Abstract
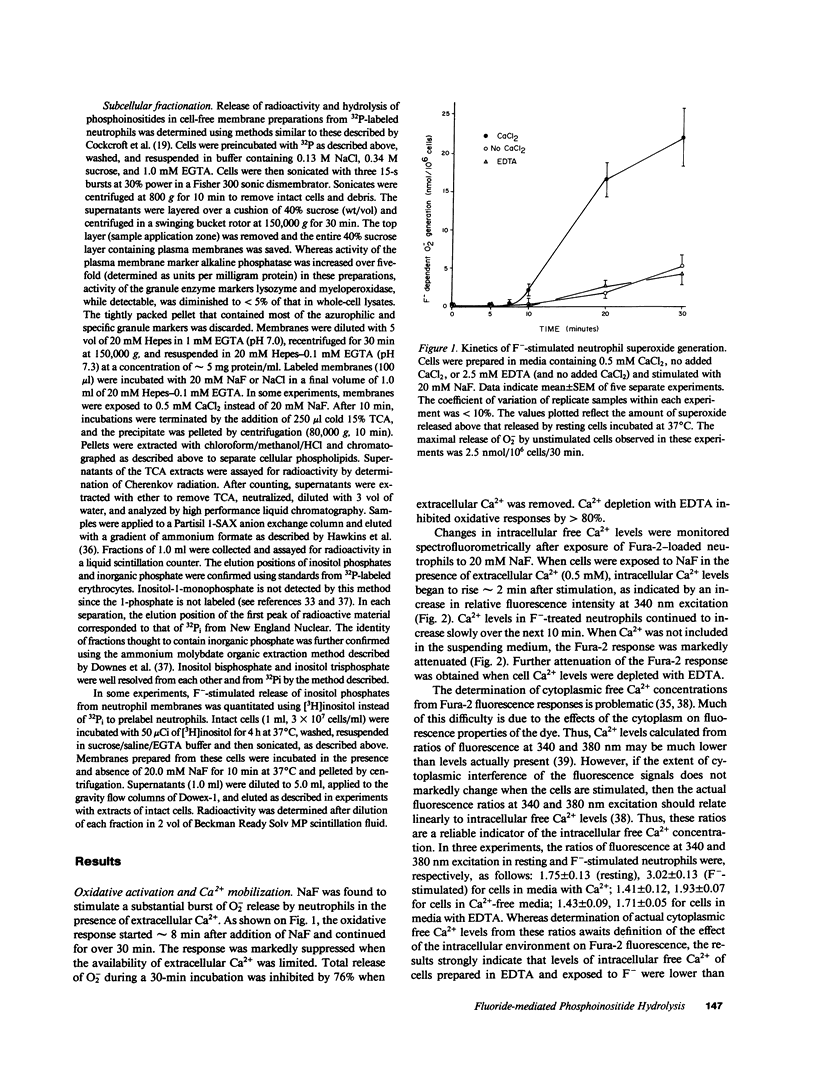
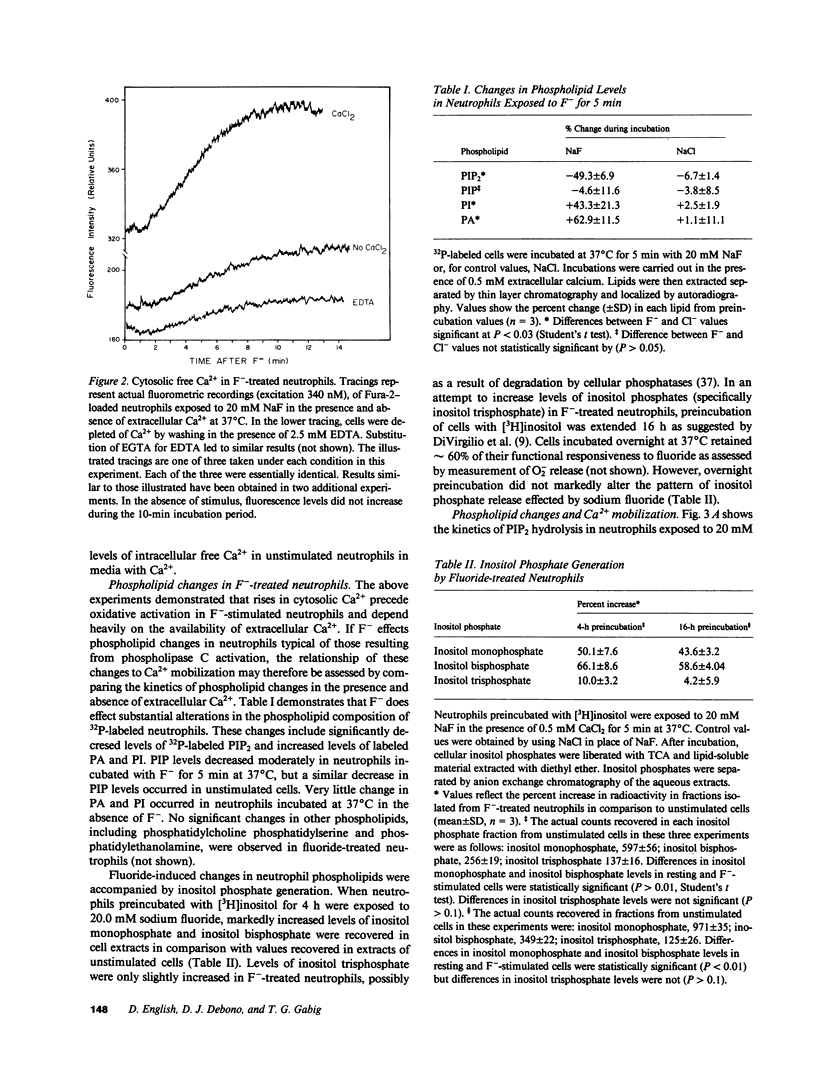
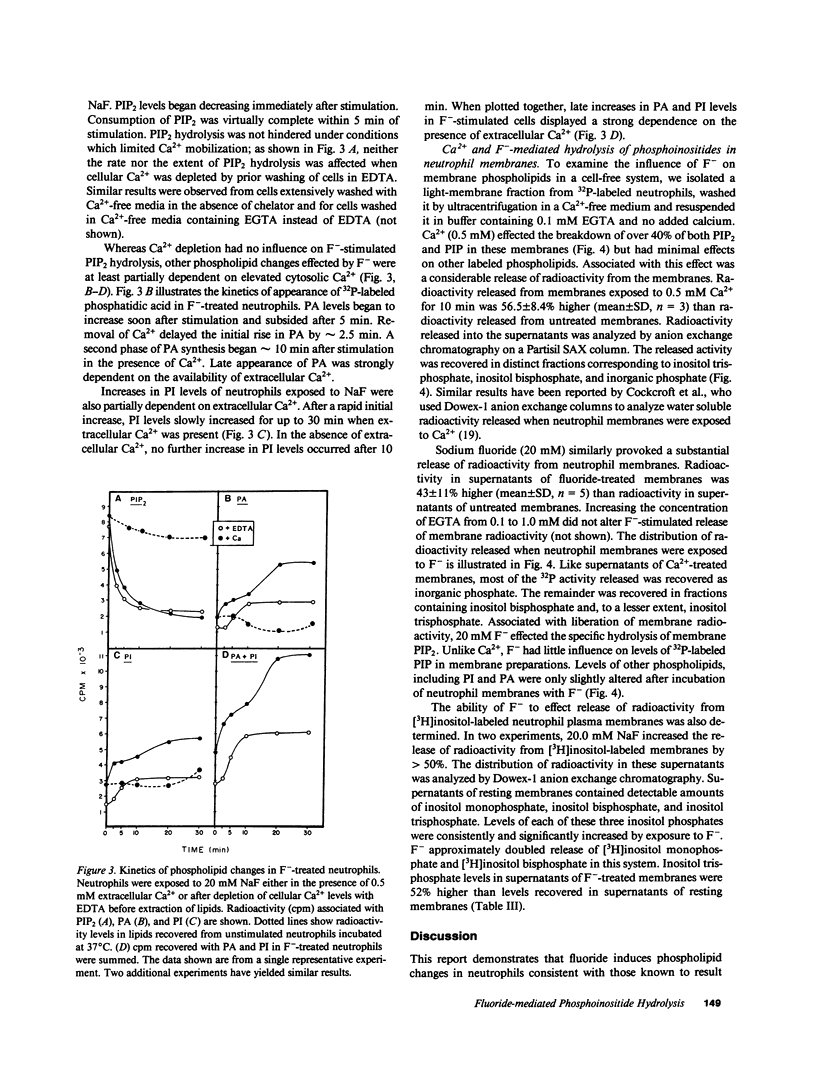
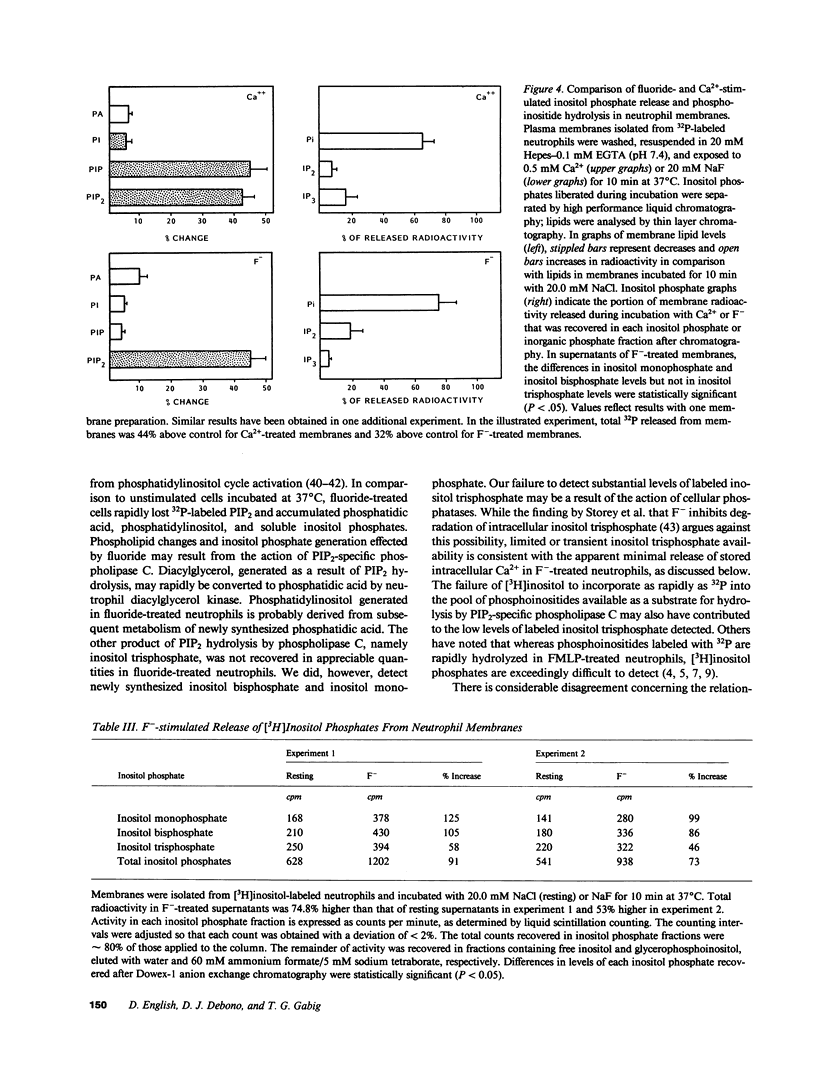
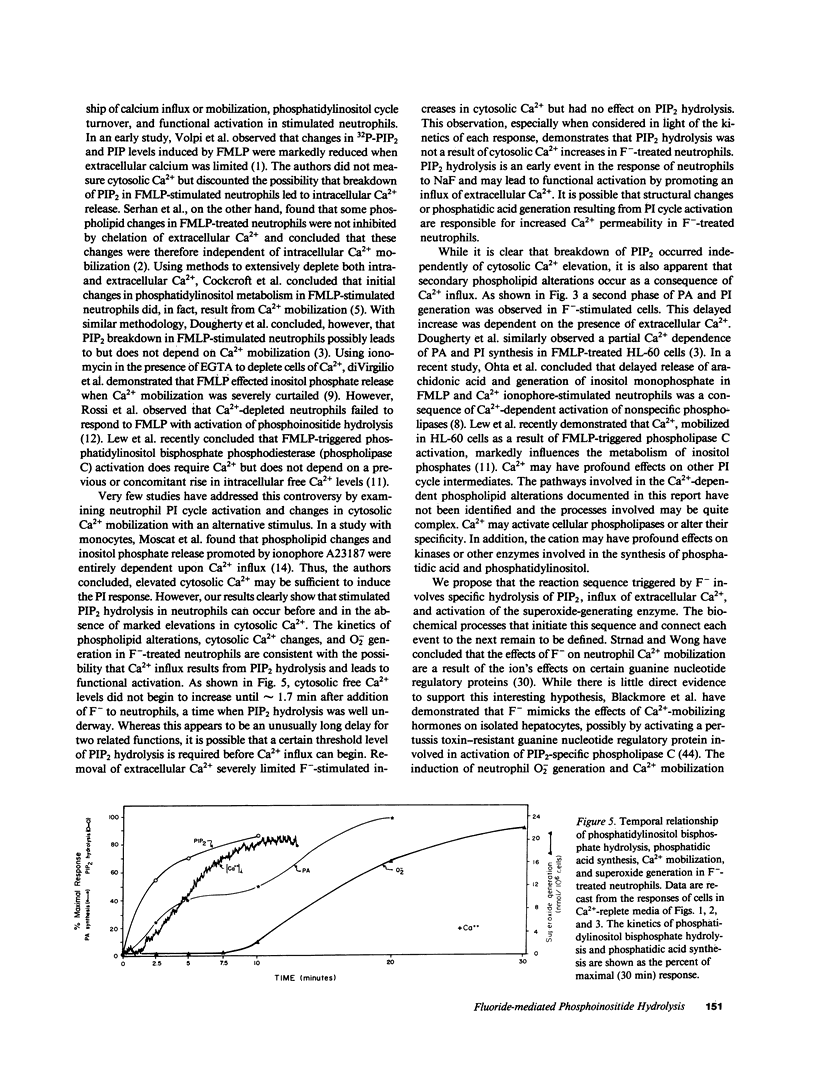
Sodium fluoride (20 mM) effected rapid hydrolysis of phosphatidylinositol bisphosphate (PIP2) in human neutrophils. Intracellular free Ca2+ levels increased after PIP2 hydrolysis but before respiratory burst activation. Both the increase in intracellular free Ca2+ levels and the extent of functional activation were dependent on the availability of extracellular Ca2+. The rate of F(-)-stimulated PIP2 hydrolysis, however, was not affected when the rise in cytosolic Ca2+ was severely limited by depletion of extracellular Ca2+. Fluoride caused the specific hydrolysis of PIP2 in isolated neutrophil plasma membranes. This effect occurred in the presence of low levels of available Ca2+ and was accompanied by the release of inositol phosphates. We conclude that PIP2 hydrolysis is an early event in the response of neutrophils to F-. This response is not Ca2+-regulated but may lead to an influx of Ca2+ from the extracellular medium. Activation of a PIP2-specific phospholipase independent of a change in cytosolic free Ca2+ levels may be the initial event in the stimulus-response pathway triggered by fluoride.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoroaluminates activate transducin-GDP by mimicking the gamma-phosphate of GTP in its binding site. FEBS Lett. 1985 Oct 28;191(2):181–185. doi: 10.1016/0014-5793(85)80004-1. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Bocckino S. B., Waynick L. E., Exton J. H. Role of a guanine nucleotide-binding regulatory protein in the hydrolysis of hepatocyte phosphatidylinositol 4,5-bisphosphate by calcium-mobilizing hormones and the control of cell calcium. Studies utilizing aluminum fluoride. J Biol Chem. 1985 Nov 25;260(27):14477–14483. [PubMed] [Google Scholar]
