Abstract
It is well known that caries invasion leads to the differentiation of dentin into zones with altered composition, collagen integrity and mineral identity. However, understanding of these changes from the fundamental perspective of molecular structure has been lacking so far. In light of this, the present work aims to utilize Fourier transform infrared spectroscopy (FTIR) to directly extract molecular information regarding collagen's and hydroxyapatite's structural changes as dentin transitions from the transparent zone (TZ) into the normal zone (NZ). Unembedded ultrathin dentin films were sectioned from carious teeth, and an FTIR imaging system was used to obtain spatially resolved FTIR spectra. According to the mineral-to-matrix ratio image generated from large-area low-spectral-resolution scan, the TZ, the NZ and the intermediate subtransparent zone (STZ) were identified. High-spectral-resolution spectra were taken from each zone and subsequently examined with regard to mineral content, carbonate distribution, collagen denaturation and carbonate substitution patterns. The integrity of collagen's triple helical structure was also evaluated based on spectra collected from demineralized dentin films of selected teeth. The results support the argument that STZ is the real sclerotic layer, and they corroborate the established knowledge that collagen in TZ is hardly altered and therefore should be reserved for reparative purposes. Moreover, the close resemblance between the STZ and the NZ in terms of carbonate content, and that between the STZ and the TZ in terms of being A-type carbonate-rich, suggest that the mineral that initially occludes dentin tubules is hydroxyapatite newly generated from odontoblastic activities, which is then transformed into whitlockite in the demineralization/remineralization process as caries progresses.
Keywords: Carbonate type, Carious dentin, Collagen cross-link, Collagen triple helix, Fourier transform infrared spectroscopy imaging, Mineral content, Subtransparent zone, Transparent zone
The formation of dentin caries is an intricate process associated with dynamic events of mineral loss and gradual denaturation of collagen fibrils [Bjørndal, 2002]. A comprehensive understanding of its prognosis and the accompanying changes to tooth structure is indispensable for the optimal management of this common oral disease. Over the past decades, researchers and clinicians have gained a great deal of knowledge on the anatomical features of carious dentin as well as on important biological and mechanical properties of the constituent mineral phase and collagen matrix [Fejerskov and Kidd, 2008]. For instance, the infected layer [Fusayama and Terachima, 1972; Fusayama, 1979], which was found to be comprised of denatured collagen that has lost the potential for remineralization, becomes the consensus target for removal. Conversely, the affected layer, which is partially demineralized and remineralizable with collagen fibrils retaining their natural structure around intact dentinal tubules, is to be preserved to maximize reparative potential and to reduce the risk of pulp exposure [Pugach et al., 2009; Alleman and Magne, 2012]. In the meantime, much knowledge of the various zones in the affected layer has been obtained, particularly the transparent zone (TZ) and the normal zone (NZ) [Ogawa et al., 1983; Marshall et al., 2001; Zheng et al., 2003, 2005; Pugach et al., 2009] using a variety of techniques including microradiography, staining, indentation and electronic microscopy.
Despite all the discoveries that have been made on carious dentin, few studies approach the subject from the perspective of the fundamental chemistry of the constituent molecules. That is, the vast majority of the work has been focused on the histological, microscopic and mechanical aspects of carious dentin, but rarely on the underlying molecular structure which is integral to a full understanding of the disease, especially its effect on the mineral content and collagen matrix. In this respect, Fourier transform infrared spectroscopy (FTIR) is a powerful tool in generating direct information about the molecules of a sample [Barer et al., 1949]. By probing the infrared absorption caused by covalent bond vibration, the structure of a chemical and its content can be readily inferred from the location and intensity of the corresponding characteristic infrared signals. Particularly, the mid-infrared region (4,000–500 cm−1) is frequently examined to produce molecule-specific vibrational signatures for biological samples [Boskey and Pleshko Camacho, 2007]. With the release of focal plane array detectors in the early 2000s, fast acquisition of large arrays of FTIR spectra in a short period of time became possible, which prompted the advance of the FTIR imaging technique [Wang et al., 2009]. Essentially a combination of light microscopy and FTIR, FTIR imaging provides spatially resolved chemical characterization of microscopic areas of a sample. It has been widely used in biological studies to elucidate the molecular differences within and between various tissues [Kazarian and Chan, 2006; Verdelis et al., 2007; Della Ventura et al., 2010; Noreen et al., 2012]. Compared to the conventional histological and microscopic methods, the FTIR imaging technique is recognized to be advantageous because it is fast, non-intrusive, stain-free, quantitative and less prone to human subjectivity [Bird et al., 2008; Noreen et al., 2012].
