye in children and young adults with hyperopic anisometropic amblyopia.
Material and Methods
This prospective study was conducted at the Ophthalmology Clinic of Kayseri Training and Research Hospital on 53 hyperopic patients with anisometropic amblyopia, defined as the presence of a BCVA difference of at least two Snellen lines in addition to a spherical difference of at least 1.5 diopters between the eyes. The study protocol was approved by our Institutional Review Board and performed according to the Declaration of Helsinki. Written informed consent to participate in the study was obtained from each patient or from the parents of minor participants. Each participant underwent a comprehensive orthoptic and ophthalmologic examination, which included the following tests: BCVA (logMAR, Early Treatment Diabetic Retinopathy Study [ETDRS] chart), ocular deviation (with cover at near [30 cm] and far [2.5 m and/or 5 m] distances), four prism diopter base-out, eccentric fixation (traditional visuoscope target, direct ophthalmoscope), binocular single vision (TNO or Titmus fly), slit-lamp, intraocular pressure (Goldmann applanation tonometry or tonopen) and cycloplegic refraction (induced with cyclopentolate 1% and tropicamide 1%, one drop each, after one drop proparacaine 1%). Autorefraction was performed using a TONOREF™ II Autoref/ Kerato/Tonometer (Nidek, Tokyo, Japan) approximately 30 minutes after the last drop and dilated funduscopy. Exclusion criteria were the presence of an organic eye disease, previous ocular trauma, or intraocular surgery, cataract, glaucoma, strabismus, eccentric fixation, laser treatment, or retinal or optic nerve disorders.
Patients were divided into two groups based on ages 5–12 years and 13–42 years. The age separation point was chosen to coincide with prior studies [12–14].
The IOL Master (Carl Zeiss Meditec, Dublin, CA) was used for ocular biometry in order to measure axial length repeating until five valid values were obtained. Axial length was measured from the corneal vertex to the retinal pigment epithelium. The results of axial length measurements were interpreted on the basis of the signal-to-noise ratio above 2.0 and the appearance of graphs.
Pentacam Scheimpflug camera (Oculus, Wetzlar, Germany) was also used in order to analyze anterior segment parameters including corneal curvature, corneal thickness, and anterior chamber depth (ACD) repeating until three valid values were obtained. ACD was measured from the posterior surface of the cornea to the anterior surface of the lens. Only the scans with quality score (QS) of >95% were chosen for analysis.
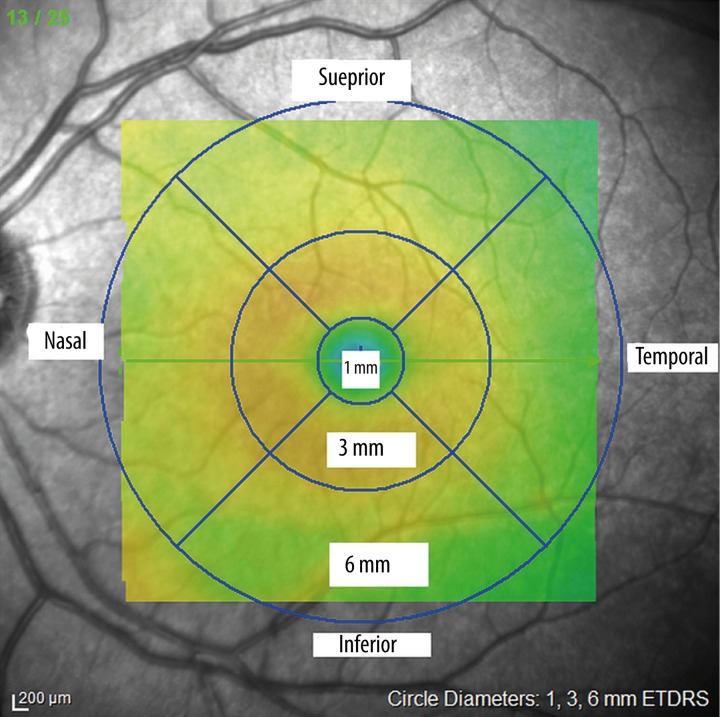
The Spectralis optical coherence tomography (OCT) device (software version 5.6.4.0; Heidelberg Engineering, Dossenheim, Germany) was used for assessing peripapillary retinal nerve fiber layer (RNFL) thickness and macular thickness. Scans for all participants were performed with pupillary dilatation under the same intensity of dim room lighting by the same experienced technician. An internal fixation target was also used in all scans with real-time eye tracking system adjusted for eye motion. Macular thickness (μm) was determined automatically and analyzed by OCT software. The Fast Macular Thickness Map included a 25-line raster volume scan 20×20 degrees centered on the fovea. The scans were obtained in high speed mode with automated real time (ART) feature enabled. The infrared scanning laser ophthalmoscope (IR SLO) scan angle was set at 30 degrees in all scans acquired. We selected the macular map analysis protocol on the Spectralis to display numeric averages of the measurements for each nine subfields as defined by the ETDRS circle grid. The diameters of the concentric circles were 1 mm, 3 mm, and 6 mm for macular scan. The results obtained from the macular scan were classified by segments as shown in Figure 1.
