Summary
Hepatitis B and C viruses (HBV, HCV) cause chronic hepatitis and hepatocellular carcinoma (HCC) by poorly understood mechanisms. We show that cytokines lymphotoxin (LT) α, β and their receptor (LTβR) are upregulated in HBV- or HCV-induced hepatitis and HCC. Liver-specific LTαβ expression in mice induces liver inflammation and HCC causally linking hepatic LT overexpression to hepatitis and HCC. Development of HCC, composed in part of A6+ oval cells, depends on lymphocytes and IKappa B kinase β expressed by hepatocytes but is independent of TNFR1. In vivo LTβR stimulation implicates hepatocytes as the major LT-responsive liver cells and LTβR inhibition in LTαβ-transgenic mice with hepatitis suppresses HCC formation. Thus, sustained LT signaling represents a pathway involved in hepatitis-induced HCC.
Introduction
A causal relationship between chronic hepatitis, hepatocellular damage, fibrosis and carcinogenesis is well established (El-Serag and Rudolph, 2007). Various etiologies, including chronic alcohol consumption, chronic drug abuse, autoimmune disorders, toxins (e.g. aflatoxin B) or infections with hepatotropic viruses (e.g. HBV, HCV) can lead to chronic hepatitis, liver fibrosis and cirrhosis. HBV- and HCV-infections are by far the most common cause of chronic hepatitis in humans (Malhi et al., 2006). Chronic HBV- and HCV-infections are frequently associated with HCC, the most prevalent primary human liver cancer (El-Serag and Rudolph, 2007), and except for HBV-infections, liver cirrhosis precedes HCC in most cases. The exact mechanisms driving hepatitis-induced liver cancer remain elusive. Among others, aberrant expression of cytotoxic cytokines is thought to be critically involved (Greten and Karin, 2004; Lee et al., 2005; Lowes et al., 2003; Vainer et al., 2008).
The pro-inflammatory and homeostatic cytokines LTα and LTβ are members of the tumor necrosis factor (TNF) superfamily. Under physiological conditions LTs are expressed by activated T-, B-, NK- and lymphoid tissue inducer cells (Fu et al., 1998; Ware, 2005) and are crucial for organogenesis and maintenance of lymphoid tissues (Tumanov et al., 2003). Whereas LTβ contains a transmembrane domain, LTα is soluble. Consequently, LT can exist as membrane bound heterotrimers (LTα1β2 or LTα2β1) interacting with LTβR or as soluble secreted homotrimers (LTα3) triggering TNF receptor 1, 2 (TNFR1, TNFR2) and the herpes virus entry mediator receptor (HVEM) (Browning et al., 1997; Ware, 2005). LTβR and TNFR1 signaling can be activated by the HCV-core protein (Chen et al., 1997; Zhu et al., 1998) involving the canonical or non-canonical NF-κB signaling pathways (Ware, 2005; You et al., 1999). Furthermore, HBV- or HCV-infections lead to increased hepatic LT expression in vivo and in vitro (Lee et al., 2005; Lowes et al., 2003) and HCV replication has been demonstrated to depend on components of the LTβR signaling pathway in vitro (Ng et al., 2007).
LTs can directly act on hepatocytes which physiologically express high levels of LTβR but little LT (Browning and French, 2002). T-cell-derived LT and LIGHT (LT-like, exhibits inducible expression, competes with HSV glycoprotein D for HVEM, expressed by T-lymphocytes) signaling to hepatocytes controls lipoprotein homeostasis (Lo et al., 2007). In addition, LT signaling is important for liver regeneration through T-cell-derived LT expression (Tumanov et al., 2008) and regulates hepatic stellate cell function and wound healing (Ruddell et al., 2008). Thus, hepatic LTβR signaling controls liver homeostasis in both health and disease.
Promotion of HCC formation by chronic inflammatory stimuli has been recapitulated in various animal models. Ablation of the multi-drug resistance gene 2 (mdr2) induces cholestatic hepatitis and liver cancer (Pikarsky et al., 2004) and administration of the chemical carcinogen diethylnitrosamine (DEN) causes acute liver injury and HCC (Maeda et al., 2005). Liver specific expression of the hepatitis B surface antigen (HBsAg) in mice demonstrates that chronic immune-mediated liver cell injury is critical for HCC formation (Nakamoto et al., 1998).
Triggering TNFR1 or LTβR induces the classical and alternative NF-κB signaling pathways, which are linked to inflammation-induced carcinogenesis (Greten and Karin, 2004). However, the precise role of these pathways in HCC pathogenesis is controversial (Vainer et al., 2008). Mice lacking IKappa B kinase β (IKKβ) specifically in hepatocytes (IkkβΔhep) exhibit a marked increase in DEN-induced HCC formation suggesting a protective function of IKKβ in HCC development (Maeda et al., 2005). In contrast, NF-κB signaling promotes HCC development in mdr2−/− mice and anti-TNFα treatment is protective (Pikarsky et al., 2004). Interestingly, mice with a hepatocyte-specific deletion of IKKγ (also called NEMO) develop steatohepatitis and HCC (Luedde et al., 2007). Consequently, the role of NF-κB signaling in hepatocarcinogenesis might depend on the mouse model and the type or degree of liver inflammation and injury (Vainer et al., 2008). Here, we investigated a possible causal relationship between sustained hepatic LTβR-signaling, chronic hepatitis and HCC development.
Results
Upregulation of LTα, LTβ and LTβR in HBV or HCV infected human livers and in HCC
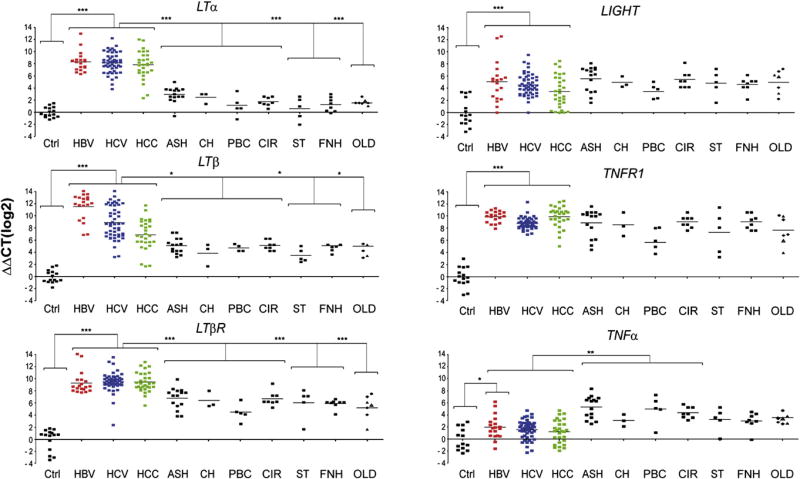
The specific role of LT signaling in the pathogenesis of virus-induced hepatitis and HCC formation is not completely defined. We analyzed transcriptional levels of LTα, LTβ, LIGHT, TNFα, LTβR and TNFR1 in human HBV- or HCV-induced chronic hepatitis and HCC or in non-viral HCC compared to healthy liver specimens (Figure 1, Figure S1). LTα, LTβ and LTβR mRNA expression was increased on average ~27 to 210 fold in HBV- or HCV-induced hepatitis and HCC (P<0.001), LIGHT transcripts were less, but still significantly, elevated (on average ~23 to 25 fold; P<0.001). Likewise, TNFR1 mRNA expression was significantly increased in HBV- or HCV-induced hepatitis and HCC (on average ~27 to 29 fold; P<0.0001). In contrast, TNFα was only slightly upregulated in HBV-induced hepatitis (P=0.04) but not in HCV-induced hepatitis (P=0.3) and HCC (P=0.4).
Figure 1. mRNA expression of TNF-superfamily members in viral (HBV, HCV induced) and non-viral liver diseases.
Analysis of hepatic LTα, LTβ, LTβR, LIGHT, TNFR1, and TNFα transcription by real-time PCR. Healthy individuals (Ctrl; n=15), patients chronically infected with HBV (n=19), HCV (n=49), affected by HCC (n=30) or suffering from various non-virus related liver disorders were investigated. Non-virus related liver diseases with hepatitis include alcoholic steatohepatitis (ASH; n=13), cholestasis (CH; n=3), primary biliary cirrhosis/ autoimmune cholangitis (PBC; n=5), end stage liver cirrhosis due to alcoholic liver disease (CIR; n=8), α1-antitrypsin deficiency (α1AT; n=1) and focal liver fibrosis (FLF; n=2). Non-virus related liver diseases without hepatitis include steatosis (ST; n=5), hemochromatosis/siderosis (HE; n=3), and Wilson’s disease (WD; n=1). Focal nodular hyperplasia (FNH; n=8) was investigated as a benign primary liver tumor. Diseases such as α1AT (●), FLF (▲), HE/SID (◆), and WD (△) are listed under “other liver diseases” (OLD). Horizontal bars represent the average mRNA expression level. The y-axis describes the ΔΔCT values on a log2 scale. *, **, *** indicate statistical significance: * = p≤0.05; ** = p<0.001; *** = p<0.0001.