As such, we undertook the current work as the first investigation of the spatial variation of carious dentin's chemical structure using an FTIR imaging system. In particular, the chemical composition of the subtransparent zone (STZ), a transition area between the TZ and the NZ discovered and apparently named by Ogawa et al. [1983] was analyzed for the first time. The mid-infrared region, including the characteristic infrared bands for the mineral phase (phosphate and carbonate ions) and organic phase (collagen amide I and amide III), was examined using undemineralized and demineralized ultrathin sections of carious dentin. The null hypothesis was that there is no difference across the TZ, the STZ and the NZ of caries-affected dentin with regard to overall mineral content, degree of collagen denaturation, and carbonate distribution and substitution patterns. The infected zone was not studied due to the limitations of our sample preparation procedure; that is, we refrained from embedding the samples in any resin to avoid alterations to the spectra caused by ethanol/glycerol fixation and resin spectrum subtraction [Aparicio et al., 2002]. As a result, the mechanically weak infected dentin could not survive the sectioning employed. Nevertheless, the present work should provide valuable information regarding dentin's molecular structural changes across the affected region, including the TZ, the STZ and the NZ.
Materials and Methods
Human molars with occlusal carious lesions were collected from the Oral Surgery Clinic at the University of Missouri-Kansas City (UMKC) School of Dentistry after obtaining the patients' informed consent under a protocol approved by the UMKC adult health sciences institutional review board. Extracted teeth were stored at 4°C in 0.96% (w/v) phosphate-buffered saline containing 0.002% sodium azide before being split in half in the occlusal-apical direction through the lesions using a slow-speed water-cooled diamond saw (Buehler, Lake Bluff, Ill., USA). A total of 7 teeth were selected (designated T1–T7), all of which had half or more of the cross-sectional area being apparently normal dentin under a light microscope, and were subsequently subjected to further processing. In more detail, sections in the same occlusal-apical direction were made with a tungsten carbide knife mounted on an SM2500S microtome (Leica, Deerfield, Ill., USA), resulting in several 2-μm-thick undemineralized dentin films per tooth. During the microtoming process, the tooth samples and the knife edge were kept moist with distilled water, and the sectioned dentin films were allowed to slide onto the knife and later collected on 13-mm-diameter, 1-mm-thick barium fluoride (BaF2) disks with a fine paintbrush. For each tooth, its corresponding undemineralized dentin films were inspected under a light microscope, from which one of the least cracked was chosen to represent the tooth and proceed with FTIR imaging. Furthermore, for four randomly selected teeth (T2, T3, T5 and T7), additional undemineralized films were harvested, demineralized in 0.5 M EDTA solution (pH = 7.4) for 12 h [Pashley et al., 2001] and then rinsed three times with deionized water before being loaded on the BaF2 disks. These demineralized dentin films were screened for integrity under a light microscope in a similar way. To ensure the flatness of the films, one additional BaF2 disk was used to sandwich each film before it was air dried in a fume hood over a week.
Each undemineralized dentin film and the BaF2 disk support were placed directly on the motorized stage of the Spectrum Spotlight FTIR imaging system (Perkin Elmer, Waltham, Mass., USA). By examining under the light microscope the dentin films as well as the remaining dentin blocks from which the films had been sectioned, the NZ of each dentin film was located. Images were then scanned, spanning the region from the top of the carious lesion down to the NZ, at a pixel resolution of 6.25 μm in the mid-infrared region of 4,000–720 cm−1 and a spectral resolution of 16 cm −1 with 8 scans per pixel. An atmospheric correction was applied to remove the contribution of the atmosphere background, primarily water vapor and carbon dioxide. The scanned area ranged from 1 to 5 mm2, providing images that contained approximately 25,000–125,000 individual spectra each. The determination of band area and band ratio was performed with the Spotlight software (Perkin Elmer) following a two-point baseline correction and band area integration. The band ratio between ∼1,035 and ∼1,655 cm−1 (A1,035/A1,655), attributed to the ν3 vibration of hydroxyapatite phosphate ion and the C=O stretching of collagen amide I, respectively, was used to represent the mineral-to-matrix (M/M) ratio [Paschalis et al., 1997]. The final visualization and color rendering of the band ratio intensity map were realized with the Python software (Python Software Foundation, Del., USA).
Based on the large-area images acquired above, the TZ, STZ and NZ of each undemineralized dentin film were identified, from which additional spectra were collected at a higher spectral resolution. In more detail, small localized FTIR images (50–100 × 50–100 μm) were obtained from each zone at the same pixel resolution as the large-area images, but the spectral resolution was raised to 4 cm −1 and 128 scans per pixel. Afterwards, 10 spectra were randomly sampled from each small image while avoiding the vicinity of obvious cracks. The spectra were exported to the Spectrum software (Perkin Elmer) and averaged to one single spectrum representing the zone from which it was taken. The average spectra were further analyzed with the Spectrum software as follows. First, A1,035/A1,655 was re-calculated, producing the M/M ratio at a higher spectral resolution. Second, A872/A1,035 was determined in a similar way to assess the distribution of carbonate content within the mineral phase. Third, the band ratio between 1,660 and 1,690 cm−1 (I1,660/I1,690) was evaluated according to published procedures [Boskey et al., 2002, 2003] to gauge the relative amount of non-reducible and reducible cross-links in collagen. Lastly, the range of 920–820 cm−1 (corresponding to the carbonate ν2 vibration) was self-deconvoluted using a Bessel filter with gamma of 1 and length of 0. The relative content of A-type and B-type carbonate was assessed from the resultant resolution-enhanced spectra [Rey et al., 1989, 1991] by calculating the band ratio between 879 and 872 cm−1 (I879/I872).
