Abstract
This review updates historical background from century-old observations on embryonic lymphatic system development through current understanding of the molecular basis of lymphvasculogenesis/lymphangiogenesis (“molecular lymphology”), highlighting similarities and differences with analogous blood vasculature processes. Topics covered include molecular mechanisms in lymphatic development, structural adaptations of the lymphatic vasculature to particulate and cellular transport and trafficking, lymphogenous route of clinical cancer spread, preservation of delineated lymphatic pathways during cancer operations, and anti-lymphangiogenesis in cancer therapy.
Keywords: anti-lymphangiogenesis, lymphogenous cancer spread, angiogenesis, lymphatic development
INTRODUCTION AND OVERVIEW
In 1997, the first review and book chapter was published on lymphangiogenesis [1]. From the outset, a clear distinction was made between lymphangiogenesis and hemangiogenesis, the exploding field of “angiogenesis” fostered by Judah Folkman [2] and occupying the remaining chapters of this book. Coincidentally, as the book went to press, the discovery of the “lymphatic growth factor” VEGF-C was reported [3], the first of the vascular endothelial growth factor (VEGF) family specifically and preferentially targeting lymphatic rather than blood vessel growth, that is, lymphvasculogenesis and lymphangiogenesis, through binding to the endothelial receptor VEGFR3/Flt 4. In 1998, the first gene mutation associated with hereditary lymphedema (Milroy disease) and disturbed lymphangiogenesis was located on the distal arm of chromosome 5 and subsequently traced to VEGFR3 [4]. In rapid succession, two other “unrelated “ transcription factor gene mutations (FOXC2) [5] and the much rarer SOX18 [6] were found by reverse genetics through lymphedema family pedigree analysis (lymphedema distichiasis and hypotrichosis–telangiectasia, respectively), and mouse analogs with similar clinical phenotypes and targeted disruptions or spontaneous mutation of these genes described [reviewed in [7]]. At the same time, the angiopoietin (Ang) family of vascular growth and remodeling factors, and specifically Ang2 and Ang1 [8] were shown to participate in the development and maturation of the lymphatic vasculature as were other members of the VEGF family, including VEGFD [9] and even the predominantly hemangiogenic VEGFA. Since then, two more human lymphedema genes (CCBE1) [10,11] and GJC2 [12] and a myriad of other transcription factors, growth factors, and endothelial receptors, some of which mark lymphatic vessels very specifically have been reported to influence non-human lymphvasculogenesis and lymphangiogenesis [reviewed in [7]]. Some of these produce distinctive lymphatic system syndromes when knocked out, under- or over-expressed in transgenic mice or zebrafish, or when mutated or duplicated/deleted in humans [7,13].
Nonetheless, although the current era of molecular lymphology is relatively recent with lymphangiogenesis erupting as a “hot topic” [14] and stimulating numerous review articles and chapters, interest in and major contributions to an understanding of lymphvasculogenesis and lymphangiogenesis date back more than 100 years [15] to the work of Florence Sabin on the embryonic development of the lymphatic vasculature [16]. Indeed, as described in further detail in the next section by Dellinger and in Kampmeier’s exhaustive treatise on the Phylogeny of the Lymphatic System [17], Sabin’s work was the subject of such intense controversy that the American Association of Anatomists was nearly disbanded because of the heated disputation on the centrifugal versus centripetal origin of the lymphatic system at their annual meetings—including controversy over Sabin’s own interpretation of her materials and observations. Over the intervening years, discontinuous threads have been picked up related to lymphangiogenesis in vivo (clinical and experimental) and subsequently, the in vitro phenomenon in lymphatic endothelial cell (LEC) culture systems [1]. Since the 1980s, the biennial meetings of the International Society of Lymphology (ISL) uniquely and regularly featured basic science and clinical presentations and symposia on lymphangiogenesis and lymphangiotumorigenesis in International Congress of Lymphology proceedings publications as supplements to the journal Lymphology [18].
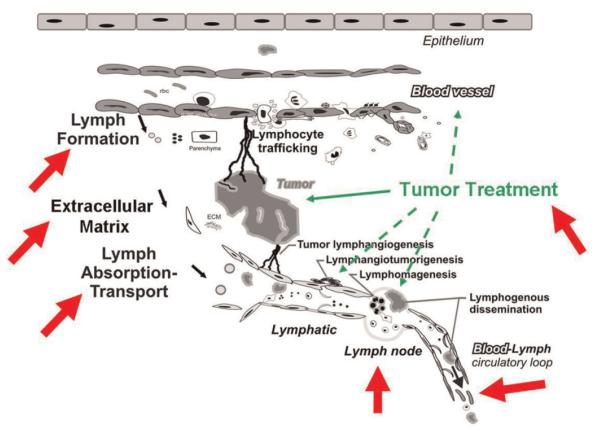
In contrast to the past neglect of the process of lymphangiogenesis and specifically downplaying of tumor lymphangiogenesis to the point of its very existence being questioned [reviewed in [19]], dating far back to the 1700s, there has been great attention and little argument about the importance of the lymphatic (lymphogenous) route—both lymphatic vessels and lymph nodes—in the spread of cancer [20]. Indeed, the lymphatic system is recognized as the stage on which most cancers play out their course (Fig. 1). Diagnosis and staging, prognosis, and therapeutic approaches to cancer are most often based on extent of involvement or lack of involvement of regional particularly recently, sentinel lymph nodes (SLNs) and, to a lesser extent, invasion of lymphatic vessels within or at some distance from the primary tumor site. Some cancers arise primarily in the lymph nodes and in lymphocyte populations (e.g., lymphomas and leukemias), and on rarer occasions, even in lymphatic vessels themselves (e.g., Stewart–Treves lymphangiosarcoma), which are not uncommonly the source of a variety of benign tumors (lymphangiotumorigenesis) particularly in childhood. Tumor immunity also resides in the lymphatic system as do clinical efforts to bolster that process, for example, dendritic cell vaccines in cancer therapeutics. And lymphedema—due to direct cancer invasion of the lymphatic trunks and nodes or secondary to obstruction from surgical extirpation or radiation treatment of the regional nodal basin—not uncommonly occurs during the course of cancer, representing a major lifelong complication diminishing quality of life and survivorship.
Fig. 1.
Structure–function interplays (arrows) between the lymphatic system and cancer. See text for further clarification. Modified with permission from Cancer Metastasis Review [20]. [Color figure can be viewed in the online issue, available at wileyonlinelibrary.com.]
Nonetheless, over the past several decades, the pendulum has switched direction from the Halstedian concepts of local lymphatic invasion and lymphogenous dissemination, and the preponderance of attention was turned to hematogenous spread of cancer [21] in a paradigm shift from earlier thinking. Intense scrutiny has been directed at steps and proximate mechanisms by which cancer cells are thought to enter blood vessels (intravasate) at the tumor site, travel through the bloodstream, and extravasate at distant metastatic sites. Therapy has increasingly emphasized systemic chemotherapy with blood vascular transport and delivery, and anti-angiogenesis therapy until very recently has exclusively targeted hemangiogenesis inhibitors using such agents as the anti-VEGF-A Avastin.
In this “Cancer Metastasis and the Lymphovascular System: Basis for Rational Therapy” Biennial Conference Series beginning in 2005, the center of attention has been returned to the lymphatic system and its role in the spread of cancer with expanded exploration of the SLN concept and its applications. In this review, we focus primarily on the lymphatic vasculature rather than the blood vasculature and on lymphangiogenesis rather than hemangiogenesis. First, Dellinger summarizes what is known and unknown about lymphatic development. McDonald then considers the recently described specialized button–zipper junctions in the lymphatic capillaries which function as convenient potential pathways for tumor cell entry. This distinctive structural feature adds to well-recognized ultrastructural and functional adaptations of lymphatic capillaries for macromolecular, particulate, and cellular transport and trafficking from the interstitium. Transcellular transport of tumor cells directly across LECs rather through intercellular junctions has also been recently documented by Azzali [22]. Furthermore, Nathanson revisits the phenomenon of lymphovascular invasion (LVI) in and around the primary tumor using highly specific lymphatic markers and finds that LVI rather than blood vessel invasion (BVI) is the key prognostic marker of aggressiveness and spread in breast and other cancers. He also suggests that access of tumor cells to the blood circulation might occur largely indirectly, that is, not by direct intravasation into blood capillaries in and around the tumor but rather predominantly by lymphatic invasion locally and only indirectly after lymphogenous transport through natural and acquired lymphatic-venous communications. The most prominent of these communications is normally located at the thoracic duct—and right lymphatic duct—subclavian venous junctions, respectively, in the left and right neck. Once in the bloodstream, tumor cells travel through the heart to reach and potentially seed the lungs and systemic organs. Boccardo and Campisi review the anatomy of the regional lymphatic drainage and lymph node basin in the axilla, breast, and arm and propose better ways to avoid interruption and obstruction of lymphatic pathways at the time of lymph nodal dissection for invasive cancer. In addition, they describe their promising results with prophylactic lymphatic-venous anastomoses to maintain lymphatic continuity and transport function when lymphatic interruption becomes unavoidable. Finally, Sleeman discusses tumor lymphangiogenesis and reviews the evidence for the use of a variety of anti-lymphangiogenic agents in cancer therapy. Whether systemic circulation of lymphatic growth factors reflect or actually govern systemic metastasis remains unclear. In the Epilogue, Gershenwald returns to the clinical arena to present a future perspective on clinical implications and therapeutic targeting applications relating to lymphangiogenesis and lymphogenous spread in human cancers.