In most cases, HCV genotype, degree of inflammation (Knodell score), fibrosis (Metavir score) and liver enzyme levels (ALT; AST) were assessed (Tables S1–S5). Levels of LTα, LTβ and LTβR mRNA did not correlate with the degree of liver inflammation (P=0.5), fibrosis (P=0.5), patient age (P=0.5), gender (P=0.5), HCV genotype or type of virus infection (HBV; HCV; HBV/HCV co-infection in the case of some HCC; P=0.5) (Figure S1; data not shown). To determine whether upregulation of LT ligands and receptors was specific for HBV- or HCV-induced liver diseases, we examined transcript levels in non-viral liver diseases. These included liver disorders with hepatitis [alcoholic steatohepatitis (ASH); cholestasis (CH); primary biliary cirrhosis/autoimmune cholangitis (PBC); end-stage liver cirrhosis due to alcoholic liver disease (CIR)] and liver diseases without inflammation [steatosis (ST), focal nodular hyperplasia (FNH)]. Additionally, other liver diseases (OLD) such as hemochromatosis/siderosis, Wilson’s disease, focal liver fibrosis, α1-antitrypsin deficiency and non-viral HCC (NVH) were investigated.
Levels of LTα, LTβ and LTβR mRNA were significantly lower in all non-viral liver diseases analyzed except NVH when compared to virus-induced chronic hepatitis and HCC (LTα: P<0.0001; LTβ: P=0.05; LTβR: P<0.0001; Figure 1, Figure S1). This was irrespective of whether non-viral liver diseases were associated with inflammation. LIGHT and TNFR1 mRNA expression in non-viral liver diseases including NVH was similar to HBV- or HCV-induced chronic hepatitis and HCC. In contrast, TNFα mRNA expression was significantly higher in non-viral liver diseases with inflammation and NVH compared to healthy livers (P<0.0001), HBV- or HCV-induced hepatitis and HCC (P<0.0001).
Increased chemokine expression in HBV- or HCV-induced hepatitis and HCC
To confirm that pro-inflammatory signaling cascades are activated during HBV- or HCV-induced hepatitis and HCC formation, chemokine mRNA levels were measured (Figure S1). CCL2, CCL3, CCL5 and CXCL10 mRNA expression was significantly higher in human HBV- (P<0.0001) or HCV- (P<0.0001) induced hepatitis and HCC (P<0.0001) compared to healthy controls. CXCL1 mRNA expression was significantly increased in HBV-induced hepatitis (P<0.0001) and HCC (P=0.02) but not in HCV-induced hepatitis (P=0.07).
Upregulation of LTα, LTβ, LIGHT in human hepatoyctes upon HCV infection in vitro
We next investigated whether LTα, LTβ, LIGHT and LTβR transcripts can be upregulated in hepatocytes as a consequence of viral infection. The human hepatocyte cell line Huh-7.5 (Blight et al., 2002) was challenged with infectious HCVcc (Pietschmann et al., 2006) and the expression of cytokines and chemokines was measured (Figure S2A). At 48 to 72 hrs post infection, transcripts of LTα (P=0.05), LTβ (P=0.05), LIGHT (P=0.05), LTβR (P=0.05) and chemokines (CCL2, CCL3, CXCL1 and CXCL10) were increased (2–32 fold) in HCVcc-infected compared to non-infected Huh-7.5 cells.
Identification of liver cells expressing LTβR and its ligands in HBV or HCV infections
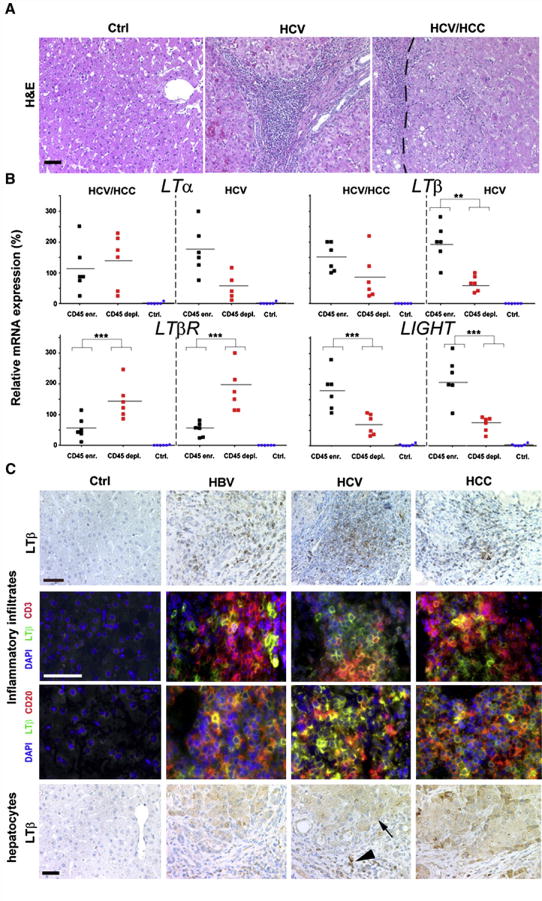
To identify the cellular source of LTα, LTβ, LTβR and LIGHT expression in human HCV-infected livers, cells were collected from HCV-induced hepatitis and HCC (Figure 2A, Figure S2B). Liver cells were sorted according to their CD45 surface expression, resulting in CD45-enriched (T-, B-cells; monocytes, macrophages/Kupffer cells; dendritic and NK-cells) or CD45-depleted (hepatocytes, oval cells, bile duct epithelial and endothelial cells) fractions. Purity of these fractions was assessed by real-time PCR for lymphocyte (CD3; CD20; CD45) or hepatocyte (cytokeratin 18) markers. CD45-depleted fractions displayed only a minor contamination with CD45 mRNA (~1–10%) whereas CD45-enriched fractions showed only a minor amount of cytokeratin 18 mRNA transcripts (~2–20%; Figure S2C; data not shown). Unsorted liver cells of healthy individuals were included as controls. Because of ethical consideration not enough human healthy liver tissue was available in order to perform cell sorting.
Figure 2. Identification of cell types expressing TNF-superfamily members in virus-infected or HCC-affected livers.
(A) Histology of representative paraffin sections of healthy controls, HCV-infected livers, and HCC with HCV etiology. HCV-infected livers (HCV) and tumors (HCV/HCC) display leukocytic infiltrates. H&E: Hematoxylin and eosin staining. The tumor border is indicated by a dashed line (scale bar: 100μm). (B) Real-time PCR analysis of sorted, CD45-enriched or CD45-depleted liver cells. For control, whole liver cell populations derived from healthy or diseased livers (HCV infected/HCC) were used. mRNA expression levels are normalized to unsorted, total cell populations of the respective liver disease and calculated as 100%. The average expression level is indicated as percentage of control (unsorted cells of the respective disease) and demarcated by horizontal bars. *, **, *** indicate statistical significance (Student’s T-test): * = p≤0.05; ** = p<0.001; *** = p<0.0001. (C) Immunohistochemical (upper and lower panel) and immunofluorescence analysis for LTβ expression in healthy, HBV- or HCV-infected and HCC-affected livers (scale bar: 50μm). Arrowhead depicts LTβ+ leukocytes, arrow LTβ+ hepatocytes.
Within HCV-induced HCC, CD45-enriched and -depleted liver cells expressed similar LTα or LTβ mRNA levels (LTα: P=0.8; LTβ: P=0.1) that were significantly higher than in controls (P<0.0001) (Figure 2A). LTβR mRNA transcript levels were significantly higher in CD45-depleted cells compared to CD45-enriched cells (P=0.006), or controls (P<0.0001). In contrast, LIGHT mRNA expression was significantly increased in CD45-enriched cells when compared to CD45-depleted cells (P=0.008), or controls (P=0.0007).
Within HCV-induced hepatitis, CD45-enriched cells exhibited a trend towards increased LTα mRNA levels (P=0.089) and a significant rise in both LTβ and LIGHT transcripts compared to CD45-depleted cells (LTβ: P=0.006; LIGHT: P=0.01), or controls. Similar to HCV-induced HCC, LTβR mRNA expression was significantly higher in CD45-depleted cells compared to CD45-enriched cells (P=0.002), or controls (P<0.0001). Thus, both CD45-enriched and CD45-depleted cell fractions express LTα, LTβ and LIGHT in HCV-induced hepatitis and HCC.
Immunohistochemical analysis for LTβ protein expression corroborated these data: CD3+, CD20+ lymphocytes and hepatocytes in HBV- or HCV-induced hepatitis and HCC, but not those in healthy liver specimens, express LTβ protein (Figure 2C).
