Abstract
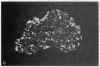
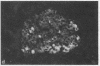
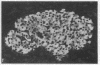
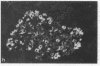
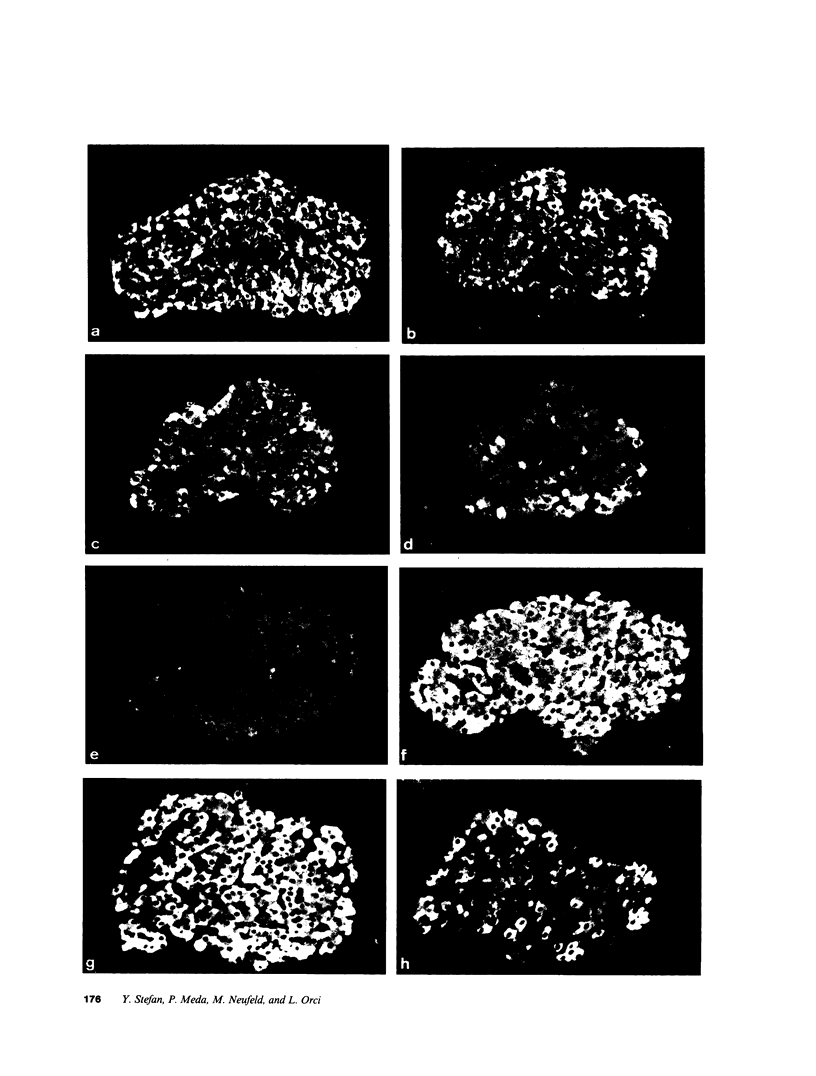
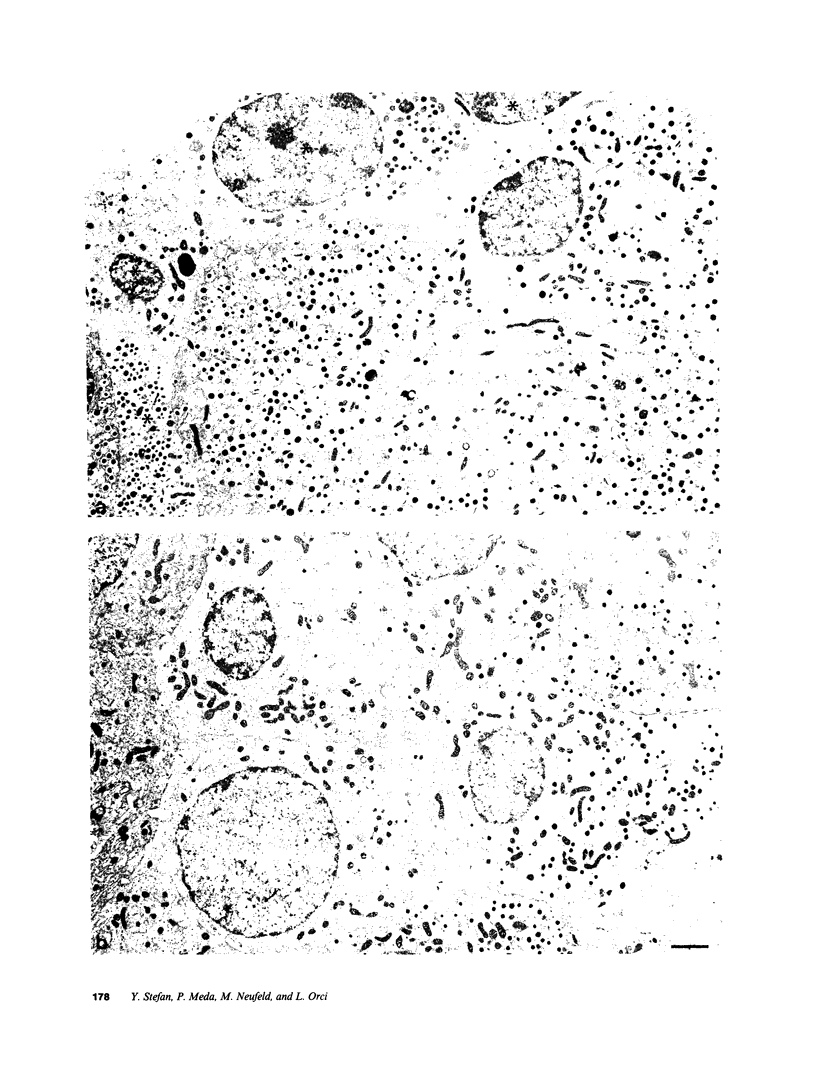
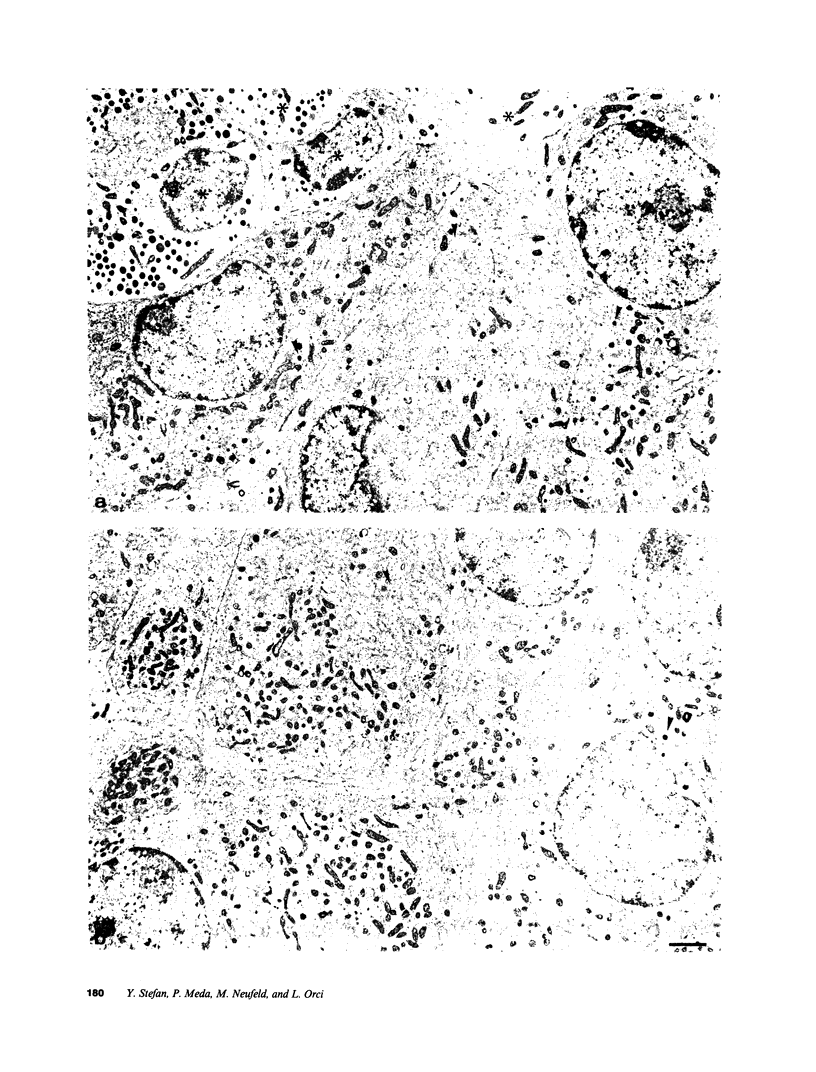
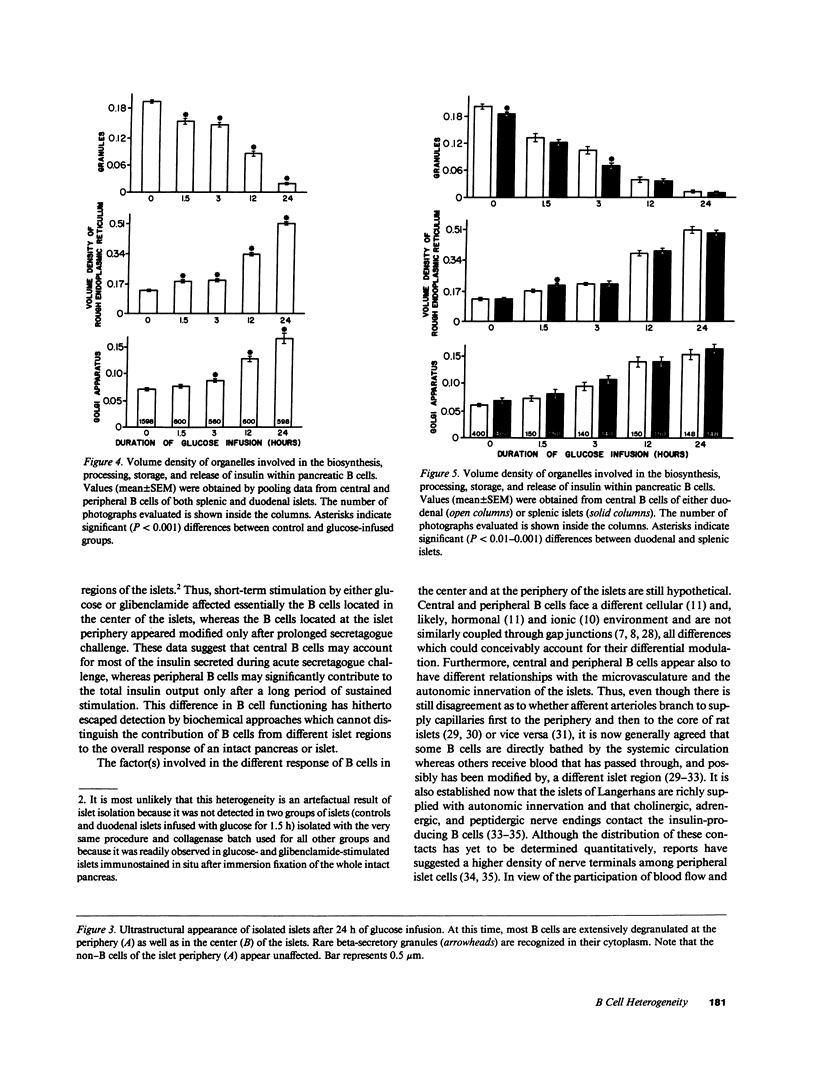
We examined the immunofluorescence and ultrastructural changes of insulin-producing B cells in the center and at the periphery of islets of Langerhans during in vivo stimulation by glucose and glibenclamide. A decreased insulin immunostaining was detected in islets from the splenic rat pancreas after 1.5 h of glucose stimulation. By contrast, immunofluorescence changes became apparent in islets from the duodenal pancreas only after greater than 3 h of hyperglycemia. In both cases, the immunolabeling of central B cells decreased before that of peripheral B cells. Similar changes were seen following in vivo stimulation of insulin secretion by glibenclamide. At the ultrastructural level, hyperglycemia decreased the volume density of B cell secretory granules and increased that of rough endoplasmic reticulum and Golgi apparatus. These changes were also detected earlier in central than in peripheral B cells and earlier in splenic than in duodenal islets. The data show that B cells form a heterogeneous population in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baetens D., Stefan Y., Ravazzola M., Malaisse-Lagae F., Coleman D. L., Orci L. Alteration of islet cell populations in spontaneously diabetic mice. Diabetes. 1978 Jan;27(1):1–7. doi: 10.2337/diab.27.1.1. [DOI] [PubMed] [Google Scholar]