Additional high-resolution spectra were collected from demineralized dentin films of four selected teeth in a way similar to their undemineralized counterparts. However, the precise selection of the STZ in the demineralized samples was not possible due to lack of mineral ‘fingerprints’ like in undemineralized dentin. Therefore, spectra were obtained from the TZ and the NZ only, which were then examined with respect to the band ratio between ∼1,235 cm−1 for amide III and ∼1,450 cm−1 for CH2 scissoring (A1,235/A1,450).
Statistical analysis was performed with Statistical Product and Service Solutions (SPSS version 20, IBM SPSS, Inc., Chicago, Ill., USA) and a nominal level of significance of 0.05. Repeated measurement analysis was used to test the overall effect of location including the TZ, the STZ and the NZ. In the procedure of repeated measurement analysis, Mauchly's W approach was utilized to test the assumption that the error covariance matrix of the ortho-normalized transformed dependent variables is proportional to an identity matrix. If the aforementioned assumption did not hold, the Huynh-Feldt test was applied to identify whether the effect of location was significant or not; otherwise, Fisher's F test was used. On the basis of the significant effect of location, the paired t test was used to detect whether two locations differed or not.
Results
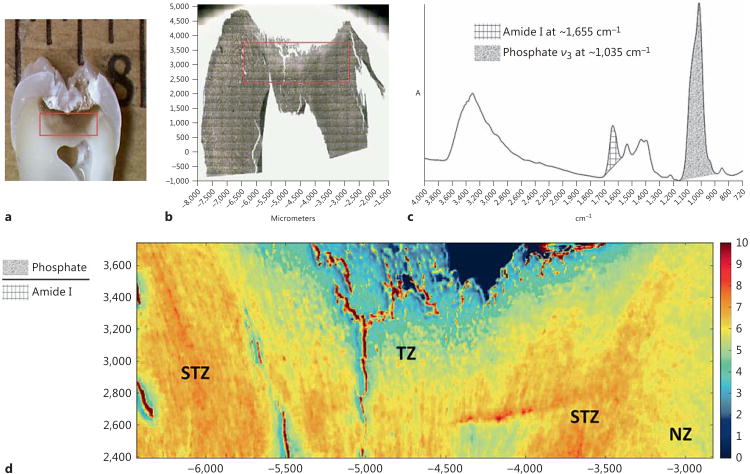
The optical and FTIR images of a representative tooth are shown in figure 1. The red rectangles in figure 1a and b demonstrate the approximate area from which the large-area FTIR image was taken. A typical spectrum from the FTIR image is given in figure 1c, where the bands of interest are illustrated in different patterns, including amide I at ∼1,655 cm−1 (grid) and phosphate ν3 vibration at ∼1,035 cm−1 (shade). The image of the M/M ratio (fig. 1d) results from the band ratio between phosphate ion and amide I. It is clear that a zone with a higher M/M ratio is sandwiched between two zones of lower M/M ratios. The three zones are identified to be the TZ, the STZ and the NZ in the order of increasing distance from the carious lesion.
Fig. 1.
a Optical image of the cross-section of a representative tooth. b Optical image of the dentin film sectioned from the same tooth. c Typical spectrum from the FTIR image. d FTIR image of the M/M ratio.
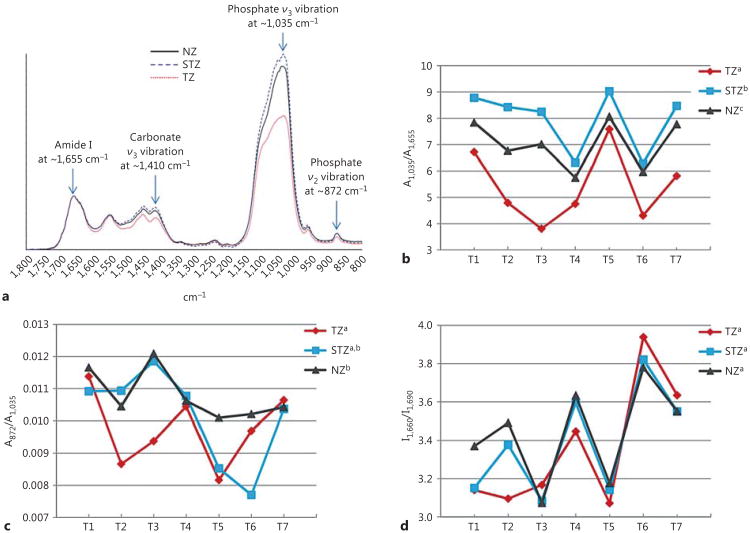
Representative spectra of the TZ, the STZ and the NZ in the range of 1,800–800 cm−1 collected at a high resolution of 4 cm−1 and 128 scans are shown in figure 2a. The spectra are normalized based on the amide I band. It can be seen that the bands due to mineral content, including carbonate ν3 vibration at ∼1,410 cm−1, phosphate ν3 vibration at ∼1,035 cm−1 and carbonate ν2 vibration at ∼872 cm−1, exhibit the most prominent changes. The M/M ratio of all 7 teeth determined at high spectral resolution, represented by A1,035/A1,655, is shown in figure 2b. Consistent with the large-area FTIR images (fig. 1d), the mineral content in the TZ, the STZ and the NZ follows the trend of STZ > NZ > TZ with statistical significance. In addition, the carbonate content within the mineral phase, represented by A872/A1,035 (fig. 2c), substantiates the transient nature of the STZ; that is, the carbonate con tent of the TZ is significantly lower than that of the NZ, while that of the STZ is not significantly different from that of either the TZ or the NZ. Moreover, with regard to the collagen phase of carious dentin, the band ratio I1,660/I1,690 (fig. 2d) indicates that there is no significant difference among the TZ, the STZ and the NZ in the relative amount of reducible cross-links.
