Abstract
Experiments on estrogen metabolism, formation of DNA adducts, mutagenicity, cell transformation and carcinogenicity have led to and supported the hypothesis that the reaction of specific estrogen metabolites, mostly the electrophilic catechol estrogen-3,4-quinones, with DNA can generate the critical mutations to initiate breast and other human cancers. Analysis of depurinating estrogen–DNA adducts in urine demonstrates that women at high risk of, or with breast cancer, have high levels of the adducts, indicating a critical role for adduct formation in breast cancer initiation. Men with prostate cancer or non-Hodgkin lymphoma also have high levels of estrogen–DNA adducts. This knowledge of the first step in cancer initiation suggests the use of specific antioxidants that can block formation of the adducts by chemical and biochemical mechanisms. Two antioxidants, N-acetylcysteine and resveratrol, are prime candidates to prevent breast and other human cancers because in various in vitro and in vivo experiments, they reduce the formation of estrogen–DNA adducts.
Keywords: adducts, cancer, cancer initiation, catechol estrogen quinines, cell transformation by estrogens, depurinating estrogen–DNA adducts, estrogen genotoxicity, estrogen metabolism balance, N-acetylcysteine, prevention, resveratrol
Estrogens in the etiology of cancer
A variety of chemicals have been demonstrated to be carcinogenic to humans. Epidemiological evidence has suggested that 30% of human cancers are due to tobacco smoking [1]. The causative factors related to diet, pollution and lifestyle remain unknown [1]. Our environment, in general, is not sufficiently polluted to account for the majority of human cancer incidence. Therefore, it has been fruitful to investigate the potential of endogenous chemicals to initiate cancer.
A few investigators had the intuition that endogenous estrogens can be cancer-causing agents, since the estrogens contain a benzene ring in their structure, and benzene and poly-cyclic aromatic hydrocarbons (PAH) are carcinogenic. Benzene has long been known to induce leukemia [2] and, more recently, lymphoma [3]. The predominant endogenous bio-molecules containing a benzene ring are the estrogens and dopamine. Benzene, estrogens and dopamine are all metabolized to electro-philic ortho-quinones, which can react with DNA to form specific depurinating DNA adducts at the N-3 of adenine (Ade) and N-7 of guanine (Gua) [4].
Testing of the endogenous estrogens, estrone (E1) and estradiol (E2), and their catechols demonstrated that they induce tumors in hormone-dependent and -independent organs [5–8]. Despite these results, the scientific community did not accept estrogens as chemical carcinogens, largely because the compounds were not found to induce mutations in bacterial and mammalian test systems [6,9–13]. These findings have led scientists to classify E1 and E2 as epigenetic carcinogens that function mainly by stimulating abnormal cell proliferation in estrogen receptor (ER)-mediated processes [10,14–18]. The stimulated cell proliferation would generate more opportunities for mutations leading to carcinogenesis [14,18,19]. The ER-mediated processes can be involved in accelerating or delaying the process of carcinogenesis, but they do not play a central role in initiation because the hypothetical mutations obtained during cell proliferation are random, whereas the intercalation of catechol estrogens and catechol estrogen quinones in DNA leads to specific mutations that can initiate cancer.
The endogenous estrogens are true carcinogens that induce mutations. Since estrogens are chemicals and are mutagenic, they must act as other carcinogens do. This line of reasoning builds on the basic principles of chemical carcinogenesis established by James and Elizabeth Miller in the late 1960s [20,21]. Most chemical carcinogens are metabolically activated to electrophilic forms that bind covalently to DNA and other cellular macromolecules. The discovery that specific oxidative metabolites of estrogens can react with DNA led to and supports the hypothesis that estrogen metabolites can become endogenous chemical carcinogens by generating the mutations leading to the initiation of cancer [22,23]. This paradigm suggests that specific, critical mutations generate abnormal cell proliferation leading to cancer, rather than ER-mediated ab normal cell proliferation giving rise to random mutations [10,14–18]. The specificity of the critical mutations is generated by an intercalating physical complex between the estrogen and DNA before the conversion to a covalent bond between them, as demonstrated with the human carcinogen diethylstilbestrol (DES) [24].
Experiments on estrogen metabolism, formation of DNA adducts, mutagenicity, cell transformation and carcinogenicity led to and support the hypothesis that reaction of specific estrogen metabolites, mostly catechol estrogen-3,4-quinones, with DNA can generate the critical mutations to initiate breast, prostate and other human cancers [4,23]. The major initiating pathway is illustrated in Figure 1. E1 and E2 can be metabolically transformed to 4-hydroxyE1(E2) (4-OHE1[E2]) by cyto-chrome P450 (CYP) 1B1. Oxidation of these catechol estrogens leads to the corresponding 3,4-quinones (E1[E2]-3,4-Q), which can react with DNA to form small amounts of stable adducts, which remain in the DNA unless removed by repair, and predominant amounts of depurinating adducts, 4-OHE1(E2)-1-N3Ade and 4-OHE1(E2)-1-N7Gua (Figure 1) , which detach from DNA, leaving behind apurinic sites. Errors in the repair of these sites can lead to the critical mutations initiating breast, prostate and other human cancers [4,23].
Figure 1.
Major metabolic pathway in cancer initiation by estrogens.
Evidence for this paradigm
Estrogen metabolism
Aromatization of androstenedione and testosterone catalyzed by CYP19 (aromatase) yields E1 and E2, respectively. E1 and E2 are inter-converted by the enzyme 17β-estradiol dehydrogenase and are metabolized by two major pathways. The first is the formation of catechol estrogens by hydroxy lation at the 2- or 4-position (Figure 2), and the second is the 16a-hydroxylation (not shown in Figure 2). CYP1A1 preferentially hydroxylates E1 and E2 at the 2-position, whereas CYP1B1 almost exclusively catalyzes the formation of 4-OHE1 and 4-OHE2 [25–27]. The two catechol estrogens are inactivated by conjugating reactions such as glucuronidation and sulfation, especially in the liver. However, the most common pathway of conjugation in extrahepatic tissues is O-methylation of the hydroxyl group, catalyzed by the ubiquitous catechol-O-methyl transferase (COMT) [28]. This conjugation pathway can become insufficient if the activity of COMT is low. In that case, the competitive oxidation of 2-OHE1(E2) and 4-OHE1(E2) to their respective semiquinones and quinones by CYP or peroxidases can increase (Figure 2).
Figure 2. Formation, metabolism and DNA adducts of estrogens.
Activating enzymes and depurinating DNA adducts are in red and protective enzymes are in turquoise. N-acetylcysteine (NAcCys, shown in blue) and resveratrol (Resv, purple) indicate the various points where NAcCys and Resv could improve the balance of estrogen metabolism and minimize the formation of depurinating DNA adducts.
The oxidation of semiquinones to quinones can also occur by molecular oxygen. The reduction of estrogen quinones to semiquinones, catalyzed by CYP reductase, completes the redox cycle. In this process, the molecular oxygen is reduced to superoxide anion radical, which is converted to H2O2. In the presence of Fe++, H2O2 yields hydroxyl radicals. The first damage by hydroxyl radicals is the formation of lipid hydroperoxides, which can act as unregulated cofactors of CYP. The lack of regulation of lipid hydroperoxides can generate an ab normal increase in the oxidation of catechol estrogens to their respective quinones. Thus, redox cycling can be one of the major contributors to the formation of catechol estrogen quinones, which are the ultimate carcinogenic metabolites of estrogens.
4-OHE1(E2) have greater carcinogenic potency than 2-OHE1(E2) [6–8]. This effect cannot be attributed to formation of hydroxyl radicals obtained by redox cycling, because 2-OHE1(E2) and 4-OHE1(E2) have the same redox potentials [29,30]. Instead, the greater carcinogenic potency of 4-OHE1(E2) must be related to the much higher levels of de purinating adducts formed by E1(E2)-3,4-Q with DNA, compared with E1(E2)-2,3-Q with DNA [31].
Once the catechol estrogen quinones are formed, they can be inactivated by conjugation with glutathione (GSH) (Figure 2). Another in activation pathway for the quinones is reduction to their respective catechols by quinone reductase [32]. Quinone reductase is a protective enzyme that can be induced by a variety of compounds [33]. If all of the inactivating (protective) processes are insufficient, the catechol estrogen quinones may react with DNA to form pre dominantly depurinating adducts.
Formation of estrogen–DNA adducts
Carcinogens react with DNA to form two types of adducts – stable adducts and depurinating adducts. Investigators in chemical carcinogenesis have always dealt with stable adducts, which remain in the DNA unless removed by repair. These adducts are routinely detected and quantified by 32P-postlabeling techniques, but their identification has rarely been accomplished. Stable adducts are formed when carcinogenic electrophiles react with the exocyclic amino group of Ade or Gua. However, formation of adducts at the N-3 and N-7 of Ade, and the N-7 of Gua destabilizes the glycosyl bond and subsequent depurination of the adduct from DNA occurs [22,34,35].