- Beigelman P. M., Ribalet B., Atwater I. Electric activity of mouse pancreatic beta-cells. II. Effects of glucose and arginine. J Physiol (Paris) 1977 Jul;73(2):201–217. [PubMed] [Google Scholar]
- Berthoud H. R., Laughton W. B., Powley T. L. A method for large volume blood sampling and transfusion in rats. Am J Physiol. 1986 Mar;250(3 Pt 1):E331–E337. doi: 10.1152/ajpendo.1986.250.3.E331. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982 Oct;31(10):883–889. doi: 10.2337/diab.31.10.883. [DOI] [PubMed] [Google Scholar]
- Gold G., Landahl H. D., Gishizky M. L., Grodsky G. M. Heterogeneity and compartmental properties of insulin storage and secretion in rat islets. J Clin Invest. 1982 Mar;69(3):554–563. doi: 10.1172/JCI110481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M. A threshold distribution hypothesis for packet storage of insulin and its mathematical modeling. J Clin Invest. 1972 Aug;51(8):2047–2059. doi: 10.1172/JCI107011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLERSTROM C., PETERSSON B., HELLMAN B. Some properties of the B cells in the islet of Langerhans studied with regard to the position of the cells. Acta Endocrinol (Copenh) 1960 Jul;34:449–456. doi: 10.1530/acta.0.xxxiv0449. [DOI] [PubMed] [Google Scholar]
- Halban P. A. Differential rates of release of newly synthesized and of stored insulin from pancreatic islets. Endocrinology. 1982 Apr;110(4):1183–1188. doi: 10.1210/endo-110-4-1183. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Leahy J. L., Cooper H. E., Deal D. A., Weir G. C. Chronic hyperglycemia is associated with impaired glucose influence on insulin secretion. A study in normal rats using chronic in vivo glucose infusions. J Clin Invest. 