Figure 1.
shows that the diameters of concentric circles were 1 mm, 3 mm, and 6 mm for macular scan. Results obtained from the ETDRS circle grid macular scan were classified by segments.
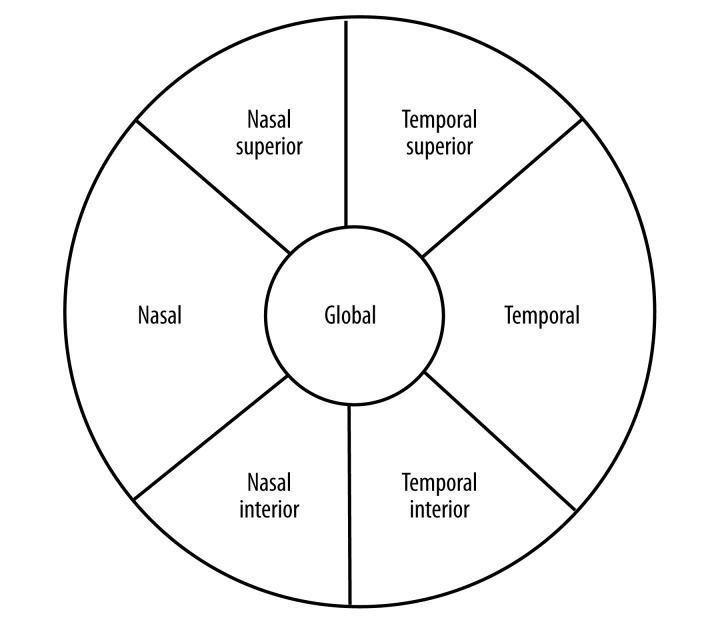
The peripapillary RNFL thickness parameters were automatically calculated by the fast RNFL mode. The scans were obtained in high speed mode with the automated real time (ART) feature enabled and set at 16 frames, and divided into sectors as shown in Figure 2. This software provided a thickness profile across the temporal-superior-nasal-inferior temporal areas of the standard 12-degree circular scan. The software also calculated average thickness values (μm) for global and each 6 sectors centered on the optic disc (temporal, temporal superior, temporal inferior, nasal, nasal inferior, nasal superior). The Spectralis uses a signal-to-noise (SNR in dB) estimate for QS. After all exposures, the non-centered scans and scans with signal strength <20 dB were excluded from the study.
Figure 2.
shows that the pie chart consists of the classification result for the 6 standard sectors of the optic disc (T, TS, TI, N, NS, NI) and global (G)
Hyperopia is known to induce errors in comparative RNFL or macular thickness OCT measurements. To compensate for magnification factors, a modified Littmann formula is commonly used in order to rescale RNFL or macular thickness measures [15]. The application of Littmann’s formula incorporates only axial length in determining the magnification factor of the eye [15,16]. Measurements of macular or RNFL thickness by SD-OCT are dependent on the optics of the eye, including anterior segment power and axial length [15]. For the Spectralis OCT, only the corneal curvature is important. It will affect the measurement of the distances and areas [17,18]. In order to compensate for this effect, The Heidelberg Eye Explorer (HEYEX) software program was used prior to the analysis [18].
Statistical analysis
We compared the differences between the amblyopic and fellow eyes. For this aim, paired sample t-tests were used for normally distributed variables, and Wilcoxon signed rank tests were used for those that were not normally distributed. Pearson correlation analyses were used to assess the relationship between the interocular differences in axial length and central macular thickness by age groups. Analyses were conducted using the Statistical Package for the Social Sciences (SPSS) for Windows software (Version 16.0, SPSS, Inc., Chicago, IL). P values less than 0.05 were considered to be statistically significant.