Evidence that depurinating DNA adducts play a major role in tumor initiation was first derived from a correlation between the levels of depurinating PAH–DNA adducts and oncogenic Harvey (H)-ras mutations in mouse skin papillomas [36–38]. The potent carcinogens 7,12-dimethylbenz[a]anthracene [39] and dibenzo[a,l]pyrene [40,41] form predominantly depurinating Ade adducts and induce A>T transversions in codon 61. By contrast, benzo[a]pyrene forms approximately twice as many Gua depurinating adducts as Ade depurinating adducts [42], and twice as many codon 13 G>T transversions as codon 61 A>T transversions [38]. A similar correlation between the sites of formation of depurinating DNA adducts and H-ras mutations was observed in mouse skin and rat mammary gland treated with E2-3,4-Q [43,44].
When E1(E2)-3,4-Q react with DNA, they predominantly form (>99%) the de purinating adducts 4-OHE1(E2) -1–N3Ade and 4-OHE1(E2)-1–N7Gua by 1,4-Michael addition (Figure 2) [22,31,34,35], whereas E1(E2)-2,3-Q forms much lower levels of 2-OHE1(E2)-6–N3Ade by 1,6-Michael addition after tautomerization of the quinone to the E1(E2)-2,3-quinone methide [31]. The levels of DNA adducts formed by the catechol estrogen quinones are concordant with the greater carcinogenic activity of 4-OHE1(E2) compared with the borderline carcinogenic potency of 2-OHE1(E2) [6–8]. The critical role of depurinating DNA adducts and the apurinic sites they generate has also been observed in the mutagenic activity of E2-3,4-Q in vivo [43,44].
Imbalances in estrogen homeostasis
Normally, estrogen metabolism occurs as a balanced set of activating and deactivating pathways (homeostasis), which minimize the oxidation of catechol estrogens to quinones and their reaction with DNA. Initiation of cancer by estrogens is based on an estrogen metabolism, in which the homeostatic balance is disrupted (Figure 2). Several factors can unbalance homeostasis. The first factor could be excessive synthesis of estrogens by overexpression of CYP19 (aromatase) in target tissues [45–47]. The observation that breast tissue can synthesize estrogens in situ suggests that much more estrogen is present in target tissues than would be predicted from plasma concentrations [47]. In fact, plasma concentrations of estrogens are 50–100-fold lower in post menopausal women than premenopausal women, but the levels of estrogens in the breast are similar in both groups of women [48,49].
A second factor that can create imbalances in estrogen metabolism is represented by high levels of 4-OHE1(E2), generated by over expression of CYP1B1, which converts estrogen predominantly to 4-OHE1(E2) (Figure 2) [26,27]. Expression of CYP1B1 can be induced by exposure to potential environmental pollutants, such as dioxin [50]. In addition, CYP1B1 with the polymorphic variation of codon 432 exhibits higher enzymatic activity, thus producing more 4-OHE1(E2) [51]. Large amounts of 4-catechol estrogens produced could generate more E1(E2)-3,4-Q. This imbalance could be further compromised by a low level of COMT activity due to polymorphic variation [52], resulting in in sufficient methylation of 4-OHE1(E2) and greater oxidation to E1(E2)-3,4-Q. Furthermore, polymorphisms in quinone reductase (NQO1) that decrease the conversion of quinones to catechols could also lead to higher levels of E1(E2)-3,4-Q [53]. For example, a proline to serine polymorphism at codon 609 creates a null phenotype [54]. Low cellular levels of GSH, which reacts with the quinones, could be an additional factor in producing higher levels of E1(E2)-3,4-Q.
However, one factor that can help maintain estrogen homeostasis is the feedback inhibition exerted by methoxy estrogens on the expression of CYP1A1 and CYP1B1 [55]. This would help to keep the level of catechol estrogens regulated.
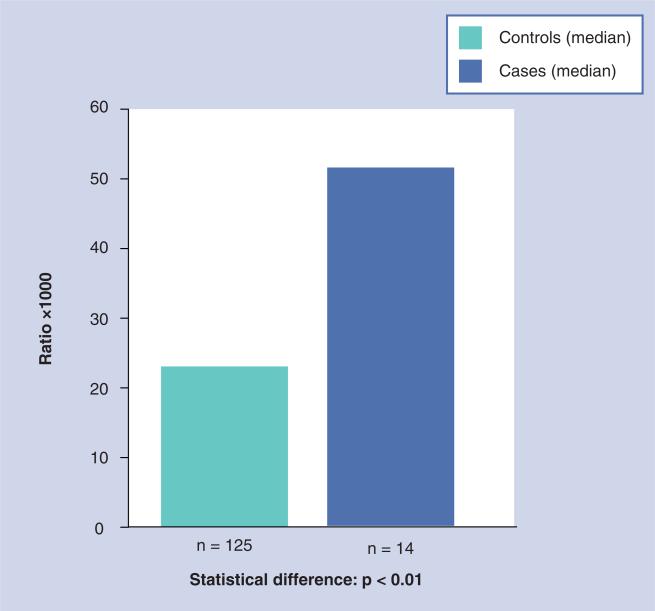
The effects of some of these factors have been observed in animal models for estrogen carcinogenicity and in the human breast. Imbalanced estrogen metabolism leading to substantial formation of catechol estrogen–GSH conjugates and depurinating catechol estrogen–DNA adducts has been observed in the kidney of male Syrian golden hamsters [56], prostate of Noble rats [57] and mammary gland of ER-α knockout mice [58]. In addition, comparison of breast tissue from women with and without breast cancer provides key evidence for imbalances in estrogen homeostasis [59]. In normal breast tissue from women with breast carcinoma, the levels of 4-OHE1(E2) were nearly four-times higher than the levels in breast tissue from women without breast cancer (p < 0.01). In addition, the levels of catechol estrogen–GSH conjugates in normal breast tissue from women with breast carcinoma were three-times the level in breast tissue from control women (p < 0.003). Thus, the levels of 4-OHE1(E2) and quinone conjugates appear to be significantly associated with breast cancer [59]. Further evidence of imbalances in estrogen homeostasis comes from the higher expression of estrogen deactivating enzymes (COMT and NQO1) in breast tissue of women without breast cancer and greater expression of estrogen activating enzymes (CYP19 and CYP1B1) in breast tissue of women with breast cancer (Figure 3) [60].
Figure 3. Expression of estrogen-metabolizing enzymes in the human breast.
Reproduced with permission from [60].
In addition to the endogenous factors that can disrupt estrogen homeostasis, there are environmental factors that can also unbalance estrogen metabolism. These factors include substances we ingest through the nose, mouth and skin. For example, pesticides and herbicides in the soil and food, pollutants in the air, cigarette smoke and other contaminants in food can affect estrogen metabolism. In fact, it is thought that certain environ mental pollutants generally induce cancer by imbalancing estrogen homeostasis and increasing formation of catechol estrogen quinones, rather than by acting as direct carcinogens themselves.
Mutagenicity of estrogens
The formation of estrogen–DNA adducts, in particular the depurinating adducts, has indicated that E1(E2)-3,4-Q are indeed the predominant carcinogenic metabolites of estrogens. The next important step in determining the role of these quinones in cancer initiation was assessing their mutagenic activity. The failure to detect estrogen-induced mutations through in vitro assays was the cornerstone of denying the genotoxicity of estrogens [61].
More recently, by determining the major pathway of metabolic activation of estrogens, using sensitive mutagenicity assays and selecting more appropriate assay conditions, we have been able to demonstrate that both 4-OHE2 and E2-3,4-Q are mutagenic [43,44,62]. The mutagenicity of 4-OHE2, under conditions in which it can be metabolized to E2-3,4-Q, and the mutagenic activity of E2-3,4-Q itself, provide solid evidence for the genotoxicity of estrogens.
Mutagenicity in SENCAR mice & ACI rats
The mutagenicity of E2-3,4-Q was first demonstrated by treating the dorsal skin of female ‘sensitivity to carcinogenesis’ (SENCAR) mice, excising the treated skin and determining both the estrogen–DNA adducts formed and the H-ras mutations generated in the skin. Although equal amounts of the de purinating 4-OHE2-1-N3Ade and 4-OHE2-1-N7Gua adducts were detected, A.T to G.C mutations were predominantly observed [43]. Similarly, when the mammary glands of female ACI rats were treated with E2-3,4-Q by intra mammillary injection, roughly equal amounts of the N3Ade and N7Gua adducts were detected and A.T to G.C. mutations in the H-ras gene predominated [44]. The high levels of mutations at A residues in the H-ras gene and small numbers of mutations at G residues may result from the rapid de purination of N3Ade adducts and much slower de purination of N7Gua adducts [31,63].