1986 Mar;77(3):908–915. doi: 10.1172/JCI112389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq-Meyer V., Marchand J., Malaisse W. J. Insulin and glucagon release from the ventral and dorsal parts of the perfused pancreas of the rat. Effects of glucose, arginine, glucagon and carbamylcholine. Horm Res. 1985;21(1):19–32. doi: 10.1159/000180021. [DOI] [PubMed] [Google Scholar]
- Logothetopoulos J., Jain K. In vivo incorporation of [3H[ leucine and [3H] tryptophan into proinsulin-insulin and other islet cell proteins in normoglycemic, hyperglycemic, and hypoglycemic rats. Diabetes. 1980 Oct;29(10):801–805. doi: 10.2337/diacare.20.10.801. [DOI] [PubMed] [Google Scholar]
- Logothetopoulos J., Valiquette N. Hormonal and non-hormonal protein biosynthesis in the pancreatic beta cell of the intact rat after prolonged hyperglycaemia. Acta Endocrinol (Copenh) 1984 Nov;107(3):382–389. doi: 10.1530/acta.0.1070382. [DOI] [PubMed] [Google Scholar]
- Loubatières A., Mariani M. M., Ribes G., de Malbosc H., Chapal J. Etude expérimentale d'un nouveau sulfamide hypoglycémiant particulièrement actif, le HB 419 ou glibenclamide. Diabetologia. 1969 Feb;5(1):1–10. doi: 10.1007/BF01212212. [DOI] [PubMed] [Google Scholar]
- Lundquist I., Ericson L. E. beta-Adrenergic insulin release and adrenergic innervation of mouse pancreatic islets. Cell Tissue Res. 1978 Oct 6;193(1):73–85. doi: 10.1007/BF00221602. [DOI] [PubMed] [Google Scholar]
- Meda P., Atwater I., Gonçalves A., Bangham A., Orci L., Rojas E. The topography of electrical synchrony among beta-cells in the mouse islet of Langerhans. Q J Exp Physiol. 1984 Oct;69(4):719–735. [PubMed] [Google Scholar]