DEVELOPMENT OF THE LYMPHATIC VASCULATURE
Historical Perspective
Although much is known about the anatomy and physiology of the lymphatic system, its precise embryonic origin has been a subject of controversy for over 100 years [23]. Much of this controversy has centered on the competing centrifugal and centripetal theories of lymphatic development. The centrifugal theory proposes that lymph sacs are the earliest anlage of the lymphatic system, arise from central embryonic veins, and give rise to the entire lymphatic vasculature of the body by a process of centrifugal sprouting [16,24,25]. The alternative, centripetal theory, proposes that numerous isolated lymphatic anlagen arise independent of veins and fuse to form lymphatic vessels [26].
Since the 19th century, the centrifugal and centripetal theories have been heavily debated. The intensity of these discussions peaked in the early 1900s when, in a series of publications, the major proponents of the centrifugal [Sabin] and centripetal [Huntington, McClure, Kampmeier] theories questioned the ability of each other’s technique to demonstrate the true origin of the lymphatic system [27–29]. Sabin performed dye and ink injections into lymphatic anlage and skin to show centrifugal sprouting of lymphatic vessels, whereas Huntington, McClure, and Kampmeier examined sectioned embryos to generate reconstructions of lymphatic vessels and lymphatic spaces. The argument against the injection method was that it would only reveal continuous lymphatic structures and not isolated lymphatic anlage in the mesenchyme [28]. On the other hand, it was argued that without dye, discontinuous tissue spaces could be mistaken for lymphatic vessels and the continuity of lymphatic vessels would be hard to determine [29]. Interestingly, this volatile discussion was sparked over the results obtained from a 23-mm pig embryo (series 23a from the Johns Hopkins Embryological Collection).
In 1910, Sabin presented a paper on the development of the thoracic duct in pig at the Annual Meeting of the American Association of Anatomists. Based on the results from her injection technique, she maintained that the thoracic duct developed by centrifugal sprouting from the jugular lymph sac. At the meeting, McClure asked Sabin if she would send him a pig embryo that she had injected with dye, then sectioned, so he could confirm the centrifugal development of the thoracic duct. Sabin agreed and sent McClure a 23-mm pig embryo, which had been processed by her. Otto Kampmeier, a trainee working under McClure, studied the sectioned embryo and found that there was multiple discontinuous anlage of the thoracic duct not filled with dye, suggesting that the thoracic duct does not develop by continual sprouting from lymph sacs, but rather by a centripetal mechanism. McClure wrote “Apparently the evidence which I sent to the Johns Hopkins Anatomical Laboratory did not meet with approval by those interested in the problem of lymphatic development at that Institution for, after receiving this evidence, two papers were published by Professor Sabin and Dr. Clark, respectively, in which no mention of this evidence is given and in which they attempt to clear up the problem of the lymphatics by a critical review of the methods employed by various investigators” [27]. Later, in the same manuscript McClure wrote, “After critical analysis of her writings, it appears to me that the present illogical position in which Professor Sabin has placed herself, with resulting confusion to all of us, is largely due to the circumstance that it is often disagreeable to acknowledge, in an emphatic and unmistakable manner, any change of opinion or modification of one’s views” [27]. To date, the origin of the thoracic duct has not been fully delineated; however, the development of the lymphatic vasculature is not as heavily contested today as it was in the early 1900s.
Currently, the most accepted model of lymphatic development conforms to the centrifugal theory and can be separated into the processes of lymphvasculogenesis, lymphangiogenesis, and remodeling.
Lymphvasculogenesis
Lymphvasculogenesis is the de novo formation of lymphatics and is the driving force behind the development of lymph sacs, the first obvious lymphatic structures in embryos. There are six lymph sacs in human embryos, two paired (jugular and posterior) and two unpaired (retroperitoneal and cisterna chyli) [25]. Of these, the jugular lymph sacs have been the most extensively characterized.
Observations on the development of the lymphatic system in pig embryos led Sabin to propose that the jugular lymph sacs arise from veins [16]. More recently, the expression pattern of the transcription factor Prox1 during murine embryonic development supports the venous origin of the jugular lymph sacs [30,31]. The transcription factor Sox18 drives Prox1 expression by a subset of venous endothelial cells that subsequently bud from the cardinal vein in response to Vegfc and form lymph sacs [32,33].
Extending the concept of lymphvasculogenesis is the lymphangioblast, a lymphatic endothelial precursor cell that arises independent of veins. Quail–Chicken chimera studies provided the first irrefutable experimental evidence that lymphangioblasts exist and integrate into lymph sacs as well as growing lymphatic vessels [34]. Descriptive studies in Xenopus laevis have also suggest that lymphangioblasts contribute to the developing lymphatic vasculature of amphibians [35]. However, the relative contribution of lymphangioblasts to the development of the mammalian lymphatic system remains controversial. Lineage tracing experiments suggest that lymphangioblasts either do not contribute to the development of the mammalian lymphatic system or serve only a minor role [36]. On the other hand, lymphangioblasts have been found to integrate into growing lymphatic vessels in mammals in pathological settings [37–39].
Developmental Lymphangiogenesis
Landmark observations on living frog larvae and transparent chambers in the rabbit ear revealed that one mechanism by which lymphatic vessel networks expand is by sprouting from pre-existing lymphatic vessels, a process termed lymphangiogenesis [40,41]. Since those observations, it has been discovered that the sprouting and circumferential growth of lymphatic vessels is mediated, in part, by lymphangiogenic growth factors. The first discovered and most extensively studied lymphatic growth factor is VEGF-C, a ligand of the receptor tyrosine kinases VEGFR2 and VEGFR3. The robust lymphangiogenic effect of VEGFC was first demonstrated by avian chorioallantoic membrane assays and transgenic overexpression in mice [42,43]. Later, it was shown that VEGF-C and VEGFR3 signaling is required for developmental lymphangiogenesis [33,44,45]. Although Vegfc is a robust and essential lymphatic growth factor, it does not act alone. The growth factors VEGF-D, IGF-1, IGF-2, PDGF-BB, HGF, Angiopoietin-1, Angiopoietin-2, and FGF-2 have all been demonstrated to stimulate lymphangiogenesis; however, the function all of these genes serve during the development of the lymphatic system has not been delineated [46–52].
Mirroring growing blood vessels, numerous filopodia radiate from growing lymphatic vessels. However, despite the striking similarities to growing blood vessels, it is unclear whether filopodia on lymphatic vessels arise from specialized lymphatic tip cells. Furthermore, it is unclear whether lymphatic vessels grow by proliferating at the tips, stalks, or both.
In order for the blood and lymphatic vascular systems to function as dual systems, it is critical for the two to be separated except at a few specific anatomical locations. There is growing evidence that signaling molecules prevent the intermingling of blood and lymphatic vessels. Mice that have mutations in Syk, SLP-76, fiaf, phospholipase C gamma 2, and T-synthase exhibit blood filled lymphatics, suggesting that genes actively participate in the repulsive mechanism that prevents the communication between blood and lymphatic vessels [reviewed by [53]].
Lymphatic Remodeling
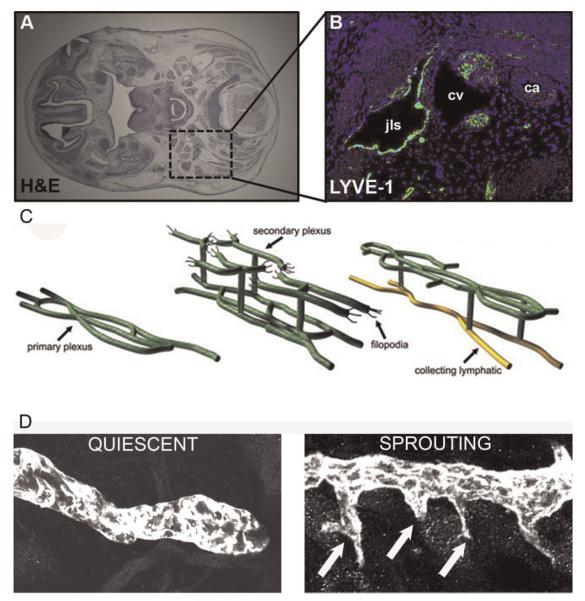
The processes of lymphvasculogenesis and lymphangiogenesis produce an immature network of lymphatic vessels that subsequently remodels into a hierarchal pattern of initial and collecting vessels (Fig. 2). Remodeling involves lymphangiogenesis and the acquisition of a collecting vessel phenotype (development of valves and recruitment of smooth muscle cells) by specific lymphatic vessels.
Fig. 2.
A: Hematoxylin and eosin stained section of a mouse embryo. The boxed region is where the left jugular lymph sac is located. B: Immunofluorescent staining for the hyaluronan receptor LYVE-1 demarks the jugular lymph sac (jls), located near the cardinal vein (cv) and carotid artery (ca) in an E12.5 mouse embryo. C: Schematic representation of postnatal remodeling of the dermal lymphatic vasculature. Numerous sprouts emerge from a primary plexus of lymphatic vessels to form a secondary plexus during postnatal development. Lymphatic vessels in the primary plexus subsequently acquire a collecting vessel phenotype. After remodeling, a hierarchal network of lymphatic vessels is present composed of initial lymphatic vessels, which transition to collecting lymphatic vessels. D: Confocal images of LYVE-1 stained lymphatic vessels show that, in marked contrast to quiescent (non-proliferating) lymphatic vessels in adult skin, lymphatic vessels exhibit numerous sprouts (arrows) during development (sprouting lymphangiogenesis). [Color figure can be viewed in the online issue, available at wileyonlinelibrary.com.]