Hepatocyte-specific LTα and β overexpression induces chronic progressive hepatitis
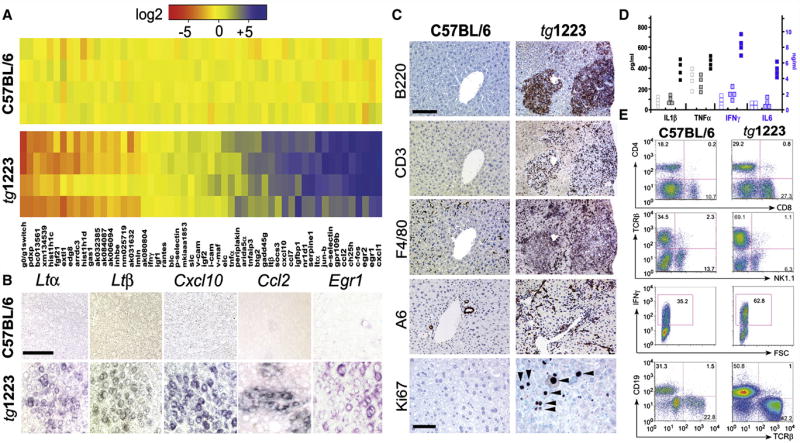
To determine whether sustained hepatic LTβR signaling is causally linked to chronic hepatitis and liver cancer, we analyzed two transgenic mouse lines that expressed LTα and β in a liver specific manner at low (tg1222) or high (tg1223) level (Heikenwalder et al., 2005). Although livers of tg1222 and tg1223 mice were histologically indistinguishable from those of control littermates at three months of age (Figure S3), the hepatic transcriptome was already considerably altered in tg1223 and to a lesser degree in tg1222 mice (Figure 3A; data not shown). Genes with the most dramatic expression changes were identified by DNA-microarray analysis and confirmed by real-time PCR (Figure 3A). As expected, Ltα and Ltβ transcripts were increased in tg1222 and tg1223 livers (Figure 3A; data not shown). Additionally, mRNA expression of chemokines (Ccl2, Ccl7, Cxcl1, Cxcl10), genes involved in early growth response (e.g. Egr1, Egr2), cholesterol metabolism (e.g. Ch25h) and immediate early response (e.g. c-Fos, Jun-b, Socs-3) were significantly (P<0.0001) elevated. In contrast, genes involved in cell cycle control, histone modifications and cell metabolism were significantly downregulated (P<0.0001) (Figure 3A; Tables S6–S8, Figure S4). In situ hybridization revealed Ltα, Ltβ, Cxcl10, Ccl2 and Egr1 mRNA transcripts in hepatocytes of 3 months old tg1223 (Figure 3B, Figure S3).
Figure 3. Characterization of tg1223 livers.
(A) Real-time PCR analysis for mRNA expression in liver of candidate genes at the age of 3 months. Data are presented in a log2 scale (blue: upregulated; red: downregulated). Rows indicate individual mice; columns represent particular genes. Each data point reflects the median expression of a particular gene resulting from 3–4 technical replicates, normalized to the mean expression value of the respective gene in C57BL/6 livers. (B) In situ hybridization of C57BL/6 and tg1223 liver sections with Ltα, Ltβ, Cxcl10, Ccl2 and Egr1 antisense probes (age of 3 months). Multiple scattered foci of hepatocyte-specific Ltα, Ltβ, Cxcl10, Ccl2 and Egr1 mRNA were detected (scale bar: 50μm). (C) Immunohistochemical analysis of representative 9 month-old C57BL/6 and tg1223 livers. B220+ stained B-cells, CD3+ T-cells, F4/80+ macrophages, Kupffer cells and A6+ oval cells (scale bar: 150μm). Ki67+ proliferating hepatocytes (arrow heads) and inflammatory cells are indicated (scale bar: 50μm). (D) ELISA for IL1β, TNFα, IFNγ and IL6 in C57BL/6 (hollow symbols), tg1223 (filled symbols) or tg1222 (dotted symbols) liver homogenates (9 months). (E) Flow cytometry of intrahepatic lymphocytes at 9 months of age. CD4 (T-helper cells), CD8 (cytotoxic T-cells), TCRβ (T-cells), CD19 (B-cells), IFNγ (Interferon γ). IFNγ expression was monitored on CD4+/CD8+ gated T-cells. Representative flow cytometry analyses are shown. Numbers in each quadrant indicate the relative percentage of cells. Staining intensity is depicted on a log scale. FSC: Forward scatter.
At the age of 4 months a slight increase in intrahepatic CD11b+, CD68+ and MHCII+ cells was detected in tg1223 mice compared to age-matched tg1222 or C57BL/6 mice (Figure S3; data not shown). At this time point, no significant increase in IL1β, IFNγ, IL6 and TNFα protein levels was found (data not shown). At 4–6 months transgenic livers started to develop strong portal and lobular (tg1223) or weak portal (tg1222) inflammation consisting of CD4+, CD8+ T-cells, B220+ B-cells and CD11c+ dendritic cells (Figure S3; Heikenwalder et al., 2005).
At ≥ 9 months of age, tg1223 livers exhibited strong portal and lobular lymphocytic infiltrates (Figure 3C). A pronounced influx of F4/80+ macrophages and proliferation of A6+ oval cells was observed. Chronic inflammation coincided with increased proliferating Ki67+ hepatocytes (tg1223: 17±5 Ki67+cells/mm2 liver section; C57BL/6: 0.5±0.3 Ki67+cells/mm2 liver section; P=0.003), which was not significant in age-matched tg1222 livers (P=0.08; Figure 3C; data not shown).
At this stage, hepatitis was accompanied by increased protein levels of IL1β (P=0.05), IFNγ (P=0.05) and IL6 (P=0.05) and, to a lesser degree, of TNFα in tg1223 livers. In tg1222 livers we observed only a slight elevation of these cytokines compared to C57BL/6 (Figure 3D). Quantitative analysis of total intrahepatic lymphocytes revealed an increase in tg1223 livers (C57BL/6: 17–24×106 cells/liver; tg1223: 35–73×106 cells/liver P<0.05). Intrahepatic lymphocytes were further characterized by flow cytometry (Figure 3E). Frequencies of CD8+ (C57BL/6: 18±11%; tg1223: 38±10%), CD4+ (C57BL/6: 16±3%; tg1223: 26±6%) and TCRβ+ T-cells (C57BL/6: 33.5±9%; tg1223: 63.5±4%) were elevated (n=4), while NK1.1+ cells (C57BL/6: 12±2%; tg1223: 7±2%) were reduced in tg1223 livers. Furthermore, an increase in the frequency of CD19+ B-cells was found in tg1223 livers (C57BL/6: 25±7%; tg1223: 52±4%). Elevated frequencies of IFNγ-producing CD4+ and CD8+ T-cells were found in tg1223 mice, while IL17-producing cells remained unchanged (Figure 3E; data not shown).
LTα and LTβ overexpression induces hepatotoxicity
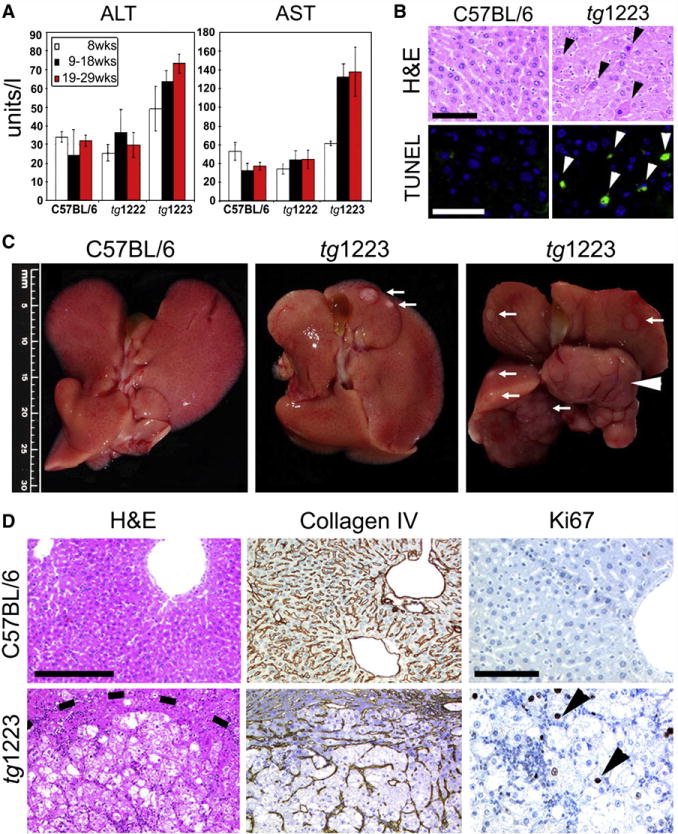
To determine whether chronic hepatitis leads to hepatocyte cell death in tg1222 or tg1223 mice, we analyzed serum transaminase levels (ALT and AST). From the age of 19 weeks on, serum ALT and AST levels were significantly elevated (P=0.05) in tg1223 but not in tg1222 mice (Figure 4A) and apoptotic hepatocytes were frequently detected in tg1223 mice (tg1223: 40.3±11.4 TUNEL+cells/mm2 liver section; C57BL/6: 3.9±6.2 TUNEL+cells/mm2 liver section; P=0.0005) but rarely in tg1222 and virtually absent in C57BL/6 mouse livers from the age of 6 months on (Figure 4B, Figure S5; data not shown for tg1222).
Figure 4. Chronic liver injury and HCC development in tg1223 mice.
(A) Significant elevation of transaminases (ALT, AST) in sera of tg1223 mice from 19 weeks of age. Standard deviation (+/− SD) is indicated by error bars. (B) Increased hepatocyte cell death in tg1223 livers documented by H&E staining and TUNEL/DAPI assay. H&E: Hematoxylin & eosin: Black arrowheads indicate apoptotic hepatocytes. TUNEL: Green TUNEL+ hepatocyte nuclei indicate apoptosis (white arrowheads; scale bars: 50μm). (C) Macroscopy of C57BL/6 (left panel) and tg1223 livers at the age of 12 (middle panel) and 18 months (right panel). White arrows indicate tumor nodules. White arrowhead indicates a liver lobe completely affected by HCC. Scale bar size is indicated. (D) Histological analysis of livers derived from C57BL/6 and tg1223 mice. Dashed line depicts the HCC border. Collagen IV staining highlights the broadening of the liver cell cords and loss of collagen IV networks indicative of HCC in tg1223 mice (scale bar: 200μm). High numbers of Ki67+ proliferating hepatocytes (arrowheads) are only found in tg1223 HCC (right column; scale bar: 100μm).