- Michaels R. L., Sheridan J. D. Islets of Langerhans: dye coupling among immunocytochemically distinct cell types. Science. 1981 Nov 13;214(4522):801–803. doi: 10.1126/science.6117129. [DOI] [PubMed] [Google Scholar]
- Miller R. E. Pancreatic neuroendocrinology: peripheral neural mechanisms in the regulation of the Islets of Langerhans. Endocr Rev. 1981 Fall;2(4):471–494. doi: 10.1210/edrv-2-4-471. [DOI] [PubMed] [Google Scholar]
- Ohtani O., Ushiki T., Kanazawa H., Fujita T. Microcirculation of the pancreas in the rat and rabbit with special reference to the insulo-acinar portal system and emissary vein of the islet. Arch Histol Jpn. 1986 Mar;49(1):45–60. doi: 10.1679/aohc.49.45. [DOI] [PubMed] [Google Scholar]
- Orci L. A portrait of the pancreatic B-cell. The Minkowski Award Lecture delivered on July 19, 1973, during the 8th Congress of the International Diabetes Federation, held in Brussels, Belgium. Diabetologia. 1974 Jun;10(3):163–187. doi: 10.1007/BF00423031. [DOI] [PubMed] [Google Scholar]
- Orci L., Baetens D., Ravazzola M., Stefan Y., Malaisse-Lagae F. Pancreatic polypeptide and glucagon : non-random distribution in pancreatic islets. Life Sci. 1976 Dec 15;19(12):1811–1815. doi: 10.1016/0024-3205(76)90112-0. [DOI] [PubMed] [Google Scholar]
- Orci L., Unger R. H. Functional subdivision of islets of Langerhans and possible role of D cells. Lancet. 1975 Dec 20;2(7947):1243–1244. doi: 10.1016/s0140-6736(75)92078-4. [DOI] [PubMed] [Google Scholar]
- Perez-Armendariz E., Atwater I., Rojas E. Glucose-induced oscillatory changes in extracellular ionized potassium concentration in mouse islets of Langerhans. Biophys J. 1985 Nov;48(5):741–749. doi: 10.1016/S0006-3495(85)83832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon D., Meda P. Heterogeneity and contact-dependent regulation of hormone secretion by individual B cells. Exp Cell Res. 1986 Feb;162(2):507–520. doi: 10.1016/0014-4827(86)90354-x. [DOI] [PubMed] [Google Scholar]
