Abstract
Study Objective
Molecular definition of disease has been changing all aspects of medical practice, from diagnosis and screening to understanding and treatment. Acute appendicitis is among many human conditions that are complicated by the heterogeneity of clinical presentation and shortage of diagnostic markers. Here, we sought to profile the urine of patients with appendicitis with the goal of identifying new diagnostic markers.
Methods
Candidate markers were identified from the urine of children with histologically proven appendicitis by using high accuracy mass spectrometry proteome profiling. These systemic and local markers were used to assess the probability of appendicitis in a blinded, prospective study of children being evaluated for acute abdominal pain in our emergency department. Tests of performance of the markers were evaluated against the pathologic diagnosis and histologic grade of appendicitis.
Results
Test performance of 57 identified candidate markers was studied in 67 patients, with median age of 11 years, 37% of whom had appendicitis. Several exhibited favorable diagnostic performance, including calgranulin A (S100-A8), α-1-acid glycoprotein 1 (orosomucoid), and leucine-rich α-2-glycoprotein (LRG), with the ROC AUC and values of 0.84 (95 % CI 0.72-0.95), 0.84 (0.72-0.95), and 0.97 (0.93-1.0), respectively. LRG was enriched in diseased appendices and its abundance correlated with severity of appendicitis.
Conclusions
High accuracy mass spectrometry urine proteome profiling allowed identification of diagnostic markers of acute appendicitis. Usage of LRG and other identified biomarkers may improve the diagnostic accuracy of clinical evaluations of appendicitis.
Introduction
Appendicitis is among many human diseases, for which the diagnosis is complicated by the heterogeneity of its clinical presentation and shortage of diagnostic markers. As such, it remains the most common surgical emergency of children, with initial diagnosis accuracy additionally challenged because of non-specific but similar symptoms of many other childhood conditions.1 Delays in accurate diagnosis lead to increased mortality, morbidity, and costs associated with the complications of appendicitis.2-4
The use of high resolution computed tomography (CT) to identify appendiceal inflammation was hoped to improve both the diagnosis and treatment of acute appendicitis. Though variable, these improvements have been modest, with rates of unnecessary appendectomies and ruptures of 3-30 % and 30-45 %, respectively.5-10 Furthermore, recently its use has been re-evaluated due to concerns of cancer risk.11
Thus, several studies sought to identify laboratory markers of acute appendicitis, by studying both markers of the acute phase response, as well as specific inflammatory mediators. The performance of both appeared to be limited,12-17 likely because of the non-specific and unrelated mechanisms of their elevation during acute appendicitis which is characterized specifically by the infiltration of neutrophils and release of distinct cytokines.18, 19
In the current study, we adopted a discovery based approach, seeking to profile molecular alterations on a proteomic scale, including molecules that may be secreted locally by the diseased tissues themselves or produced systemically in response to local disease. We chose to study urinary markers because urine is abundant, obtained frequently and non-invasively, and as a result of being a serum filtrate, relatively simple in its composition.
Recently, advanced mass spectrometry (MS) has been used effectively to discover the protein composition of human urine,20-22 and to identify markers of diseases affecting the kidney23 and the urogenital tract.24 Similarly, MS studies of urine have been used to study proteins produced by distal organs such as the brain25 and the intestine,26 and to relate them to brain injury and inflammatory bowel disease, respectively.
The goal of our study was to discover and validate urinary biomarkers of acute appendicitis in a prospective pediatric cohort. By using high accuracy mass spectrometry proteome profiling of urine specimens routinely collected from children and young adults evaluated for acute abdominal pain, we analyzed the differences in individual urine proteomes and employed pattern recognition class prediction and gene expression profiling of diseased appendices to discover candidate diagnostic markers. By carrying out a blinded, prospective study of these candidate markers, we assessed their diagnostic performance.
Methods
Setting
The study was conducted at an urban tertiary care pediatric emergency department with 68,000 visits per year. This investigation was approved by the Children's Hospital Boston Committee on Clinical Investigation, began in November of 2006, and ended in May of 2008.
Participants and Enrollment Procedure
Patients aged less than 18 years who were being evaluated for possible appendicitis were enrolled, based on clinical history and physical examination. Surgical consultation or advanced imaging for the primary evaluation of appendicitis was required in order to be considered for study enrollment. The pediatric emergency medicine attending physician caring for the child obtained written consent from caregivers and assent for children over the age of 7 years. For the discovery phase, urines from 6 patients with abdominal pain and histologically proven appendicitis were compared to urines from 6 patients without appendicitis, including 3 intra-individual control specimens collected from patients with appendicitis after undergoing appendectomies. The number of patients required to sufficiently power the discovery phase was estimated using the Pearson statistic.27
For the validation phase, we enrolled ED patients prior to knowledge of the final diagnosis. Patients were excluded if they had pre-existing autoimmune, neoplastic, renal or urologic disease or were pregnant. Urine was collected as clean catch, mid stream samples. Urine specimens were labeled with a study number such that all further analysis was blinded. The urine was stored at -80 °C within 6 hours of collection.