Results
This study included 106 eyes of 53 patients with hyperopic anisometropic amblyopia, of whom 25 (47.2%) were female and 28 (52.8%) were male. The mean age was 20.40±11.40 years (range, 5–42). Participant characteristics are displayed in Table 1. In all participants, we found significant differences in mean BCVA (0.61±0.33 logMAR vs. 0.01±0.03 logMAR P<.001), spherical equivalent refraction (4.75±2.20 D. vs. 1.35±1.1 D. P<.001), mean anterior corneal curvature (42.55±1.35 D. vs. 42.75±1.26 P=0.025), ACD (2.91±0.27 mm vs. 2.96±0.27 P=.001) and axial length (21.63±0.84 vs. 22.82±0.79 P<0.001) between the amblyopic and fellow eyes; however, we found no significant difference in corneal thickness (557.87±42.25 vs. 556.25±35.44 P>.05). Comparisons between the age groups for visual acuity, refraction and the IOL Master and Pentacam parameters are shown in Table 2.
Table 1.
Participant characteristics.
| Variables | All participants n=53 | Participants aged 5 to 12 years n=18 | Participants aged 13 to 42 years n=35 |
|---|---|---|---|
| Age (years) | 20.40±11.40 | 8.56±1.92 | 26.49±9.20 |
| Range | (5–42) | (5–12) | (13–42) |
| Gender | |||
| Male | 28 (52.8%) | 10 (55.6%) | 18 (51.4%) |
| Female | 25 (47.2%) | 8 (44.4%) | 17 (48.6%) |
| Amblyobic eye | |||
| Right | 27 (50.9%) | 11 (61.1%) | 16 (45.7%) |
| Left | 26 (49.1%) | 7 (38.9%) | 19 (54.3%) |
Values are expressed as n(%) or mean ±SD.
Table 2.
Comparison of age groups for visual acuity, refraction, IOL master, and pentacam parameters.
| Variables | All participants | Participants aged 5 to 12 years | Participants aged 13 to 42 years | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Amplyopic eye (n=53) | Fellow eye (n=53) | p | Amplyopic eye (n=18) | Fellow eye (n=18) | p | Amplyopic eye (n=35) | Fellow eye (n=35) | p | |
| Best corrected visual acuity, (logMAR) | 0.61±0.33 | 0.01±0.03 | <0.001 | 0.49±0.31 | 0.01±0.04 | <0.001 | 0.67±0.32 | 0.01±0.03 | <0.001 |
| Refraction, D Spherical equivalant | 4.75±2.20 | 1.35±1.1 | <0.001 | 5.16±2.98 | 1.27±0.83 | 0.011 | 4.61±1.95 | 1.38±1.18 | <0.001 |
| Axial length (mm) | 21.63±0.84 | 22.82±0.79 | <0.001 | 21.39±0.87 | 22.49±0.68 | <0.001 | 21.72±0.82 | 22.95±0.8 | <0.001 |
| Km (Pentacam), D | 42.55±1.35 | 42.75±1.26 | 0.025 | 42.64±1.39 | 42.98±1.11 | 0.135 | 42.50±1.34 | 42.63±1.33 | 0.073 |
| ACD (Pentacam), mm | 2.91±0.27 | 2.96±0.27 | 0.001 | 2.91±0.26 | 2.99±0.20 | 0.033 | 2.90±0.28 | 2.94±0.30 | 0.010 |
| Corneal thickness (Pentacam) μm | 557.87±42.25 | 556.25±35.44 | 0.560 | 577.76±50.17 | 575.54±44.28 | 0.295 | 547.31±33.67 | 548.22±30.17 | 0.753 |
Values are expressed as mean ±SD.
Among the younger group, the mean central macular thickness in the amblyopic eyes (260.71±14.48 μm) was significantly greater than that in the fellow eyes (254.29±14.79 μm), P=.001. In this group, the thickness of the superior inner quadrant was also greater in the amblyopic eyes (350.54±13.05 μm vs. 345±12.01 μm, P=.002). Among the older group, there were no significant differences except in the outer inferior quadrant, which was thicker in the amblyopic eyes (298±12.79 μm vs. 295.32±13.78 μm, P=.027). Table 3 shows a comparison of the mean macular thickness in the amblyopic and fellow eyes by age groups.
Table 3.
