Abstract
Inhibition of platelet derived growth factor (PDGF) can increase the efficacy of other cancer therapeutics, but the cellular mechanism is incompletely understood. We examined the cellular effects on tumor vasculature of a novel DNA oligonucleotide aptamer (AX102) that selectively binds PDGF-B. Treatment with AX102 led to progressive reduction of pericytes, identified by PDGF receptor β, NG2, desmin, or α-smooth muscle actin immunoreactivity, in Lewis lung carcinomas. The decrease ranged from 35% at 2 days, 63% at 7 days, to 85% at 28 days. Most tumor vessels that lacked pericytes at 7 days subsequently regressed. Overall tumor vascularity decreased 79% over 28 days, without a corresponding decrease in tumor size. Regression of pericytes and endothelial cells led to empty basement membrane sleeves, which were visible at 7 days, but only 54% remained at 28 days. PDGF-B inhibition had a less pronounced effect on pancreatic islet tumors in RIP-Tag2 transgenic mice, where pericytes decreased 47%, vascularity decreased 38%, and basement membrane sleeves decreased 21% over 28 days. Taken together, these findings show that inhibition of PDGF-B signaling can lead to regression of tumor vessels, but the magnitude is tumor specific and does not necessarily retard tumor growth. Loss of pericytes in tumors is an expected direct consequence of PDGF-B blockade, but reduced tumor vascularity is likely to be secondary to pericyte regression.
Introduction
Endothelial cells secrete platelet-derived growth factor B (PDGF-B) and pericytes (mural cells) express PDGF receptor β (PDGFR-β). This relationship is important for recruitment of pericytes to blood vessels during development (1–3). Mice lacking PDGF-B or PDGFR-β have blood vessels with few pericytes, develop an abnormal vasculature, and die in late gestation (4–6). Pericytes have important roles in maintaining the microvasculature, including contributions to blood vessel maturation and stabilization, normal endothelial cell function, and blood flow regulation (5, 7–10). PDGF-B is also integral to pericyte and endothelial cell interactions in tumors. Tumors engineered to overexpress PDGF-B have increased pericyte density associated with tumor vessels (11, 12). Moreover, inhibition of PDGFR-β signaling can loosen, detach, or even eliminate pericytes from tumor vessels (11, 13–17).
In addition to the direct effects that inhibition of PDGFR-β signaling has on pericytes, there is an intricate association between pericytes and endothelial cells. Previous reports describe paracrine regulation of growth factors such as PDGF-B from endothelial cells and vascular endothelial growth factor (VEGF) from pericytes (15, 18). Thus, regression of pericytes may affect vessels. It is still unclear whether pericyte presence protects tumor vessels from regression or whether removal of pericytes makes tumor vessels vulnerable to regression.
The role of PDGF inhibition on tumor growth is incompletely understood. PDGF inhibition alone has no effect on some tumor models (14, 19), whereas it reduces tumor growth in others (20). Nonetheless, PDGF inhibitors enhance the effects of antiangiogenic inhibitors (14) and chemotherapeutics on tumor growth (19, 21).
Most of the agents used to study PDGFR-β inhibition are nonspecific. Thus, questions remain about the direct therapeutic benefit that is attributable to targeting PDGF-B in tumors (22). Studies using PDGF-B retention motif–deficient mice (11), PDGF-B aptamer (19), or PDGFR-β antibodies (16) showed pericyte reduction, enlargement of tumor vessels, decreased tumor interstitial pressure, and increased endothelial cell apoptosis, but no apparent reduction in tumor vascularity was observed (11, 16, 19).
The present study sought to use a novel and specific PDGF-B inhibitor to obtain a more complete understanding of the cellular effects of selective PDGF-B blockade. DNA aptamer AX102, which blocks the action of PDGF-B, was used in two murine tumor models, spontaneous islet tumors in RIP-Tag2 transgenic mice (RIP-Tag2 tumors) and Lewis lung carcinoma (LLC tumors). Aptamers are backbone-modified ssDNA or RNA oligonucleotides that form well-defined three-dimensional shapes and bind to target proteins with high affinity and specificity (23). AX102 is a modified version of the DNA aptamer NX1975/ARC126 (19, 24) that is highly selective for PDGF-B. Experiments focused on the effects of AX102 on pericytes, endothelial cells, and tumor vascular integrity. Our studies revealed that inhibition of PDGF-B signaling by AX102 rapidly reduced pericyte coverage of tumor vessels. Removal of pericytes subsequently caused regression of vessels. Basement membrane sleeves also disappeared. Tumor vessels that survived 28 days of treatment had tight pericyte coverage and a normalized phenotype.
Materials and Methods
Aptamer synthesis
AX102 is a 34-nucleotide aptamer modified on the 5′ terminus with a 40-kDa polyethylene glycol moiety to reduce renal filtration and prolong plasma half-life. The 3′ terminus was modified with an inverted-dT cap. The nucleotide core sequence of AX102 is 5′-NH2-dC-dC-dC-dA-dG-dG-dC-T-dA-dC-mG-HEG-dC-dG-T-dA-mG-dA-mG-dC-dA-mU-mC-mA-HEG-T-dG-dA-T-mC-dC-T-mG-mG-mG-3′dT-3′. AX104 is an inactive variant with the following sequence: 5′-dC-dA-dG-fC-mG-{fU}-dA-fC-mG-HEG-dC-dG-T-dA-dC-dC-mG-dA-T-fU-fC-mA-HEG-T-dG-dA-dA-dG-fC-fU-mG-3′dT-3′. Abbreviations for the aptamer core sequence represent backbone modifications directed to the 2′-ribose position of the nucleoside such that dC, dA, dG, and dT signify 2′-deoxycytidine, 2′-deoxyadenosine, 2′-deoxyguanosine, and 2′-deoxythymidine, respectively; mC, mA, mG, and mU signify 2′-O-methoxycytidine, 2′-O-methoxyadenosine, 2′-O-methoxyguanosine, and 2′-O-methoxyuridine, respectively; dT signifies 5′-5′ inverted dT; HEG signifies hexaethylene glycol; and fU and fC signify 2′-fluorocytidine and 2′-fluorouridine, respectively. Aptamers were synthesized as described in the Supplementary Methods.
In vitro assays of functional PDGF-B–binding aptamer
Aptamers were tested for effect on PDGF-driven proliferation of 3T3 mouse fibroblasts (American Type Culture Collection) using the methylthiazoletetrazolium (MTT) cell proliferation kit (Sigma). Cells were plated (3,000 per well) in 96-well plates 1 day before the start of the assay in DMEM with 10% FCS. Cells were washed with 100 μL of DMEM and incubated for 3 days in 75-μL DMEM/0.8% FCS containing various concentrations of PDGF-B aptamer and human, rat, or mouse PDGF-B to a final concentration of 2 nmol/L. On day 3, 10-μL MTT was added to each well. After 1.5 h, the medium was removed, cells were lysed with 200 μL isopropanol, and absorbance was measured with a SpectraMax Plus plate reader (Molecular Devices).