Mutagenicity in Big Blue® rats
The Big Blue® (BB) rat is a Fischer 344 rat with about 80 copies of the λ-LIZ vector in every cell. The transgene is not expressed and has no effect on the biochemistry or physiology of the rat. Female BB rats were implanted with E2, 4-OHE2 or both compounds, and, after 20 weeks mutations in the cII gene in the mammary cells, were analyzed. The mutational spectra in rats treated with E2 were similar to those in untreated control rats. By contrast, only in the rats treated with 4-OHE2 (with or without E2) were A.T to G.C mutations detected [23]. These results demon strate the mutagenicity of 4-OHE2 under conditions in which the catechol can be metabolized to E2-3,4-Q in vivo.
Mutagenicity in BB rat2 embryonic cells
Cells in the BB rat2 embryonic cell line contain approximately 60 copies of the λ-LIZ vector per cell. Treatment of these cells with 4-OHE2 or E2-3,4-Q generates mutations, but six treatments with either compound are needed to obtain statistically significant results [62]. By contrast, no mutagenic activity could be demonstrated with 2-OHE2. The mutational spectrum obtained with 4-OHE2 contains predominant mutations at A.T base pairs, presumably due to rapid de purination of N3Ade adducts.
In summary, 4-OHE2 and E2-3,4-Q have been demonstrated to be mutagenic both in vitro and in vivo under appropriate assay conditions, whereas 2-OHE2 was not mutagenic in the BB rat2 cells under the same assay conditions. The mutational spectra, both in vitro and in vivo, were consistent with those expected from the DNA adducts formed by E2-3,4-Q and enzyme-activated 4-OHE2. These results provide further support for the hypothesis that estrogens are carcinogenic through their genotoxicity.
Transformation of human cells lacking ER-α
Further evidence for the initiation of cancer by estrogen–DNA adducts has been acquired by the use of cultured human breast epithelial cells. MCF-10F cells are an immortalized, non transformed ER-α-negative cell line. When these cells are treated with E2 or 4-OHE2 at doses of 0.007 nM to 1 ng/ml, transformation of the cells is detected by their ability to form colonies in soft agar and their aggressiveness [64,65]. Transformation of the cells is not affected by the inclusion of the antiestrogens tamoxifen or ICI-182,780 [66], supporting the induction of transformation by genotoxic effects of estrogens. 2-OHE2 also induces these changes, but to a much lesser extent. Implantation of estrogen-transformed MCF-10F cells, selected on the basis of their invasiveness, into severely-compromised immune-deficient mice leads to the development of tumors [67]. The depurinat ing 4-OHE2-1-N3Ade and 4-OHE2-1-N7Gua adducts were detected following treatment of the MCF-10F cells with 4-OHE2 [68].
These results demonstrate that ER-negative breast epithelial cells can be transformed by genotoxic effects of estrogen metabolites. Thus, they support the hypothesis that formation of specific estrogen–DNA adducts is the critical event in the initiation of estrogen-induced cancer.
Carcinogenicity in estrogen-receptor-α knockout mice
The carcinogenicity of catechol estrogens was first demonstrated in hamsters [5–7] and mice [8]. 4-OHE1(E2) was carcinogenic in both species, while 2-OHE2 had borderline carcinogenic activity only in the mice.
Studies of transgenic mice with ER-α knocked-out (ERKO)/wnt-1 mice provide further important evidence for the role of estrogen-induced genotoxic events in the initiation of cancer. Bocchinfuso et al. found that the wnt-1 transgene in female ERKO/wnt-1 mice induced mammary tumors in 100% of the mice, despite the lack of ER-α and ER-β [69,70]. The mammary tissue of these mice was found to contain 4-OHE1(E2) and GSH conjugates formed by E1(E2)-3,4-Q, but no methoxycatechol estrogens [58], indicating that estrogen metabolism is unbalanced toward excess activation and reduced protection. When the mice were ovariectomized at 15 days of age to remove the major source of estrogens and implanted with E2, the E2-treated mice developed mammary tumors in a dose-dependent manner [71,72]. Furthermore, mammary tumors developed when the mice were implanted with both E2 and the anti estrogen ICI-182,780 [73]. These results provide strong evidence for tumor initiation by estrogen-induced genotoxic events, and not by ER-mediated processes.
Unifying mechanism of cancer initiation by natural & synthetic estrogens
The oxidation of estrogens to catechols and then to semiquinones and quinones is the pre dominant metabolic pathway that can initiate cancer by natural estrogens (Figure 2). Reaction of the estrogen quinones with DNA by 1,4-Michael addition yields N3Ade and N7Gua adducts, and depurination of the N7Gua adducts occurs rather slowly [31,63]. Synthetic estrogens, such as the human carcinogen DES [74] and its hydrogenated derivative hexestrol, are similar to the natural estrogens:
■ They are carcinogenic in the Syrian golden hamster [5,75];
■ The catechol quinones of DES and hexestrol have similar properties to those of E1(E2)-3,4-Q and form their N3Ade and N7Gua adducts by 1,4-Michael addition after reaction with DNA (Figure 4);
■ Analogously to the natural estrogens, depuri-nation of the N7Gua adducts occurs rather slowly [24,63,79,80].
These strong similarities between natural and synthetic estrogens suggest that the catechol quinones are the ultimate carcinogenic metabolites for the synthetic estrogens and substantiate the hypothesis that E1(E2)-3,4-Q are the endogenous initiators of breast, prostate and other human cancers.
Figure 4. Structures of the depurinating N3Ade and N7Gua adducts formed by reaction of the catechol quinones of hexestrol and DES with DNA.
DES: Diethylstilbestrol; HES: Hexestrol.
Analysis of estrogen–DNA adducts in human subjects with & without cancer
If formation of estrogen–DNA adducts is the critical event in the initiation of cancer by estrogens, one would expect to observe increased levels of estrogen–DNA adduct formation in tissues at risk for cancer. When depurinating estrogen–DNA adducts are released from DNA, they are shed from cells and tissues, pass into the bloodstream and are eventually excreted in urine. Therefore, ana lysis of urine or serum samples could provide information on the presence or risk of developing cancer.
High levels of estrogen–DNA adducts have been observed in analyses of urine from women who are at high risk of breast cancer or have the disease (Figure 5) [81,82]. These analyses are conducted by using ultraperformance liquid chromatography/tandem mass spectrometry, a methodology developed in our laboratory [81]. In two studies of such women, highly significant differences in relative levels of estrogen–DNA adducts were observed when urine samples from normal-risk women were compared with those from high-risk women or those with breast cancer (Figure 5) [81,82]. Subject characteristics did not affect these highly significant differences. These studies demonstrate that elevation of estrogen– DNA adducts is associated with high risk of developing breast cancer. In addition, ana lysis of urine samples from men with and without prostate cancer demonstrated that men with the disease have relatively high levels of estrogen–DNA adducts in their urine samples [83]. These results have been confirmed in a second study of men with and without prostate cancer (Figure 6) [84].
Figure 5. Depurinating estrogen–DNA adducts in urine of healthy women, high-risk women and women with breast cancer.
(A) First study. (B) Second study [81,82]. In the ratio of depurinating estrogen–DNA adducts to the sum of their respective estrogen metabolites and conjugates, the 4-catechol estrogen–DNA adducts account for more than 98% of the value of the ratio.
Figure 6. Levels of the estrogen–DNA adducts in urine samples from men with and without prostate cancer.
Reproduced with permission from [84].
Most recently, we have found that men diagnosed with non-Hodgkin lymphoma have significantly higher levels of depurinating estrogen–DNA adducts in their urine, when compared with healthy control men [85]. Thus, formation of estrogen–DNA adducts may play a critical role in the etiology of non-Hodgkin lymphoma, and these adducts could be potential biomarkers of risk for this disease.