The demonstration that defective lymph vessel remodeling underlies the syndrome, lymphedema-distichiasis, has fueled intense research efforts to identify the molecular mechanisms regulating the maturation of lymphatics. Lymphedema-distichiasis is a dominantly inherited disorder due to mutations in the transcription factor FOXC2 [5]. Interestingly, Foxc2+/− and Foxc2−/− exhibit spectrum of phenotypes mice a similar to patients with lymphedemadistichiasis [54,55]. Detailed characterization of Foxc2−/− lymphatics revealed that they are abnormally covered with pericytes/smooth muscle cells (PCs/SMCs) and lack valves, suggesting that Foxc2 regulates the formation of a collecting vessel phenotype [55]. More recently, the spatial–temporal expression pattern of Foxc2 in the mesentery supports a role of Foxc2 in lymph vessel remodeling [56]. During embryonic development, mesenteric lymphatics undergo a dramatic morphological change from a highly branched meshwork into collecting trunks [56]. During this time, the expression of Foxc2 and Prox1 by LECs diminishes except in areas where valves develop. Lymphatics in the mesentery of Foxc2−/− embryos fail to specify regions that will develop into valves, evidenced by the uniform expression of Prox1 by lymphatics [56].
Despite the identification of mice that lack lymphatic valves, the process of valve morphogenesis is poorly understood. Ultrastructural analysis of lymphatic valves demonstrated a close association between LECs in valve leaflets and the extracellular matrix (ECM). Recently, integrin alpha 9 was shown to be upregulated by lymphatic valve leaflets in the mesentery and regulate valve morphogenesis by coordinating the organization of the ECM in the core of lymphatic valve leaflets [57]. Kampmeier (1928) [58] has also reported three distinct mechanisms of valve morphogenesis; however, his models have not been revisited and confirmed with molecular markers of the lymphatic system.
In addition to the mesentery, the maturation of lymphatic vessels in the skin has been examined. Sabin first described the remodeling of the dermal lymphatics in pig embryos [24]. She demonstrated that a primary plexus of valveless lymphatics form, from which, sprouts emerge and give rise to a secondary plexus of lymphatics. During this time, vessels of the primary plexus mature into collecting vessels [24]. More recently, the same remodeling process was shown to occur postnatally in the skin of mice and depend on ephrinb2 [59]. Dermal lymphatics fail to mature in mice expressing a form of eprhinb2 lacking a domain that interacts with PDZ domain containing proteins [59].
Interestingly, Angiopoietin-2 mutant mice exhibit similar defects to ephrinb2 mutant mice. Ang2−/− mice display chylous ascites, peripheral lymphedema, and lymphatic hypoplasia [8]. Furthermore, lymphatic vessels in Ang2−/− mice fail to mature resulting in a profound deficiency of collecting lymphatics in adult mice [60]. Nearly all of the lymphatic vessels in the skin of Ang2−/− mice express the initial lymphatic marker LYVE-1 and exhibit a deficiency of valves [60]. These findings suggest that defective lymphangiogenic remodeling underlie the mutant phenotype of Ang2−/− mice. Indeed, a hypoplastic primary plexus of lymphatic vessels that has prematurely recruited PCs/SMCs forms in Ang2−/− mice during postnatal development [60]. Later in development, a hypoplastic secondary plexus forms and lymphatics in the primary plexus fail to mature [60]. The premature recruitment of PCs/SMCs by lymphatic vessels in Ang2−/− mice, and subsequent failure in maturation, suggests that PCs/SMCs can inhibit the maturation of lymphatics [60].
ULTRASTRUCTURE OF THE LYMPHATIC SYSTEM: REGULATION OF FLUID AND CELL ENTRY INTO LYMPHATIC VESSELS BY FUNCTIONALLY SPECIALIZED JUNCTIONS
Entry of tumor cells into lymphatic vessels is the first step in metastasis to lymph nodes and beyond. Despite recent advances in understanding lymphatic function, the cellular features responsible for entry of fluid and cells into lymphatics are incompletely understood. A common view is that fluid and cells enter lymphatics passively through openings between unattached LECs. However, Baluk et al. [61] and Dejana et al. [62] have found highly specialized button-like junctions between endothelial cells at sites where fluid and cells enter initial lymphatics.
Overlapping flaps at the border of oak leaf-shaped endothelial cells of initial lymphatics lacked junctions at the tip but were anchored on the sides by discontinuous button-like junctions [Fig. 3, [62]]. The discontinuous nature of button-like junctions distinguished them from conventional, continuous, zipper-like junctions in collecting lymphatics and blood vessels. Button-like junctions were composed of specialized adherens junctions and tight junctions and were disrupted by a function-blocking antibody to VE-cadherin but not by deletion of PECAM-1.
Fig. 3.
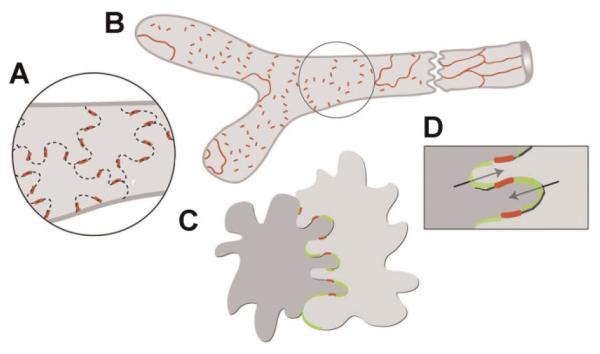
Buttons in initial lymphatics border sites of fluid entry. A: Schematic diagram showing distinctive, discontinuous buttons in endothelium of initial lymphatics and continuous zippers in collecting lymphatics. Both types of junction consist of proteins typical of adherens junctions and tight junctions. B: More detailed view showing the oak leaf shape of endothelial cells (dashed lines) of initial lymphatics. Buttons (red) appear to be oriented perpendicular to the cell border but are in fact parallel to the sides of flaps. In contrast, most PECAM-1 expression is at the tips of flaps. C,D: Enlarged views of buttons show that flaps of adjacent oak leaf-shaped endothelial cells have complementary shapes with overlapping edges. Adherens junctions and tight junctions at the sides of flaps direct fluid entry (arrows) to the junction-free region at the tip without repetitive disruption and reformation of junctions. Reproduced with permission from Journal of Experimental Medicine [61]. [Color figure can be viewed in the online issue, available at wileyonlinelibrary.com.]
These findings indicate that endothelial cells of initial lymphatics have specialized button-like junctions that regulate the entry of fluid and cells. In disease such as chronic inflammation or tumors, abnormalities in these specialized junctions of the initial lymphatics can increase or decrease entry of fluid and cells. Thus, changes in these junctions in intratumoral lymphatics may alter the entry of tumor cells into lymphatics but further work is needed to document this phenomenon and the conditions under which it occurs.
PATHOPHYSIOLOGIC/BIOLOGIC ASPECTS OF LYMPHOGENOUS SPREAD: ARE LYMPHATICS THE MAIN INVASION SITES INTO THE SYSTEMIC CIRCULATION OF PRIMARY MALIGNANT TUMORS OF EPITHELIAL ORIGIN?
Extensive preclinical, clinical, and in vitro study of systemic cancer spread has yielded important information about the key steps in the process of metastasis. Tumor cells invade host parenchymal tissue, a process requiring proteolysis, adhesion receptors, and directional motility, but little is known about the initial steps whereby the cells interact with tumoral or peritumoral vessels and gain access to the systemic circulation. While tumors can enter the blood directly by intravasating into blood vessel capillaries (BVCs) or indirectly via lymphatics the relative contributions of both pathways as routes of egress from the primary site is still debated. Descriptions and diagrams of the anatomic pathways of systemic metastasis in textbooks, scientific articles, and reviews demonstrate direct invasion of blood vessels by primary malignant tumors of epithelial origin [63]. Most observers assume that angiogenesis not only produces more blood vessels, providing a greater blood flow to the primary tumor, but that angiogenesis also creates a larger surface “window” of BVC endothelium for tumor cells to invade and metastasize to systemic sites [64].
Twentieth century pathologists alluded to the process of peritumoral and/or intratumoral invasion into capillaries as “lymphovascular invasion” (LVI) or used the synonymous term “angiolymphatic invasion,” electing to avoid the more direct terminology “lymphatic invasion,” or “blood vessel invasion,” because it is not easy to assuredly distinguish microscopically between lymphatic capillaries (LC) and BVC using standard morphological criteria after hematoxylin and eosin staining. The term “LVI” accents “vascular” and creates the impression that the invaded vessels are blood vessels and has thus contributed to the assumption of peri/intratumoral BVC invasion although direct observation of such a phenomenon is rare. Additional contributions to the belief that direct BVI must be the pre-eminent mechanism for creation of systemic metastases comes from experimental metastasis studies in animal models [63], with an emphasis on systemic metastases while ignoring the initial steps of invasion in the primary organ.