The in vitro binding affinity and specificity of the PDGF aptamer were evaluated by nitrocellulose filter-binding assay. Recombinant human and rodent PDGF-BB and its various human isoforms were purchased from R&D Systems or from Biosource. Recombinant human VEGF165 was from R&D Systems. Binding reactions were prepared with radiolabeled AX102 (≤10 pmol/L) and increasing concentrations (0.001–60 nmol/L) of recombinant PDGF or VEGF in PBS (Invitrogen) containing 0.1 mg/mL bovine serum albumin (EMD Biosciences) and 0.01 mg/mL tRNA (Sigma). Nitrocellulose filtration and analysis was done essentially as described (25) to obtain estimates of the dissociation constant (KD). The pharmacokinetic analysis and pharmacodynamic assay were done as described in Supplementary Methods.
Animals and treatment
LLC and RIP-Tag2 tumor models were used essentially as described (26). LLC tumors were allowed to grow for 5 to 7 days before treatment. During the treatment, LLC tumor volume was measured every other day using calipers and applying the formula [volume = 0.52 × (width)2 × (length)] as previously described (14). Aptamer AX102, AX104, or their vehicle (0.9% NaCl) was injected at a dose of 0.5 to 50 mg/kg i.p. once daily for 1 to 28 days. These experimental procedures were approved by the University of California, San Francisco Institutional Animal Care and Use Committee.
Fixation by vascular perfusion and immunohistochemistry
Following treatment, mice were fixed by vascular perfusion and the tumors were processed for and stained by immunohistochemistry as previously described (26). Trachea vasculature was analyzed according to Supplementary Methods. Endothelial cells, pericytes, and basement membrane were identified by staining sections with combinations of two or three primary antibodies. Endothelial cells were labeled with CD31; pericytes with α-smooth muscle actin (α-SMA), desmin, NG2 proteoglycan or PDGFR-β; and basement membrane with type IV collagen, laminin, or entactin/nidogen, as described in detail in the Supplementary Methods.
Imaging and analysis
Specimen were examined with a Zeiss Axiophot fluorescence microscope and a Zeiss LSM 510 laser scanning confocal microscope. Area densities were calculated from digital fluorescence microscopic images using an empirically determined threshold value of 30 to 50 essentially as previously described (26).
Overall pericyte-endothelial cell colocalization
As an estimate of pericyte coverage, the amount of colocalization of pericytes (PDGFR-β) and endothelial cells (CD31) was measured on 20-μm sections of LLC tumors. Digital images of the red channel (PDGFR-β) and green channel (CD31) were captured separately under a Axiophot fluorescence microscope (10× objective, 1× Optovar; ref. 27). The Colocalization plug-in function of ImageJ was used to identify pixels that had fluorescence intensity in both red and green channels equal to or greater than a threshold value, ranging from 30 to 45 in different experiments. Amount of colocalization was expressed as a percentage calculated from the number of pixels with above-threshold CD31 staining that colocalized with above-threshold PDGFR-β staining divided by the total number of above-threshold pixels in the CD31 image.
Pericyte-endothelial cell colocalization in individual vessels
Pericyte coverage was measured in fluorescence microscopic images of 100 randomly selected blood vessels in 20-μm sections of LLC tumors stained for CD31 and PDGFR-β. Individual vessels were outlined with the freehand selection in ImageJ, and the percentage of PDGFR-β pixels that colocalized with CD31 pixels was determined in the same manner as overall colocalization. After calculating the frequency distribution of values for 100 vessels, the number of vessel profiles per bin was scaled for the vascular density of the experimental group and expressed as vessel profiles per square millimeter of section.
Transmission electron microscopy
Mice implanted with LLC tumors were prepared for and examined by transmission electron microscopy as previously described (26, 28).
Statistics
The significance of differences between groups was assessed by ANOVA followed by Bonferroni-Dunn or Fisher’s post hoc tests. Multiple comparisons were used where appropriate. Values are expressed as mean ± SE. N = 4 to 5 mice per group. P < 0.05 was considered statistically significant.
Results
Affinity and specificity of AX102 for PDGF-B
The selectivity of AX102 for different PDGF subtypes was tested by nitrocellulose filtration assay. AX102 bound with highest affinity to PDGF-BB homodimer (KD = 0.046 ± 0.0037 nmol/L) and PDGF-AB heterodimer (KD = 0.092 ± 0.0065 nmol/L). The affinity for PDGF-AA (KD = 2.2 ± 0.43 nmol/L) was only 2% of that for PDGF-BB, whereas little or no binding of PDGF-CC or VEGF165 was detected (Fig. 1A-i). Binding measurements showed that AX102 had similar affinity for PDGF-BB from human (KD = 0.046 ± 0.0037 nmol/L), rat (KD = 0.045 ± 0.0035 nmol/L), and mouse (KD = 0.061 ± 0.0053 nmol/L; Fig. 1A-ii). Pharmacokinetic studies in mice indicated a half-life of 6 to 10 h (Supplementary Fig. S1A). Noncompartmental estimates for clearance ranged from 10 to 20 mL/h/kg, whereas those for Vss ranged from 73 to 104 mL/kg. The presence of active aptamer in plasma samples was verified by a competitive nitrocellulose filtration assay. Although AX102 seemed to lose some binding activity at 24 h, the concentration of AX102 in each plasma sample determined by this method was similar to that determined by OliGreen (Supplementary Fig. S1B; ref. 29).
Figure 1.
Specificity of AX102 and AX102-mediated reduction in tumor pericytes. A, in nitrocellulose filtration assays, AX102 bound with high affinities to PDGF-BB and PDGF-AB, but not to PDGF-AA, PDGF-CC, or VEGF165 (i). Cross-reactivity to rodent PDGF was shown in binding assays in which AX102 bound to mouse and rat PDGF-BB with equal affinity to human (ii). B, fluorescence microscopic images showing pericytes in LLC tumors, stained for α-SMA immunoreactivity, after 7 d of vehicle (i) or 50 mg/kg AX102 (ii). Measurements show a dose-dependent decrease after 7 d of AX102 but no change after scrambled aptamer AX104 (iii). *, P < 0.05, different from corresponding value for vehicle; †, P < 0.05, different from AX104. C, fluorescence microscopic images of LLC stained for α-SMA show pericytes in the vehicle group (i) and few pericytes remaining after AX102 (50 mg/kg) for 28 d (ii). Measurements show the large, rapid decrease in α-SMA immunoreactive pericytes after AX102 in LLC tumors (iii). D, fluorescence microscopic images of RIP-Tag2 tumors stained for α-SMA show abundant pericytes under baseline conditions (i) and a conspicuous decrease after AX102 for 28 d (ii). Quantification illustrates a smaller, more gradual decrease in RIP-Tag2 tumors (iii) than in LLC tumors (C-iii). *, P < 0.05, different from corresponding value for vehicle. †, P < 0.05, different from corresponding value for 1-d AX102. Bar in (D-ii) is 110 μm in (B), 125 μm in (C), and 150 μm in (D).