Novel approach to cancer prevention
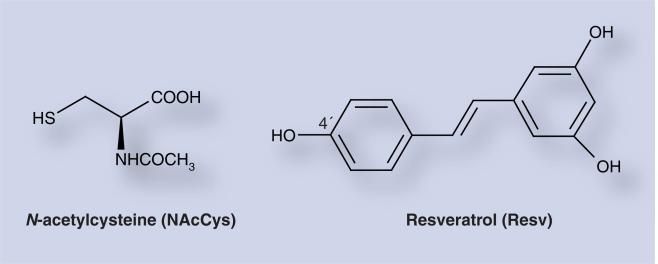
We hypothesize that prevention of breast and other cancers can arise from blocking the first critical step in initiation, reaction of catechol estrogen quinones with DNA. Such prevention could be obtained with natural compounds, such as N-acetylcysteine (NAcCys) and resveratrol (Resv) (Figure 7). These compounds can prevent oxidative and/or electrophilic damage to DNA by inhibiting formation of the electro-philic quinones and/or reacting with them [86]. The antimutagenic and anticarcinogenic properties of NAcCys are attributed to multiple protective mechanisms, including its nucleophilicity, antioxidant activity and inhibition of DNA adduct formation. Hydrolysis of NAcCys by acylase in the liver and gut yields cysteine (Cys), the precursor to GSH biosynthesis, guaranteeing replenishment of this critical tri-peptide. GSH has similar chemical properties to Cys and NAcCys. The use of Cys as a preventive agent is limited by its toxicity in humans. By contrast, NAcCys has very low toxicity [87]. NAcCys reacts with E1(E2)-3,4-Q [88] to prevent their reaction with DNA (Figure 2). NAcCys, like Cys, also operates as an antioxidant in reducing catechol estrogen semiquinones to catechol estrogens (Figure 2) [89].
Figure 7.
Structures of antioxidant compounds, N-acetylcysteine and resveratrol.
A normal mouse mammary epithelial cell line, E6, can be transformed by a single treatment with 4-OHE2 or E2-3,4-Q [90]. NAcCys inhibited the oxidation of 4-OHE2 to its quinone and consequent formation of the DNA adducts 4-OHE1(E2)-1–N3Ade and 4-OHE1(E2)-1–N7Gua. It also highly reduced the level of estrogen-induced transformation of the E6 cells. These results implicate formation of the estrogen–DNA adducts in the malignant transformation of these mammary cells. These results also provide a proof-of-principle and warrant exploration of NAcCys in the prevention of breast cancer.
Resv, 3,5,4′-3-OH-trans-stilbene (Figure 7), is found in foods, including grapes, peanuts and wine. This compound exerts chemo-preventive effects in diverse in vivo and in vitro systems [91]. Resv was first demonstrated to function as an antioxidant and antimutagenic agent, thus acting as an anti-initiation agent in cancer [91]. These properties are attributed to the easy hydrogen abstraction from the 4′-hydroxy bond with formation of a hydroxyl radical [92]. This easy abstraction is a consequence of the greater resonance stabilization of the intermediate. Resv is also an inducer of the protective enzyme NQO1 and modulator of the activating enzyme CYP1B1 (Figure 2) [93,94]. Furthermore, Resv prevents formation of depurinating estrogen–DNA adducts and neoplastic transformation in human breast epithelial cells treated with E2 [93].
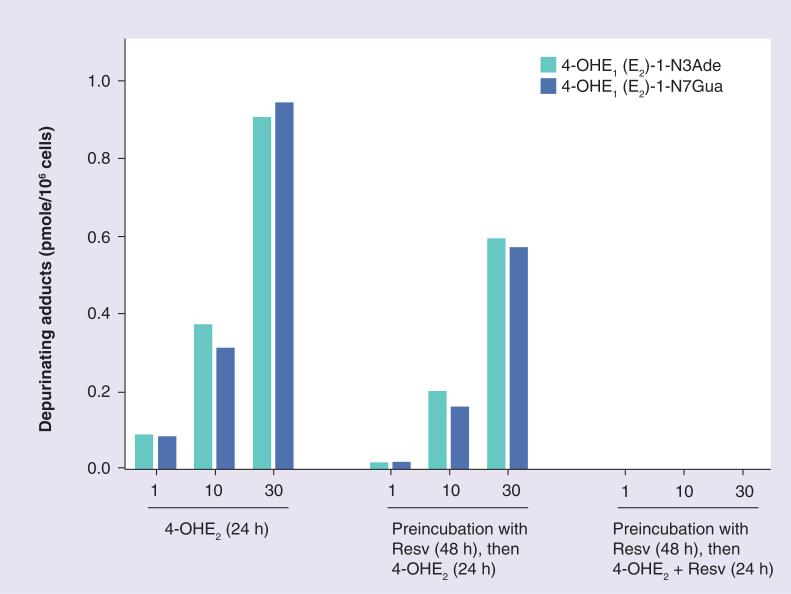
Resv inhibits formation of estrogen–DNA adducts both biochemically and chemically. First, it induces NQO1 [93], which reduces E1(E2)-3,4-Q to 4-OHE1(E2) [32], thus minimizing reaction of the quinones with DNA. Second, Resv reacts with estrogen semi-quinones, reducing them to their catechol estrogens [93]. These effects were observed in MCF-10F cells treated with 4-OHE2 and Resv (Figure 8). When the cells were incubated with 4-OHE2, 4-OHE1(E2)-1–N3Ade and 4-OHE1(E2)-1–N7Gua were formed in a dose-dependent manner. When the cells were preincubated with Resv for 48 h to induce NQO1 before 4-OHE2 was added, formation of the N3Ade and N7Gua adducts was reduced at all doses of 4-OHE2. When fresh Resv was also added to the cells along with the 4-OHE2, formation of the estrogen–DNA adducts was no longer detected (Figure 8). Therefore, the preventing effects of Resv, its modulating effect on estrogen-activating enzymes, and its protective effect by inducing quinone reductase suggest that this compound should be an excellent candidate for protection against estrogen-initiated cancer.
Figure 8. Levels of depurinating estrogen–DNA adducts in culture medium from cells treated with 4-OHE2, with or without Resv.
The levels of DNA adducts in Resv-treated cells were significantly lower than those in the cells not treated with Resv (p < 0.05) as determined by analysis of variance.
Reproduced with permission from [94].
When MCF-10F cells were cultured in the presence of 4-OHE2, and Resv and/or NAcCys were added to the culture medium at different doses, the formation of estrogen–DNA adducts by the cells was greatly inhibited in a dose-dependent manner [Cavalieri EL, Rogan EG et al. Unpublished data]. The same methods were used as in the published studies with MCF-10F cells [93,94]. Inhibition of estrogen–DNA adduct formation was greater in the presence of both compounds at all doses.
We have also conducted a small pilot study of the ability of one natural antioxidant compound, NAcCys, to reduce the level of depurinating estrogen–DNA adducts in the urine of healthy subjects. The same analytical procedures were used as in previous studies with human subjects [81,82,84]. Healthy people at normal risk of developing cancer have low, but detectable, levels of adducts (Figures 5 & 6) [81–84]. After 1 month of daily ingestion of NAcCys, the level of estrogen–DNA adducts in urine decreased an average of 55%, and 60% of the subjects experienced an average decrease of 84% [Cavalieri EL, Rogan EG et al. Unpublished data]. People at elevated risk of cancer are anticipated to have elevated levels of estrogen–DNA adducts, based on the higher levels observed in women at high risk for breast cancer (Figure 5) [81,82]. The results of this pilot study suggest that selected antioxidant compounds can reduce formation of estrogen–DNA adducts, presumably decreasing the risk of initiating and, thus, developing breast and other human cancers.
Conclusion
Evidence has been obtained for a unifying mechanism of initiation of breast and prostate cancer, as well as non-Hodgkin lymphoma. We think that other prevalent types of human cancer are also initiated by this same mechanism. This mechanism entails the metabolic formation of endogenous catechol estrogen-3,4-quinones that can react with DNA to yield predominantly the depurinating N3Ade and N7Gua adducts. The N3Ade adducts de purinate instantaneously, while the N7Gua adducts depurinate with a half-life of a few hours. These adducts leave apurinic sites in the DNA that can generate mutations leading to the initiation of cancer.
The genotoxicity and mutagenicity of 4-OHE2 and E2-3,4-Q have been demonstrated in cultured mammalian cells and in animal models.
Treatment of ER-α-negative human breast epithelial cells, MCF-10F cells, with E2 or 4-OHE2 in culture, generates not only estrogen–DNA adducts, but also malignant trans formation of the cells. Injection of these transformed cells into severely compromised immuno deficient mice results in the growth of tumors. These results are consistent with estrogen induction of malignant transformation of the cells beginning with reaction of the catechol estrogen-3,4-quinones with DNA.
Catechol estrogens have also been demonstrated to be carcinogenic in Syrian golden hamsters and SENCAR mice. The 4-OHE1(E2) are carcinogenic in both animal models, but the 2-OHE2 are noncarcinogenic or borderline carcinogenic in these models. In addition, ERKO/wnt-1 mice develop mammary tumors, despite the lack of ERs. Once again, these results indicate that estrogen genotoxic effects initiate the series of events leading to the development of cancer.
The synthetic estrogen DES, a human carcinogen, and its dihydrogenated derivative, hexestrol, are metabolized to their catechols and quinones and react with DNA to form N3Ade and N7Gua adducts analogous to those of the natural E1(E2)-3,4-Q.