- Samols E., Bonner-Weir S., Weir G. C. Intra-islet insulin-glucagon-somatostatin relationships. Clin Endocrinol Metab. 1986 Feb;15(1):33–58. doi: 10.1016/s0300-595x(86)80041-x. [DOI] [PubMed] [Google Scholar]
- Sando H., Borg J., Steiner D. F. Studies on the secretion of newly synthesized proinsulin and insulin from isolated rat islets of Langerhans. J Clin Invest. 1972 Jun;51(6):1476–1485. doi: 10.1172/JCI106944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata A., Ludvigsen C. W., Jr, Naber S. P., McDaniel M. L., Lacy P. E. Standardization fo a digestion-filtration method for isolation of pancreatic islets. Diabetes. 1976 Aug;25(8):667–672. doi: 10.2337/diab.25.8.667. [DOI] [PubMed] [Google Scholar]
- Trimble E. R., Halban P. A., Wollheim C. B., Renold A. E. Functional differences between rat islets of ventral and dorsal pancreatic origin. J Clin Invest. 1982 Feb;69(2):405–413. doi: 10.1172/JCI110464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble E. R., Renold A. E. Ventral and dorsal areas of rat pancreas: islet hormone content and secretion. Am J Physiol. 1981 Apr;240(4):E422–E427. doi: 10.1152/ajpendo.1981.240.4.E422. [DOI] [PubMed] [Google Scholar]
- Zucker P., Logothetopoulos J. Persisting enhanced proinsulin-insulin and protein biosynthesis (3H-leucine incorporation) by pancreatic islets of the rat after glucose exposure. Diabetes. 1975 Feb;24(2):194–200. doi: 10.2337/diab.24.2.194. [DOI] [PubMed] [Google Scholar]