Fig. 2.
Representative high-resolution FTIR spectra collected from the NZ, the STZ and the TZ of a tooth (a); A1,035/A1,655 (b), A872/A1,035 (c) and I 1,660/I1,690 (d) ratios of all 7 teeth calculated from high-resolution spectra. Different superscripts of legends indicate significant difference.
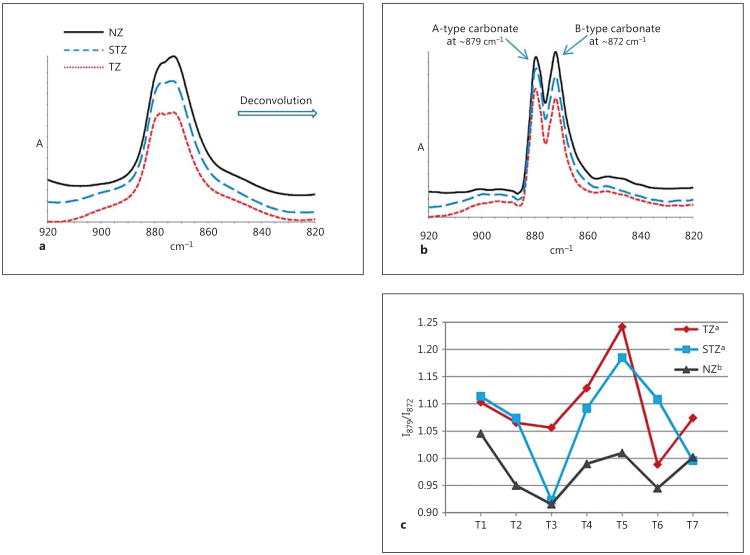
Shown in figure 3a is the magnified region of figure 2a (920–820 cm−1), representing carbonate ν2 vibration before self-deconvolution. After self-deconvolution, the bands attributed to A-type (897 cm−1) and B-type (872 cm−1) carbonate ions are resolved (fig. 3b), and the band ratio I879/I872 (fig. 3c) as calculated from the resolution-enhanced spectra (fig. 3b) shows that the relative amount of A-type carbonate follows the trend TZ = STZ > NZ.
Fig. 3.
a Magnified domain of carbonate ν2 vibration of high-resolution spectra taken from the NZ, the TZ and the STZ of a representative tooth. b Resolution-enhanced spectra after self-deconvolution. c I879/I872 ratios of all 7 teeth calculated from resolution-enhanced spectra. Different superscripts of legends indicate significant difference.
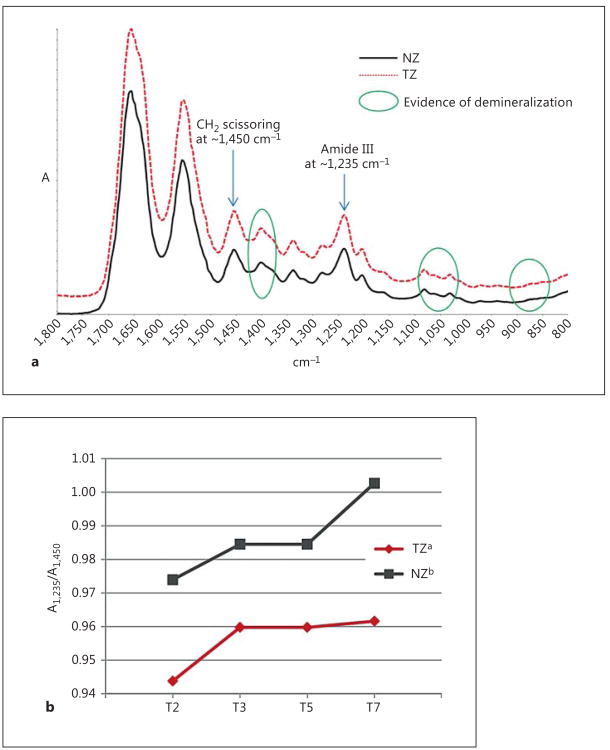
Representative spectra collected from four demineralized dentin films are shown in figure 4a. The bands due to mineral content including phosphate and carbonate ions are clearly removed due to demineralization (green circles). The band ratio (A1,235/A1,450) of the TZ is significantly lower than that of the NZ (fig. 4b), although both are very close to unity.