Outcome measures
Final diagnosis was determined by the presence or absence of appendicitis on gross and histologic examination. All appendectomy specimens were reviewed by a clinical pathologist, and their disease assignments were confirmed by an independent, blinded review. One patient with perforated appendicitis underwent an interval appendectomy, and was not included in the histologic review. Assessment of the histologic severity of appendicitis was done by classifying the specimens as having: no inflammatory changes (normal); foci of neutrophilic infiltration in mucosa or wall (focal); scattered transmural infiltration (mild); dense transmural infiltration with tissue distortion (moderate); dense transmural infiltration with tissue necrosis or wall perforation (severe).13, 15, 18, 28 For patients who did not undergo appendectomies, the outcome was confirmed via telephone 6-8 weeks after the ED evaluation; parents responded to scripted questions to ascertain if the patient had any subsequent related medical care, including any operative care. All patients enrolled in the study received a final outcome.
Discovery urine proteome profiling and candidate validation targeted mass spectrometry
For the discovery of candidate markers, thawed 10 ml urine aliquots were fractionated by using ultracentrifugation, cation exchange chromatography, protein precipitation, polyacrylamide gel electrophoresis, and reverse phase liquid chromatography. Their protein composition was discovered by using liquid chromatography tandem mass spectrometry (LC-MS/MS) using a nanoflow HPLC system (Eksigent) coupled to a recently developed hybrid linear ion trap-Orbitrap (LTQ-Orbitrap) mass spectrometer (Thermo Scientific). The LTQ-Orbitrap enables an unprecedented combination of high detection sensitivity in the attomolar (10-18 M) range, and high mass accuracy of less than 2 parts per million (0.001 Da for a typical 500 Da peptide), as described in detail in the Online Appendix. Validation of candidate markers was performed using 1 ml aliquots of coded specimens that were blinded to the final outcome, as described in the Online Appendix. The entire experimental procedure is schematized in Figure 1.
Figure 1.
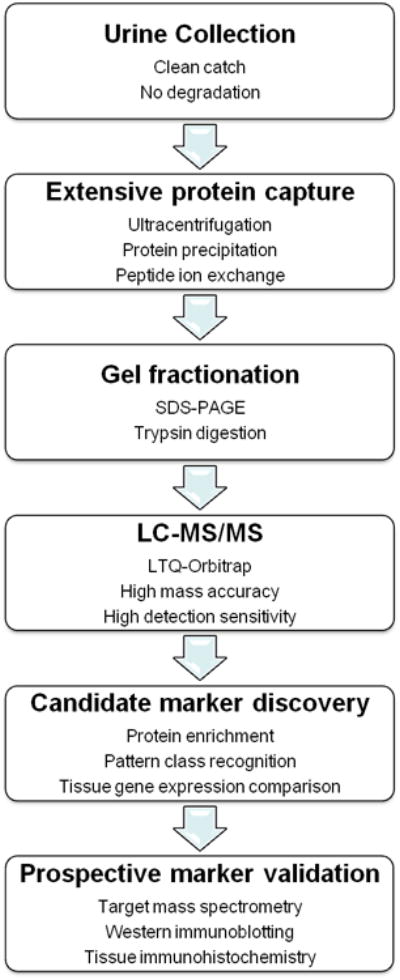
Experimental scheme, outlining methods used for protein capture and fractionation, identification for discovery urine proteomics, and validation of candidate diagnostic markers. The discovery phase of the study involved comparisons of 12 specimens obtained from 9 patients (6 patients without appendicitis, and 3 patients with appendicitis before and after they underwent appendectomies), whereas the validation phase of the study involved all 67 patients.
Analysis
During the discovery phase, candidate urine markers were ranked by calculating relative enrichment ratios (RER) of detection in appendicitis versus non-appendicitis groups by summing individual protein spectral counts normalized to the spectral counts of albumin to account for small differences in total protein abundance,29 where , with Cp and Ca denoting spectral counts of candidate protein markers and albumin, respectively. Candidate markers were additionally ranked by assessing the prevalence of their detection among different specimens by using a uniformity parameter (U), calculated by dividing the number of appendicitis cases in which they were detected by the total number of appendicitis cases. Candidate markers were filtered to have U > 0.7 and RER > 5 to remove those that were variably detected or insufficiently enriched, respectively. Support vector machine analysis and comparison of urine protein candidate markers with tissue gene expression profiles of diseased appendices were carried as described in the Online Appendix. The latter was based on a previous study.30 For the validation phase, the test performance of the candidate marker was compared against the binary outcome of appendicitis or no appendicitis. Receiver operating characteristics were calculated using standard methods (SPSS Version 14.0, Chicago, Ill).