From all of the similarities described above for the natural and synthetic estrogens, we foresee a common mechanism of initiation for many types of cancer.
Estrogen metabolites, conjugates and de purinating DNA adducts can be analyzed in human urine samples by using ultra performance liquid chromatography/tandem mass spectrometry. Such analyses have demonstrated that women with breast cancer or at high risk for the disease, have significantly higher levels of estrogen–DNA adducts than healthy women at normal risk for breast cancer. In addition, men with prostate cancer or non-Hodgkin lymphoma have significantly higher levels of estrogen–DNA adducts in their urine than do healthy men. The higher levels of depurinating estrogen–DNA adducts observed in subjects with cancer and women at high risk of breast cancer suggest that these adducts could serve as biomarkers of susceptibility that can potentially be used to monitor the risk of breast, prostate and other prevalent types of human cancer.
Having understood how estrogens can initiate a variety of types of cancer and knowing what to target, cancer prevention could be tackled. By preventing the first critical step in cancer initiation, the formation of estrogen–DNA adducts, the series of events leading to the development of cancer can be blocked. Two suitable candidates for cancer prevention in humans are the natural antioxidants NAcCys and Resv. These agents act by inhibiting formation of catechol quinones and/or reacting with the quinones themselves. They may also induce expression of protective enzymes such as quinone reductase (Figure 2), and/or inhibit activating enzymes such as CYP19 (aromatase) and CYP1B1 (Figure 2) Both NAcCys and Resv inhibit formation of catechol estrogen quinones and their reaction with DNA in cultured mammary cells, as well as the malignant transformation of these cells. Thus, this approach to cancer prevention can be widely applicable and successful.
Future perspective
We have clear and compelling evidence that specific oxidized metabolites of estrogens react with DNA to form predominantly depurinating estrogen–DNA adducts. These adducts are critical in the initiation of cancer by estrogens. We can capitalize on the fact that the depurinating adducts responsible for cancer initiation by estrogens can be detected in tissues and body fluids, as we have already found in people with breast and prostate cancer. The levels of these adducts in urine are significantly higher than the levels in healthy people without cancer. Therefore, these adducts can become important biomarkers for assessing the risk for cancer before tumors are detected.
The future of this research has a two-pronged approach – screening of people with various types of cancer to discover the levels of estrogen–DNA adducts in their urine, and development of targeted strategies for cancer prevention. To screen various types of cancer, subject populations with the following types of cancer need to be identified and their urine samples analyzed. These cancers include brain, colon, endometrium, kidney, leukemia, lymphoma, lung of nonsmokers, melanoma, myeloma, ovary, pancreas, testis and thyroid. Such studies will provide new information related to the possible role of estrogen–DNA adducts in the initiation of these types of cancer.
The strategy of using estrogen–DNA adduct bio-markers is not limited to the assessment of cancer risk, but it is also a very important tool for studying cancer prevention. If the levels of estrogen–DNA adducts are reduced by a preventive compound, the likelihood of cancer initiation should also decrease. This strategy of prevention does not require knowledge of the genes involved or the complex series of events that occur after initiation of cancer.
The study of cancer prevention begins with short-term trials (a few months) assessing the ability of the selected agents to reduce the levels of estrogen–DNA adducts in urine samples from healthy subjects. In addition, a study of women at high risk for breast cancer, for example, women with 5-year Gail Model scores greater than 1.66% would provide a very useful target population in which to discover the ability of the selected antioxidants to reduce formation of estrogen–DNA adducts. An added benefit for these women is that reduction in the level of estrogen–DNA adducts would presumably lower their risk of actually developing breast cancer. If populations at high risk for other types of cancer can be identified, they also could be targeted in these short-term studies of reduction in the levels of estrogen–DNA adducts.
Following these short-term studies, the next effective step would be to conduct a medium-term and medium-size study of the ability of the selected antioxidants to prevent breast cancer in women at high risk for breast cancer. In this population, sufficient numbers of breast cancer cases could be anti cipated to develop in 4–5 years so that the ability of the antioxidants to decrease the incidence of breast cancer could be determined.
Finally, a long-term, large clinical trial of cancer prevention should be conducted in both men and women to determine the efficacy of the selected antioxidants, presumably NAcCys and Resv, to prevent the initiation and, thus, development of breast, prostate and other prevalent types of human cancer. In such a trial, subjects would ingest one or more of the selected anti-oxidants in comparison with placebo, and regular, periodic medical monitoring and assessments would be conducted.
Executive summary.
Estrogens in the etiology of cancer
■ Experiments on estrogen metabolism, formation of DNA adducts, mutagenicity, cell transformation and carcinogenicity led to and support the hypothesis that reaction of specific estrogen metabolites, mostly catechol estrogen-3,4-quinones with DNA, can generate the critical mutations to initiate breast and other human cancers.
■ Estrogen metabolism is normally a balanced set of activating and deactivating pathways that minimizes formation of the quinones and their reaction with DNA.
■ When this balance is disrupted, more formation of the adducts 4-OHE1(E2)-1–N3Ade and 4-OHE1(E2)-1–N7Gua can occur, generating mutations that can lead to cancer initiation.
■ The estrogens are genotoxic and mutagenic and induce malignant transformation of human breast epithelial cells, as well as tumors in estrogen-receptor knockout mice.
■ Estrogen metabolites, conjugates and DNA adducts can be analyzed in human urine samples. Women with breast cancer or at high risk have significantly higher levels of estrogen–DNA adducts than healthy, normal-risk women. Men diagnosed with prostate cancer or non-Hodgkin lymphoma also have significantly higher levels of urinary adducts than healthy men.
■ It is thought that the most prevalent human cancers are initiated by mutations generated by depurinating estrogen–DNA adducts.
Novel approach to cancer prevention
■ Specific, selected natural antioxidants, through chemical and biochemical mechanisms, can reduce formation of catechol estrogen quinones and their reaction with DNA.
■ Both N-acetylcysteine and resveratrol reduce the formation of estrogen–DNA adducts and malignant transformation of cultured breast epithelial cells. In combination, they are even more effective in reducing adduct formation in human breast cells.
■ Pilot data indicate that ingestion of these two natural antioxidants reduces formation of estrogen–DNA adducts in humans.
■ This is a novel approach to prevention of human cancer based on the etiology of the disease.
Acknowledgements
The progress in research on the etiology and prevention of cancer is due to the efforts, dedication, creativity and accomplishments of the fine scientists with whom we have worked. We especially acknowledge our long-term collaborations with Prof. R Jankowiak of Kansas State University and Dr D Chakravarti of our research group. In addition, we thank our postdoctoral scientists, N Gaikwad, K-M Li, M Saeed, S Singh, D Venugopal and M Zahid, our graduate student L Yang, and our technical support, P Mailander.
Dr Cavalieri and Dr Rogan are two of the partners in Prevention LLC. Preparation of this article was supported by Prevention LLC. Core support at the Eppley Institute was supported by Grant CA P30 36727 from the National Cancer Institute.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Ercole L Cavalieri, Eppley Institute for Research in Cancer and Allied Diseases, University of Nebraska Medical Center, 986805 Nebraska Medical Center, Omaha, NE 68198-6805, USA.