Hepatitis persisted in both transgenic lines for 18 months. Phenotypes were much milder in tg1222 mice, implying that the LT expression level determined the severity of inflammation and liver injury. Therefore, tg1223 mice were selected for additional experiments and further key results were obtained from this mouse line.
Microarray and real-time PCR analyses revealed elevated mRNA expression of genes involved in embryogenesis (e.g. Dmrta1), liver inflammation (e.g. Pbfe1), carcinogenesis (e.g. Phlda3, Thrsp; Kawase et al., 2009), glucose homeostasis and insulin sensitivity (e.g. Fgf21) and reduced mRNA expression of genes responsible for cell cycle control (Gadd45g) and protease inhibition (SerpinA9) in nine month-old tg1223 livers compared to C57BL/6 livers (Figure S5; Tables S9–S11). Several genes strongly up- or downregulated in 3 month-old tg1223 livers (Figure 3A) returned to normal levels at 9 months, except that Ltα and β mRNAs remained at high levels. On the other hand, genes involved in cell division, liver inflammation, lipid metabolism, wound healing and tumorigenesis were significantly upregulated (P<0.001) whereas genes involved in growth arrest and apoptosis were significantly downregulated (P<0.001) in 9 month-old compared to 3 month-old tg1223 livers (Figure S5; Tables S12–S17).
HCC development in tg1223 mice
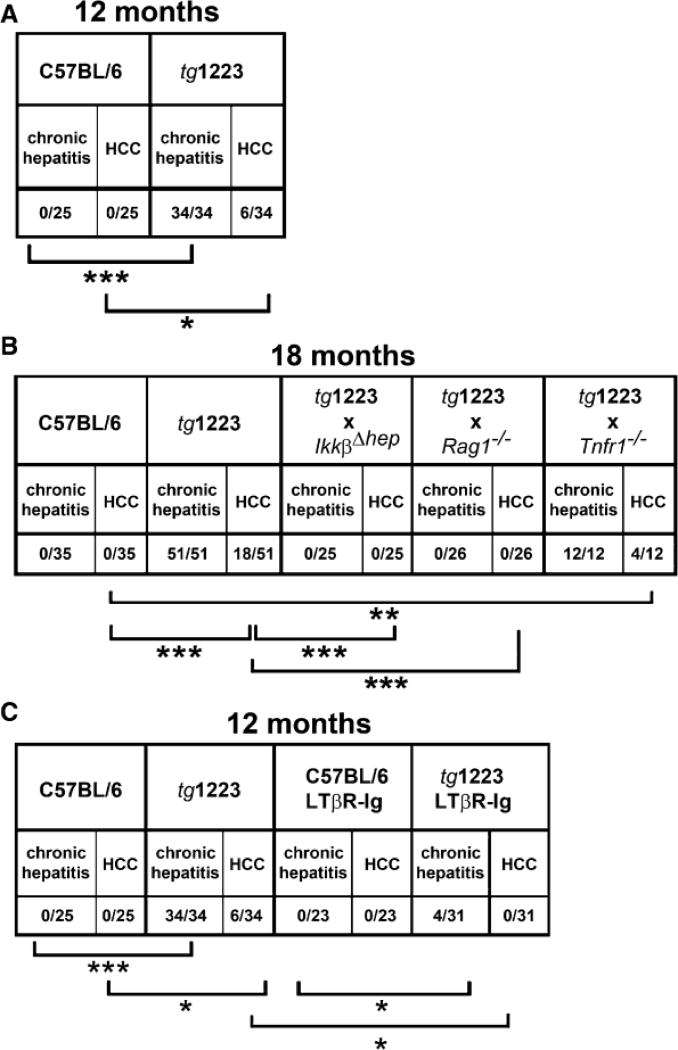
At 12 months of age, about 20% (6/34) of tg1223 mice developed macroscopically visible nodules that classified histologically as HCC, including broadening of liver cell cords, loss of collagen IV networks and increased proliferative activity. In contrast, age-matched C57BL/6 livers lacked HCC (0/20; P=0.05) (Figures 4C, 4D, and 5). Tumor frequency increased with age reaching ~35% (18/51) by 18 months, whereas C57BL/6 mice did not develop HCC (0/35; P<0.0001) (Figure 5, Figure S6). Tumors varied in size (1–25mm), histology and affected both genders with similar frequencies (males:females = 13:11; P=0.3) (Figure 4; Tables S18 and S19).
Figure 5. Chronic hepatitis and HCC incidence in tg1223 mice, tg1223 mice intercrossed with various knockout mice and LTβR-Ig treated tg1223 mice.
(A) Chronic hepatitis and HCC incidence in 12 month-old tg1223 and C57BL/6 mice. (B) Chronic hepatitis and HCC incidence in 18 month-old tg1223 and intercrossed tg1223 mice. (C) Reduced chronic hepatitis and HCC incidence in 12 month-old tg1223 mice treated with LTβR-Ig. Statistical evaluation: *, **, *** indicate the degree of statistical significance: * = p<0.05; ** = p<0.001; *** = p<0.0001.
A6+ oval cells (Engelhardt et al., 1990) were focally (8/24) or diffusely (2/24) distributed within some tg1223 HCC. The remaining tg1223 HCC (14/24) lacked A6+ cells but were surrounded by them at the border zone of HCC (Figure S6).
Chromosomal aberrations and local spread of HCC in tg1223 mice
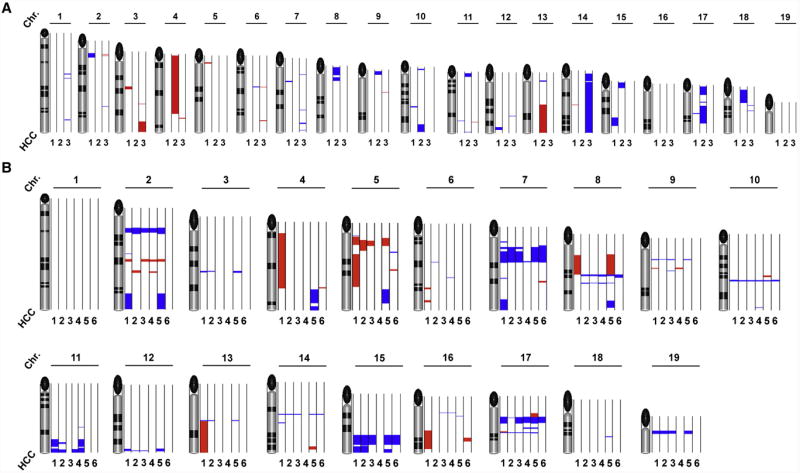
We further investigated micro-dissected tg1223 HCC (n=9) and age-matched C57BL/6 livers (n=5) for chromosomal aberrations. Array comparative genomic hybridization analysis (aCGH) revealed chromosomal aberrations in all tg1223 HCC (Figure 6). Amplifications and deletions of chromosomal regions ranged from ≤ 1 mega-base (MB) to 160 MB and were detected in most autosomes of all analyzed tg1223 HCC. Of note, the pattern of chromosomal aberrations varied in HCC from different individual tg1223 mice (P=0.34). aCGH analysis of independent C57BL/6 liver DNA samples did not reveal significant chromosomal aberrations.
Figure 6. aCGH analysis of tg1223 HCC.
The q-arm of each chromosome is shown and chromosome numbers are indicated. Black ellipses on the top of each q-arm represent the centromere. Dark horizontal bars within the symbolised chromosomes represent G bands. Chromosomal deletions are indicated in blue, amplifications in red (see methods for details). (A) HCC of individual tg1223 mice were hybridized against liver tissue of age matched C57BL/6 mice and analyzed by aCGH analysis. Columns next to each chromosome represent individual HCC (1; 2; 3) with numerous chromosomal aberrations on the q-arm of various autosomes. No common pattern of chromosomal aberrations could be detected. (B) aCGH analysis of six representative HCC (1, 2, 3, 4, 5 and 6) taken from different lobes of the same tg1223 liver.
We did not detect lung metastases but often saw multifocal intrahepatic disease in 18 month-old tg1223 mice. We therefore investigated whether multifocal tg1223 HCC represented intrahepatic spread of clonal tumors. Independent HCC (n=6) from different lobes of the same tg1223 liver were micro-dissected and subsequently analyzed by aCGH. All HCC taken from the same liver displayed significantly overlapping chromosomal aberrations throughout the entire genome (P<0.05), suggesting a clonal relationship of a tumor that has locally spread within the liver (Figure 6B).