The in vivo activity of AX102 was assayed in implanted LLC tumors in mice using pericytes immunoreactive for α-SMA as the readout (28). After 7-day treatment with vehicle or AX104, blood vessels in these tumors had abundant α-SMA–positive pericytes (Fig. 1B-i). AX102 caused a dose-dependent reduction in α-SMA (Fig. 1B-ii). AX102 had no effect at 0.5 mg/kg, but it reduced α-SMA–positive pericytes by 60% at 2 mg/kg, 80% at 10 mg/kg, and 82% at 50 mg/kg (Fig. 1B-iii). No adverse effects were detected at any dose.
Time course of pericyte loss from tumor vessels
Blood vessels in LLC tumors of vehicle-treated mice had abundant α-SMA-positive pericytes (Fig. 1C-i); treatment with AX102 dramatically reduced α-SMA immunoreactivity (Fig. 1C-ii). In fact, α-SMA–positive pericytes were reduced by 55% at 1 day and 84% at 7 days. Prolonging the treatment to 28 days caused no further reductions (Fig. 1C-iii).
Blood vessels in untreated RIP-Tag2 tumors had even more abundant α-SMA-positive pericytes (Fig. 1D-i). AX102 reduced α-SMA immunoreactivity in RIP-Tag2 tumors, but the changes were smaller than in LLC tumors (Fig. 1D-ii). In RIP-Tag2 tumors, α-SMA immunoreactivity was reduced by 28% at 7 days and 47% at 14 and 28 days (Fig. 1D-iii).
The reduction in α-SMA immunoreactivity on tumor vessels could reflect a loss of pericytes, a decrease in α-SMA expression, or both. This issue was addressed by comparing α-SMA staining with staining of LLC tumors for three other pericyte markers, NG2, PDGFR-β, and desmin (Fig. 2A and B). Under baseline conditions, the staining for all four markers was similar (Fig. 2A). After 1 day of AX102, α-SMA immunoreactivity was reduced by 55% and NG2 by 69%, but PDGFR-β was reduced only by 13% and desmin only by 26%. After 7 days, however, all four pericyte markers were reduced by 63% to 89% (Fig. 2B).
Figure 2.
Reduction of four pericyte markers in LLC tumors after AX102. A, confocal microscopic images of LLC tumors comparing pericytes stained for α-SMA (i), NG2 (ii), PDGFR-β (iii), or desmin (iv). v, area densities of the four markers in untreated LLC tumors. B, comparison of α-SMA, NG2, desmin, and PDGFR-β immunoreactivities in LLC tumors after vehicle, 1 d of AX102, and 7 d of AX102. *, P < 0.05, different from corresponding value for vehicle. C, fluorescence microscopic images of LLC tumors showing a pericyte with both PDGFR-β and NG2 immunoreactivities in the vehicle group (i); a pericyte with PDGFR-β but not NG2 immunoreactivity after 1 d of AX102 (ii); and a tumor vessel that lacks pericytes with either marker after 7 d of AX102 (iii). D, transmission electron micrographs showing pericyte (red) envelopment of endothelial cells (green) in vehicle-treated LLC tumor (i) and absence of pericytes in LLC tumor vessels after AX102 for 7 d (ii). Bar in (D-ii) is 10 μm in (A), 15 μm in (C), and 1.9 μm in (D).
Differential effects of AX102 on pericyte markers were further examined by comparing in the same sections immunoreactivities of NG2, the marker showing the greatest reduction, and PDGFR-β, the marker having the least reduction. Both markers colocalized in most pericytes under baseline conditions (Fig. 2C-i); after AX102 for 1 day, some cells expressed PDGFR-β but lacked NG2 (Fig. 2C-ii). After 7 days, many tumor vessels had neither marker (Fig. 2C-iii), consistent with absence of pericytes. Examination of tumor vessels in LLC tumors by transmission electron microscopy confirmed the presence of pericytes under baseline conditions (Fig. 2D-i) and the reduction in pericytes after AX102 for 7 days (Fig. 2D-ii). Measurements of electron micrographs showed an 84% reduction in endothelial cell perimeter covered by pericytes.
Regression of tumor vessels after pericyte loss
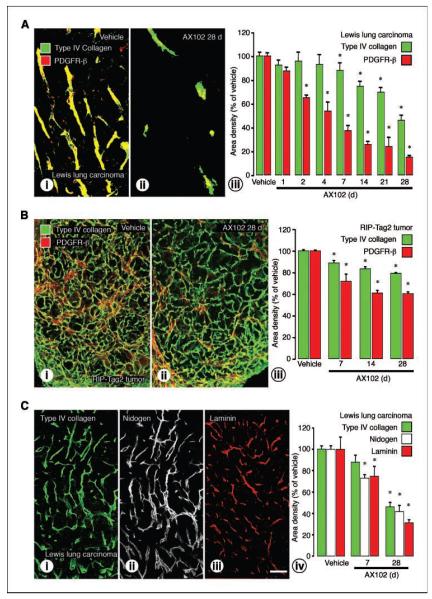
Because AX102 had such a large effect on tumor pericytes, we examined the vascularity of LLC and RIP-Tag2 tumors after treatment. Compared with the baseline vascularity reflected by CD31 immunoreactivity, no reduction in tumor vessels was evident after 1 or 2 days of AX102, but longer treatments led to increasing loss of tumor vessels: 31% reduction at 4 days, 47% reduction at 7 days, and 79% reduction at 28 days in LLC tumors (Fig. 3A).
Figure 3.