Eleanor G Rogan, Eppley Institute for Research in Cancer and Allied Diseases and Department of Environmental, Agricultural and Occupational Health, College of Public Health, University of Nebraska Medical Center, NE, USA.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J. Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 2.IARC Monographs on the evaluation of the carcinogenic risk of chemicals to humans. IARC. 1982;29:93–148. [PubMed] [Google Scholar]
- 3.Mehlman MA. Casual relationship between non-Hodgkin's lymphoma and exposure to benzene and benzene-containing solvents. Ann. NY Acad. Sci. 2006;1076:120–128. doi: 10.1196/annals.1371.004. [DOI] [PubMed] [Google Scholar]
- 4.Cavalieri E, Rogan E. Catechol quinones of estrogens in the initiation of breast, prostate, and other human cancers: keynote lecture. Ann. NY Acad. Sci. 2006;1089:286–301. doi: 10.1196/annals.1386.042. [DOI] [PubMed] [Google Scholar]
- 5.Li JJ, Li SA, Klicka JK, Parsons JA, Lam LK. Relative carcinogenic activity of various synthetic and natural estrogens in the Syrian hamster kidney. Cancer Res. 1983;43:5200–5204. [PubMed] [Google Scholar]
- 6.Liehr JG, Fang WF, Sirbasku DA, Ari-Ulubelen A. Carcinogenicity of catechol estrogens in syrian hamsters. J. Steroid Biochem. 1986;24:353–356. doi: 10.1016/0022-4731(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 7.Li JJ, Li SA. Estrogen carcinogenesis in Syrian hamster tissues: role of metabolism. Fed. Proc. 1987;46:1858–1863. [PubMed] [Google Scholar]
- 8.Newbold RR, Liehr JG. Induction of uterine adenocarcinoma in CD-1 mice by catechol estrogens. Cancer Res. 2000;60:235–237. [PubMed] [Google Scholar]
- 9.Nandi S. Role of hormones in mammary neoplasia. Cancer Res. 1978;38:4046–4049. [PubMed] [Google Scholar]
- 10.Li JJ. Estrogen carcinogenesis in hamster tissues: update. Endocr. Rev. 1993;14:94–95. doi: 10.1210/edrv-11-4-524. [DOI] [PubMed] [Google Scholar]
- 11.Lang R, Redmann U. Non-mutagenicity of some sex hormones in the Ames salmonella/microsome mutagenicity test. Mutat. Res. 1979;67:361–365. doi: 10.1016/0165-1218(79)90033-8. [DOI] [PubMed] [Google Scholar]
- 12.Lang R, Reimann R. Studies for a genotoxic potential of some endogenous and exogenous sex steroids. I. Communication: examination for the induction of gene mutations using the Ames Salmonella/microsome test and the HGPRT test in V79 cells. Environ. Mol. Mutagen. 1993;21:272–304. doi: 10.1002/em.2850210311. [DOI] [PubMed] [Google Scholar]
- 13.Drevon C, Piccoli C, Montesano R. Mutagenicity assays of estrogenic hormones in mammalian cells. Mutat. Res. 1981;89:83–90. doi: 10.1016/0165-1218(81)90134-8. [DOI] [PubMed] [Google Scholar]
- 14.Feigelson HS, Henderson BE. Estrogens and breast cancer. Carcinogenesis. 1996;17:2279–2284. doi: 10.1093/carcin/17.11.2279. [DOI] [PubMed] [Google Scholar]
- 15.Dickson RB, Stancel GM. Estrogen receptor-mediated processes in normal and cancer cells. J. Natl. Cancer Inst. Monogr. 2000;27:135–145. doi: 10.1093/oxfordjournals.jncimonographs.a024237. [DOI] [PubMed] [Google Scholar]
- 16.Furth J. Hormones as etiological agents in neoplasia. In: Becker FF, editor. Cancer. A Comprehensive Treatise. 1. Etiology: Chemical and Physical Carcinogenesis. Vol. 4. Cancer Plenum Press; NY, USA: 1982. pp. 89–134. [Google Scholar]
- 17.Li JJ, Li SA. Estrogen carcinogenesis in hamster tissues: a critical review. Endocr. Rev. 1990;11:524–531. doi: 10.1210/edrv-11-4-524. [DOI] [PubMed] [Google Scholar]
- 18.Nandi S, Guzman RC, Yang J. Hormones and mammary carcinogenesis in mice, rats, and humans: a unifying hypothesis. Proc. Natl Acad. Sci. USA. 1995;92:3650–3657. doi: 10.1073/pnas.92.9.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn WC, Weinberg RA. Rules for making human tumor cells. N. Engl. J. Med. 2002;347:1593–1603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 20.Miller JA. Carcinogenesis by chemicals: an overview – GHA Clowes memorial lecture. Cancer Res. 1970;30:559–576. [PubMed] [Google Scholar]
- 21.Miller EC, Miller JA. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer. 1981;47:2327–2345. doi: 10.1002/1097-0142(19810515)47:10<2327::aid-cncr2820471003>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 22.Cavalieri EL, Stack DE, Devanesan PD, et al. Molecular origin of cancer: catechol estrogen-3,4-quinones as endogenous tumor initiators. Proc. Natl Acad. Sci. USA. 1997;94:10937–10942. doi: 10.1073/pnas.94.20.10937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23■.Cavalieri E, Chakravarti D, Guttenplan J, et al. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim. Biophys. Acta. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [Reviews the evidence until 2006 indicating that estrogens can become endogenous cancer initiators when catechol estrogen-3,4-quinones react with DNA to form specific depurinating adducts.] [DOI] [PubMed] [Google Scholar]
- 24■■.Saeed M, Rogan E, Cavalieri E. Mechanism of metabolic activation and DNA adduct formation by the human carcinogen diethylstilbestrol: the defining link to natural estrogens. Int. J. Cancer. 2009;124:1276–1284. doi: 10.1002/ijc.24113. [The synthetic human carcinogen diethylstilbestrol and the natural estrogens estrone and estradiol have in common the metabolic formation of catechol quinones, which react with DNA to afford the depurinating N3Ade and N7Gua adducts.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spink DC, Hayes CL, Young NE, et al. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on estrogen metabolism in MCF-7 breast cancer cells: evidence for induction of a novel 17 β-estradiol 4-hydroxylase. J. Steroid Biochem. Mol. Biol. 1994;51:251–258. doi: 10.1016/0960-0760(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 26.Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. 17β-estradiol hydroxylation catalyzed by human cytochrome P4501B1. Proc. Natl Acad. Sci. USA. 1996;93:9776–9781. doi: 10.1073/pnas.93.18.9776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spink DC, Spink BC, Cao JQ, et al. Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis. 1998;19:291–298. doi: 10.1093/carcin/19.2.291. [DOI] [PubMed] [Google Scholar]
- 28.Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol. Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- 29.Mobley JA, Bhat AS, Brueggemeier RW. Measurement of oxidative DNA damage by catechol estrogens and analogues in vitro. Chem. Res. Toxicol. 1999;12:270–277. doi: 10.1021/tx980128i. [DOI] [PubMed] [Google Scholar]
- 30.Cavalieri E. Minisymposium on endogenous carcinogens: the catechol estrogen pathway. An introduction. Polycycl. Aromat. Compd. 1994;6:223–228. [Google Scholar]
- 31■■.Zahid M, Kohli E, Saeed M, Rogan E, Cavalieri E. The greater reactivity of estradiol-3,4-quinone vs estradiol-2,3-quinone with DNA in the formation of depurinating adducts: implications for tumor-initiating activity. Chem. Res. Toxicol. 2006;19:164–172. doi: 10.1021/tx050229y. [The greater reactivity of estradiol-3,4-quinone versus estradiol-2,3-quinone with DNA to form depurinating adducts correlates with the carcinogenicity, mutagenicity and cell-transforming activity of their precursors, the catechol estrogens 4-hydroxyestradiol and 2-hydroxyestradiol.] [DOI] [PubMed] [Google Scholar]
- 32.Gaikwad NW, Rogan EG, Cavalieri EL. Evidence from ESI-MS for NQO1-catalyzed reduction of estrogen ortho-quinones. Free Radic. Biol. Med. 2007;43:1289–1298. doi: 10.1016/j.freeradbiomed.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talalay P, Dinkova-Kostova AT, Holtzclaw WD. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv. Enzyme Regul. 2003;43:121–134. doi: 10.1016/s0065-2571(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 34.Stack DE, Byun J, Gross ML, Rogan EG, Cavalieri EL. Molecular characteristics of catechol estrogen quinones in reactions with deoxyribonucleosides. Chem. Res. Toxicol. 1996;9:851–859. doi: 10.1021/tx960002q. [DOI] [PubMed] [Google Scholar]
- 35.Li KM, Todorovic R, Devanesan P, et al. Metabolism and DNA binding studies of 4-hydroxyestradiol and estradiol-3,4-quinone in vitro and in female ACI rat mammary gland in vivo. Carcinogenesis. 2004;25:289–297. doi: 10.1093/carcin/bgg191. [DOI] [PubMed] [Google Scholar]