Comparison of the mean macular thickness (μm) of the amblyopic and fellow eyes in age groups.
| Variables | All participants | Participants aged 5 to 12 years | Participants aged 13 to 42 years | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Amplyopic eye (n=53) | Fellow eye (n=53) | p | Amplyopic eye (n=18) | Fellow eye (n=18) | p | Amplyopic eye (n=35) | Fellow eye (n=35) | p | |
| Central macular thickness (1 mm) | 263.76±19.76 | 262.26±22.09 | 0.342 | 260.71±14.48 | 254.29±14.79 | 0.001 | 265.61±22.42 | 267.11±24.52 | 0.483 |
| Temporal inner macula | 341.12±14.23 | 341.39±15.04 | 0.807 | 338.31±9.03 | 335.38±12.18 | 0.128 | 342.43±16.07 | 344.18±15.62 | 0.188 |
| Superior inner macula | 350.9±15.09 | 349.54±16.04 | 0.281 | 350.54±13.05 | 345±12.01 | 0.002 | 351.07±16.17 | 351.64±17.39 | 0.716 |
| Nasal inner macula | 340.68±19.48 | 339.85±19.15 | 0.600 | 337.77±19.76 | 336.77±15.45 | 0.787 | 342.04±19.56 | 341.29±20.75 | 0.648 |
| Inferior inner macula | 345.54±13.51 | 344.41±14.27 | 0.221 | 343.54±10.55 | 340.46±9.91 | 0.083 | 346.46±14.77 | 346.25±15.72 | 0.841 |
| Temporal outer macula | 313.76±21.81 | 309.8±18.53 | 0.068 | 309±17.48 | 307±17.77 | 0.605 | 315.96±23.52 | 311.11±19.04 | 0.070 |
| Superior outer macula | 306.78±12.69 | 306.71±13.92 | 0.950 | 307.92±10.32 | 307.31±10.23 | 0.779 | 306.25±13.79 | 306.43±15.5 | 0.898 |
| Nasal outer macula | 305.73±30.67 | 307.63±25.27 | 0.585 | 312.08±24.4 | 311.38±21.04 | 0.903 | 302.79±33.17 | 305.89±27.19 | 0.486 |
| Inferior outer macula | 299.83±13.6 | 296.78±13.66 | 0.035 | 303.77±14.97 | 299.92±13.39 | 0.327 | 298±12.79 | 295.32±13.78 | 0.027 |
1,3, 6 mm EDTRS circle grid. Values are expressed as mean ±SD.
A comparison of the peripapillary RNFL thickness of the amblyopic and fellow eyes by age groups is shown in Table 4. It was slightly thicker in the amblyopic eyes than the fellow eyes for most examined sectors, but the differences were not significant for any comparison group (in contrast, average thickness was less in the younger group at temporal superior and temporal inferior sectors).
Table 4.
Comparison of the Peripapillary retinal nerve fiber layer (RNFL) thickness (μm) of the amblyopic and fellow eyes in age groups.
| Variables | All participants | Participants aged 5 to 12 years | Participants aged 13 to 42 years | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Amplyopic eye (n=53) | Fellow eye (n=53) | p | Amplyopic eye (n=18) | Fellow eye (n=18) | p | Amplyopic eye (n=35) | Fellow eye (n=35) | p | |
| RNFL-G | 106.13±13.2 | 102.42±10.44 | 0.680 | 111±19.23 | 105.67±14.77 | 0.848 | 103.69±8.3 | 100.92±7.61 | 0.363 |
| RNFL-N | 82.74±28.85 | 77.34±18.68 | 0.309 | 93.92±45.29 | 84.42±28.49 | 0.281 | 77.15±13.55 | 74.08±11.14 | 0.777 |
| RNFL-NS | 136.08±27.7 | 129.08±22.92 | 0.616 | 149.31±38.26 | 131.5±24.58 | 0.739 | 129.46±18.08 | 127.96±22.52 | 0.389 |
| RNFL-TS | 127.26±23.11 | 122.89±31.5 | 0.256 | 129.69±15.81 | 131.75±46.5 | 0.452 | 126.04±26.21 | 118.81±21.5 | 0.399 |
| RNLF-T | 73.74±9.9 | 71.05±12.73 | 0.499 | 72.08±8.37 | 69.75±13.58 | 0.748 | 74.58±10.64 | 71.65±12.55 | 0.299 |
| RNLF-TI | 133.74±22.16 | 129.08±26.29 | 0.650 | 128.15±18.38 | 130.92±30.15 | 0.939 | 136.54±23.66 | 128.23±24.91 | 0.588 |
| RNLF-NI | 140.23±34.97 | 141.32±24.99 | 0.085 | 149.08±46.72 | 144.17±29.39 | 0.695 | 135.81±27.39 | 140±23.21 | 0.058 |
In the TSNIT graph. Values are expressed as mean ±SD.