Nineteenth century pathologists identified spontaneous metastasis to lymph nodes and their anatomic observations prompted surgeons to design operations that resected the primary cancer and its originating organ plus the draining regional lymph nodes (RLNs) in one piece [65] based on the belief that such radical surgery would sometimes cure cancer patients. The paradigm of thinking changed because of ideas promulgated by Fisher and Fisher [21] based on laboratory animal models and partly confirmed by carefully designed clinical studies. Breast cancer metastasis to the RLNs was seen as an indicator but not necessarily a promoter of systemic metastasis. Radical surgical procedures for breast cancer slowly became outmoded and smaller operations, such as lumpectomy and sentinel lymph node biopsy (SLNB) became the accepted standard of surgical care. However, breast cancer clinicians have observed long-term cures in patients treated by radical loco-regional therapy without systemic therapy in patients with RLN metastasis, suggesting that systemic metastasis is not always inevitable when RLN metastasis is present [66]. The evidence from such studies also identified groups of patients without RLN metastasis who were cured by loco-regional therapies alone and where there was no systemic metastasis and presumably no direct invasion of BVCs.
Invading tumor cells may gain access to the systemic circulation via LC invasion, passage through peripheral lymphatic trunks, progressing anatomically through the sentinel (SLN) and other RLNs, the large lymphatic ducts, and the existing lymphaticovenous anastomoses (LVA) in the neck [67]. Systemic metastases established primarily through LC invasion and metastasis to the RLNs supports the Halstedian hypothesis, with a predominant pattern of orderly lymphatic and lymph node progression; systemic metastasis would follow in some patients [65].
Direct evidence that LC invasion is the predominant method whereby cancer gains access to the systemic circulation is provided by spontaneous metastasis studies in animals [68] and by specialized lymphatic endothelial stains in breast cancer. In a mouse spontaneous metastasis model green fluorescent protein-stained tumor cells were observed by video-microscopy entering peritumoral lymphatics and the SLN and not the peritumoral angiogenic BVCs. The Nottingham group used specific lymphatic endothelial stains to demonstrate that breast cancer cells in endothelial-lined spaces (LVI) were in lymphatics 96% of the time; invasion of BVCs was quite rare in this human breast cancer study [69].
Indirect evidence for LC invasion is provided by animal models of spontaneous metastasis, and the recent evolution of the SLN concept, and by astute observations of clinical patterns of metastasis in every human cancer of epithelial origin, and by our recent investigations into the interactions between LVI, systemic and regional lymph node metastasis, and by evaluation of the pathophysiology of interstitial fluid pressure (IFP) in tumors and the interplay with lymphatic anatomy. Lymphatic capillaries lack interendothelial tight junctions, pericytes, and basement membranes seen in blood vessels making the mechanics of entry into lymphatics theoretically easier for an invading cancer cell to accomplish. In a mouse footpad melanoma model lung metastases occurred only after metastasis to the SLN and never without lymph node metastases [70]. A sizeable number of animal spontaneous metastasis studies have reported the association and interaction between peritumoral lymphangiogenesis and sentinel node metastasis [67]. The orderly anatomic progression of spontaneous metastasizing human tumors, observed initially by pathologists 160 years ago as left supra-clavicular lymph node metastases from infra-diaphragmatic malignancies (the so-called Virchow–Trosier node), and more recently in sentinel node metastasis from melanoma [71], exemplifies the argument in favor of LC invasion.
While specific immunohistochemical stains that distinguish LECs from blood vessel endothelial cells (BECs) strongly suggest that lymphatic invasion is the preferred method by which tumor cells metastasize to the RLNs [69], this breast cancer study failed to also conclude that the initial lymphatic invasion was also the predominant method by which tumor cells gained access to the systemic circulation. We addressed this question interrogating our breast cancer SLN database [72], providing a 14-year follow-up of patients, to determine whether the relationships among LVI, RLN, and systemic metastases support direct peritumoral and/or intratumoral BVC or LC invasion as the predominant pattern of metastasis. LVI was significantly associated with both RLN and systemic metastases in this study of 1,668 clinically N0 breast cancer patients offered SLNB and/or complete axillary lymph node dissection (ALND) and followed for 1–14 years (median 3.62 years). Analysis of the data also showed that systemic metastasis in the presence of LVI required metastasis to the RLNs for the association to be true, supporting the likelihood that the invaded vessels were lymphatics rather than blood vessels.
The mechanical aspects of lymph node metastasis include the effects of abnormal fluid mechanics in the primary tumor. VEGFs induce brisk endothelial proliferation, sprouting, and an abundance of new blood vessels that are leaky, expanding interstitial volume and creating increased IFP [67]. IFP is elevated in malignant tumors and the extent of the increase correlates with the size of the tumor [73]. The resulting peritumoral edema is often observed by radiologic imaging studies and is visible during surgical resection of tumors. The direction of flow of interstitial fluid is towards the perimeter of the primary tumor where newly formed initial lymphatics are able to absorb the increased volume of lymph. The unique micro-anatomy of initial lymphatics, with “button” interendothelial openings [62], aided by tethering filaments to the ECM allows these vessels to remain open even when the IFP is raised. In contrast, angiogenic BVCs, lacking tethering filaments between the endothelial cells, and with tightly closed interendothelial junctions are compressed by increased extracellular pressures exerted by increased IFP and by crowded growth of tumor cells [74]. Figure 4 depicts the collapse of blood capillaries exposed to increased external pressure; Figure 5 demonstrates the likely path of a tumor cell invading a lymphatic rather than a blood capillary.
Fig. 4.
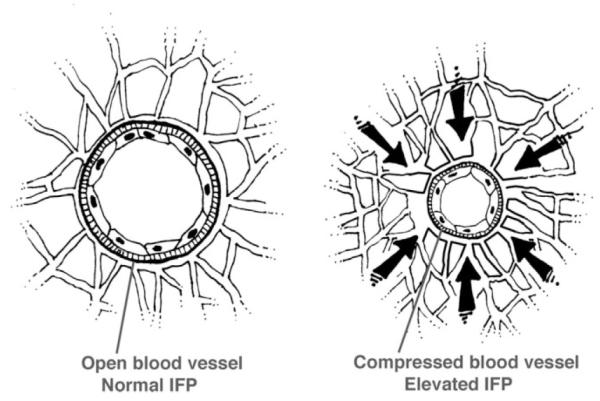
The diagram shows blood vessels with intact endothelium and basement membrane. The blood vessel on the right is collapsed by rising interstitial fluid pressure (IFP) compared to the vessel on the left under normal IFP.
Fig. 5.
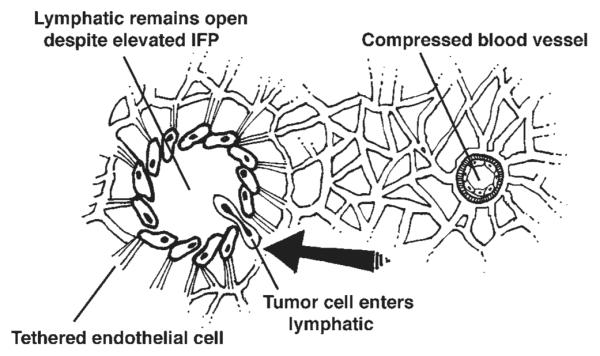
The microstructure of the lymphatic wall (on the left), with patchy basement membrane, tethering of endothelial cells to surrounding stroma, and “button-like” interendothelial openings [13] remains open to the inflow of fluid and tumor cells despite increased IFP. In contrast, the collapsed blood vessel (right) probably is more difficult for a tumor cell to negotiate.
In summary, our recently published study [72] supports the hypothesis, previously studied both directly and indirectly, in animals and humans, that spontaneous metastasis from a primary tumor site to the regional draining lymph nodes is the major pathway to progression into the systemic circulation and to distant organ sites. Invasion into newly formed tumor blood vessels in or around the primary tumor is an unusual event in most common tumors of epithelial origin, although soft tissue sarcomas appear uniquely different in that RLN metastasis in these rare malignancies is unusual while systemic metastasis occurs relatively frequently. Future studies of the mechanisms of RLN metastasis might well benefit by focusing on these differences.
LYMPHATIC PATHWAYS, BREAST CANCER, AND SURGICAL MANAGEMENT
Anatomical and Pathophysiologic Aspects
A side effect of axillary lymph node excision and radiotherapy for breast cancer is arm lymphedema in about 25% (ranging from 13% to 52%). SLN biopsy has reduced the severity of swelling to nearly 6% (from 2% to 7%) and, in the case of positive SLN, complete axillary dissection (AD) is still required. That is why the axillary reverse mapping (ARM) method was developed aimed at identifying and preserving lymphatics draining the arm. It consists of injecting intradermally and subcutaneously a small quantity (1–2 ml) of blue dye at the medial surface of the arm which helps in locating the draining arm lymphatic pathways. The ARM technique delineated the variable clinical anatomical conditions already generally known, identifying the most common location of arm lymphatics below and around the axillary vein. In about one-third of the cases, blue lymphatics can be found up to 3–4 cm below the vein, a site where SLN can easily be located, justifying the occurrence of lymphedema after only SLN biopsy. The ARM procedure showed that blue nodes were almost always placed at the lateral part of the axilla, under the vein and above the second intercostal brachial nerve. Leaving in place lymph nodes related to arm lymphatic drainage would decrease the risk of arm lymphedema, but by not retrieving all nodes, the main risk is to leave metastatic disease in the axilla. Conversely, arm lymphatic pathways when they enter the axilla are not a source of breast tumoral disease, and their preservation would surely bring about a significant decrease of lymphedema occurrence rate [75–88] (Fig. 6).