- Bokoch G. M., Gilman A. G. Inhibition of receptor-mediated release of arachidonic acid by pertussis toxin. Cell. 1984 Dec;39(2 Pt 1):301–308. doi: 10.1016/0092-8674(84)90008-4. [DOI] [PubMed] [Google Scholar]
- Bradford P. G., Rubin R. P. Pertussis toxin inhibits chemotactic factor-induced phospholipase C stimulation and lysosomal enzyme secretion in rabbit neutrophils. FEBS Lett. 1985 Apr 22;183(2):317–320. doi: 10.1016/0014-5793(85)80801-2. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Allan D. The fatty acid composition of phosphatidylinositol, phosphatidate and 1,2-diacylglycerol in stimulated human neutrophils. Biochem J. 1984 Sep 1;222(2):557–559. doi: 10.1042/bj2220557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Baldwin J. M., Allan D. The Ca2+-activated polyphosphoinositide phosphodiesterase of human and rabbit neutrophil membranes. Biochem J. 1984 Jul 15;221(2):477–482. doi: 10.1042/bj2210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Barrowman M. M., Gomperts B. D. Breakdown and synthesis of polyphosphoinositides in fMetLeuPhe-stimulated neutrophils. FEBS Lett. 1985 Feb 25;181(2):259–263. doi: 10.1016/0014-5793(85)80271-4. [DOI] [PubMed] [Google Scholar]
- Cockcroft S. Ca2+-dependent conversion of phosphatidylinositol to phosphatidate in neutrophils stimulated with fMet-Leu-Phe or ionophore A23187. Biochim Biophys Acta. 1984 Aug 15;795(1):37–46. doi: 10.1016/0005-2760(84)90102-4. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M., Karnovsky M. L. Fluoride-mediated activation of the respiratory burst in human neutrophils. A reversible process. J Clin Invest. 1979 Apr;63(4):637–647. doi: 10.1172/JCI109346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Bianca V., Grzeskowiak M., Cassatella M. A., Zeni L., Rossi F. Phorbol 12, myristate 13, acetate potentiates the respiratory burst while inhibits phosphoinositide hydrolysis and calcium mobilization by formyl-methionyl-leucyl-phenylalanine in human neutrophils. Biochem Biophys Res Commun. 