Expression of tumor markers GP73, GS and AFP in tg1223 HCC
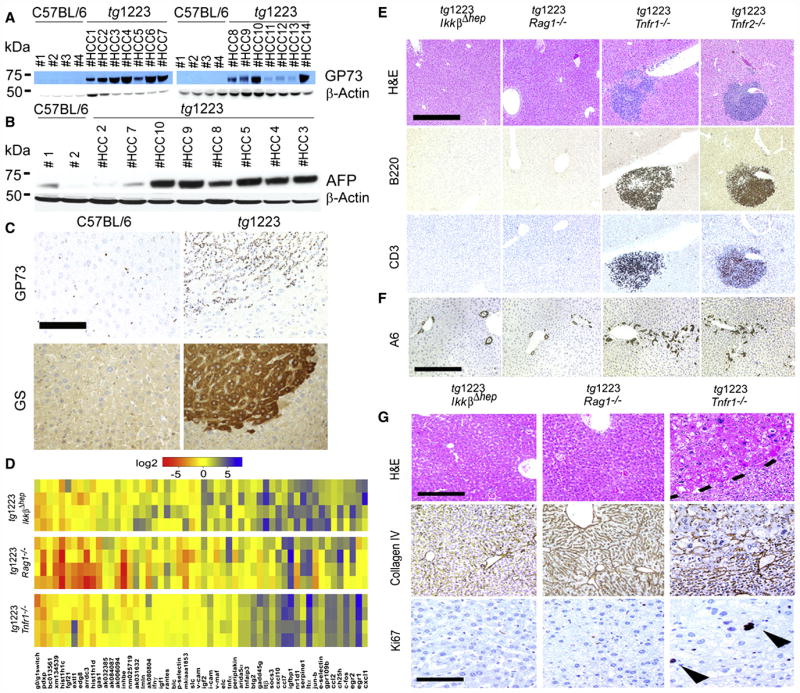
We then evaluated expression of human liver tumor markers golgi protein 73 (GP73), glutamine synthetase (GS) and α-fetoprotein (AFP) in tg1223 livers (Bachert et al., 2007; Marrero and Lok, 2004; Sakamoto, 2009). GP73, GS and AFP protein expression was elevated in most tg1223 HCC as detected by immunohistochemistry and immunoblot analysis compared to C57BL/6 livers or unaffected liver regions adjacent to HCC (Figure 7A–7C; data not shown).
Figure 7. Expression of tumor markers in tg1223 HCC and mechanistic characterization of liver carcinogenesis in tg1223 mice.
(A) Immunoblot analysis of C57BL/6 and tg1223 HCC homogenates for GP73. Strong to moderate signal intensities were detected in all tg1223 HCC, but not in C57BL/6 livers. (B) Immunoblot analysis of C57BL/6 and tg1223 HCC homogenates for AFP. β-Actin served as a loading control (kDa: kilo Dalton). (C) Immunohistochemistry for GP73 and GS in a representative tg1223 HCC and age-matched C57BL/6 control (scale bar: 100μm). (D) mRNA expression of candidate genes in livers of 3 month-old tg1223/IkkβΔhep, tg1223/Rag1−/− and tg1223/Tnfr1−/− mice. Data are presented in a log 2 scale (blue: upregulated; red: downregulated). Rows indicate individual mice; columns represent particular genes. Each data point reflects the median expression of a particular gene resulting from 3–4 technical replicates, normalized to the mean expression value of the respective gene in C57BL/6 livers. (E) Histological analysis of tg1223/IkkβΔhep, tg1223/Rag1−/−, tg1223/Tnfr1−/−, and tg1223/Tnfr2−/− livers at 9 months of age. H&E, B220 for B-cells, CD3 for T-cells (scale bar: 500μm). (F) Immunohistochemical analysis of A6+ cells (oval cells) in livers of tg1223/IkkβΔhep, tg1223/Rag1−/−, tg1223/Tnfr1−/−, and tg1223/Tnfr2−/− mice at 9 months of age (scale bar: 500μm). (G) Immunohistochemical analysis of tg1223/IkkβΔhep, tg1223/Rag1−/−, and tg1223/Tnfr1−/− livers (18 months of age). Dashed line depicts the HCC border (upper row; scale bar: 200μm). Collagen IV staining highlights the broadening of liver cell cords and loss of collagen IV networks in tg1223/Tnfr1−/− HCC. Ki67+-proliferating hepatocytes are indicated by arrowheads (lower row; scale bar: 50 μm).
Mechanisms driving LTαβ-induced chronic hepatitis and liver cancer
To identify other receptors and molecular mediators potentially involved in LT-induced chronic hepatitis and HCC development, we intercrossed tg1223 with Tnfr1−/−, Tnfr2−/− or IkkβΔhep mice. The requirement of lymphocytes in chronic hepatitis and HCC formation was investigated by intercrossing with Rag1−/− mice, which lack mature lymphocytes.
The absence of IKKβ, TNFR1 or lymphocytes per se did not appear to influence transgenic Ltα and β mRNA expression (Figures 3A and 7D). Initially, at three months of age, tg1223/IkkβΔhep, tg1223/Tnfr1−/−, tg1223/Tnfr2−/− and tg1223/Rag1−/− mice lacked histological evidence of hepatitis similar to tg1223 mice (data not shown). The aberrant hepatic gene expression pattern described for 3 month-old tg1223 mice developed only partially in tg1223/IkkβΔhep and tg1223/Rag1−/− mice, whereas tg1223/Tnfr1−/− livers displayed an expression profile rather similar to that of tg1223 mice (Figure 7D, Figure S7). At 9 months of age tg1223/Rag1−/− (n=26) and tg1223/IkkβΔhep (n=18) livers lacked hepatitis, hepatocyte or oval-cell proliferation (Figure S4), whereas tg1223/Tnfr1−/− (n=8) or tg1223/Tnfr2−/− (n=8) livers were indistinguishable from those of tg1223 mice (Figures 7E and 7F, Figure S7).
At the age of 18 months, tg1223/Rag1−/− (n=26) and tg1223/IkkβΔhep (n=25) mice were devoid of hepatitis and HCC (P<0.0001) (Figures 5 and 7G) suggesting that both lymphocytes and hepatocyte-specific IKKβ expression are required for LT-induced chronic hepatitis and HCC development.
Notably, tg1223/Tnfr1−/− mice displayed HCC (4/12) with an incidence similar to tg1223 mice (Figures 5 and 7G, Figure S8; Table S18 and S19) indicating that TNFR1 signaling is not essential for LT-induced HCC formation in tg1223 mice.
Hepatocytes are the major responsive liver cells to agonistic LTβR antibody treatment
To investigate whether hepatocytes represent the major LT-responsive liver cells and to investigate LTβR signaling in Tnfr1−/− and IkkβΔhep livers, TNFα (positive control), agonistic LTβR antibody (3C8) and appropriate negative controls (PBS; rat IgG) were administered intravenously (i.v.) to C57BL/6 and various knock-out mice (Figure 8, Figure S8). Nuclear p65 (RelA) translocation in hepatocytes and non-parenchymal cells (NPC: e.g. Kupffer cells, lymphocytes), alterations in the hepatic transcriptome and protein expression of selected chemokines were examined.
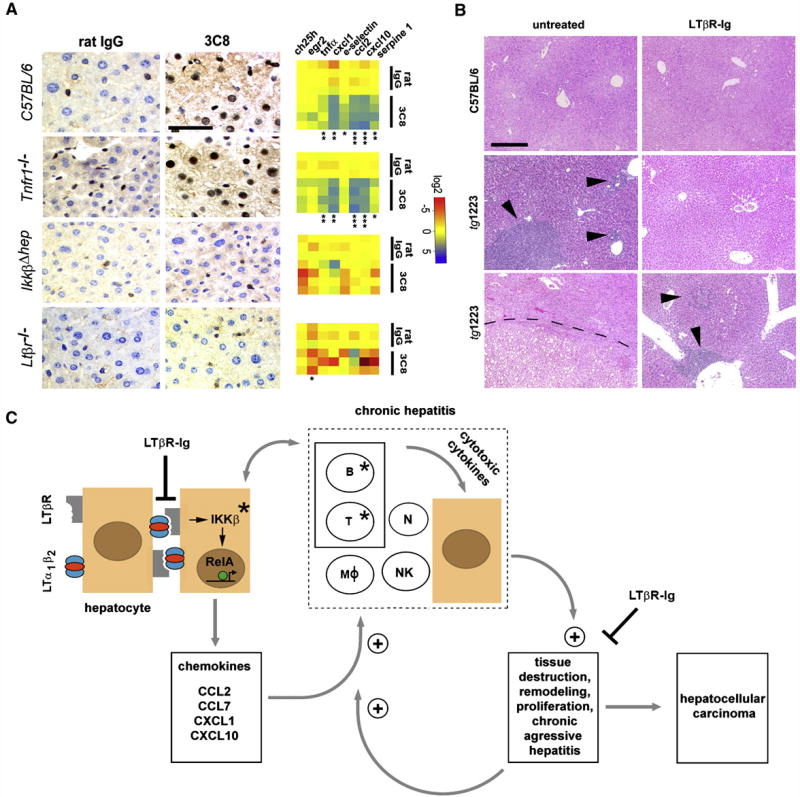
Figure 8. Effects of acute 3C8 and long-term LTβR-Ig treatment and a model of chronic inflammation-induced hepatocarcinogenesis in tg1223 mice.
(A) Immunohistochemical analysis of nuclear p65 translocation and real-time PCR for mRNA expression of selected NF-κB target genes in livers of C57BL/6 and various knock-out mice treated with 3C8. Data are presented on a log 2 scale (blue: upregulated; red: downregulated). Rows indicate individual mice; columns represent particular genes. Each data point reflects the median expression value of a particular gene resulting from 3–4 technical replicates, normalized to the mean expression value of the respective gene in C57BL/6 livers. (scale bar: 50μm). Expression data are depicted according to treatment group: rat IgG (control) or 3C8 (LTβR agonist). Statistical significance was evaluated by t-test: * = p≤0.05; ** = p<0.001; *** = p<0.0001.