Fig. 4.
a Representative high-resolution spectra taken from a demineralized dentin film. b A1,235/A1,450 ratios of demineralized dentin films. Different superscripts of legends indicate significant difference.
Discussion
The band ratio between phosphate ion at ∼1,035 cm−1 and collagen amide I at ∼1,655 cm-1(A1,035/A1,655) has been widely used in the spectroscopic studies of calcified tissues to analyze the distribution of mineral content [Gourion-Arsiquaud et al., 2008]. Using this band ratio, the generated FTIR image (fig. 1d) clearly demonstrated the heterogeneous nature of caries-affected dentin in terms of mineral distribution. Three zones were distinct, across which the transition of mineral content in the order of increasing distance from caries lesion was not monotonic; that is, a zone with more mineral was sandwiched between two zones with less mineral. This apparent up-and-down distribution of mineral content corresponds well with a previous mechanical characterization of carious dentin. Thus, Ogawa et al. [1983] discovered that the Knoop hardness of carious dentin gradually increased from the discolored layer, the transparent layer to the subtransparent layer, and then dropped in sound dentin. Ogawa et al.'s work actually seems to be the first that designated the name ‘subtransparent layer’ to the transition zone between transparent and normal dentin. No other studies have been dedicated to the characterization of the subtransparent layer, but a few groups did also observe a non-monotonic pattern in terms of intertubular dentin's mechanical properties as it transitioned from the TZ to the NZ. For example, Marshall et al. [2001] examined carious dentin's hardness and elastic modulus at the hydrated state using a nano-indentation technique, and they found that values of intertubular dentin increased slightly from near the pulp into the TZ before staying constant or slightly decreasing through the TZ. Similarly, Zheng et al. [2003] used nano-indentation to determine the mechanical properties of carious zones identified by caries detector staining. Although the staining did not reveal the STZ, the hardness and elastic modulus profiles of intertubular dentin as a function of distance to carious lesion indicated a continuous increase of these two values into what was assumed to be normal dentin before starting to decrease. Moreover, in both Ogawa et al.'s and Zheng et al.'s studies, the depth of this layer with higher mechanical strength appeared to be a few tenths of a millimeter, in line with the FTIR images in the present work. As such, the three zones in our study were identified as TZ, STZ and NZ in the order of increasing distance to the carious lesion.
As an effort to study the fine molecular structure of hydroxyapatite and collagen in the TZ, the STZ and the NZ, spectra at a higher spectral resolution were obtained from each zone. Note that the collection of large-area FTIR images at the same high spectral resolution was not practical due to the constraints of time and computation power. As seen in figure 2a, all spectra exhibited the typical features of mineralized dentin, and the absorbance of bands attributed to hydroxyapatite was obviously affected by the zone from which the spectrum was taken. It is worth mentioning that in all spectra, the strongest signal (phosphate ν3 vibration at ∼1,035 cm−1) was integral with no sign of artificial shape change, which is only possible for dentin films ≤2 μm. Thicker films generally rendered a saturated absorbance, manifested by truncation of this band.
Spectroscopic analyses revealed that the null hypothesis had to be rejected. In more detail, the mineral content in the TZ, the STZ and the NZ was ranked according to the M/M ratio (as determined by A 1,035/A1,655) re-calculated at high spectral resolution (fig. 2b), and it was found that the STZ has the highest mineral content, followed by the NZ and the TZ. With regard to the NZ and the TZ, it has been long discovered that the mineral content in the TZ is lower than that in the NZ [Ogawa et al., 1983; Zheng et al., 2003; Pugach et al., 2009], despite the fact that tubules in the TZ are filled or partially filled with mineral whereas those in the NZ are not [Zheng et al., 2003]. The evidence included not only circumstantial one, such as lower mechanical properties of the TZ than the NZ [Ogawa et al., 1983; Zheng et al., 2003], but also direct measurement of mineral content using transverse microradiography [Pugach et al., 2009]. Our results agree with these findings and corroborate the argument that the TZ is not sclerotic [Fusayama, 1991]. When it comes to the STZ, only mechanical tests were done with outcomes implying a higher mineral content in the STZ than the NZ (and the TZ) [Ogawa et al., 1983]. Thus, the FTIR analysis presented here serves as the direct proof that the STZ contains more mineral than the NZ and is therefore the real sclerotic layer. As the transient area between the TZ (which is tubule-filled and demineralized) and the NZ (which is tubule-unfilled and undemineralized), the formation of the STZ likely results from the combined effects of tubular occlusion (like the TZ) and absence of demineralization (like the NZ).