- 36.Cavalieri E, Rogan E. Mechanisms of tumor initiation by polycyclic aromatic hydrocarbons in mammals. In: Neilson AH, editor. The Handbook of Environmental Chemistry. PAHs and Related Compounds. 3J. Springer-Verlag; Heidelberg, Germany: 1998. pp. 81–117. [Google Scholar]
- 37.Chakravarti D, Pelling JC, Cavalieri EL, Rogan EG. Relating aromatic hydrocarbon-induced DNA adducts and H-ras mutations in mouse skin papillomas: the role of apurinic sites. Proc. Natl Acad. Sci. USA. 1995;92:10422–10426. doi: 10.1073/pnas.92.22.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jankowiak R, Rogan EG, Cavalieri EL. The role of fluorescence line-narrowing spectroscopy and related luminescence-based techniques in the elucidation of mechanisms of tumor initiation by polycyclic aromatic hydrocarbons and estrogens. J. Phys. Chem. B. 2004;108:10266–10283. [Google Scholar]
- 39.Devanesan PD, RamaKrishna NVS, Padmavathi NS, et al. Identification and quantitation of 7,12-dimethylbenz[a] anthracene-DNA adducts formed in mouse skin. Chem. Res. Toxicol. 1993;6:364–371. doi: 10.1021/tx00033a018. [DOI] [PubMed] [Google Scholar]
- 40.Cavalieri EL, Rogan EG, Li KM, et al. Identification and quantification of the depurinating DNA adducts formed in mouse skin treated with dibenzo[a,l]pyrene (DB[a,l]P), or its metabolites and in rat mammary gland treated with DB[a,l]P. Chem. Res. Toxicol. 2005;18:976–983. doi: 10.1021/tx049682k. [DOI] [PubMed] [Google Scholar]
- 41.Todorovic R, Devanesan P, Rogan E, Cavalieri E. Identification and quantification of stable DNA adducts of dibenzo[a,l]pyrene or its metabolites in vitro, and in mouse skin and rat mammary gland. Chem. Res. Toxicol. 2005;18:984–990. doi: 10.1021/tx049681s. [DOI] [PubMed] [Google Scholar]
- 42.Rogan EG, Devanesan PD, RamaKrishna NV, et al. Identification and quantitation of benzo[a]pyrene–DNA adducts formed in mouse skin. Chem. Res. Toxicol. 1993;6:356–363. doi: 10.1021/tx00033a017. [DOI] [PubMed] [Google Scholar]
- 43■.Chakravarti D, Mailander P, Li KM, et al. Evidence that a burst of DNA depurination in SENCAR mouse skin induces error-prone repair and forms mutations in the H-ras gene. Oncogene. 2001;20:7945–7953. doi: 10.1038/sj.onc.1204969. [Treatment of SENCAR mouse skin with estradiol-3,4-quinone produces A to G mutations in the H-ras gene after 6 h to 3 days. These results confirm the genotoxicfff effects of catechol estrogen quinones..] [DOI] [PubMed] [Google Scholar]
- 44.Mailander PC, Meza JL, Higginbotham S, Chakravarti D. Induction of A.T to G.C mutations by erroneous repair of depurinated DNA following estrogen treatment of the mammary gland of ACI rats. J. Steroid Biochem. Mol. Biol. 2006;101:204–215. doi: 10.1016/j.jsbmb.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Miller WR, O'Neill J. The importance of local synthesis of estrogen within the breast. Steroids. 1987;50:537–548. doi: 10.1016/0039-128x(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 46.Simpson ER, Mahendroo MS, Means GD, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 47.Jefcoate CR, Liehr JG, Santen RJ, et al. Tissue-specific synthesis and oxidative metabolism of estrogens. J. Natl. Cancer Inst. Monogr. 2000;27:95–112. doi: 10.1093/oxfordjournals.jncimonographs.a024248. [DOI] [PubMed] [Google Scholar]
- 48.Pasqualini JR, Chetrite G, Blacker C, et al. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J. Clin. Endocrinol. Metab. 1996;81:1460–1464. doi: 10.1210/jcem.81.4.8636351. [DOI] [PubMed] [Google Scholar]
- 49.Chetrite GS, Cortes-Prieto J, Philippe JC, Wright F, Pasqualini JR. Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J. Steroid Biochem. Mol. Biol. 2000;72:23–27. doi: 10.1016/s0960-0760(00)00040-6. [DOI] [PubMed] [Google Scholar]
- 50.Lu F, Zahid M, Saeed M, Cavalieri EL, Rogan EG. Estrogen metabolism and formation of estrogen–DNA adducts in estradiol-treated MCF-10F cells. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction and catechol-O-methyltransferase inhibition. J. Steroid Biochem. Mol. Biol. 2007;105:150–158. doi: 10.1016/j.jsbmb.2006.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paracchini V, Raimondi S, Gram IT, et al. Meta- and pooled analyses of the cytochrome P-450 1B1 Val432Leu polymorphism and breast cancer: a HuGE-GSEC review. Am. J. Epidemiol. 2006;165:115–125. doi: 10.1093/aje/kwj365. [DOI] [PubMed] [Google Scholar]
- 52.Mitrunen K, Hirvonen A. Molecular epidemiology of sporadic breast cancer. The role of polymorphic genes involved in oestrogen biosynthesis and metabolism. Mutat. Res. 2003;544:9–41. doi: 10.1016/s1383-5742(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 53.Singh S, Zahid M, Saeed M, et al. NAD(P)H:quinone oxidoreductase 1 Arg139Trp and Pro187Ser polymorphism imbalance estrogen metabolism towards DNA adduct formation in human mammary epithelial cells. J. Steroid Biochem. Mol. Biol. 2009;117:56–66. doi: 10.1016/j.jsbmb.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem. Biol. Interact. 2000;129:77–97. doi: 10.1016/s0009-2797(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 55.Dawling S, Roodi N, Parl FF. Methoxyestrogens exert feedback inhibition on cytochrome P450 1A1 and 1B1. Cancer Res. 2003;3:3127–3132. [PubMed] [Google Scholar]
- 56.Cavalieri EL, Kumar S, Todorovic R, Higginbotham S, Badawi AF, Rogan EG. Imbalance of estrogen homeostasis in kidney and liver of hamsters treated with estradiol: implications for estrogen-induced initiation of renal tumors. Chem. Res. Toxicol. 2001;14:1041–1050. doi: 10.1021/tx010042g. [DOI] [PubMed] [Google Scholar]
- 57.Cavalieri EL, Devanesan P, Bosland MC, Badawi AF, Rogan EG. Catechol estrogen metabolites and conjugates in different regions of the prostate of Noble rats treated with 4-hydroxyestradiol: implications for estrogen-induced initiation of prostate cancer. Carcinogenesis. 2002;23:329–333. doi: 10.1093/carcin/23.2.329. [DOI] [PubMed] [Google Scholar]
- 58.Devanesan P, Santen RJ, Bocchinfuso WP, Korach KS, Rogan EG, Cavalieri EL. Catechol estrogen metabolites and conjugates in mammary tumors and hyperplastic tissue from estrogen receptor α knock out (ERKO)/Wnt 1 mice; implications for initiation of mammary tumors. Carcinogenesis. 2001;22:1573–1576. doi: 10.1093/carcin/22.9.1573. [DOI] [PubMed] [Google Scholar]
- 59.Rogan EG, Badawi AF, Devanesan PD, et al. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24:697–702. doi: 10.1093/carcin/bgg004. [DOI] [PubMed] [Google Scholar]
- 60.Singh S, Chakravarti D, Edney JA, et al. Relative imbalances in the expression of estrogen-metabolizing enzymes in the breast tissue of women with breast carcinoma. Oncol. Rep. 2005;14:1091–1096. [PubMed] [Google Scholar]
- 61.Liehr JG. Is estradiol a genotoxic mutagenic carcinogen? Endocr. Rev. 2000;21:40–54. doi: 10.1210/edrv.21.1.0386. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Z, Kosinska W, Khmelnitsky M, et al. Mutagenic activity of 4-hydroxyestradiol, but not 2-hydroxyestradiol, in BB rat2 embryonic cells, and the mutational spectrum of 4-hydroxyestradiol. Chem. Res. Toxicol. 2006;19:475–479. doi: 10.1021/tx0502645. [DOI] [PubMed] [Google Scholar]
- 63.Saeed M, Zahid M, Gunselman SJ, Rogan E, Cavalieri E. Slow loss of deoxyribose from the N7deoxyguanosine adducts of estradiol-3,4-quinone and hexestrol-3′,4′-quinone. Implications for mutagenic activity. Steroids. 2005;70:29–35. doi: 10.1016/j.steroids.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 64.Russo J, Hasan Lareef M, Balogh G, Guo S, Russo IH. Estrogen and its metabolites are carcinogenic agents in human breast epithelial cells. J. Steroid Biochem. Mol. Biol. 2003;87:1–25. doi: 10.1016/s0960-0760(03)00390-x. [DOI] [PubMed] [Google Scholar]
- 65.Russo J, Russo IH. Genotoxicity of steroidal estrogens. Trends Endocrinol. Metab. 2004;15:211–214. doi: 10.1016/j.tem.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Lareef MH, Garber J, Russo PA, Russo IH, Heulings R, Russo J. The estrogen antagonist ICI-182,780 does not inhibit the transformation phenotypes induced by 17-β-estradiol and 4-OH estradiol in human breast epithelial cells. Int. J. Oncol. 2005;26:423–429. [PubMed] [Google Scholar]