Fig. 6.
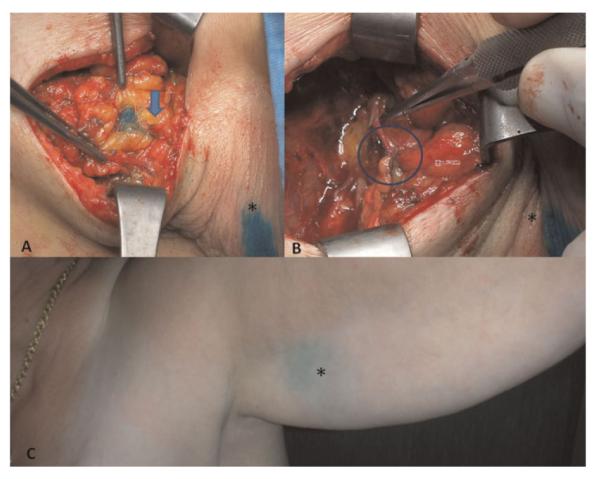
A: Blue lymphatic pathways (arrow) highlighted in the arm during axillary lymph nodal dissection for breast cancer treatment; (B) lymphatic-venous anastomoses (circle) to prevent arm lymphedema (LYMPHA); (C) blue dye (*) injected at the upper third of the volar surface of the arm almost disappeared after only 4 weeks. [Color figure can be viewed in the online issue, available at wileyonlinelibrary.com.]
Lymphangiogenesis and Other Local Changes
Another important aspect to point out is that, in the axilla, new lymphatic vessel formation (lymphangiogenesis) occurs in response to ligation of lymphatic vessels during lymph node retrieval. Lymphangiogenesis and lymphatic hypertension have been demonstrated experimentally in cases of lymphatic drainage obstruction. And, in response to lymphatic hypertension, lymphovenous shunts open and provide alternative lymphatic pathways when the main ones are obstructed. These mechanisms represent an adaptive response to lymphatic hypertension but are not sufficient to restore normal flow parameters. Furthermore, chronic obstruction to lymph flow progressively leads to reduced lymphatic contractility, lymphatic thrombosis, and fibrotic changes to different degrees according to variable constitutional predisposition [79,80].
Surgical Preventive Procedures
Recent advances in the treatment of breast cancer, specifically concerning prevention of lymphatic complications following SLNB and AD led to the proposal of a new technique to primarily prevent lymphedema by microsurgical lymphatic-venous anastomoses. The ARM technique allows identification of arm lymphatics and lymph nodes, which can then be preserved even though there is still the risk to leave undetected metastatic disease in the axilla. But it is almost impossible to preserve efferent lymphatics from the blue nodes because they join the common axillary nodal basin draining the breast. Thus, not preserving afferent lymphatics makes preservation of arm lymphatic flow practically impossible. So, based on our wide experience in the treatment of lymphedema by microsurgical lymphatic-venous anastomoses (LVA), we proposed performing LVA immediately after finishing nodal axillary excision. The surgical technique for patients with operable breast cancer requiring AD consisted of carrying out LVA between arm lymphatics identified by injecting blue dye in the arm and an axillary vein branch simultaneously (lymphatic microsurgical preventive healing approach—LYMPHA) [81]. It is almost always possible to find blue lymphatics and also to find a vein branch long enough to be connected to arm lymphatics which are usually located very laterally.
Patients are followed up both clinically by volumetric assessment and by lymphangioscintigraphy performed before surgery and after 18 months. Blue nodes in relation to lymphatic arm drainage can be identified in almost all patients after blue dye injection at the arm. All blue nodes must be resected and 2–4 main afferent lymphatics from the arm can be prepared and used for anastomoses. Lymphatics are introduced inside the vein cut-end by a U-shaped stitch. A few stitches are added to fix the lymphatic adventitia to the vein wall. The operation takes only 15–20 min on average, since both lymphatics and the vein are prepared during nodal dissection. LVA proved not only to prevent lymphedema but also to reduce early lymphatic complications (i.e., lymphorrhea, lymphocele) because of the reduced regional intralymphatic pressure. Drain tubes can be removed after about 7–10 days at the utmost. Postoperative lymphangioscintigraphy documents demonstration of patency of microvascular anastomoses after over 1 year and a half from operation.
Final Considerations
Disruption of the axillary nodes and closure of arm lymphatics explains the high risk of early and late lymphatic complications after AD, especially the most serious complication, that is, arm lymphedema. The use of the blue dye and of LVA helps to prevent secondary arm lymphedema while maintaining the optimal oncological control. LYMPHA, therefore, represents a rational approach to the prevention of lymphedema and reduction of other lymphatic complications after axillary surgery in the therapy of breast cancer.
ANTI-LYMPHANGIOGENESIS
A large number of growth factors have been discovered to be able to promote lymphangiogenesis by activating their cognate receptors on the surface of LECs. The most well studied of these are members of the VEGF/VEGFR family, including the archetypal regulator of lymphangiogenesis VEGFR-3 and its ligands VEGF-C and VEGF-C, and also VEGF-A and its receptor VEGFR-2 [82]. Many of these growth factors are produced and released by tumors, and there is a large body of literature that shows that a wide variety of human tumors exhibit augmented expression of these factors [83]. In animal models, increased expression of such pro-lymphangiogenic factors in the tumor can induce lymphangiogenesis within and/or at the periphery of tumors. Conversely, inhibition of the activity of these factors reduces the lymphatic vessel density in and/or in the vicinity of the tumor [84].
It is now well established that tumor-induced lymphangiogenesis is a mechanism that can promote metastasis to RLNs. It is thought to act by promoting the entry of tumor cells into the lymphatic vasculature, and/or by increasing interstitial fluid flow to the draining lymph nodes, thereby facilitating their access to and entry into the regional nodes. VEGF-A, -C and -D, COX-2, and PDGF-BB [48,85–87] have all been functionally implicated in the promotion of tumor-associated lymphangiogenesis and the metastasis of experimental tumors. Furthermore, in the majority of animal models published to date, tumor-induced lymphangiogenesis induces metastasis not only to lymph nodes but also to other organs, although the mechanism behind this observation remains to be determined [84]. Concordantly, many studies on tumor material from human cancer patients have correlated expression of pro-lymphangiogenic factors and lymphatic vessel density in and around primary tumors with lymph node metastasis formation and poor prognosis [83].
Although pro-lymphangiogenic factors are produced in the primary tumor environment, there is mounting evidence to suggest that they may act systemically in addition to their local effects on the tumor-associated lymphatics (Fig. 7). For example, several studies have demonstrated that tumors can remotely induce lymphangiogenesis in their draining lymph nodes, even before tumor cells colonize the nodes [88–92]. Furthermore, a number of studies have reported increased levels of pro-lymphangiogenic factors in the blood of cancer patients that correlates with poor prognosis. For example, increased levels of VEGF-C in the blood of gastric and papillary thyroid cancer patients is associated with reduced survival [93,94]. Thus, tumor-derived prolymphangiogenesis factors have in principle the potential to act even more distantly. VEGF-A, for example, is able to mobilize bone marrow precursor cells at concentrations typically found in the blood of cancer patients [95]. These or other effects on organs in which tumors ultimately metastasize may contribute to the observed increase in distant organ metastasis in animal models in response to prolymphangiogenic factor production by the primary tumor, as well as to the poor prognosis reported to be connected with pro-lymphangiogenic factor expression in human primary tumors. However, this remains to be investigated experimentally.
Fig. 7.
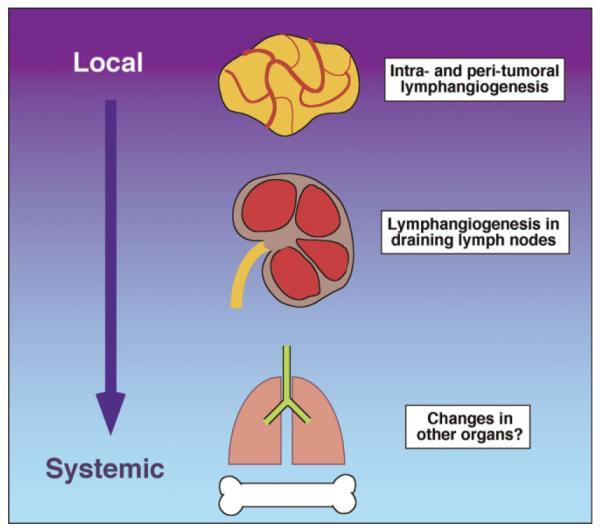
Schematic diagram showing the different levels at which prolymphangiogenesis factors produced by tumors can potentially contribute to metastasis. Highest levels of these factors, represented by the darkest background color, are found closest to the tumor that produces them. However, factors released systemically also have the potential to act distantly, as indicated in the diagram. [Color figure can be viewed in the online issue, available at wileyonlinelibrary.com.]