Blood vessel loss in LLC and RIP-Tag2 tumors after AX102, leaving tumor vessels with closely associated pericytes. A, fluorescence microscopic images of LLC tumors treated with vehicle (i) and AX102 (ii) for 28 d stained for CD31 (endothelial cells; green) and PDGFR-β (pericytes; red). Measurements of LLC tumors show a significant reduction in PDGFR-β at 2 d, but no reduction in CD31 until 4 d. CD31 decreased more slowly than PDGFR-β, but by 28 d both had decreased by the same amount (iii). B, fluorescence microscopic images of RIP-Tag2 tumors stained for CD31 and PDGFR-β compare the abundant blood vessels under baseline conditions (i) and marked reduction after 28 d of AX102 (ii). Measurements of RIP-Tag2 tumors compare reductions in PDGFR-β and CD31 after 7, 14, and 28 d of AX102 (iii). *, P < 0.05, different from corresponding value for vehicle. C, confocal microscopic images of endothelial cells (CD31; green) and pericytes (α-SMA; red) on tumor vessels. Most pericytes in LLC tumors were loosely associated with blood vessels under baseline conditions (i); after AX102, pericytes were tightly apposed to endothelial cells (ii). Similarly, most pericytes in RIP-Tag2 tumors were loosely associated with tumor vessels under baseline conditions (iii); after AX102 for 7 d, pericytes were absent on some tumor vessels and closely associated with others (iv). Bar in (C-iv) represents 110 μm in (A), 150 μm in (B), and 12 μm in (C).
AX102 had smaller but significant effects on the vascularity of RIP-Tag2 tumors, which were densely vascular under baseline conditions. Tumor vascularity decreased during treatment with AX102 in a time-dependent manner: 14% reduction at 7 days, 27% reduction at 14 days, and 38% reduction at 28 days (Fig. 3B).
Tumor vessels that remained after AX102 were changed. Most pericytes in untreated LLC and RIP-Tag2 tumors were detached or loosely associated with the tumor blood vessels (Fig. 3C-i and iii). After 7 or 28 days of AX102 treatment, although the pericytes and tumor vessels were reduced in number, the remaining tumor vessels had closely associated pericytes (Fig. 3C-ii and iv).
To investigate whether the loss of pericytes led to regression of tumor vessels, we compared the relative abundance of pericytes (PDGFR-β) and endothelial cells (CD31) in the same sections of LLC tumors. Under baseline conditions, tumor vessels had dense pericyte coverage; about half of the CD31 immunoreactivity colocalized with PDGFR-β (Fig. 4A-i and B). After AX102 for 7 days, PDGFR-β was reduced much more than CD31, and colocalization was reduced to 19%, indicative of reduced pericyte coverage (Fig. 4A-ii and B). From 7 to 28 days, the progressive loss of tumors vessels (CD31) outpaced further decreases in PDGFR-β. By 28 days, CD31 and PDGFR-β immunoreactivities were decreased equally, and about half of CD31 colocalized with PDGFR-β (Fig. 4A-iii and B). These changes are consistent with a rapid loss of pericytes during the first week and subsequent loss of endothelial cells, with both decreasing ~80% over 28 days (Figs. 1C and 3A).
Figure 4.
Changes in pericyte-endothelial cell colocalization in tumor vessels after AX102. A, confocal microscopic images showing CD31 (green), PDGFR-β (red), and colocalization of CD31 and PDGFR-β (white) in LLC tumors. Under baseline conditions, about half of the CD31 immunoreactivity colocalized with PDGFR-β (i), but after AX102 for 7 d, the colocalization was reduced, and many vessels lacked PDGFR-β (ii). After 28 d, endothelial cell staining decreased to the low value for pericytes, and the colocalization of CD31 with PDGFR-β increased compared with the value at 7 d (iii). B, measurements show a significant reduction in colocalization after AX102 for 7 d followed by an increase toward baseline at 28 d. *, P < 0.05, different from corresponding value for vehicle. C, confocal microscopic images showing blood vessels in LLC tumors stained for PDGFR-β (red) and CD31 (green) with 10% colocalization (i), 40% colocalization (ii), and 90% colocalization (iii). D, frequency distribution of pericyte-endothelial cell colocalization, expressed as vessel profiles per square millimeter of section, based on measurements of 100 individual blood vessels in LLC tumors in each of the three treatment groups. Top, colocalization pattern in vehicle-treated tumors where most vessels had 40% to 90% coverage. Middle, a very different colocalization pattern after AX102 for 7 d, where most vessels had little or no coverage. Bottom, another pattern after AX102 for 28 d, where pericyte coverage was about the same across the entire range. Differences in bar height under the three treatment conditions reflect differences in tumor vascularity. Bar in (C-iii) is 70 μm in (A) and 11 μm in (C).
The extent of pericyte coverage varied from vessel to vessel in LLC tumors (Fig. 4C). To examine if the loss of pericyte coverage led to a reduction of tumor vessels, we compared the distribution of CD31 and PDGFR-β colocalization on individual vessels. Measurements showed that the amount of colocalization was 40% to 90% for most vessels under baseline conditions (Fig. 4D, top), but after AX102 for 7 days, 80% of vessels had <40% coverage and 41% had <10% coverage (Fig. 4D, middle). Surprisingly, this abundance of tumor vessels with few or no pericytes was transient. After treatment for 28 days, pericyte coverage spanned the range from none to complete; only 42% had <40% coverage and 9% had <10% coverage (Fig. 4D, bottom).
The effect of AX102 was also analyzed in normal blood vessels by examining the microvasculature of the trachea (30). Despite a moderate reduction of pericyte coverage, capillaries did not undergo noticeable regression, nor did empty sleeves of vascular basement membrane appear, indicative of regressing capillaries (Supplementary Fig. S2).
Reduction in empty basement membrane sleeves
Because pericytes and endothelial cells are thought to produce the extracellular matrix proteins which compose the basement membrane (31), we asked whether AX102-induced loss of pericytes and blood vessels was accompanied by corresponding changes in basement membrane. Under baseline conditions, tumor vessels were enveloped by type IV collagen immunoreactive basement membrane. In LLC and RIP-Tag2 tumors, treatment with AX102 led to time-dependent reductions in basement membrane. In LLC tumors treated with AX102, type IV collagen was unchanged at 1 day, but was reduced by 12% at 7 days and by 54% at 28 days (Fig. 5A). The network of type IV collagen was more dense and complex in RIP-Tag2 tumors. Treatment with AX102 caused a smaller but significant reduction in type IV collagen (11% at 7 days and 21% at 28 days) in RIP-Tag2 tumors (Fig. 5B).
Figure 5.
Reduction of vascular basement membrane after AX102. A, fluorescence microscopic images of tumors stained for basement membrane (type IV collagen; green) and pericytes (PDGFR-β; red). Regions of colocalization are in yellow. In vehicle-treated LLC tumors, most type IV collagen colocalized with pericytes (i). AX102 for 28 d led to a reduction in type IV collagen (ii). Time course analysis revealed that after AX102, the total amount of type IV collagen was essentially unchanged at 1 d, but was reduced by 12% at 7 d, and by 54% at 28 d (iii). B, in vehicle-treated RIP-Tag2 tumors, most type IV collagen was associated with pericytes (i), but after AX102, type IV collagen was reduced by 11% at 7 d and by 21% at 28 d (ii and iii). Measurements of LLC tumors (A-iii) and RIP-Tag2 tumors (B-iii) show that pericytes decreased more rapidly and to a greater extent than type IV collagen. C, staining of basement membrane in the same section of LLC tumors showed complete colocalization of type IV collagen (i) and nidogen (ii). Laminin had a similar distribution (iii). All three basement membrane proteins in LLC tumors decreased by about the same amount after AX102 for 7 or 28 d (iv). *, P < 0.05, different from corresponding vehicle group. Bar in (C-iii) represents 110 μm in (A), 150 μm in (B), and 120 μm in (C).