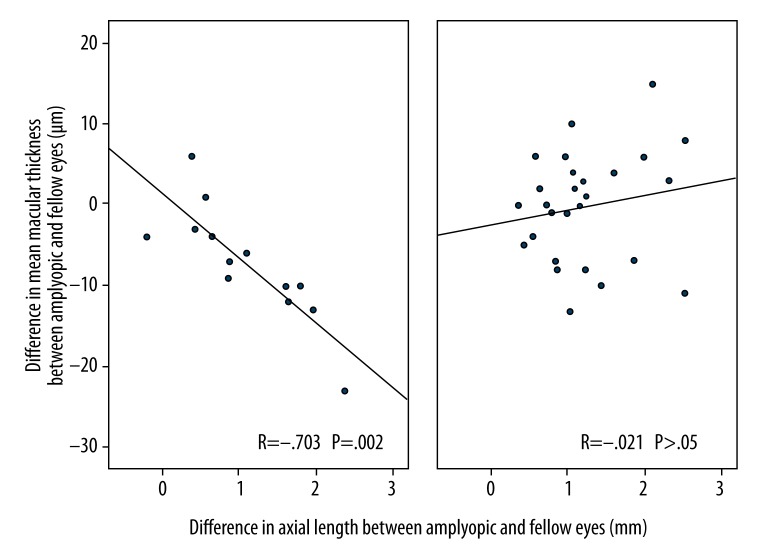
In hyperopic anisometropic amblyopia, the interocular differences in axial lengths seemed to be related to the differences in the central macular thickness among those aged 5 to 12 years (R=−.703, P=.002). We found no such correlation among those aged 13 to 42 years (R=−.021, P>.05). Figure 3 shows this relationship by age groups.
Figure 3.
shows the relationship between axial length and central macular thickness in age groups. (A) Refers to the 5 to 12-year-old group whereas; (B) refers to the age group of 13 years and above.
Discussion
To our knowledge, this is the first study that compared the differences in the anterior and posterior ocular structures between different age groups with hyperopic anisometropic amblyopia.
We found significant differences in the mean anterior corneal curvature and ACD between the amblyopic and fellow eyes in all participants using both Pentacam and IOL Master. These results are consistent with those of other studies [19–21]. In addition, the axial lengths of the amblyopic eyes were significantly shorter than those of the fellow eyes according to the IOL Master. Similarly, Cass and Tromans [19] found that axial length on an anisometropic amblyopic eye was significantly smaller than that of the fellow eye in 27 patients as measured by the A-scan ultrasound biometry through-the-lid technique. Patel et al. [20] also found axial length to be significantly shorter in amblyopic eyes compared to fellow eyes in nine patients with hyperopic anisometropic amblyopia.
Retinal involvement in amblyopia is controversial. Previous studies investigating peripapillary RNFL thickness and/or macular thickness in patients with amblyopia have reported various results. Some studies [9,10,25,32] found greater RNFL thickness and/or macular thickness in amblyopic eyes, but others [11–14,21–24] reported no significant differences.
Contrary to previous studies [9,10] we found no significant interocular difference in the peripapillary RNFL thickness of none of the participants or age groups. Yen et al. [9] and Yoon et al. [10] reported that RNFL thickness was significantly greater in eyes with refractive amblyopia compared to fellow eyes. In addition, Yoon et al. [10] found no difference in mean macular retinal thickness between anisometropic amblyopic eyes and normal eyes in hyperopic anisometropic children. They did not apply a compensation formula to OCT measurements in order to compensate for ocular magnification prior to analysis. Yen et al. [9] hypothesized that amblyopia affected postnatal maturation of the retina, including postnatal reduction of retinal ganglion cells, would lead to a measurable increase in the thickness of the RNFL in amblyopic eyes [9].
Similar to our findings, Repka et al. [13,14] and Walker et al. [22] found no difference in RNFL thickness between amblyopic and sound eyes. Walker et al. [22] also found no significant differences in macular thickness between amblyopic and fellow eyes in patients older than 18 years of age with amblyopia.
Huynh et al. [12] reported that amblyopic eyes had slightly greater foveal minimum thickness than fellow eyes, and this was more pronounced in 6-year-old children than 12-year-old children. Amblyopic eyes were also slightly thicker in the central macula (1 mm diameter region) in both comparisons, although these differences were not statistically significant to the P>.05 level. The inner macular rings (outer radii 1.5 mm) were thinner in the amblyopic eyes, but the peripapillary RNFL thickness was not significantly different. In both age groups, the central macular thickness was greater in those eyes with amblyopia, although it is uncertain if this preceded or followed the development of amblyopia. The IODs in foveal minimum thickness were greater among children who did not receive treatment for unilateral amblyopia. The peripapillary RNFL thickness was not significantly different between the amblyopic and normal fellow eyes or normal eyes of non-amblyopic children.