Regardless of the mechanism of action, there is much interest in inhibiting the activity of tumor-induced pro-lymphangiogenesis factors as a means of suppressing the formation of metastases. A variety of lymphangiogenesis inhibitors have been defined and developed. In our own work, we have reported a novel indoline [MAZ51] that is able to inhibit activation of VEGFR-3 in response to ligand [96,97]. We have also shown that hyperforin, a natural product derived from St. John’s Wort, has potent anti-tumor, anti-angiogenesis, and anti-lymphangiogenesis properties [98–100]. A synthetic hyperforin derivative we have developed with enhanced pharmacological properties has even more potent effects [100,101]. At low concentrations of both substances, LECs enter cell-cycle arrest, whereas higher concentrations induce apoptosis [100]. This translates into an inhibition of lymphangiogenesis in both a novel ex vivo lymphangiogenesis assay [102] as well as in the tumor context in vivo [100]. Other natural compounds that we have found to potentially inhibit lymphangiogenesis through inhibiting the ligand-induced activation of VEGFR-3 include anthocyanidin derivatives found widely in fruits and vegetables [103,104].
EPILOGUE
Lymphatic metastases represent the first site of tumor dissemination in many solid tumors. The presence of lymphatic metastasis is often an important predictor of distant recurrence and adverse survival compared to patients with similar primary tumor prognostic features but without lymphatic metastasis. Despite the well-established prognostic association of lymphatic metastasis and adverse outcome, strategies to prevent formation or effectively treat subsequent clinically evident distant metastasis—the major cause of death in most cancer patients who succumb to their disease—for many of these “high-risk” patients, remains an all too elusive goal. It is likely that several factors account for the failure to adequately treat metastases. It is well appreciated that neoplasms are biologically heterogeneous and contain subpopulations of cells with different angiogenic, invasive, and metastatic properties. Moreover, the metastatic process selects for a small subpopulation of cells that pre-exist within a parental neoplasm. Perhaps the most important obstacle to effective “anti-metastasis” therapy is the inability to effectively control the often-redundant aberrant signal transduction pathways in tumor cells and, in particular, the multiple interactions between metastatic cells and homeostatic mechanisms that the tumor cells usurp [105]. A better understanding of the molecular events that lead to metastasis and of the complex interactions between metastatic cells and host factors is essential for the design of more effective cancer therapies. To produce a metastasis, tumor cells must complete a series of sequential, interrelated steps. These include growth; neovascularization and lymphangiogenesis (development of new lymphatic vessels); invasion of the host stroma, blood vessels, and lymphatic system; survival in the circulation; arrest in small blood vessels; extravasation (migration out of blood vessels) into the parenchyma of organs; and continuous proliferation, which depends on establishing an adequate blood supply (hemangiogenesis) [106,107].
The clinical importance of the lymphatic system has been recognized for centuries [107]; however, until recently, its involvement in the metastatic cascade has taken a back seat to the longstanding and “explosive” interest surrounding the formation of tumor-associated blood vessels. As highlighted in this chapter, relatively recent work by a multitude of investigators is beginning to unravel the fundamental biological mechanisms of lymphangiogenesis and lymphatic metastases.
Although the controversy of whether cancer cells in a primary tumor are transported to RLNs through intratumor or peritumoral lymphatic vessels remains unresolved, significant advances in our understanding of the lymphatic system, including (1) the ultrastructure, development, biology, and pathobiology of the lymphatics; (2) lymphatic pathways in breast cancer, melanoma, and other solid tumors; (3) new lymphatic imaging agents based on improved understanding of lymphatic vessel biology (e.g., Lymphoseek); and (4) an appreciation of potential therapeutic targets in lymphangio/hemangiogenesis have together created a “mini-revolution” in our quest for a better understanding of this important element of the global metastatic cascade [106]. Techniques such as intradermal administration of vital blue dye and radiolabeled colloid at the periphery of primary tumors that reliably identify the specific lymph nodes (i.e., the SLNs) that receive afferent lymphatic drainage from the primary tumor site are now used in widespread clinical practice for patients with breast cancer, melanoma, and other solid tumors. These SLNs are the most likely to contain tumor metastases, which are often harbingers of future tumor development at sites distant from the lymph node [108].
Over the past several years, various members of the VEGF family (including VEGF-A, VEGF-C, and VEGF-D), as well as other growth factors, have been implicated as inducing lymphangiogenesis—that is, the formation of new lymphatic vessels. At least conceptually, blockade of lymphangiogenic factors is of potential therapeutic interest for treating lymphatic tumor metastases, but there is still much to learn, since the specific contributions to promote the lymphatic metastatic cascade are not fully resolved.
Targeting lymphangiogenic factors to prevent lymphatic metastasis should be approached with caution because in many patients with solid tumors, occult metastasis may have already occurred by the time of diagnosis [106]. Moreover, fluid homeostasis requires an intact lymphatic system; functioning lymphangiogenic factors very likely play at least some role. From a clinical perspective, surgical excision of tumor-containing lymph nodes, a mainstay of treatment for patients with regional nodal disease can further upset this dynamic biologic equilibrium. The subsequent disruption of lymphatic drainage may result in the accumulation of fluid in the affected extremity, that is, lymphedema. Interestingly, in a mouse model of congenital lymphedema caused by an inactivating mutation in VEGFR-3, symptoms were abrogated by treatment with VEGF-C gene therapy, which induced lymphangiogenesis. In addition, inhibition of lymphangiogenesis in transgenic mice expressing a soluble form of the same receptor produced severe lymphedema. These studies provide a conceptual framework for a potential concern that systemic targeting of lymphangiogenic molecules could increase the risk of lymphedema in patients. Therefore, clinical trials involving targeted therapy that may disrupt this delicate balance (i.e., “anti-lymphangiogenic” therapy) should include methods to identify the potential increased risk of undesirable side effects such as lymphedema. Similarly, any trial designed to assess the efficacy of a systemic treatment for reversing lymphedema in cancer patients must account for the theoretic possibility that lymphatic metastasis may be induced. A better understanding of lymphangiogenesis remains a prerequisite for developing effective targeted therapy for treating cancer patients [106].
ACKNOWLEDGMENTS
Dr. Witte and Dr. Dellinger acknowledge the contributions of Michael Bernas, Kim Jones, and many colleagues and collaborators in the International Society of Lymphology. Dr. McDonald would like to acknowledge the contributions of Peter Baluk, Jonas Fuxe, Barbara Sennino, and Hiroya Hashizume to the research described. Dr. Witte acknowledges grants from Arizona Disease Control Research Commission Contracts 8277-000000-1-1-AT-6625, ZB-7492, I-103, and 9-056; Better Than Ever Program (BTE FY-10); NIH Science Education Partnership Award (1R25RR022720); and Short-Term Institutional Research Training Program (#T35HL07479). Dr. Dellinger acknowledges support for postdoctoral fellowship from the Department of Defense Breast Cancer Research Program (BC087509). Donald McDonald would like to acknowledge research funding from National Institutes of Health grants HL24136, HL59157, and HL96511 from the National Heart, Lung, and Blood Institute and grant CA82923 from the National Cancer Institute, and funding from AngelWorks Foundation. Dr. Nathanson’s work was supported by Nathanson-Rands Chair in Breast Cancer Research. Dr. Sleeman’s work was supported by grants from the Deutsche Forschungsgemeinschaft under the auspices of the Schwerpunkt Program SPP1190 (Tumor-vessel interface), and from the European Union under the auspices of the FP7 collaborative project TuMIC, contract no. HEALTH-F2-2008-201662. Dr. Gershenwald’s work was supported by an American Cancer Society Research Scholar Grant (RSG-05-250-01-CSM).
Grant sponsor: Arizona Disease Control Research Commission Contracts; Grant numbers: 8277-000000-1-1-AT-6625, ZB-7492, I-103, 9-056.