1986 Mar 13;135(2):556–565. doi: 10.1016/0006-291x(86)90030-6. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Vicentini L. M., Treves S., Riz G., Pozzan T. Inositol phosphate formation in fMet-Leu-Phe-stimulated human neutrophils does not require an increase in the cytosolic free Ca2+ concentration. Biochem J. 1985 Jul 15;229(2):361–367. doi: 10.1042/bj2290361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty R. W., Godfrey P. P., Hoyle P. C., Putney J. W., Jr, Freer R. J. Secretagogue-induced phosphoinositide metabolism in human leucocytes. Biochem J. 1984 Sep 1;222(2):307–314. doi: 10.1042/bj2220307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The polyphosphoinositide phosphodiesterase of erythrocyte membranes. Biochem J. 1981 Jul 15;198(1):133–140. doi: 10.1042/bj1980133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D., Roloff J. S., Lukens J. N. Chemotactic factor enhancement of superoxide release from fluoride and phorbol myristate acetate stimulated neutrophils. Blood. 1981 Jul;58(1):129–134. [PubMed] [Google Scholar]
- English D., Roloff J. S., Lukens J. N. Regulation of human polymorphonuclear leukocyte superoxide release by cellular responses to chemotactic peptides. J Immunol. 1981 Jan;126(1):165–171. [PubMed] [Google Scholar]
- English D., Schell M., Siakotos A., Gabig T. G. Reversible activation of the neutrophil superoxide generating system by hexachlorocyclohexane: correlation with effects on a subcellular superoxide-generating fraction. J Immunol. 1986 Jul 1;137(1):283–290. [PubMed] [Google Scholar]