In both RIP-Tag2 and LLC tumors, the reduction in type IV collagen after AX102 was significantly less than the reduction in pericytes. To determine whether type IV collagen was representative of other basement membrane proteins, we examined nidogen and laminin in the same or sequential sections and found comparable amounts and distributions in LLC tumors (Fig. 5C). Area density measurements showed that the three markers decreased to a similar extent after AX102 (Fig. 5C-iv).
Effect of AX102 on tumor growth
Treatment with AX102 did not have an antitumor effect. Until day 16, the growth of LLC tumors treated with AX102 was similar to vehicle-treated tumors. Following longer treatments, AX102-treated tumors grew significantly larger than vehicle-treated tumors (Fig. 6). The effect of AX102 on tumor growth of RIP-Tag2 tumor was not analyzed because these tumors are orthotopic.
Figure 6.

Increased growth of LLC tumors after AX102. Growth curves of LLC tumors after vehicle or AX102 (50 mg/kg) treatment for 26 d. The curves were obtained by combining data from two different experiments (N = 10 for each group). P < 0.05 between the two curves by repeated measures ANOVA.
Discussion
The goal of the present study was to determine the cellular effects of selective inhibition of PDGF-B by the DNA oligonucleotide aptamer AX102 on the vasculature of tumors. Experiments revealed that AX102 rapidly reduced the pericyte coverage of tumor vessels. In LLC tumors, changes in pericytes were evident within 24 h. Immunoreactivity for some pericyte markers was reduced at 1 day; overall pericyte number was reduced by 35% at 2 days, 63% at 7 days, and 85% at 28 days. Pericyte loss predisposed the tumor vessels to regression. Tumor vascularity was decreased by 31% at 4 days, 47% at 7 days, and 79% at 28 days. Pericyte-free tumor vessels were abundant at 7 days but largely disappeared with further treatment. By 28 days, most surviving tumor vessels had a more normal appearance. Empty basement membrane sleeves were reduced in amount but not eliminated. AX102 had qualitatively similar but smaller effects on RIP-Tag2 tumors. Taken together, these results show that inhibition of PDGF-B results in pericyte loss followed by pruning of pericyte-free tumor blood vessels. At 4 weeks, the tumor vasculature and empty basement membrane sleeves were more sparse, but surviving tumor vessels had a more normal phenotype.
Selectivity of PDGF-B inhibition by AX102
AX102 had selective binding for PDGF-BB or PDGF-AB, but not PDGF-AA, PDGF-CC, or VEGF165. The scrambled version (AX104) had no such binding. The affinity for the heterodimer PDGF-AB was half of the affinity of the homodimer PDGF-BB. This highlights the high selectivity of AX102 for PDGF-B. AX102 had similar affinities for mouse, rat, and human PDGF-B; thus, it may be used across species. Pharmacokinetic studies of AX102 showed a half-life in blood of 6 to 10 h, which justified daily dosing. The mean observed Vss was approximately equal to the expected blood volume of a mouse (32), indicating that for healthy mice with normal vasculature, the aptamer is not widely distributed outside the vasculature. The observed estimates for clearance represented only 1% to 2% of the glomerular filtration rate for mice (32), suggesting that renal elimination does not play a major role in the overall clearance of intact AX102 from plasma. Similar PDGF-B binding aptamers improve the efficacy of chemotherapeutic agents in tumors, decrease ocular neovascularization, and ameliorate glomerulonephritis in experimental models (19, 24, 33). AX102, however, has a greater affinity for PDGF-B than previously used aptamers (24, 34).
Reduced pericyte coverage of tumor vessels after PDGF-B inhibition
Inhibition of PDGFR-β signaling by genetic mutation, anti-receptor antibodies, and small-molecule receptor tyrosine kinase blockers reduce pericyte coverage of blood vessels in tumors (11, 13–16). Because pericytes respond to PDGFR-β signaling inhibition, pericytes were used as a reference for assessing effects of treatment. Pericytes are present on >97% of vessels in LLC and RIP-Tag2 tumors (28). Different human tumors have strikingly variable pericyte coverage (35). The differences in expression of immunohistochemical markers used for pericyte identification complicate this determination as none of the markers used to identify pericytes are expressed by all pericytes (11, 16, 28). Therefore, absence of a single marker may not indicate the absence of pericytes. For this reason, we used four different pericyte markers: two cytoskeleton proteins, α-SMA and desmin, and two membrane proteins, NG2 and PDGFR-β (36, 37).
Measurements showed that the four pericyte markers had different immunoreactivities in LLC tumors under baseline conditions. NG2 was most abundant and desmin the least. These differences may reflect a combination of different cellular distributions of the marker proteins, levels of expression, antibody affinities, or presence of multiple pericyte populations. After LLC tumors were treated with AX102 for 1 day, NG2 and α-SMA were reduced by >50%, desmin was reduced by 26%, and PDGFR-β was unchanged. At 2 days, PDGFR-β was significantly reduced. At 7 days, all four markers were reduced by >60%, and by at least 80% after 28 days. Electron microscopic studies confirmed the reduction of pericytes at 7 days. We interpret these findings as evidence that the reduction of markers may initially reflect variable down-regulation of protein expression in pericytes, but after the second day, most of the changes reflected loss of pericytes.
Regression of tumor vessels after pericyte loss
Pericytes play an important role in blood vessel growth and maturation by providing survival and stabilization signals for endothelial cells (10, 18, 36, 38–40). Whereas pericyte loss may lead to endothelial cell apoptosis, extensive regression of endothelial cells was not observed in tumors after inhibition of PDGFR-β signaling (11, 16). Imatinib (STI571, Gleevec), which targets PDGFRs and certain other receptor tyrosine kinases, did not reduce vascular density when given alone but did augment the effects of VEGF inhibitors (14). Treatment of RIP-Tag2 tumors with anti–PDGFR-β antibody for 3 weeks reduces pericytes, increases endothelial cell apoptosis, and enlarges tumor vessels but does not seem to reduce tumor vascular density (16). AX102 treatment of LLC and RIP-Tag2 tumors elicited a robust effect on tumor vessels. These differences in effects may be due to duration of inhibition, differences in tumor models, different methods of PDGF inhibition, or varying methods of analyzing tumor vessel density. We used methods that have previously shown reductions in tumor vessels after VEGF inhibition (26, 27, 30). In addition, we took advantage of the potent and selective binding of PDGF-B by AX102 to assess the effect of pericyte loss on endothelial cell viability in two very different murine tumor models. After 7 to 28 days of AX102, blood vessel loss was modest in RIP-Tag2 tumors but extensive in LLC tumors. Accordingly, many tumor vessels lacked pericytes after 7 days of AX102, but this vessel population was absent after 28 days of treatment.