Meanwhile, the mineral phase of the three zones was examined regarding the overall carbonate content with respect to phosphate. As shown in figure 2c, the mineral of the STZ was found to contain an equivalent amount of carbonate to that of the NZ. In contrast, the mineral of the TZ has a significantly lower carbonate level compared to that of the NZ, which reflects the intratubular mineral of the TZ being significantly altered from carbonated hydroxyapatite. Indeed, Frank et al. [1964] reported that whitlockite instead of carbonated hydroxyapatite occludes carious dentin tubules based on the rhombohedral shape of mineral crystals. Nevertheless, little is known about the origin of whitlockite, i.e. whether it is produced ‘as is’ by the pulpo-dentinal complex or transformed from hydroxyapatite in the cariogenic environment. Our results here apparently support the latter notion because the carbonate level in the STZ resembles that in the NZ, suggesting that the mineral in the tubules of the STZ has a chemical composition similar to the hydroxyapatite of normal intertubular dentin. Therefore, it should be hydroxyapatite (or carbonated hydroxyapatite to be exact) that is initially accreted to block dentin tubules as a defense against caries invasion. However, as caries progresses, hydroxyapatite is demineralized in the area closer to lesion (the TZ), where remineralization takes place concomitantly, albeit at a slower rate than demineralization. It is during remineralization that whitlockite is precipitated in preference to hydroxyapatite since it is more stable than hydroxyapatite in an acidic environment [ten Cate et al., 2008].
The intactness of organic phase in dentin, predominantly type I collagen [Linde, 1989] was examined (fig. 2d) by comparing the band intensities of 1,660 and 1,690 cm−1 (I1,660/I1,690). Paschalis et al. [2001] determined that the former intensity was attributed to non-reducible cross-links in collagen whereas the latter to reducible cross-links. Therefore, the value of I1,660/I1,690 has been widely used to assess the alteration of collagen molecular structure in a variety of studies [Krafft and Sergo, 2006; Saito and Marumo, 2010]. Based on the same rationale, the molecular structure of collagen was apparently unchanged across the TZ, the STZ and the NZ since the difference in I1,660/I1,690 was not significant (fig. 2d). This is consistent with the established knowledge that collagen in the TZ is intact and remineralizable, and thus should be reserved for reparative purposes [Alleman and Magne, 2012].
Additional information could be extracted from FTIR spectra regarding carbonate ions' substitution pattern within the hydroxyapatite crystal lattice. Two types of substitution occur, A-type and B-type, the former of which is designated to carbonate ions occupying the positions of hydroxide whereas the latter phosphate ions [Rey et al., 1989]. Due to the slight difference in the chemical environment of these two types of carbonates, they give rise to subtle variations in the infrared bands attributed to carbonate ν2 vibration (fig. 3a). Following deconvolution, the resolution-enhanced spectra (fig. 3b) featured two distinctive bands at 879 and 872 cm−1, attributed to A-type and B-type carbonate ions, respectively [Rey et al., 1991]. The band intensity ratio (I879/I872) determined from the resolution-enhanced spectra demonstrated that the hydroxyapatite of the STZ is significantly richer in A-type carbonate than that of the NZ (fig. 3c). Since the difference between the STZ and the NZ is tubular occlusion as discussed earlier, this points to a higher relative level of A-type carbonate in the intratubular hydroxyapatite as compared to intertubular dentin, which in turn implies that hydroxyapatite freshly generated by the pulpo-dentinal complex is richer in A-type carbonate. Such age-related variation in the A-type/B-type carbonate ratio of dentin was spotted in gestation calf incisors [Verdelis et al., 2007]. It was displayed that the circumpulpal dentin closest to the cervix (therefore the newest) has a substantially higher A-type carbonate level, matching our theory of freshly generated hydroxyapatite from odontoblastic activities being A-type-rich. Back to our results, the average I 879/I872 ratio of the TZ (1.094 ± 0.078) is slightly higher than that of the STZ (1.070 ± 0.086), but the difference is not significant (fig. 3c). This further verifies the speculation that the tubules in the TZ should be filled with the newly-deposited A-type-rich hydroxyapatite at first (like the STZ), which is then gradually transformed to whit-lockite during caries progression. The major difference between the STZ and the TZ is demineralization, as evidenced by the M/M ratio (fig. 2b), and demineralization could be the cause for the slight elevation of the relative A-type carbonate level in the TZ as compared to the STZ (fig. 3c). That is, computational simulations have shown that it is thermodynamically more favorable to substitute the hydroxide ion of a hydroxyapatite crystal lattice than to substitute the phosphate ion with carbonate ion [Peroos et al., 2006]. In other words, from the chemical point of view A-type substitution is more stable than B-type substitution, and thus may be more inert to acid.
The integrity of the triple helical structure of collagen in the TZ and the NZ was evaluated with FTIR spectra taken from demineralized dentin films (fig. 4a). Demineralization was necessary because this evaluation would require the calculation of the band ratio between collagen's amide III at ∼1,235 cm−1 and CH2 scissoring at ∼1,450 cm−1(A1,235/A1,450), the latter of which is greatly interfered by carbonate ν3 vibration (∼1,410 cm−1) of the mineral phase. Collagen with integral triple helical structure has a A1,235/A1,450 value close to unity, whereas a ratio around 0.5 suggests that the triple helix is destroyed like gelatin [Sylvester et al., 1989; Liu and Wang, 2013]. As shown in figure 4b, the triple helical structure of collagen in the TZ is significantly but diminutively less integral than in the NZ, far from breakdown. In fact, the A1,235/A1,450 ratio of demineralized caries-infected dentin was found to be <0.7 (data not shown). This is again consistent with the consensus that transparent dentin should be saved for reparative purposes.