- Goldman D. W., Chang F. H., Gifford L. A., Goetzl E. J., Bourne H. R. Pertussis toxin inhibition of chemotactic factor-induced calcium mobilization and function in human polymorphonuclear leukocytes. J Exp Med. 1985 Jul 1;162(1):145–156. doi: 10.1084/jem.162.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Grzeskowiak M., Della Bianca V., Cassatella M. A., Rossi F. Complete dissociation between the activation of phosphoinositide turnover and of NADPH oxidase by formyl-methionyl-leucyl-phenylalanine in human neutrophils depleted of Ca2+ and primed by subthreshold doses of phorbol 12,myristate 13,acetate. Biochem Biophys Res Commun. 1986 Mar 28;135(3):785–794. doi: 10.1016/0006-291x(86)90997-6. [DOI] [PubMed] [Google Scholar]
- Grzeskowiak M., Della Bianca V., De Togni P., Papini E., Rossi F. Independence with respect to Ca2+ changes of the neutrophil respiratory and secretory response to exogenous phospholipase C and possible involvement of diacylglycerol and protein kinase C. Biochim Biophys Acta. 1985 Jan 18;844(1):81–90. doi: 10.1016/0167-4889(85)90237-x. [DOI] [PubMed] [Google Scholar]
- Guillon G., Mouillac B., Balestre M. N. Activation of polyphosphoinositide phospholipase C by fluoride in WRK1 cell membranes. FEBS Lett. 1986 Aug 18;204(2):183–188. doi: 10.1016/0014-5793(86)80808-0. [DOI] [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. Effects of acetylcholine on phospholipides in the pancreas. J Biol Chem. 1954 Aug;209(2):549–558. [PubMed] [Google Scholar]
- Hawkins P. T., Stephens L., Downes C. P. Rapid formation of inositol 1,3,4,5-tetrakisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate. Biochem J. 1986 Sep 1;238(2):507–516. doi: 10.1042/bj2380507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. B., Edelson H. S., Friedman R., Weissmann G. The roles of degranulation and superoxide anion generation in neutrophil aggregation. Biochim Biophys Acta. 1982 Sep 13;721(1):55–63. doi: 10.1016/0167-4889(82)90023-4. [DOI] [PubMed] [Google Scholar]