- 67.Fernandez SV, Russo PA, Fernbough R, et al. 17β-Estradiol induces transformations and tumorigenesis in human breast epithelial cells. FASEB J. 2006;20:1622–1634. doi: 10.1096/fj.05-5399com. [DOI] [PubMed] [Google Scholar]
- 68.Saeed M, Rogan E, Fernandez SV, Sheriff F, Russo J, Cavalieri E. Formation of depurinating N3Adenine and N7Guanine adducts by MCF-10F cells cultured in the presence of 4-hydroxyestradiol. Int. J. Cancer. 2007;120:1821–1824. doi: 10.1002/ijc.22399. [DOI] [PubMed] [Google Scholar]
- 69.Bocchinfuso WP, Korach KS. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J. Mammary Gland Biol. Neoplasia. 1997;2:323–334. doi: 10.1023/a:1026339111278. [DOI] [PubMed] [Google Scholar]
- 70.Bocchinfuso WP, Hively WP, Couse JF, Varmus HE, Korach KS. A mouse mammary tumor virus-wnt-1 transgene induces mammary gland hyperplasia and tumorigenesis in mice lacking estrogen receptor-α. Cancer Res. 1999;59:1869–1876. [PubMed] [Google Scholar]
- 71.Yue W, Santen RJ, Wang JP, et al. Genotoxic metabolites of estradiol in breast: potential mechanism of estradiol induced carcinogenesis. J. Steroid Biochem. Mol. Biol. 2003;86:477–486. doi: 10.1016/s0960-0760(03)00377-7. [DOI] [PubMed] [Google Scholar]
- 72.Santen RJ, Yue W, Bocchinfuso W, et al. Estradiol-induced carcinogenesis via formation of genotoxic metabolites. In: Ingle JN, Dowsett M, editors. Advances in Endocrine Therapy of Breast Cancer. Marcel Dekker; NY, USA: 2003. pp. 163–177. [Google Scholar]
- 73.Santen R, Cavalieri E, Rogan E, et al. Estrogen mediation of breast tumor formation involves estrogen receptor-dependent, as well as independent, genotoxic effects. Ann. NY Acad. Sci. 2009;1155:132–140. doi: 10.1111/j.1749-6632.2008.03685.x. [DOI] [PubMed] [Google Scholar]
- 74.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 75.Liehr JG, Ballatore AM, Dague BB, Ulubelen AA. Carcinogenicity and metabolic activation of hexestrol. Chem. Biol. Interact. 1985;55:157–176. doi: 10.1016/s0009-2797(85)80125-3. [DOI] [PubMed] [Google Scholar]
- 76.Haaf H, Metzler M. In vitro metabolism of diethylstilbestrol by hepatic, renal and uterine microsomes of rats and hamsters. Effects of different inducers. Biochem. Pharmacol. 1985;34:3107–3115. doi: 10.1016/0006-2952(85)90155-8. [DOI] [PubMed] [Google Scholar]
- 77.Blaich G, Gottlicher M, Cikryt P, Metzler M. Effects of various inducers on diethylstilbestrol metabolism, drug-metabolizing enzyme activities and the aromatic hydrocarbon (Ah) receptor in male Syrian golden hamster liver. J. Steroid Biochem. 1990;35:201–204. doi: 10.1016/0022-4731(90)90275-w. [DOI] [PubMed] [Google Scholar]
- 78.Metzler M, McLachlan JA. Oxidative metabolism of the synthetic estrogens hexestrol and dienestrol indicates reactive intermediates. Adv. Exp. Med. Biol. 1981;136(Pt A):829–837. doi: 10.1007/978-1-4757-0674-1_65. [DOI] [PubMed] [Google Scholar]
- 79.Jan ST, Devanesan PD, Stack DE, et al. Metabolic activation and formation of DNA adducts of hexestrol, a synthetic nonsteroidal carcinogenic estrogen. Chem. Res. Toxicol. 1998;11:412–419. doi: 10.1021/tx970141n. [DOI] [PubMed] [Google Scholar]
- 80.Saeed M, Gunselman SJ, Higginbotham S, Rogan E, Cavalieri E. Formation of the depurinating N3adenine and N7guanine adducts by reaction of DNA with hexestrol-3′,4′-quinone or enzyme-activated 3′-hydroxyhexestrol. Implications for a unifying mechanism of tumor initiation by natural and synthetic estrogens. Steroids. 2005;70:37–45. doi: 10.1016/j.steroids.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 81.Gaikwad NW, Yang L, Muti P, et al. The molecular etiology of breast cancer: evidence from biomarkers of risk. Int. J. Cancer. 2008;122:1949–1957. doi: 10.1002/ijc.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82■■.Gaikwad NW, Yang L, Pruthi S, et al. Urine biomarkers of risk in the molecular etiology of breast cancer. Breast Cancer: Basic & Clinical Research. 2009;3:1–8. doi: 10.4137/bcbcr.s2112. [Validates the finding that women with breast cancer or at high risk for the disease have significantly higher levels of depurinating estrogen–DNA adducts in their urine than healthy, normal-risk women. These data predict that depurinating estrogen–DNA adducts could become biomarkers for early detection of breast cancer risk and be used in prevention strategies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Markushin Y, Gaikwad N, Zhang H, et al. Potential biomarker for early risk assessment of prostate cancer. Prostate. 2006;66:1565–1571. doi: 10.1002/pros.20484. [DOI] [PubMed] [Google Scholar]
- 84■.Yang L, Gaikwad N, Meza J, et al. Novel biomarkers for risk of prostate cancer. Results from a case–control study. Prostate. 2009;69:41–48. doi: 10.1002/pros.20850. [Men with prostate cancer have significantly higher levels of depurinating estrogen–DNA adducts in their urine compared with healthy men. These data suggest that depurinating estrogen–DNA adducts could serve as potential biomarkers to predict risk for prostate cancer.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gaikwad N, Yang L, Weisenburger DD, et al. Urinary biomarkers suggest that estrogen–DNA adducts may play a role in the etiology of non-Hodgkin lymphoma. Biomarkers. 2009;14:502–512. doi: 10.3109/13547500903121715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zahid M, Gaikwad N, Rogan EG, Cavalieri EL. Inhibition of depurinating estrogen–DNA adducts formation by natural compounds. Chem. Res. Toxicol. 2007;20:1947–1953. doi: 10.1021/tx700269s. [DOI] [PubMed] [Google Scholar]
- 87.De Flora S, Cesarone CF, Balansky RM, et al. Chemopreventive properties and mechanisms of N-acetylcysteine. The experimental background. J. Cell Biochem. Suppl. 1995;22:33–41. doi: 10.1002/jcb.240590806. [DOI] [PubMed] [Google Scholar]
- 88.Cao K, Stack DE, Ramanathan R, Gross ML, Rogan EG, Cavalieri EL. Synthesis and structure elucidation of estrogen quinones conjugated with cysteine, N-acetylcysteine and glutathione. Chem. Res. Toxicol. 1998;11:909–916. doi: 10.1021/tx9702291. [DOI] [PubMed] [Google Scholar]
- 89.Samuni AM, Chuang EY, Krishna MC, et al. Semiquinone radical intermediate in catecholic estrogen-mediated cytotoxicity and mutagenesis: chemoprevention strategies with antioxidants. Proc. Natl Acad. Sci. USA. 2003;100:5390–5395. doi: 10.1073/pnas.0930078100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90■■.Venugopal D, Zahid M, Mailander PC, et al. Reduction of estrogen-induced transformation of mouse mammary epithelial cells by N-acetylcysteine. J. Steroid Biochem. Mol. Biol. 2008;109:22–30. doi: 10.1016/j.jsbmb.2007.12.003. [4-hydroxyestradiol and estradiol-3,4-quinone transform the normal mouse mammary epithelial cell line E6. N-acetylcysteine, a common antioxidant, inhibited both formation of depurinating estrogen–DNA adducts and malignant transformation of the cells. These results suggest that N-acetylcysteine can be an effective cancer prevention agent.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jang M, Cai L, Udeani O, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 92.Stivala LA, Savio M, Carafoli F, et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 2001;276:22586–22594. doi: 10.1074/jbc.M101846200. [DOI] [PubMed] [Google Scholar]
- 93■■.Lu F, Zahid M, Wang C, Saeed M, Cavalieri EL, Rogan EG. Resveratrol prevents estrogen–DNA adduct formation and neoplastic transformation in MCF-10F cells. Cancer Prev. Res. 2008;1:135–145. doi: 10.1158/1940-6207.CAPR-08-0037. [The antioxidant resveratrol blocked formation of depurinating estrogen–DNA adducts and neoplastic transformation in estrogen receptor-negative human breast epithelial, MCF-10F, cells. These results suggest that resveratrol can be an effective cancer prevention agent.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zahid M, Gaikwad NW, Ali MF, et al. Prevention of estrogen–DNA adduct formation in MCF-10F cells by resveratrol. Free Radic. Biol. Med. 2008;45:136–145. doi: 10.1016/j.freeradbiomed.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]