Wang and Taranath [21] found no interocular differences in peripapillary RNFL thickness, central macular thickness, or macular volumes.
Dickmann et al. [23–25] reported no statistically significant differences in RNFL or macular thickness in patients with anisometropic amblyopia, yet Alotaibi et al. [26] found significantly thicker RNFLs in the overall amblyopic group and no significant differences in macular or foveal thickness. There was slightly greater macular and foveal thickness only in the anisometropic amblyopic group. Based on their findings, Alotaibi [26] and Dickmann [23–25] suggested that amblyopia of different etiologies involve the loss of different neural cells. None of them measured axial length in their studies.
Wu et al. [27] found that the mean peripapillary RNFL and macular foveola thickness of amblyopic eyes were significantly thicker than those of fellow eyes. However, there were no significant differences in the 1 mm, 3 mm, or 6 mm macular thickness between them. They used no compensation formula for ocular magnification which could have influenced their results.
Andalib et al. [28] reported that the mean age of the patients in the anisometropic group was 10±3.1 years and that their mean macular thickness was significantly greater in the amblyopic eyes versus fellow eyes. There were no significant interocular differences between the prepapillary RNFLs.
Firat et al. [29] found no significant differences in the mean global RNFL thickness or the mean macular thickness between the amblyopic and fellow eyes in 19 patents with anisometropic amblyopia.
Leone et al. [30] reviewed the literature on measuring macular thickness in amblyopes and proposed that the increased macular thickness found in several studies was due to inadvertent measurement of a parafoveal eccentric point in amblyopia. To address this issue, central fixation was confirmed in each participant in our study by ensuring that the foveal depression was located at the center of the macular scan.
Kok et al. [31] observed that there is a relationship between the axial lengths and the pericentral retinal thickness in both amblyopic and their fellow eyes. In all participants or age groups, our findings are consistent with theirs (R=.692, P<.0001; R=.883, P<.0001 in all participants, respectively). We also observed that the interocular differences in axial lengths seemed to be related to the interocular differences in central macular thickness among those aged 5 to 12 years (R=−.703, P=.002). However, we found no such correlation among those aged 13 to 42 years (R=−.021, P>.05).
Among all participants, the amblyopic eyes had slightly greater mean macular thickness in the 1-mm diameter areas. In the older group, they were similar, but in the younger group, they were significantly different. Findings for the younger group are consistent with those of Al-haddad et al. [32,33] This result could be explained by the fact that the arrest of the physiological postnatal ganglion cell reduction in amblyopia can lead to increased retinal thickness in amblyopic eyes [9]; however, the mean RNFL thickness was similar in both eyes. In a subsequent study using high definition SD-OCT, Al-haddad et al. [33] concluded that amblyopic eyes demonstrated differences in their macula, possibly representing signs of immaturity. Our study was unable to find a significant difference in the mean macular thickness between the amblyopic and fellow eyes in a group aged 13 to 42 years or in the overall participant group. It is difficult to explain this result, but it might be related to multiple pathogenetic mechanism or treatment of amblyopia [12,34].
Song et al. [35] found that axial length correlated negatively with average macular thickness and macular volume. We could not demonstrate differences in foveal retinal thickness. Our findings of anomalous relationships between axial length and pericentral retinal thickness in both amblyopic and fellow eyes of children could be due to a disturbed development of the retina in those eyes, although a hypothetical arrest of the normal postnatal ganglion cell reduction would be expected to lead to an abnormally thick retinal thickness, especially pericentral retinal thickness.
A substantial number of previous studies about comparative retinal OCT measurements between amblyopic and fellow eyes have not taken into account this potential effect of anterior segment power and axial length. Measurements of macular or RNFL thickness by SD-OCT are dependent on the optics of the eye, including anterior segment power and axial length [15]. If a compensation formula is not applied to measurements prior to analysis for ocular magnification, it can affect the measurement of distances and areas inadvertently [17,18]. Hyperopia is known to induce errors in comparative RNFL and macular thickness in OCT measurements. Therefore, the HEYEX software was applied to measurements prior to analysis in order to compensate for ocular magnification effect.