- Kruskal B. A., Shak S., Maxfield F. R. Spreading of human neutrophils is immediately preceded by a large increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1986 May;83(9):2919–2923. doi: 10.1073/pnas.83.9.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad P. M., Olson C. V., Smiley P. A. Association of the N-formyl-Met-Leu-Phe receptor in human neutrophils with a GTP-binding protein sensitive to pertussis toxin. Proc Natl Acad Sci U S A. 1985 Feb;82(3):869–873. doi: 10.1073/pnas.82.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Krause K. H., Waldvogel F. A., Biden T. J., Schlegel W. The role of cytosolic free calcium in the generation of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in HL-60 cells. Differential effects of chemotactic peptide receptor stimulation at distinct Ca2+ levels. J Biol Chem. 1986 Oct 5;261(28):13121–13127. [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Dewald B., Baggiolini M., Pozzan T. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986 Jun;102(6):2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Moscat J., Aracil M., Diez E., Balsinde J., Garcia Barreno P., Municio A. M. Intracellular Ca2+ requirements for zymosan-stimulated phosphoinositide hydrolysis in mouse peritoneal macrophages. Biochem Biophys Res Commun. 1986 Jan 14;134(1):367–371. doi: 10.1016/0006-291x(86)90572-3. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Ohta H., Okajima F., Ui M. Inhibition by islet-activating protein of a chemotactic peptide-induced early breakdown of inositol phospholipids and Ca2+ mobilization in guinea pig neutrophils. J Biol Chem. 1985 Dec 15;260(29):15771–15780. [PubMed] [Google Scholar]
- Okamura N., Uchida M., Ohtsuka T., Kawanishi M., Ishibashi S. Diverse involvements of Ni protein in superoxide anion production in polymorphonuclear leukocytes depending on the type of membrane stimulants. Biochem Biophys Res Commun. 1985 Aug 15;130(3):939–944. doi: 10.1016/0006-291x(85)91705-x. [DOI] [PubMed] [Google Scholar]