The regression of tumor vessels is likely not a direct effect of PDGF inhibition. Tumor vessel regression was slower than pericyte reduction. Whereas AX102 reduced tumor pericytes after 2 days, tumor vessels were not affected until after 4 days of treatment. Studies examining the effects of VEGF inhibition, which targets tumor vessels, observed regression as early as 1 day (26). In addition, PDGFR-α or PDGFR-β expression is absent in endothelial cells (14). These results indicate that pericytes are necessary for tumor vessel survival because reductions of ~50% of tumor pericytes lead to reductions in tumor vessels. Pericyte coverage alone, however, cannot protect tumor vessels from regression. Despite 97% of tumor vessels being covered by pericytes, VEGF inhibition can eliminate tumor vessels without removing pericytes (27, 28). This indicates that removal of pericyte coverage leads to exposed tumor vessels, which may explain the enhanced effect of combining inhibitors that target both tumor vessels and pericytes (14, 41).
Despite the strong effect on tumor vasculature, AX102 had only a modest effect (19% reduction) on pericyte coverage of normal tracheal capillaries in adult mice. No regression of tracheal capillaries was evident. These findings indicate that pericytes in this vasculature have only modest dependence on PDGF-B and that these capillaries can survive for 4 weeks after a small reduction in pericyte coverage.
Regression of empty basement membrane sleeves
Blood vessels in tumors are enveloped by basement membrane. This provides a substrate for endothelial cell and pericyte attachment as well as a storage site for VEGF and other growth factors (27, 42–44). When tumor vessels regress after VEGF inhibition, empty sleeves of basement membrane are left behind (26, 27). These sleeves provide a scaffold for blood vessel regrowth after VEGF inhibition ends (27). Inhibition of PDGF-B by AX102 for 28 days was accompanied by loss of 54% of basement membrane sleeves in LLC tumor, but this reduction was significantly less than the ~80% loss of pericytes and endothelial cells. Similarly, in RIP-Tag2 tumors, the reduction in the empty sleeves at 28 days was less than the loss of blood vessels. The time course of the reduction suggests that AX102 reduces the basement membrane by eliminating pericytes and endothelial cells, which contribute to the synthesis of the basement membrane components (31). Although not eliminated, the reductions in basement membrane sleeves may prevent or slow vascular regrowth after cessation of AX102.
Differences between LLC and RIP-Tag2 tumors
Comparison of effects of AX102 on pericytes, blood vessels, and basement membrane revealed larger and more rapid effects on LLC tumors than RIP-Tag2 tumors. The lower sensitivity of RIP-Tag2 tumors to AX102 may reflect a lesser dependency on pericytes because VEGF is highly expressed by the tumor cells (26, 45). Alternatively, the greater reductions observed in LLC tumors may indicate a more immature vasculature that greatly depends on PDGF and pericytes for stabilization of newly formed vessels. This immaturity is reflected by the differences in tumor vascularity whereby RIP-Tag2 tumors have a vessel area density of 55% and LLC tumors, 16%. Nonetheless, treatment of RIP-Tag2 tumors with AX102 for 28 days resulted in a 47% decrease in pericytes, a 38% decrease in blood vessels, and a 21% decrease in basement membrane sleeves.
Normalization of tumor vessels by AX102
Unlike untreated tumors where most of the blood vessels had loosely attached pericytes (28), tumors treated with AX102 for 7 to 28 days had blood vessels with pericytes tightly associated with endothelial cells. Surviving tumor vessels had fewer sprouts, less branching, and more uniform caliber. Whereas the normalized tumor vessels may be the result of pruning vessels with loosely attached pericytes, we cannot eliminate the possibility that tumor vessels go through a gradual process that leads to this normalization. However, many vessels in tumors treated for 7 days had few or no pericytes. This population of bare vessels was eliminated with further treatment. More efficient blood flow and drug delivery, attributed to normalization of tumor vessels (46), may contribute to the increased efficiency of chemotherapeutics or other agents administered in combination with inhibitors of PDGFR-β signaling (14, 15, 19, 47).
Effect of AX102 on tumor growth
Despite reductions of endothelial cells, pericytes, and basement membrane, AX102 did not reduce the growth of LLC tumors. Instead, beyond 16 days, LLC tumors treated with AX102 were as large or even larger than those treated with vehicle. The mechanism of the observed increase in tumor volume remains to be determined. Contributing factors may include (a) expanded tumor growth supported by improved blood flow through normalized, albeit much less numerous, tumor vessels; (b) reduced tumor cell death; (c) impaired clearance of necrotic tumor tissue; or (d) increased tumor edema due to greater plasma leakage or reduced fluid clearance. Although not found in LLC tumors treated with AX102, PDGF inhibition is efficacious in reducing growth of tumors that overexpress PDGFR-β in stroma (20). Inhibition of PDGF also augments the reduction in tumor growth and metastasis produced by other inhibitors of angiogenesis (14, 15, 17), chemotherapeutic agents (19, 21, 48), and irradiation (49).
In conclusion, experiments with aptamer AX102 highlight the importance of PDGFR-β signaling in the structure, function, and survival of the tumor vasculature. Many pericytes in LLC tumors depend on PDGF-B for survival, with only 15% remaining after PDGF-B is inhibited for 4 weeks. Fewer pericytes in RIP-Tag2 tumors are PDGF-B dependent, as reflected by persistence of 53% after 4 weeks of PDGF-B inhibition. Loss of pericyte coverage leads to loss of endothelial cells and regression of tumor vessels. This leads to formation of empty basement membrane sleeves, which eventually regress (Supplementary Fig. S3). Tumor vessels that survive PDGF-B inhibition are more normal, which may improve drug delivery to adjacent tumor cells despite reduced tumor vascularity.
Supplementary Material
Supplementary Figure 1. Pharmacokinetic-pharmacodynamic profile of AX102.
Pharmacokinetic profile of AX102 after iv injection of a dose of 10 mg/kg into mice (n=3 per time point) (A). Pharmacokinetic-pharmacodynamic profile of AX102 after iv injection of a dose of 10mg/kg into mice (B). Pharmacokinetic and pharmacodynamic profiles were measured from serum samples obtained at different time points and then assayed in vitro for total aptamer and functional aptamer as described in the Methods.