In conclusion, we have demonstrated that FTIR imaging and subsequent spectra analyses are highly useful in probing the molecular structure of carious dentin. Based on the characteristic infrared bands of hydroxyapatite and collagen, various zones are identified in good agreement with the knowledge we already possessed by means of other techniques. In addition, the fine chemical features of both inorganic and organic phases, including mineral distribution, carbonate substitution, collagen cross-link alteration and triple helical structure are evaluated and compared between the different zones. With such information we could further understand the progression of dentin caries and ultimately improve our treatment of this common disease.
Acknowledgments
This investigation was supported in part by USPHS Research Grant R01-DE021431 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Authors' Contributions: X. Yao, Y. Liu and Y. Wang conceived and designed the experiments; X. Yao and Y. Liu performed the experiments; Y. Liu and Y.W. Liu analyzed the data; Y. Liu and Y. Wang wrote the paper.
Disclosure Statement: There is no potential conflict of interest in the research conducted and presented herewith.
References
- Alleman DS, Magne P. A systematic approach to deep caries removal end points: the peripheral seal concept in adhesive dentistry. Quintessence Int. 2012;43:197–208. [PubMed] [Google Scholar]
- Aparicio S, Doty SB, Camacho NP, Paschalis EP, Spevak L, Mendelsohn R, Boskey AL. Optimal methods for processing mineralized tissues for Fourier transform infrared microspectroscopy. Calcif Tissue Int. 2002;70:422–429. doi: 10.1007/s00223-001-1016-z. [DOI] [PubMed] [Google Scholar]
- Barer R, Cole ARH, Thompson HW. Infra-red spectroscopy with the reflecting microscope in physics, chemistry and biology. Nature. 1949;163:198–201. doi: 10.1038/163198a0. [DOI] [PubMed] [Google Scholar]
- Bird B, Miljkovic M, Romeo MJ, Smith J, Stone N, George MW, Diem M. Infrared micro-spectral imaging: distinction of tissue types in axillary lymph node histology. BMC Clin Pathol. 2008;8:8. doi: 10.1186/1472-6890-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørndal L. Dentin caries: progression and clinical management. Oper Dent. 2002;27:211–217. [PubMed] [Google Scholar]
- Boskey AL, Moore DJ, Amling M, Canalis E, Delany AM. Infrared analysis of the mineral and matrix in bones of osteonectin-null mice and their wildtype controls. J Bone Miner Res. 2003;18:1005–1011. doi: 10.1359/jbmr.2003.18.6.1005. [DOI] [PubMed] [Google Scholar]
- Boskey A, Pleshko Camacho N. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials. 2007;28:2465–2478. doi: 10.1016/j.biomaterials.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- Della Ventura G, Bellatreccia F, Marcelli A, Cestelli Guidi M, Piccinini M, Cavallo A, Piochi M. Application of micro-FTIR imaging in the Earth sciences. Anal Bioanal Chem. 2010;397:2039–2049. doi: 10.1007/s00216-010-3811-8. [DOI] [PubMed] [Google Scholar]
- Fejerskov O, Kidd E, editors. Dental Caries: The Disease and Its Clinical Management. Ames, Iowa: Blackwell Munksgaard; 2008. [Google Scholar]
- Frank RM, Wolff F, Gutmann B. Microscopie électronique de la carie au niveau de la dentine humaine. Arch Oral Biol. 1964;9:181–188. doi: 10.1016/0003-9969(64)90006-8. [DOI] [PubMed] [Google Scholar]
- Fusayama T. Two layers of carious dentin; diagnosis and treatment. Oper Dent. 1979;4:63–70. [PubMed] [Google Scholar]
- Fusayama T. Intratubular crystal deposition and remineralization of carious dentin. J Biol Buccale. 1991;19:255–262. [PubMed] [Google Scholar]
- Fusayama T, Terachima S. Differentiation of two layers of carious dentin by staining. J Dent Res. 1972;51:866. doi: 10.1177/00220345720510032601. [DOI] [PubMed] [Google Scholar]
- Gourion-Arsiquaud S, West PA, Boskey AL. Fourier transform-infrared microspectroscopy and microscopic imaging. Methods Mol Biol. 2008;455:293–303. doi: 10.1007/978-1-59745-104-8_20. [DOI] [PubMed] [Google Scholar]
- Kazarian SG, Chan KL. Applications of ATR-FTIR spectroscopic imaging to biomedical samples. Biochim Biophys Acta. 2006;1758:858–867. doi: 10.1016/j.bbamem.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Krafft C, Sergo V. Biomedical applications of Raman and infrared spectroscopy to diagnose tissues. Spectroscopy. 2006;20:195–218. [Google Scholar]