- Parkos C. A., Cochrane C. G., Schmitt M., Jesaitis A. J. Regulation of the oxidative response of human granulocytes to chemoattractants. No evidence for stimulated traffic of redox enzymes between endo and plasma membranes. J Biol Chem. 1985 Jun 10;260(11):6541–6547. [PubMed] [Google Scholar]
- Rossi F., Della Bianca V., Grzeskowiak M., De Togni P., Cabrini G. Relationships between phosphoinositide metabolism, Ca2+ changes and respiratory burst in formyl-methionyl-leucyl-phenylalanine-stimulated human neutrophils. The breakdown of phosphoinositides is not involved in the rise of cytosolic free Ca2+. FEBS Lett. 1985 Feb 25;181(2):253–258. doi: 10.1016/0014-5793(85)80270-2. [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Broekman M. J., Korchak H. M., Smolen J. E., Marcus A. J., Weissmann G. Changes in phosphatidylinositol and phosphatidic acid in stimulated human neutrophils. Relationship to calcium mobilization, aggregation and superoxide radical generation. Biochim Biophys Acta. 1983 Jun 2;762(3):420–428. doi: 10.1016/0167-4889(83)90007-1. [DOI] [PubMed] [Google Scholar]
- Sha'afi R. I., White J. R., Molski T. F., Shefcyk J., Volpi M., Naccache P. H., Feinstein M. B. Phorbol 12-myristate 13-acetate activates rabbit neutrophils without an apparent rise in the level of intracellular free calcium. Biochem Biophys Res Commun. 1983 Jul 29;114(2):638–645. doi: 10.1016/0006-291x(83)90828-8. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Lane B. C., Kusaka I., Verghese M. W., Snyderman R. Chemoattractant receptor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate in human polymorphonuclear leukocyte membranes. Requirement for a guanine nucleotide regulatory protein. J Biol Chem. 1985 May 25;260(10):5875–5878. [PubMed] [Google Scholar]