Supplementary Figure 2. Modest reduction of pericytes on normal capillaries after AX102.
Confocal microscopic images of mouse trachea mucosa show fewer desmin-positive pericytes (red) on tracheal capillaries. In tracheas of vehicle-treated mice, desmin-positive pericytes were present along 94% of the length of capillaries (Ai), but after AX102 for 7 days (Aii) or 28 days (Aiii), pericyte coverage decreased to 76% of capillary length. By comparison, total length of tracheal capillaries, made visible by CD31 staining (green), did not decrease after AX102, nor did empty basement membrane sleeves (nidogen without CD31) appear, as would be expected if endothelial cells regressed (Bi-Biii) (26). Measurements show a modest but significant reduction in pericyte coverage (percent of capillary length accompanied by pericytes) of tracheal capillaries after AX102 (Ci), but no significant reduction in tracheal capillaries after AX102 for 7 or 28 days (Cii). Indeed, a small but significant increase in vascularity was found at 7 days but not at 28 days. Nidogen-positive nerves (arrows), which were easily distinguished from blood vessels, were excluded from measurements. * Different from corresponding vehicle group (P < 0.05). Scale bar in Biii represents 50 μm in A-B.
Supplementary Figure 3. Summary of effects of AX102 on blood vessels in tumors. Under baseline conditions, pericytes are loosely associated with tumor blood vessels. Both endothelial cells (green) and pericytes (red) are surrounded by basement membrane (blue) (A). Inhibition of PDGF-B signaling by AX102 induces sequential changes in the tumor vasculature. Initially, pericyte coverage is reduced leaving some vessels bare (B). Loss of pericytes is followed by loss of endothelial cells, which eliminates tumor vessels and leaves behind empty basement membrane sleeves (C). The number of empty basement membrane sleeves decreases with longer treatment but not as much as pericytes and endothelial cells (D).
Acknowledgments
Grant support: NIH grants HL24136 and HL59157 from the National Heart, Lung, and Blood Institute and NIH grant CA82923 from the National Cancer Institute; a grant from Archemix Corp.; funding from AngelWorks Foundation and the Vascular Mapping Project (D.M. McDonald); a postdoctoral fellowship from the Tobacco-Related Disease Research Program, no. 14FT-0152 (B.L. Falcón).
We thank Douglas Hanahan for breeding pairs of RIP-Tag2 mice; Jie Wei for genotyping; Rachel Davis, Shaun O’Brien, and Scott Norberg for technical help; Alaric Falcón for critical review of the manuscript; Ryan Boomer and Markus Kurz for aptamer preparation; and Anna Zoltoski and Jeff Preiss for in vitro biochemical characterization.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/). B. Sennino and B.L. Falcón contributed equally to this work.
References
- 1.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 2.Betsholtz C, Lindblom P, Gerhardt H. Role of pericytes in vascular morphogenesis. EXS. 2005;(94):115–25. doi: 10.1007/3-7643-7311-3_8. [DOI] [PubMed] [Google Scholar]
- 3.Enge M, Bjarnegard M, Gerhardt H, et al. Endothelium-specific platelet-derived growth factor-B ablation mimics diabetic retinopathy. EMBO J. 2002;21:4307–16. doi: 10.1093/emboj/cdf418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellstrom M, Gerhardt H, Kalen M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–53. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellstrom M, Kal NM, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–55. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 6.Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- 7.Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–98. [PubMed] [Google Scholar]
- 8.Lindahl P, Hellstrom M, Kalen M, Betsholtz C. Endothelial-perivascular cell signaling in vascular development: lessons from knockout mice. Curr Opin Lipidol. 1998;9:407–11. doi: 10.1097/00041433-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 10.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–9. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112:1142–51. doi: 10.1172/JCI18549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuhashi M, Sjoblom T, Abramsson A, et al. Platelet-derived growth factor production by B16 melanoma cells leads to increased pericyte abundance in tumors and an associated increase in tumor growth rate. Cancer Res. 2004;64:2725–33. doi: 10.1158/0008-5472.can-03-1489. [DOI] [PubMed] [Google Scholar]
- 13.Shaheen RM, Tseng WW, Davis DW, et al. Tyrosine kinase inhibition of multiple angiogenic growth factor receptors improves survival in mice bearing colon cancer liver metastases by inhibition of endothelial cell survival mechanisms. Cancer Res. 2001;61:1464–8. [PubMed] [Google Scholar]
- 14.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18:338–40. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 16.Song S, Ewald AJ, Stallcup W, Werb Z, Bergers G. PDGFRβ+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat Cell Biol. 2005;7:870–9. doi: 10.1038/ncb1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoi K, Sasaki T, Bucana CD, et al. Simultaneous inhibition of EGFR, VEGFR, and platelet-derived growth factor receptor signaling combined with gemcitabine produces therapy of human pancreatic carcinoma and prolongs survival in an orthotopic nude mouse model. Cancer Res. 2005;65:10371–80. doi: 10.1158/0008-5472.CAN-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reinmuth N, Liu W, Jung YD, et al. Induction of VEGF in perivascular cells defines a potential paracrine mechanism for endothelial cell survival. FASEB J. 2001;15:1239–41. doi: 10.1096/fj.00-0693fje. [DOI] [PubMed] [Google Scholar]
- 19.Pietras K, Rubin K, Sjoblom T, et al. Inhibition of PDGF receptor signaling in tumor stroma enhances antitumor effect of chemotherapy. Cancer Res. 2002;62:5476–84. [PubMed] [Google Scholar]
- 20.Kitadai Y, Sasaki T, Kuwai T, Nakamura T, Bucana CD, Fidler IJ. Targeting the expression of platelet-derived growth factor receptor by reactive stroma inhibits growth and metastasis of human colon carcinoma. Am J Pathol. 2006;169:2054–65. doi: 10.2353/ajpath.2006.060653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SJ, Uehara H, Yazici S, et al. Simultaneous blockade of platelet-derived growth factor-receptor and epidermal growth factor-receptor signaling and systemic administration of paclitaxel as therapy for human prostate cancer metastasis in bone of nude mice. Cancer Res. 2004;64:4201–8. doi: 10.1158/0008-5472.CAN-03-3763. [DOI] [PubMed] [Google Scholar]
- 22.Fabian MA, Biggs WH, III, Treiber DK, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–36. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 23.James W. Aptamers. In: Meyers RA, editor. Encyclopedia of analytical chemistry. John Wiley & Sons Ltd.; Chichester: 2000. pp. 4848–71. [Google Scholar]