(B) Histological analysis (H&E) of livers from untreated (left column) and LTβR-Ig treated (right column) C57BL/6 or tg1223 mice (12 months of age). Representative sections show no hepatitis or HCC in untreated or LTβR-Ig-treated C57BL/6 livers (upper row). Untreated tg1223 livers display hepatitis in 34/34 (middle panel, left column) and HCC in 6/34 cases (lower panel, left column). LTβR-Ig treatment reduces the incidence of hepatitis (middle and lower panel, right column) and prevents HCC formation in LTβR-Ig treated tg1223 mice. Arrowheads indicate inflammatory foci. Tumor border is indicted by a dashed line (scale bar: 200μm).
(C) Scheme of chronic inflammation-induced liver carcinogenesis in tg1223 mice: Transgenic hepatocytes (brown) express LTα, β and induce chemokine production (e.g. CCL2, CCL7, CXCL1, CXCL10) in the presence of IKKβ and intrahepatic lymphocytes. Chemoattraction and activation of myeloid cells and lymphocytes expressing particular chemokine receptors (e.g. CXCR3, CXCR2, CCR2, CCR1) causes hepatitis: CXCL10 attracts CXCR3+ T- and NK-cells, CXCL1 CXCR2+ T-, B-cells and neutrophils, CCL2 CCR2+ macrophages and CCL7 attracts CCR1+ monocytes. Activated, infiltrating immune cells secrete cytotoxic cytokines (e.g. IL6; IL1β, TNFα, IFNγ, LTαβ) that cause tissue destruction, hepatocyte proliferation, cell death and tissue remodeling. In such an environment, hepatocytes are susceptible to chromosomal aberrations leading to HCC. Tissue destruction and remodeling supports the infiltration of activated inflammatory cells (e.g. myeloid cells) leading to a feed-forward loop towards chronic aggressive hepatitis. Asterisks indicate that genetic depletion of those components (IKKβ; T- and B-cells) blocks chronic hepatitis development and HCC. Blocking LTβR signaling with LTβR-Ig in 9 month-old tg1223 mice reduces chronic hepatitis incidence and prevents HCC. +: indicates the fortification of a described process. ┤: indicates the suppression of a described process. The transcription factor RelA is schematically depicted as a green circle, inducing transcription of NF-κB target genes (e.g. chemokines) (arrow). B, T: B- and T-cells. MØ: macrophages. N: neutrophils. NK: NK-cells.
Administration of 3C8 induced nuclear p65 translocation primarily in hepatocytes and some NPC of C57BL/6 livers (Figure 8A), as well as transcriptional changes and upregulation of selected chemokines reminiscent of those observed in 3 month-old tg1223 livers (Figure 8A, Figure S9). Similar results were obtained after 3C8 treatment of Tnfr1−/− mice, in contrast to IkkβΔhep livers which were devoid of nuclear p65 translocation in hepatocytes and NPC (Table S20 and S21). Furthermore, upregulation of selected NF-κB target genes could not be detected. Control Ltβr−/− mice treated with 3C8 lacked nuclear p65 translocation in hepatocytes or NPC as well as upregulation of selected NF-κB target genes.
To examine whether lack of functional IKKα on hepatocytes and NPC would suppress LTβR induced upregulation of selected NF-κB responsive genes, we investigated livers of mice expressing a non-phosphorylatable IKKαAA knock-in allele (IkkαAA/AA; Cao et al., 2001). Upon 3C8 treatment Ikkα AA/AA mice upregulated selected NF-κB responsive genes (Figure S8). The degree of mRNA upregulation in liver was similar to 3C8-treated C57BL/6 mice. In contrast, control treated (rat IgG) Ikkα AA/AA mice lacked upregulation of selected NF-κB responsive genes. This suggests that 3C8-mediated hepatic LTβR signaling is mainly integrated by hepatocytes involving canonical NF-κB pathway.
Inhibition of LTβR signaling reduces chronic hepatitis and carcinogenesis
We further investigated the involvement of LTβR signaling in the transition of chronic hepatitis to HCC by long-term LTβR-Ig administration in tg1223 mice. Nine month-old tg1223 mice with chronic hepatitis (n=31) or age-matched C57BL/6 mice (n=23) were treated with LTβR-Ig for 2 months, remained untreated for another 4 weeks and were then sacrificed. LTβR-Ig treatment significantly reduced chronic hepatitis incidence in tg1223 mice compared to untreated tg1223 mice (treated: 4/31; untreated: 34/34; P<0.0001). Furthermore, LTβR-Ig treatment suppressed chronic hepatitis-driven HCC formation (treated: 0/31; untreated: 6/34; P<0.05) (Figures 5 and 8B). LTβR-Ig treatment did not lead to overt histopathological alterations in C57BL/6 livers or overt changes in lymphocyte (B-, T-cells) or macrophage populations within spleens of C57BL/6 or tg1223 mice (data not shown). Efficiency of LTβR-Ig treatment was ascertained by the loss of LTβR-dependent follicular dendritic cells (FDCs) within C57BL/6 and tg1223 spleens (Figure S9). Thus, our results imply that long-term suppression of LTβR reduces chronic hepatitis incidence and can prevent the transition from chronic hepatitis to HCC in tg1223 mice.
Discussion
This study uncovered drastic and robust mRNA upregulation of LTβR, LTα and LTβ in HBV- or HCV-induced hepatitis and HCC. LT and LIGHT transcripts were mainly expressed by CD3+ T- and CD20+ B-cells; a significant proportion of LTα and LTβ expression was also attributable to hepatocytes. Notably, upregulation of LTβR, LTα and LTβ transcripts was also detected in non-virus related HCC, which could stem from activated, tumor infiltrating lymphocytes and/or from neoplastic hepatocytes that have upregulated LT, possibly in response to IL6. It was demonstrated that HCC derived cell lines express IL6 (Baffet et al., 1991) and that LT levels are increased in response to IL6 in the latter (Subrata et al., 2005). LT signaling induces both canonical and non-canonical NF-κB signaling pathways, whose role in controlling liver cancer formation remains controversial (Vainer et al., 2008). In a mouse model with acute DEN exposure, depletion of functional NF-κB signaling (IkkβΔhep mice) increased hepatocyte cell death, enhanced Kupffer-cell activation and elevated HCC incidence (Maeda et al., 2005). In contrast, NF-κB signaling promotes HCC development in mdr2−/− mice (Pikarsky et al., 2004) and hepatocyte-specific depletion of IKKβ prevents HCC formation in tg1223 mice. How can this contradictory role of IKKβ signaling in HCC formation be reconciled? On the one hand IKKβ signaling might be required for hepatocytes to appropriately respond to and survive carcinogenic stimuli and acute liver injury (e.g. DEN exposure). On the other hand, IKKβ signaling might enable chemokine expression by hepatocytes leading to hepatitis and HCC. Consistent with this hypothesis, tg1223/Rag1−/− mice were devoid of chronic hepatitis, hepatocyte or oval-cell proliferation and failed to develop HCC.
Why could immune cells contribute to liver tumorigenesis? One explanation might be that CD4+ or CD8+ T-cells expressing inflammatory cytokines (e.g. IL1β, TNFα, IFNγ) as well as cytolytic proteins (e.g. Granzyme B) contribute to hepatocyte cell death, tissue remodeling and transformation, finally leading to HCC (Budhu and Wang, 2006; Nakamoto et al., 1998). Intrahepatic lymphocytes may also influence the production of inflammatory mediators, as 3 month-old tg1223/Rag1−/− livers displayed markedly reduced cytokine and chemokine levels. We propose that rather than directly acting as a cell-autonomous oncogene on hepatocytes or A6+ oval cells, hepatic LTαβ expression induces local upregulation of chemokines (e.g. Ccl2; Cxcl10, Cxcl1, Ccl7) by hepatocytes. This leads to the attraction of circulating inflammatory cells and a hyperproliferative, hepatotoxic environment stochastically leading to HCC formation (Figure 8C). It is worth mentioning that some chemokines found in this study (e.g. CXCL10) have been reported to be mainly expressed by human hepatocytes in chronic hepatitis C (Zeremski et al., 2007).
Ablation of TNFR1 signaling did not prevent chronic hepatitis and HCC formation in tg1223 mice although anti-TNFα antibody treatment prevents HCC development in mdr2−/− mice (Pikarsky et al., 2004). We investigated the mode of LT signaling in Tnfr1−/− livers upon 3C8 treatment. This induced analogous hepatic changes seen in tg1223 mice at 3 months of age. Similar to our results with tg1223/Tnfr1−/− mice, this suggests that heterotrimeric LT causes p65 translocation in hepatocytes and induces a TNFR1-independent signaling cascade via LTβR, presumably contributing to chronic hepatitis and HCC. Most probably, HCC formation in mdr2−/− mice depends on pathways involving TNFR1 distinct from the LTβR-dependent pathways described in our study.