- Storey D. J., Shears S. B., Kirk C. J., Michell R. H. Stepwise enzymatic dephosphorylation of inositol 1,4,5-trisphosphate to inositol in liver. Nature. 1984 Nov 22;312(5992):374–376. doi: 10.1038/312374a0. [DOI] [PubMed] [Google Scholar]
- Strnad C. F., Wong K. Calcium mobilization in fluoride activated human neutrophils. Biochem Biophys Res Commun. 1985 Nov 27;133(1):161–167. doi: 10.1016/0006-291x(85)91855-8. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Ishitoya J., Homma Y., Kato M., Nagai Y. Role of enhanced inositol phospholipid metabolism in neutrophil activation. Biochem Pharmacol. 1985 Jun 1;34(11):1931–1935. doi: 10.1016/0006-2952(85)90311-9. [DOI] [PubMed] [Google Scholar]
- Verghese M., Uhing R. J., Snyderman R. A pertussis/choleratoxin-sensitive N protein may mediate chemoattractant receptor signal transduction. Biochem Biophys Res Commun. 1986 Jul 31;138(2):887–894. doi: 10.1016/s0006-291x(86)80579-4. [DOI] [PubMed] [Google Scholar]
- Volpi M., Naccache P. H., Molski T. F., Shefcyk J., Huang C. K., Marsh M. L., Munoz J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits fMet-Leu-Phe- but not phorbol ester-stimulated changes in rabbit neutrophils: role of G proteins in excitation response coupling. Proc Natl Acad Sci U S A. 1985 May;82(9):2708–2712. doi: 10.1073/pnas.82.9.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi M., Yassin R., Naccache P. H., Sha'afi R. I. Chemotactic factor causes rapid decreases in phosphatidylinositol,4,5-bisphosphate and phosphatidylinositol 4-monophosphate in rabbit neutrophils. Biochem Biophys Res Commun. 1983 May 16;112(3):957–964. doi: 10.1016/0006-291x(83)91711-4. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Cannell M. B., Berlin J. R., Marban E., Lederer W. J. Cellular and subcellular heterogeneity of [Ca2+]i in single heart cells revealed by fura-2. Science. 1987 Jan 16;235(4786):325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]