- Linde A. Dentin matrix proteins: composition and possible functions in calcification. Anat Rec. 1989;224:154–166. doi: 10.1002/ar.1092240206. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Y. Proanthocyanidins' efficacy in stabilizing dentin collagen against enzymatic degradation: MALDI-TOF and FTIR analyses. J Dent. 2013;41:535–542. doi: 10.1016/j.jdent.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall GW, Habelitz S, Gallagher R, Balooch M, Balooch G, Marshall SJ. Nanomechanical properties of hydrated carious human dentin. J Dent Res. 2001;80:1768–1771. doi: 10.1177/00220345010800081701. [DOI] [PubMed] [Google Scholar]
- Noreen R, Moenner M, Hwu Y, Petibois C. FTIR spectro-imaging of collagens for characterization and grading of gliomas. Biotechnol Adv. 2012;30:1432–1446. doi: 10.1016/j.biotechadv.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Yamashita Y, Ichijo T, Fusayama T. The ultrastructure and hardness of the transparent layer of human carious dentin. J Dent Res. 1983;62:7–10. doi: 10.1177/00220345830620011701. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Betts F, DiCarlo E. FTIR microspectroscopic analysis of normal human cortical and trabecular bone. Calcif Tissue Int. 1997;61:480–486. doi: 10.1007/s002239900371. [DOI] [PubMed] [Google Scholar]
- Paschalis EP, Verdelis K, Doty SB, Boskey AL, Mendelsohn R, Yamauchi M. Spectroscopic characterization of collagen cross-links in bone. J Bone Miner Res. 2001;16:1821–1828. doi: 10.1359/jbmr.2001.16.10.1821. [DOI] [PubMed] [Google Scholar]
- Pashley DH, Agee KA, Nakajima M, Tay FR, Carvalho RM, Terada RS, Harmon FJ, Lee WK, Rueggeberg FA. Solvent-induced dimensional changes in EDTA-demineralized dentin matrix. J Biomed Mater Res. 2001;56:273–281. doi: 10.1002/1097-4636(200108)56:2<273::aid-jbm1095>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Peroos S, Du Z, De Leeuw NH. A computer modelling study of the uptake, structure and distribution of carbonate defects in hydroxy-apatite. Biomaterials. 2006;27:2150–2161. doi: 10.1016/j.biomaterials.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pugach MK, Strother J, Darling CL, Fried D, Gansky SA, Marshall SJ, Marshall GW. Dentin caries zones: mineral, structure, and properties. J Dent Res. 2009;88:71–76. doi: 10.1177/0022034508327552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey C, Collins B, Goehl T, Dickson IR, Glimcher MJ. The carbonate environment in bone mineral: a resolution-enhanced Fourier transform infrared spectroscopy study. Calcif Tissue Int. 1989;45:157–164. doi: 10.1007/BF02556059. [DOI] [PubMed] [Google Scholar]
- Rey C, Renugopalakrishnan V, Shimizu M, Collins B, Glimcher MJ. A resolution-enhanced Fourier transform infrared spectroscopic study of the environment of the CO32− ion in the mineral phase of enamel during its formation and maturation. Calcif Tissue Int. 1991;49:259–268. doi: 10.1007/BF02556215. [DOI] [PubMed] [Google Scholar]
- Saito M, Marumo K. Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporosis Int. 2010;21:195–214. doi: 10.1007/s00198-009-1066-z. [DOI] [PubMed] [Google Scholar]
- Sylvester MF, Yannas IV, Salzman EW, Forbes MJ. Collagen banded fibril structure and the collagen-platelet reaction. Thromb Res. 1989;55:135–148. doi: 10.1016/0049-3848(89)90463-5. [DOI] [PubMed] [Google Scholar]
- ten Cate JM, Larsen MJ, Pearce EIF, Fejerskov O. Chemical interactions between the tooth and oral fluids. In: Fejerskov O, Kidd E, editors. Dental Caries: The Disease and Its Clinical Management. Ames, Iowa: Blackwell Munksgaard; 2008. pp. 209–230. [Google Scholar]
- Verdelis K, Lukashova L, Wright JT, Mendelsohn R, Peterson MGE, Doty S, Boskey AL. Maturational changes in dentin mineral properties. Bone. 2007;40:1399–1407. doi: 10.1016/j.bone.2006.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yao X, Parthasarathy R. Characterization of interfacial chemistry of adhesive/dentin bond using FTIR chemical imaging with univariate and multivariate data processing. J Biomed Mater Res A. 2009;91:251–262. doi: 10.1002/jbm.a.32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Hilton JF, Habelitz S, Marshall SJ, Marshall GW. Dentin caries activity status related to hardness and elasticity. Eur J Oral Sci. 2003;111:243–252. doi: 10.1034/j.1600-0722.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Zheng L, Nakajima M, Higashi T, Foxton RM, Tagami J. Hardness and Young's modulus of transparent dentin associated with aging and carious disease. Dent Mater J. 2005;24:648–653. doi: 10.4012/dmj.24.648. [DOI] [PubMed] [Google Scholar]