I.v. administration of TNFα into IkkβΔhep mice did not cause p65 translocation in hepatocytes but upregulated NF-κB target genes, presumably through TNFα-activated NPC. In contrast, 3C8 treatment in IkkβΔhep mice had no effect. Therefore, hepatocytes but not NPC are likely to be the major liver cells integrating LT signaling. Interestingly, upon 3C8 treatment IkkαAA/AA livers upregulated selected NF-κB target genes similar to C57BL/6 mice (Figure S8). Therefore, absence of IKKα in hepatocytes and NPC, allows NF-κB target gene expression upon 3C8 treatment, suggesting the involvement of the classical NF-κB pathway in LTβR-induced hepatic signaling.
LTβR signaling was reported to induce oval-cell proliferation (Akhurst et al., 2005), which is thought to contribute to the development of liver tumors (Lee et al., 2006). We observed proliferation of A6+ oval cells in chronically inflamed tg1223 livers at the age of 9 months and found A6+ cells within and at the border of tg1223 HCC. Whether those A6+ cells represent transformed oval cells contributing to liver carcinogenesis or whether A6 is upregulated on aberrant hepatocytes within HCC remains to be determined.
Lack of lymphocytes or chronic hepatitis prevented oval-cell proliferation, although LTα, β transgene expression was unaltered. Therefore, it is conceivable that activated, infiltrating lymphocytes or Kupffer cells may contribute to oval cell proliferation by providing further LT or other cytokines in tg1223 livers. Based on the presented data, a sequence of events leading to chronic hepatitis and HCC in tg1223 mice can be proposed (Figure 8C).
What are the possible clinical implications of our findings? It has recently been demonstrated that pharmacological inhibition of LTβR signaling reduces virus-, bacteria- and concavalin A-induced liver injury (An et al., 2006; Anand et al., 2006; Puglielli et al., 1999), while triggering LTβR signaling on hepatocytes appears beneficial during liver regeneration (Tumanov et al., 2008). Moreover, siRNA knock-down of various components of the LTβR signaling pathway (e.g. LTβ; Rel A) were shown to interfere with HCV replication in vitro (Ng et al., 2007). Therefore, inhibition of LTβR signaling might also impede the efficiency of HCV replication. But what are the possible side-effects of blocking LTβR signaling? Effects include alterations in the microarchitecture of white pulp follicles and disappearance of FDC networks in non-human primates (Gommerman et al., 2002). Of note, despite the loss of FDCs and a reduced capacity to trap immune complexes the primary antibody response to keyhole limpet hemocyanin was not significantly altered (Gommerman et al., 2002).
Accordingly, we have investigated a possible beneficial effect of blocking LTβR signaling in tg1223 mice with chronic hepatitis. This partially reverted inflammation and prevented HCC formation, suggesting that LTβR-Ig treatment might be beneficial in liver pathologies with sustained LT signaling.
Our results show that LT signaling is critically involved in hepatitis and subsequent HCC development and imply that blocking LTβR signaling might become a beneficial therapeutic approach in the context of HBV- or HCV-induced chronic hepatitis and other liver diseases displaying sustained hepatic LTβR signaling.
Experimental Procedures
Human liver tissue
Human liver biopsies were obtained from University Hospitals Zurich, Freiburg, Grenoble, Heidelberg and Graz. Biopsies were registered in the respective biobanks and kept anonymous. The research project was authorized by the ethical committees of the “Gesundheitsdirektion Kanton Zürich” (Ref. Nr. StV 26-2005), Freiburg (Nr. 299/2001), Heidelberg (Prof. Bannasch), Graz (Ref. Nr. 1.0 24/11/2008) and Grenoble (Ref. Nr. 03/APTF/1). The study protocol was in accordance with the ethical guidelines of the Helsinki declaration. Patients were enrolled after giving their written informed consent. HBV- or HCV infected patients with chronic hepatitis were not treated with ribavirin or other immunomodulatory drugs at the time point of needle biopsy.
Mice
Animals were maintained under specific pathogen-free conditions and experiments were approved and conform with the guidelines of the Swiss Animal Protection Law, Veterinary office, Canton Zurich. Mouse experiments were performed under licences 198/2007; 83/2007; 30/2005 according to the regulations of the Veterinary office of the Canton Zurich. Tg1223, tg1222, Tnfr1−/−, Tnfr2−/−, Rag1−/−, Ltβr−/−, IkkαAA/AA and IkkβΔhep mice were generated as previously published (Bluethmann et al., 1994; Cao et al., 2001; Futterer et al., 1998; Heikenwalder et al., 2005; Maeda et al., 2005; Mombaerts et al., 1992).
TNFα and 3C8 treatment
Twelve to fourteen week-old male mice (C57BL/6 and knock-out mice) were i.v. injected with either PBS, murine recombinant TNFα (50μg/kg bodyweight; R&D Systems), agonistic LTβR antibody (50μg/mouse; clone 3C8; eBioscience) or rat IgG (50μg/mouse; eBioscience) and sacrificed for analysis 45 min post injection. All substances were injected at a total volume of 100μl dissolved in PBS.
Isolation of intrahepatic murine lymphocytes (IHL)
Mice were anesthetized and liver was perfused with PBS to remove circulating leukocytes, then isolated liver tissue was minced and digested in medium containing collagenase (1mg/ml) and DNase (25μg/ml) at 37°C for 40 min. Cells were centrifuged at 300rpm for 3 min to sediment the majority of hepatocytes. Supernatant was removed and centrifuged again at 1200 rpm for 10 min. Cell pellet was resuspended in the 40% fraction of a 40:80 Percoll gradient. Upon centrifugation at 2500rpm for 20 min, IHL were collected at the interface. IHL were analyzed for surface marker expression by staining with anti-CD4, anti-CD8, anti-TCR-β, anti-NK1.1 or anti-CD19 Abs, and for cytokine production capacity by intracellular staining with anti-IFNγ and anti-IL17 Abs (all from eBioscience) upon PMA/Ionomycin stimulation by using a two-laser FACScalibur (BD). Analysis was executed with CellQuest and FlowJo software. Cytokine production by IHL was determined by intracellular staining with anti-IFNγ and anti-IL17 antibodies (all from eBioscience) after 4 hrs stimulation with PMA and Ionomycin.
Measurement of aminotransferases
The analysis for AST and ALT was performed with mouse serum on a Roche Modular System (Roche Diagnostics) with a commercially available automated colorimetric system at the Institute of Clinical Chemistry at the University Hospital Zurich using a Hitachi P-Modul (Roche).
Supplementary Material
Significance.
Pharmacological inhibition of LTβR signaling reduces pathogen- and concavalin A-induced liver injury while LTβR signaling on hepatocytes appears beneficial during liver regeneration. We demonstrate that sustained hepatic LT expression in mice can be injurious causing chronic hepatitis and HCC. Enhanced hepatic LTβR signaling might be of potential clinical relevance since LTβR and its ligands are drastically increased in human HBV- and HCV-induced hepatitis and HCC when compared to normal livers or non-viral, benign liver diseases. Thus, hepatic LT signaling might be advantageous if transiently active during liver regeneration, but detrimental if chronically triggered. We propose that suppression of hepatic LTβR signaling might be beneficial in liver diseases with chronic LTα, LTβ or LIGHT overexpression.
Acknowledgments
We thank Birgit Riepl, Mareike Schroff, Manja Barthel, Petra Schwarz, Marianne König, Rita Moos, Udo Ungethüm, Mirzet Delic, Urs Egli, Silvia Behnke, André Fitsche, Andrea Patrignani, Marie-Ange Thelu, Gitta Seleznik for excellent technical assistance. We thank the members of the LT team at Biogen Idec for LT reagents, Annette Schmitt-Graeff for performing Knodell score analysis, Wolfram Jochum, Tracy O’Connor, Stefan Zoller, Thomas Fuchs, Holger Moch, Glen Kristiansen, Peter Schraml, Thomas Ried, Claude Carnaud, Helmut Denk, Christopher Soll, Evelyne Jouvin-Marche and Burkhardt Seifert for discussions. We thank Peter Bannasch for providing human tissues, Valentina Factor for antibodies (A6) and Alexei Tumanov (mLtα, mLtβ), Wolf-Dietrich Hardt (mCxcl10), Barrett Rollins (mCcl2) and Volkhard Lindner (mEgr1) for providing plasmids. We thank Jean-Pierre Zarski, Vincent Leroy and Christian Letoublon from Michallon Hospital in Grenoble. This study was supported by grants of the Oncosuisse foundation OCS 02113-08-2007 (MH; AW; MOK), the Bonizzi-Theler foundation (MH), the “Stiftung zur Schweizerischen Krebsbekämpfung” (MH), the research foundation at the Medical Faculty Zurich (JB, MH), the “Kurt und Senta Hermann Stiftung” (MH, JH), the Austrian Genome Programme GEN-AU (KZ), the Swiss National Science Foundation (AA), the Agence Nationale pour la Recherche sur le Sida (ANRS) and from Pole de Compétitivité LyonBiopole (PNM). MJW was supported by a grant of the Roche Research Foundation. SN is HHMI International Research Scholar. MR was supported by the Higher Education Commission of Pakistan. MH is a fellow of the Prof. Dr. Max Cloëtta foundation.