REFERENCES
- 1.Witte MH, Way DL, Witte CL, et al. Lymphangiogenesis: Mechanisms, significance and clinical implications. In: Goldberg ID, Rosen EM, editors. Regulation of angiogenesis. Birkhauser Verlag; Basel, Switzerland: 1997. pp. 65–112. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 3.Joukov V, Pajusola K, Kaipanen A, et al. A novel vascular endothelial growth factor, VEGFC, is a ligand for the Flt 4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:1951. [PMC free article] [PubMed] [Google Scholar]; EMBO J. 1996;15:290–298. Erratum for. [PMC free article] [PubMed] [Google Scholar]
- 4.Karkkainen MJ, Ferrell RE, Lawrence EC, et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet. 2000;25:153–159. doi: 10.1038/75997. [DOI] [PubMed] [Google Scholar]
- 5.Fang JM, Dagenais SL, Erickson RP, et al. Mutations in Foxc2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am J Hum Genet. 2000;67:1382–1388. doi: 10.1086/316915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irrthum A, Devriendt K, Chitayat D, et al. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis–lymphedema–telangiectasia. Am J Hum Genet. 2003;72:1470–1478. doi: 10.1086/375614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dellinger M, Bernas M, Witte M. Lymphatic biology and pathobiology. In: Dieter R, Dieter RA Jr., Dieter RA III, editors. Venous and lymphatic disorders. McGraw Hill; New York, NY: Chapter 3. (in press) [Google Scholar]
- 8.Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 9.Stacker S, Caesar C, Baldwin M, et al. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 10.Alders M, Hogan BM, Gjini E, et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat Genet. 2009;41:1272–1274. doi: 10.1038/ng.484. [DOI] [PubMed] [Google Scholar]
- 11.Connell F, Kalidas K, Ostergaard P, et al. Lymphoedema Consortium. Linkage and sequence analysis indicate that CCBE1 is mutated in recessively inherited generalised lymphatic dysplasia. Hum Genet. 2010;127:231–241. doi: 10.1007/s00439-009-0766-y. [DOI] [PubMed] [Google Scholar]; Hum Genet. 2010;127:243. Erratum appears in. [Google Scholar]
- 12.Ferrell RE, Baty CJ, Kimak MA, et al. GJC2 missense mutations cause human lymphedema. Am J Hum Genet. 2010;86:943–948. doi: 10.1016/j.ajhg.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witte MH, Dellinger M, Bernas MJ, et al. Molecular lymphology and genetics of lymphedema-angiodysplasia syndromes. In: Földi M, Földi E, editors. Textbook of lymphology. 3rd edition. Urban & Fischer Verlag; München, Germany: 2010. Chapter 16. (in press) [Google Scholar]
- 14.Brown P. Unlocking the drains. Nature. 2005;436:456–458. doi: 10.1038/436456a. [DOI] [PubMed] [Google Scholar]
- 15.Witte MH, Ohkuma M, Andrade M, et al. Nature’s historic gap: The 20th century of lymphology. Lymphology. 2005;38:157–158. [PubMed] [Google Scholar]
- 16.Sabin FR. On the origin and development of the lymphatic system from the veins and the development of the lymph hearts and the thoracic duct in the pig. Am J Anat. 1902;1:367–389. [Google Scholar]
- 17.Kampmeier OF. Evolution and comparative morphology of the lymphatic system. Charles C. Thomas; Springfield, IL: 1969. [Google Scholar]
- 18.XI-XXII International Congress of Lymphology Proceedings. Lymphology (suppl) 1987–2009:27–42. [Google Scholar]
- 19.Witte M, Witte C. On tumor (and other) lymphangiogenesis. Lymphology. 1997;30:1–2. [PubMed] [Google Scholar]
- 20.Witte MH, Jones K, Wilting J, et al. Structure–function relationships in the lymphatic system and implications for cancer biology. Cancer Metastasis Rev. 2006;25:159–184. doi: 10.1007/s10555-006-8496-2. [DOI] [PubMed] [Google Scholar]
- 21.Fisher B, Fisher ER. The interrelationship of hematogenous and lymphatic tumor cell dissemination. Surg Gynecol Obstet. 1966;122:791–798. [PubMed] [Google Scholar]
- 22.Azzali G. Tumor cell transendothelial passage in the absorbing lymphatic vessel of transgenic adenocarcinoma mouse prostate. Am J Pathol. 2007;170:334–346. doi: 10.2353/ajpath.2007.060447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witte MH, Bernas MJ, Martin CP, et al. Wilting J, editor. Lymphangiogenesis and lymphangiodysplasia: From molecular to clinical lymphology. The biology of lymphangio-genesis. Microsc Res Tech. 2001;55:122–145. doi: 10.1002/jemt.1163. [DOI] [PubMed] [Google Scholar]
- 24.Sabin FR. On the development of the superficial lymphatics in the skin of the pig. Am J Anat. 1904;3:183–195. [Google Scholar]
- 25.Sabin FR. The lymphatic system in human embryos, with a consideration of the morphology of the system as a whole. Am J Anat. 1909;9:43–91. [Google Scholar]
- 26.Kampmeier OF. The development of the thoracic duct in the pig. Am J Anat. 1912;13:401–475. [Google Scholar]
- 27.McClure CFW. A few remarks relative to Mr. Kampmeier’s paper on “The value of the injection method in the study of lymphatic development.”. Anat Rec. 1912;6:233–246. [Google Scholar]
- 28.Kampmeier OF. The value of the injection method in the study of lymphatic development. Anat Rec. 1912;6:223–232. [Google Scholar]
- 29.Sabin FR. A critical study of the evidence presented in several recent articles on the development of the lymphatic system. Anat Rec. 1911;5:417–446. [Google Scholar]
- 30.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 31.Wigle JT, Harvey N, Detmar M, et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francois M, Caprini A, Hosking B, et al. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 33.Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 34.Wilting J, Aref Y, Huang R, et al. Dual origin of avian lymphatics. Dev Biol. 2006;292:165–173. doi: 10.1016/j.ydbio.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 35.Ny A, Koch M, Schneider M, et al. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nat Med. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan RS, Dillard ME, Lagutin OV, et al. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kerjaschki D, Huttary N, Raab I, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 38.Jiang S, Bailey A, Goldman DC, et al. Hematopoietic stem cells contribute to lymphatic endothelium. PLoS ONE. 2008;3:e3812. doi: 10.1371/journal.pone.0003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zumsteg A, Baeriswyl V, Imaizumi N, et al. Myeloid cells contribute to tumor lymphangiogenesis. PLoS ONE. 2009;4:e7067. doi: 10.1371/journal.pone.0007067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark ER. Observations on living growing lymphatics in the tail of frog larva. Anat Rec. 1909;3:183–198. [Google Scholar]
- 41.Clark ER, Clark EL. Observations on the new growth of lymphatic vessels as seen in transparent chambers introduced into the rabbit’s ear. Am J Anat. 1932;51:49–87. [Google Scholar]
- 42.Oh SJ, Jeltsch MM, Birkenhäger R, et al. VEGF and VEGF-C: Specific induction of angiogenesis and lymphangiogenesis in the differentiated avian chorioallantoic membrane. Dev Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- 43.Jeltsch M, Kaipainen A, Joukov V, et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 44.Dellinger MT, Hunter RJ, Bernas MJ, et al. Chy-3 mice are Vegfc haploinsufficient and exhibit defective dermal superficial to deep lymphatic transition and dermal lymphatic hypoplasia. Dev Dyn. 2007;236:2346–2355. doi: 10.1002/dvdy.21208. [DOI] [PubMed] [Google Scholar]
- 45.Karkkainen MJ, Saaristo A, Jussila L, et al. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bjorndahl M, Cao R, Nissen LJ, et al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao R, Bjorndahl MA, Religa P, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic meta-stasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 48.Kajiya K, Hirakawa S, Ma B, et al. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J. 2005;24:2885–2895. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kubo H, Cao R, Brakenhielm E, et al. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci USA. 2002;99:8868–8873. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morisada T, Oike Y, Yamada Y, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–4656. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 51.Tammela T, Saaristo A, Lohela M, et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642–4648. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 52.Veikkola T, Jussila L, Mäkinen T, et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 2001;20:1223–1231. doi: 10.1093/emboj/20.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliver G, Srinivasan RS. Endothelial cell plasticity: How to become and remain a lymphatic endothelial cell. Development. 2010;137:363–372. doi: 10.1242/dev.035360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kriederman BM, Myloyde TL, Witte MH, et al. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome. Hum Mol Genet. 2003;12:1179–1185. doi: 10.1093/hmg/ddg123. [DOI] [PubMed] [Google Scholar]
- 55.Petrova TV, Karpanen T, Norrmén C, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 56.Norrmén C, Ivanov KI, Cheng J, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol. 2009;185:439–457. doi: 10.1083/jcb.200901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bazigou E, Xie S, Chen C, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kampmeier OF. Further observations on the numerical variability, position, function and fate of the valves in the human thoracic duct. Anat Rec. 1928;38:225–231. [Google Scholar]