Conclusions
The interocular differences in axial length parameters seemed to be related to the central macular thickness differences between the amblyopic and fellow eyes in the younger group. We found significantly greater mean central macular thickness in anisometropic amblyopic eyes among participants aged 5 to 12 years, but not in those who were older. All participants showed significant differences in mean anterior corneal curvatures and ACDs between amblyopic and fellow eyes. Further study to investigate and clarify the reproducibility and significance of these observations is needed.
Acknowledgments
The authors would like to thank Ayse Unal Ersonmez for professional language editing.
Footnotes
Source of support: Departmental sources
Statement
None of the authors has conflict of interest.
References
- 1.von Noorden GK. Amblyopia: Multidisciplinary approach. Proctor lecture. Invest Ophthalmol Vis Sci. 1985;26:1704–16. [PubMed] [Google Scholar]
- 2.Daw NW. Critical periods and amblyopia. Arch Ophthalmol. 1998;116:502–5. doi: 10.1001/archopht.116.4.502. [DOI] [PubMed] [Google Scholar]
- 3.Wong AMF. New concepts concerning the neural mechanisms of amblyopia and their clinical implications. Can J Ophthalmol. 2012;47:399–409. doi: 10.1016/j.jcjo.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Leon A, Donahue SP, Morrison DG, et al. The age-dependent effect of anisometropia magnitude on anisometropic ambliyopia severity. J AAPOS. 2008;12:150–56. doi: 10.1016/j.jaapos.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Spencer R, Leffler JN, Birch EE. Characteristics of peripapillary retinal nerve fiber layer in preterm children. Am J Ophthalmol. 2012;153:850–53. doi: 10.1016/j.ajo.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Pineles SL, Demer JL. Bilateral abnormalities of optic nerve size and eye shape in unilateral amblyopia. Am J Ophthalmol. 2009;148:551–57. doi: 10.1016/j.ajo.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lempert P. The axial length/ disc area ratio in anisometropic hyperopic amblyopia. A hypothesis for decreased unilateral vision associated with hyperopic anisometropia. Ophthalmology. 2004;111:304–8. doi: 10.1016/j.ophtha.2003.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Lempert P. Retinal area and optic disc rim area in amblyopic, fellow, and normal hyperopic eyes: A hypothesis for decreased acuity in amblyopia. Ophthalmology. 2008;115:2259–61. doi: 10.1016/j.ophtha.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 9.Yen MY, Cheng CY, Wang AG. Retinal nerve fiber layer thickness in unilateral amblyopia. Invest Ophthalmol Vis Sci. 2004;45:2224–30. doi: 10.1167/iovs.03-0297. [DOI] [PubMed] [Google Scholar]
- 10.Yoon SW, Park WH, Baek SH, Kong SM. Thicknesses of macular retinal layer and peripapillary retinal nerve fiber layer in patients with hyperopic anisometropic amblyopia. Korean J Ophthalmol. 2005;19:62–67. doi: 10.3341/kjo.2005.19.1.62. [DOI] [PubMed] [Google Scholar]
- 11.Altintas Ö, Yüksel N, Özkan B, Çaglar Y. Thickness of the retinal nerve fiber layer, macular thickness, and macular volume in patients with strabismic amblyopia. J Pediatr Ophthalmol Strabismus. 2005;42:216–21. doi: 10.3928/01913913-20050701-03. [DOI] [PubMed] [Google Scholar]
- 12.Huynh SC, Samarawickrama C, Wang XY, et al. Macular and nerve fiber layer thickness in amblyopia. Ophthalmology. 2009;116:1604–9. doi: 10.1016/j.ophtha.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Repka MX, Goldenberg-Cohen N, Edwards AR. Retinal nerve fiber layer thickness in amblyopic eyes. Am J Ophthalmol. 2006;142:247–51. doi: 10.1016/j.ajo.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Repka MX, Kraker RT, Tamkins SM, et al. Retinal nerve fiber layer thickness in amblyopic eyes. Am J Ophthalmol. 2009;148:143–47. doi: 10.1016/j.ajo.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel NB, Garcia B, Harwerth RS. Influence of anterior segment power on the scan path and RNFL thickness using SD-OCT. Invest Ophthalmol Vis Sci. 2012;53:5788–98. doi: 10.1167/iovs.12-9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann’s method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 1994;232:361–67. doi: 10.1007/BF00175988. [DOI] [PubMed] [Google Scholar]