Footnotes
Accession numbers: Gene expression microarray data are deposited in the Arrayexpress database: accession number E-MEXP-1998. aCGH data are deposited in the GEO database, accession number GSE 14467.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akhurst B, Matthews V, Husk K, Smyth MJ, Abraham LJ, Yeoh GC. Differential lymphotoxin-beta and interferon gamma signaling during mouse liver regeneration induced by chronic and acute injury. Hepatology. 2005;41:327–335. doi: 10.1002/hep.20520. [DOI] [PubMed] [Google Scholar]
- An MM, Fan KX, Cao YB, Shen H, Zhang JD, Lu L, Gao PH, Jiang YY. Lymphtoxin beta receptor-Ig protects from T-cell-mediated liver injury in mice through blocking LIGHT/HVEM signaling. Biol Pharm Bull. 2006;29:2025–2030. doi: 10.1248/bpb.29.2025. [DOI] [PubMed] [Google Scholar]
- Anand S, Wang P, Yoshimura K, Choi IH, Hilliard A, Chen YH, Wang CR, Schulick R, Flies AS, Flies DB, et al. Essential role of TNF family molecule LIGHT as a cytokine in the pathogenesis of hepatitis. J Clin Invest. 2006;116:1045–1051. doi: 10.1172/JCI27083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachert C, Fimmel C, Linstedt AD. Endosomal trafficking and proprotein convertase cleavage of cis Golgi protein GP73 produces marker for hepatocellular carcinoma. Traffic. 2007;8:1415–1423. doi: 10.1111/j.1600-0854.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- Baffet G, Braciak TA, Fletcher RG, Gauldie J, Fey GH, Northemann W. Autocrine activity of interleukin 6 secreted by hepatocarcinoma cell lines. Mol Biol Med. 1991;8:141–156. [PubMed] [Google Scholar]
- Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76:13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluethmann H, Rothe J, Schultze N, Tkachuk M, Koebel P. Establishment of the role of IL-6 and TNF receptor 1 using gene knockout mice. J Leukoc Biol. 1994;56:565–570. doi: 10.1002/jlb.56.5.565. [DOI] [PubMed] [Google Scholar]
- Browning JL, French LE. Visualization of lymphotoxin-beta and lymphotoxin-beta receptor expression in mouse embryos. J Immunol. 2002;168:5079–5087. doi: 10.4049/jimmunol.168.10.5079. [DOI] [PubMed] [Google Scholar]
- Browning JL, Sizing ID, Lawton P, Bourdon PR, Rennert PD, Majeau GR, Ambrose CM, Hession C, Miatkowski K, Griffiths DA, et al. Characterization of lymphotoxin-alpha beta complexes on the surface of mouse lymphocytes. J Immunol. 1997;159:3288–3298. [PubMed] [Google Scholar]
- Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol. 2006;80:1197–1213. doi: 10.1189/jlb.0506297. [DOI] [PubMed] [Google Scholar]
- Cao Y, Bonizzi G, Seagroves TN, Greten FR, Johnson R, Schmidt EV, Karin M. IKKalpha provides an essential link between RANK signaling and cyclin D1 expression during mammary gland development. Cell. 2001;107:763–775. doi: 10.1016/s0092-8674(01)00599-2. [DOI] [PubMed] [Google Scholar]
- Chen CM, You LR, Hwang LH, Lee YH. Direct interaction of hepatitis C virus core protein with the cellular lymphotoxin-beta receptor modulates the signal pathway of the lymphotoxin-beta receptor. J Virol. 1997;71:9417–9426. doi: 10.1128/jvi.71.12.9417-9426.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- Engelhardt NV, Factor VM, Yasova AK, Poltoranina VS, Baranov VN, Lasareva MN. Common antigens of mouse oval and biliary epithelial cells. Expression on newly formed hepatocytes. Differentiation. 1990;45:29–37. doi: 10.1111/j.1432-0436.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Fu YX, Huang G, Wang Y, Chaplin DD. B lymphocytes induce the formation of follicular dendritic cell clusters in a lymphotoxin alpha-dependent fashion. J Exp Med. 1998;187:1009–1018. doi: 10.1084/jem.187.7.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Gommerman JL, Mackay F, Donskoy E, Meier W, Martin P, Browning JL. Manipulation of lymphoid microenvironments in nonhuman primates by an inhibitor of the lymphotoxin pathway. J Clin Invest. 2002;110:1359–1369. doi: 10.1172/JCI15975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Karin M. The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 2004;206:193–199. doi: 10.1016/j.canlet.2003.08.029. [DOI] [PubMed] [Google Scholar]
- Heikenwalder M, Zeller N, Seeger H, Prinz M, Klohn PC, Schwarz P, Ruddle NH, Weissmann C, Aguzzi A. Chronic lymphocytic inflammation specifies the organ tropism of prions. Science. 2005;307:1107–1110. doi: 10.1126/science.1106460. [DOI] [PubMed] [Google Scholar]
- Kawase T, Ohki R, Shibata T, Tsutsumi S, Kamimura N, Inazawa J, Ohta T, Ichikawa H, Aburatani H, Tashiro F, Taya Y. PH domain-only protein PHLDA3 is a p53-regulated repressor of Akt. Cell. 2009;136:535–550. doi: 10.1016/j.cell.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- Lee SH, Park SG, Lim SO, Jung G. The hepatitis B virus X protein up-regulates lymphotoxin alpha expression in hepatocytes. Biochim Biophys Acta. 2005;1741:75–84. doi: 10.1016/j.bbadis.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Lo JC, Wang Y, Tumanov AV, Bamji M, Yao Z, Reardon CA, Getz GS, Fu YX. Lymphotoxin beta receptor-dependent control of lipid homeostasis. Science. 2007;316:285–288. doi: 10.1126/science.1137221. [DOI] [PubMed] [Google Scholar]
- Lowes KN, Croager EJ, Abraham LJ, Olynyk JK, Yeoh GC. Upregulation of lymphotoxin beta expression in liver progenitor (oval) cells in chronic hepatitis C. Gut. 2003;52:1327–1332. doi: 10.1136/gut.52.9.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- Marrero JA, Lok AS. Newer markers for hepatocellular carcinoma. Gastroenterology. 2004;127:S113–119. doi: 10.1053/j.gastro.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Nakamoto Y, Guidotti LG, Kuhlen CV, Fowler P, Chisari FV. Immune pathogenesis of hepatocellular carcinoma. J Exp Med. 1998;188:341–350. doi: 10.1084/jem.188.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TI, Mo H, Pilot-Matias T, He Y, Koev G, Krishnan P, Mondal R, Pithawalla R, He W, Dekhtyar T, et al. Identification of host genes involved in hepatitis C virus replication by small interfering RNA technology. Hepatology. 2007;45:1413–1421. doi: 10.1002/hep.21608. [DOI] [PubMed] [Google Scholar]
- Pietschmann T, Kaul A, Koutsoudakis G, Shavinskaya A, Kallis S, Steinmann E, Abid K, Negro F, Dreux M, Cosset FL, Bartenschlager R. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci U S A. 2006;103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Puglielli MT, Browning JL, Brewer AW, Schreiber RD, Shieh WJ, Altman JD, Oldstone MB, Zaki SR, Ahmed R. Reversal of virus-induced systemic shock and respiratory failure by blockade of the lymphotoxin pathway. Nat Med. 1999;5:1370–1374. doi: 10.1038/70938. [DOI] [PubMed] [Google Scholar]
- Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddell RG, Mann DA, Ramm GA. The function of serotonin within the liver. J Hepatol. 2008;48:666–675. doi: 10.1016/j.jhep.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Sakamoto M. Early HCC: diagnosis and molecular markers. J Gastroenterol. 2009;44(Suppl 19):108–111. doi: 10.1007/s00535-008-2245-y. [DOI] [PubMed] [Google Scholar]
- Subrata LS, Lowes KN, Olynyk JK, Yeoh GC, Quail EA, Abraham LJ. Hepatic expression of the tumor necrosis factor family member lymphotoxin-beta is regulated by interleukin (IL)-6 and IL-1beta: transcriptional control mechanisms in oval cells and hepatoma cell lines. Liver Int. 2005;25:633–646. doi: 10.1111/j.1478-3231.2005.01080.x. [DOI] [PubMed] [Google Scholar]
- Tumanov AV, Koroleva EP, Christiansen PA, Khan MA, Ruddy MJ, Burnette B, Papa S, Franzoso G, Nedospasov S, Fu YX, Anders RA. T Cell-Derived Lymphotoxin Regulates Liver Regeneration. Gastroenterology. 2008;136:694–704. doi: 10.1053/j.gastro.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumanov AV, Kuprash DV, Nedospasov SA. The role of lymphotoxin in development and maintenance of secondary lymphoid tissues. Cytokine Growth Factor Rev. 2003;14:275–288. doi: 10.1016/s1359-6101(03)00026-1. [DOI] [PubMed] [Google Scholar]
- Vainer GW, Pikarsky E, Ben-Neriah Y. Contradictory functions of NF-kappaB in liver physiology and cancer. Cancer Lett. 2008;267:182–8. doi: 10.1016/j.canlet.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- You LR, Chen CM, Lee YH. Hepatitis C virus core protein enhances NF-kappaB signal pathway triggering by lymphotoxin-beta receptor ligand and tumor necrosis factor alpha. J Virol. 1999;73:1672–1681. doi: 10.1128/jvi.73.2.1672-1681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeremski M, Petrovic LM, Talal AH. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J Viral Hepat. 2007;14:675–687. doi: 10.1111/j.1365-2893.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- Zhu N, Khoshnan A, Schneider R, Matsumoto M, Dennert G, Ware C, Lai MM. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J Virol. 1998;72:3691–3697. doi: 10.1128/jvi.72.5.3691-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.