- 59.Mäkinen T, Adams RH, Bailey J, et al. PDZ interaction site in ephrinb2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dellinger M, Hunter R, Bernas M, et al. Defective remodeling and maturation of the lymphatic vasculature in angiopoietin-2 deficient mice. Dev Biol. 2008;319:309–320. doi: 10.1016/j.ydbio.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baluk P, Fuxe J, Hashizume H, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dejana E, Orsenigo F, Molendini C, et al. Organization and signaling of endothelial cell-to-cell junctions in various regions of the blood and lymphatic vascular trees. Cell Tissue Res. 2009;335:17–25. doi: 10.1007/s00441-008-0694-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fidler IJ, Baker CH, Yokoi K, et al. Targeting the tumor microenvironment for treatment of metastasis. In: Figg WD, Folkman J, editors. Angiogenesis: An integrative approach from science to medicine. Springer; New York: 2008. pp. 259–270. [Google Scholar]
- 64.Liotta LA, Kleinerman J, Saidel GM. Quantitative relationships of intravascular tumor cells, tumor vessels and pulmonary metastases following tumor implantation. Cancer Res. 1974;34:997–1004. [PubMed] [Google Scholar]
- 65.Halsted W. The results of operations for the cure of carcinoma of the breast. Ann Surg. 1907;46:1–9. doi: 10.1097/00000658-190707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hellman S. Karnovsky Memorial Lecture. Natural history of small breast cancers. J Clin Oncol. 1994;12:2229–2234. doi: 10.1200/JCO.1994.12.10.2229. [DOI] [PubMed] [Google Scholar]
- 67.Nathanson SD. Insights into the mechanisms of lymph node metastasis. Cancer. 2003;98:413–423. doi: 10.1002/cncr.11464. [DOI] [PubMed] [Google Scholar]
- 68.Hoshida T, Isaka N, Hagendoorn J, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: Therapeutic implications. Cancer Res. 2006;66:8065–8075. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- 69.Mohammed RAA, Martin SG, Gill MS, et al. Improved methods of detection of lymphovascular invasion demonstrate that it is the predominant method of vascular invasion in breast cancer and has important clinical consequences. Am J Surg Pathol. 2007;31:1825–1833. doi: 10.1097/PAS.0b013e31806841f6. [DOI] [PubMed] [Google Scholar]
- 70.Nathanson SD, Westrick P, Anaya P, et al. Relationship of spontaneous regional lymph node metastases to dose of local irradiation of primary B16 melanomas. Cancer Res. 1989;49:4412–4416. [PubMed] [Google Scholar]
- 71.Reintgen D, Cruse CW, Wells K. The orderly progression of melanoma nodal metastases. Ann Surg. 1994;220:759–767. doi: 10.1097/00000658-199412000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nathanson SD, Kwon D, Kapke A, et al. The role of lymph node metastasis in the systemic dissemination of breast cancer. Ann Surg Oncol. 2009;16:3396–3405. doi: 10.1245/s10434-009-0659-2. [DOI] [PubMed] [Google Scholar]
- 73.Nathanson SD, Nelson LT. Interstitial fluid pressure in breast cancer, benign breast conditions and breast parenchyma. Ann Surg Oncol. 1994;1:333–338. doi: 10.1007/BF03187139. [DOI] [PubMed] [Google Scholar]
- 74.Padera TP, Stoll BR, Tooredman JB, et al. Pathology: Cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 75.Thompson M, Korourian S, Henry-Tillman R, et al. Axillary reverse mapping (ARM): A new concept to identify and enhance lymphatic preservation. Ann Surg Oncol. 2007;14:1890–1895. doi: 10.1245/s10434-007-9412-x. [DOI] [PubMed] [Google Scholar]
- 76.Sakorafasa GH, Perosa G, Cataliotti L, et al. Lymphedema following axillary lymph node dissection for breast cancer. Surg Oncol. 2006;15:153–165. doi: 10.1016/j.suronc.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Nos C, Lesieur B, Clough KB, et al. Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary dissection. Ann Surg Oncol. 2007;14:2490–2496. doi: 10.1245/s10434-007-9450-4. [DOI] [PubMed] [Google Scholar]
- 78.Ponzone R, Mininanni P, Cassina E, et al. Axillary reverse mapping in breast cancer: Can we spare what we find? Ann Surg Oncol. 2007;15:390–391. doi: 10.1245/s10434-007-9663-6. [DOI] [PubMed] [Google Scholar]
- 79.Jila A, Kim H, Nguyen VP, et al. Lymphangiogenesis following obstruction of large postnodal lymphatics in sheep. Microvasc Res. 2007;73:214–223. doi: 10.1016/j.mvr.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 80.Modi S, Stanton AWB, Svensson WE, et al. Human lymphatic pumping measured in healthy and lymphoedematous arms by lymphatic congestion lymphoscintigraphy. J Physiol. 2007;583:271–285. doi: 10.1113/jphysiol.2007.130401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Boccardo F, Casabona F, De Cian F, et al. Lymphedema microsurgical preventive healing approach: A new technique for primary prevention of arm lymphedema after mastectomy. Ann Surg Oncol. 2009;16:703–708. doi: 10.1245/s10434-008-0270-y. [DOI] [PubMed] [Google Scholar]
- 82.Karpanen T, Alitalo K. Molecular biology and pathology of lymphangiogenesis. Annu Rev Pathol. 2008;3:367–397. doi: 10.1146/annurev.pathmechdis.3.121806.151515. [DOI] [PubMed] [Google Scholar]
- 83.Sleeman J, Thiele W. Tumor metastasis and the lymphatic vasculature. Int J Cancer. 2009;125:2747–2756. doi: 10.1002/ijc.24702. [DOI] [PubMed] [Google Scholar]
- 84.Sleeman J, Schmid A, Thiele W. Tumor lymphatics. Semin Cancer Biol. 2009;19:285–297. doi: 10.1016/j.semcancer.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 85.Krishnan J, Kirkin V, Steffen A, et al. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713–722. [PubMed] [Google Scholar]
- 86.Iwata C, Kano MR, Komuro A, et al. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res. 2007;67:10181–10189. doi: 10.1158/0008-5472.CAN-07-2366. [DOI] [PubMed] [Google Scholar]
- 87.Shibata MA, Morimoto J, Shibata E, et al. Combination therapy with short interfering RNA vectors against VEGF-C and VEGF-A suppresses lymph node and lung metastasis in a mouse immunocompetent mammary cancer model. Cancer Gene Ther. 2008;15:776–786. doi: 10.1038/cgt.2008.43. [DOI] [PubMed] [Google Scholar]
- 88.Hirakawa S, Kodama S, Kunstfeld R, et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirakawa S, Brown LF, Kodama S, et al. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109:1010–1017. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ruddell A, Kelly-Spratt KS, Furuya M, et al. p19/Arf and p53 suppress sentinel lymph node lymphangiogenesis and carcinoma metastasis. Oncogene. 2008;27:3145–3155. doi: 10.1038/sj.onc.1210973. [DOI] [PubMed] [Google Scholar]
- 92.Van den Eynden GG, Van der Auwera I, Van Laere SJ, et al. Induction of lymphangiogenesis in and around axillary lymph node metastases of patients with breast cancer. Br J Cancer. 2006;95:1362–1366. doi: 10.1038/sj.bjc.6603443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang TB, Deng MH, Qiu WS, et al. Association of serum vascular endothelial growth factor-C and lymphatic vessel density with lymph node metastasis and prognosis of patients with gastric cancer. World J Gastroenterol. 2007;13:1794–1797. doi: 10.3748/wjg.v13.i12.1794. discussion 1797–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu XM, Lo CY, Lam AK, et al. Serum vascular endothelial growth factor C correlates with lymph node metastases and high-risk tumor profiles in papillary thyroid carcinoma. Ann Surg. 2008;247:483–489. doi: 10.1097/SLA.0b013e31815fa447. [DOI] [PubMed] [Google Scholar]
- 95.Spring H, Schuler T, Arnold B, et al. Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci USA. 2005;102:18111–18116. doi: 10.1073/pnas.0507158102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kirkin V, Mazitschek R, Krishnan J, et al. Characterization of indolinones which preferentially inhibit VEGF-C- and VEGF-D-induced activation of VEGFR-3 rather than VEGFR-2. Eur J Biochem. 2001;268:5530–5540. doi: 10.1046/j.1432-1033.2001.02476.x. [DOI] [PubMed] [Google Scholar]
- 97.Kirkin V, Thiele W, Baumann P, et al. MAZ51, an indolinone that inhibits endothelial cell and tumor cell growth in vitro, suppresses tumor growth in vivo. Int J Cancer. 2004;112:986–993. doi: 10.1002/ijc.20509. [DOI] [PubMed] [Google Scholar]
- 98.Schempp CM, Kirkin V, Simon-Haarhaus B, et al. Inhibition of tumour cell growth by hyperforin, a novel anticancer drug from St. John’s wort that acts by induction of apoptosis. Oncogene. 2002;21:1242–1250. doi: 10.1038/sj.onc.1205190. [DOI] [PubMed] [Google Scholar]
- 99.Schempp CM, Kiss J, Kirkin V, et al. Hyperforin acts as an angiogenesis inhibitor. Planta Med. 2005;71:999–1004. doi: 10.1055/s-2005-871303. [DOI] [PubMed] [Google Scholar]
- 100.Rothley M, Schmid A, Thiele W, et al. Hyperforin and aristoforin inhibit lymphatic endothelial cell proliferation in vitro and suppress tumor-induced lymphangiogenesis in vivo. Int J Cancer. 2009;125:34–42. doi: 10.1002/ijc.24295. [DOI] [PubMed] [Google Scholar]
- 101.Gartner M, Muller T, Simon JC, et al. Aristoforin, a novel stable derivative of hyperforin, is a potent anticancer agent. Chem-BioChem. 2005;6:171–177. doi: 10.1002/cbic.200400195. [DOI] [PubMed] [Google Scholar]
- 102.Bruyère F, Melen-Lamalle L, Blacher S, et al. Modeling lymphangiogenesis in a three-dimensional culture system. Nat Methods. 2008;5:431–437. doi: 10.1038/nmeth.1205. [DOI] [PubMed] [Google Scholar]
- 103.Teller N, Thiele W, Boettler U, et al. Delphinidin inhibits a broad spectrum of receptor tyrosine kinases of the ErbB and VEGFR family. Mol Nutr Food Res. 2009;53:1075–1083. doi: 10.1002/mnfr.200800524. [DOI] [PubMed] [Google Scholar]
- 104.Teller N, Thiele W, Marczylo TH, et al. Suppression of the kinase activity of receptor tyrosine kinases by anthocyanin-rich mixtures extracted from bilberries and grapes. J Agric Food Chem. 2009;57:3094–3101. doi: 10.1021/jf803094a. [DOI] [PubMed] [Google Scholar]
- 105.Fidler J. Clinical oncology. 2nd edition Churchill Livingstone; New York: 2000. pp. 29–53. [Google Scholar]
- 106.Gershenwald JE, Fidler IJ. Cancer. Targeting lymphatic meta-stasis. Science. 2002;296:1811–1812. doi: 10.1126/science.10731318. [DOI] [PubMed] [Google Scholar]
- 107.Foster RS., Jr. General anatomy of the lymphatic system. Surg Oncol Clin N Am. 1996;5:1–13. [PubMed] [Google Scholar]
- 108.Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: The prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17:976–983. doi: 10.1200/JCO.1999.17.3.976. [DOI] [PubMed] [Google Scholar]