- 24.Akiyama H, Kachi S, Silva RL, et al. Intraocular injection of an aptamer that binds PDGF-B: a potential treatment for proliferative retinopathies. J Cell Physiol. 2006;207:407–12. doi: 10.1002/jcp.20583. [DOI] [PubMed] [Google Scholar]
- 25.McCauley TG, Kurz JC, Merlino PG, et al. Pharmacologic and pharmacokinetic assessment of anti-TGFβ2 aptamers in rabbit plasma and aqueous humor. Pharm Res. 2006;23:303–11. doi: 10.1007/s11095-005-9305-2. [DOI] [PubMed] [Google Scholar]
- 26.Inai T, Mancuso M, Hashizume H, et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–21. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reyderman L, Stavchansky S. Quantitative determination of short single-stranded oligonucleotides from blood plasma using capillary electrophoresis with laser-induced fluorescence. Anal Chem. 1997;69:3218–22. doi: 10.1021/ac970280+. [DOI] [PubMed] [Google Scholar]
- 30.Baffert F, Le T, Sennino B, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290:H547–59. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 31.Mandarino LJ, Sundarraj N, Finlayson J, Hassell HR. Regulation of fibronectin and laminin synthesis by retinal capillary endothelial cells and pericytes in vitro. Exp Eye Res. 1993;57:609–21. doi: 10.1006/exer.1993.1166. [DOI] [PubMed] [Google Scholar]
- 32.Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–5. doi: 10.1023/a:1018943613122. [DOI] [PubMed] [Google Scholar]
- 33.Ostendorf T, Kunter U, Grone HJ, et al. Specific antagonism of PDGF prevents renal scarring in experimental glomerulonephritis. J Am Soc Nephrol. 2001;12:909–18. doi: 10.1681/ASN.V125909. [DOI] [PubMed] [Google Scholar]
- 34.Green LS, Jellinek D, Jenison R, Ostman A, Heldin CH, Janjic N. Inhibitory DNA ligands to platelet-derived growth factor B-chain. Biochemistry. 1996;35:14413–24. doi: 10.1021/bi961544+. [DOI] [PubMed] [Google Scholar]
- 35.Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–93. [PubMed] [Google Scholar]
- 36.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 37.McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713–25. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 38.Darland DC, Massingham LJ, Smith SR, Piek E, Saint-Geniez M, D’Amore PA. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–88. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Shih SC, Ju M, Liu N, Mo JR, Ney JJ, Smith LE. Transforming growth factor h1 induction of vascular endothelial growth factor receptor 1: mechanism of pericyte-induced vascular survival in vivo. Proc Natl Acad Sci U S A. 2003;100:15859–64. doi: 10.1073/pnas.2136855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levanon K, Varda-Bloom N, Greenberger S, et al. Vascular wall maturation and prolonged angiogenic effect by endothelial-specific platelet-derived growth factor expression. Pathobiology. 2006;73:149–58. doi: 10.1159/000095561. [DOI] [PubMed] [Google Scholar]
- 41.Pietras K, Hanahan D. A multitargeted, metronomic, and maximum-tolerated dose “chemo-switch” regimen is antiangiogenic, producing objective responses and survival benefit in a mouse model of cancer. J Clin Oncol. 2005;23:939–52. doi: 10.1200/JCO.2005.07.093. [DOI] [PubMed] [Google Scholar]
- 42.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 44.Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163:1801–15. doi: 10.1016/S0002-9440(10)63540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D. VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic β cell carcinogenesis. Cancer Cell. 2002;1:193–202. doi: 10.1016/s1535-6108(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 46.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 47.Apte SM, Fan D, Killion JJ, Fidler IJ. Targeting the platelet-derived growth factor receptor in antivascular therapy for human ovarian carcinoma. Clin Cancer Res. 2004;10:897–908. doi: 10.1158/1078-0432.ccr-1151-3. [DOI] [PubMed] [Google Scholar]
- 48.Garofalo A, Naumova E, Manenti L, et al. The combination of the tyrosine kinase receptor inhibitor SU6668 with paclitaxel affects ascites formation and tumor spread in ovarian carcinoma xeno-grafts growing orthotopically. Clin Cancer Res. 2003;9:3476–85. [PubMed] [Google Scholar]
- 49.Griffin RJ, Williams BW, Wild R, Cherrington JM, Park H, Song CW. Simultaneous inhibition of the receptor kinase activity of vascular endothelial, fibroblast, and platelet-derived growth factors suppresses tumor growth and enhances tumor radiation response. Cancer Res. 2002;62:1702–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Pharmacokinetic-pharmacodynamic profile of AX102.
Pharmacokinetic profile of AX102 after iv injection of a dose of 10 mg/kg into mice (n=3 per time point) (A). Pharmacokinetic-pharmacodynamic profile of AX102 after iv injection of a dose of 10mg/kg into mice (B). Pharmacokinetic and pharmacodynamic profiles were measured from serum samples obtained at different time points and then assayed in vitro for total aptamer and functional aptamer as described in the Methods.
Supplementary Figure 2. Modest reduction of pericytes on normal capillaries after AX102.
Confocal microscopic images of mouse trachea mucosa show fewer desmin-positive pericytes (red) on tracheal capillaries. In tracheas of vehicle-treated mice, desmin-positive pericytes were present along 94% of the length of capillaries (Ai), but after AX102 for 7 days (Aii) or 28 days (Aiii), pericyte coverage decreased to 76% of capillary length. By comparison, total length of tracheal capillaries, made visible by CD31 staining (green), did not decrease after AX102, nor did empty basement membrane sleeves (nidogen without CD31) appear, as would be expected if endothelial cells regressed (Bi-Biii) (26). Measurements show a modest but significant reduction in pericyte coverage (percent of capillary length accompanied by pericytes) of tracheal capillaries after AX102 (Ci), but no significant reduction in tracheal capillaries after AX102 for 7 or 28 days (Cii). Indeed, a small but significant increase in vascularity was found at 7 days but not at 28 days. Nidogen-positive nerves (arrows), which were easily distinguished from blood vessels, were excluded from measurements. * Different from corresponding vehicle group (P < 0.05). Scale bar in Biii represents 50 μm in A-B.
Supplementary Figure 3. Summary of effects of AX102 on blood vessels in tumors. Under baseline conditions, pericytes are loosely associated with tumor blood vessels. Both endothelial cells (green) and pericytes (red) are surrounded by basement membrane (blue) (A). Inhibition of PDGF-B signaling by AX102 induces sequential changes in the tumor vasculature. Initially, pericyte coverage is reduced leaving some vessels bare (B). Loss of pericytes is followed by loss of endothelial cells, which eliminates tumor vessels and leaves behind empty basement membrane sleeves (C). The number of empty basement membrane sleeves decreases with longer treatment but not as much as pericytes and endothelial cells (D).