- 17.Yang B, Ye C, Yu M, et al. Optic disc imaging with spectral-domain optical coherence tomography Variability and agreement study with Heidelberg retinal tomograph. Ophthalmology. 2012;119:1852–57. doi: 10.1016/j.ophtha.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 18.Spectralis HRA+OCT User Guide software version 5.1 Heidelberg Engineering. 2009. p. 14. [Google Scholar]
- 19.Cass K, Tromans C. A biometric investigation of ocular components in amblyopia. Ophthal Physiol Opt. 2008;28:429–40. doi: 10.1111/j.1475-1313.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- 20.Patel VS, Simon JW, Schultze RL. Anisometropic amblyopia: Axial length versus corneal curvature in children with severe refractive imbalance. J AAPOS. 2010;14:396–98. doi: 10.1016/j.jaapos.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Wang BZ, Taranath D. A comparison between the amblyopic eye and normal fellow eye ocular architecture in children with hyperopic anisometropic amblyopia. J AAPOS. 2012;16:428–30. doi: 10.1016/j.jaapos.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 22.Walker RA, Rubab S, Voll ARL, et al. Macular and peripapillary retinal fibre layer thickness in adults with amplyopia. Can J Ophthalmol. 2011;46:425–27. doi: 10.1016/j.jcjo.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Dickmann A, Petroni S, Salerni A, et al. Unilateral amblyopia: An optical coherence tomography study. J AAPOS. 2009;13:148–50. doi: 10.1016/j.jaapos.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Dickmann A, Petroni S, Perrotta V, et al. A morpho-functional study of amblyopic eyes with the use of optical coherence tomography and microperimetry. J AAPOS. 2011;15:338–41. doi: 10.1016/j.jaapos.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Dickmann A, Petroni S, Perrotta V, et al. Measurement of retinal fiber layer thickness, macular thickness, and foveal volume in amblyopic eyes using spectral-domain optical coherence tomography. J AAPOS. 2012;16:86–88. doi: 10.1016/j.jaapos.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Alotaibi AG, Al Enazi B. Unilateral amblyopia: Optical coherence tomography findings. Saudi J Ophthalmol. 2011;25:405–9. doi: 10.1016/j.sjopt.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu SQ, Zhu LW, Xu QB, et al. Macular and peripapillary retinal nerve fiber layer thickness in children with hyperopic anisometropic amblyopia. Int J Ophthalmol. 2013;6:85–89. doi: 10.3980/j.issn.2222-3959.2013.01.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andalib D, Javadzadeh A, Nabai R, Amizadeh Y. Macular and retinal nerve fiber layer thickness in unilateral anisometropic or strabismic amblyopia. J Pediar Ophthalmol Strabismus. 2013;50:218–21. doi: 10.3928/01913913-20130319-02. [DOI] [PubMed] [Google Scholar]
- 29.Firat PG, Ozsoy E, Demirel S, et al. Evaluation of peripapillary retinal nerve fiber layer, macula and ganglion cell thickness in amblyopia using spectral optical coherence tomography. Int J Ophthalmol. 2013;6:90–94. doi: 10.3980/j.issn.2222-3959.2013.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leone J, Koklanis K, Georgievski Z, Wilkinson R. Macular and retinal nerve fiber layer thickness in strabismus and anisometropia. Binocul Vis Strabismus Q. 2008;23:227–34. [PubMed] [Google Scholar]
- 31.Kok PH, de Kinkelder R, Braaksma-Besselink YC, et al. Anomalous relation between axial length and retinal thickness in amblyopic children. J AAPOS. 2013;17:598–602. doi: 10.1016/j.jaapos.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Al-haddad CE, Mollayess GMEL, Cherfan CG, et al. Retinal nerve fibre layer and macular thickness in amblyopia as measured by spectral-domain optical coherence tomography. Br J Ophthalmol. 2011;95:1696–99. doi: 10.1136/bjo.2010.195081. [DOI] [PubMed] [Google Scholar]
- 33.Al-haddad CE, Mollayess GMEL, Mahfoud ZR, et al. Macular ultrastructural features in amblyopia using high-defination optical coherence tomography. Br J Ophthalmol. 2013;97:318–22. doi: 10.1136/bjophthalmol-2012-302434. [DOI] [PubMed] [Google Scholar]
- 34.Vasileios K, Theodosios C, Stylianos V, et al. A peculiar case of persistent pupillary membrane associated with excellent visual function despite the refractive error. Am J Case Rep. 2009;10:176–78. [Google Scholar]
- 35.Song WK, Lee SC, Lee ES, et al. Macular thickness variations with sex, age, and axial length in healthy subjects: a spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:3913–18. doi: 10.1167/iovs.09-4189. [DOI] [PubMed] [Google Scholar]