Tissue immunohistochemistry and urine immunoblotting
To prove the presence of the actual protein marker in diseased appendices, immunohistochemical staining of formalin fixed, paraffin embedded appendices was performed for the most promising marker by using the rabbit anti-LRG polyclonal antibody at 1:750 dilution (Atlas Antibodies), OmniMap DAB anti-rabbit HRP detection kit and the Ventana Discovery XT automated slide processing platform, according to the manufacturer's instructions (Ventana Medical Systems).
To confirm the detectability of specific protein marker in urine, immunoblotting was performed on a sample of urine specimens. Specimens were precipitated and resolved by SDS-PAGE as described for targeted mass spectrometry (Online Appendix). Western blotting was done blinded to final outcome, as described previously,31 using the rabbit anti-LRG polyclonal antibody at 1:2000 dilution, and the SuperSignal West Pico chemiluminescent reagent (Thermo).
Results
Over the 18 month course of this study, we enrolled 67 patients who presented to our ED and underwent evaluation for possible acute appendicitis. In agreement with earlier studies of the epidemiology and presentation of acute appendicitis in pediatric EDs, the mean age of our study population was 11 years, with presenting signs and symptoms described in Table 1. Twenty five patients (37 %) received a final diagnosis of appendicitis. All patients with appendicitis underwent appendectomies, 16 % of which were found to have a perforation. Two patients (7.4 %) who received a pre-operative diagnosis of appendicitis was found to have no gross or histologic evidence of appendicitis upon undergoing appendectomy. Twenty four percent of patients were found to have no specific cause of their abdominal pain, with the remaining patients found to have a variety of common and rare mimicking conditions (Table 2).
Table 1. Presenting signs, symptoms and diagnostic studies of 67 patients with acute abdominal pain.
| Final Diagnosis | ||
|---|---|---|
| Appendicitis | Non-appendicitis | |
| Number | 25 | 42 |
| Gender (% male) | 56 | 40 |
| Age (years) | 11 ± 3.5 | 11 ± 4.2 |
| Duration of symptoms (days) | 2 ± 1 | 2 ± 1 |
| Nausea or vomiting (%) | 72 | 52 |
| Fever (%) | 52 | 48 |
| Pain migration (%) | 36 | 14 |
| RLQ pain or tenderness (%) | 100 | 95 |
| Temperature at triage (° C) | 36.9 ± 0.6 | 36.6 ± 0.9 |
| Peripheral white blood cell count (K cells/mm3) | 15.7 ± 5.2 | 11.0 ± 6.4 |
| Absolute neutrophil count (K cells/mm3) | 12.8 ± 5.4 | 8.5 ± 6.6 |
| US imaging (%) | 88 | 74 |
| US diagnosis of appendicitis (%) | 64 | 0 |
| CT imaging (%) | 60 | 64 |
| CT diagnosis of appendicitis (%) | 93 | 7.4 |
Values are reported as mean ± standard deviation, where appropriate, except for duration of symptoms which is reported as median ± quartile. RLQ (right lower quadrant), US (ultrasound), CT (computed tomography).
Table 2. Final diagnosis of the 67 study patients.
| Number of patients | |
|---|---|
| Appendicitis | 25 |
| Non specific abdominal pain | 16 |
| Ovarian cyst or torsion | 5 |
| Constipation | 5 |
| Pyelonephritis or Urinary Tract Infection | 5 |
| Renal calculus | 2 |
| Mesenteric adenitis | 2 |
| Gastroenteritis or gastritis | 2 |
| Influenza or scarlet fever | 2 |
| Intussusception | 1 |
| Inflammatory bowel disease | 1 |
| Diverticulitis | 1 |
Discovery of diagnostic markers by using urine proteomic profiling
Candidate urine markers of appendicitis were identified from the analysis of 12 specimens, collected at the onset of the study, and distributed equally between patients with and without appendicitis (Online Appendix). Table 3 lists the 32 candidate markers, identified by ranking their relative enrichment ratios (Methods). These candidate proteins include known components of the acute phase response such as α-1-acid glycoprotein (orosomucoid), plasminogen, carbonic anhydrase, angiotensin converting enzyme, and lipopolysaccharide binding protein, consistent with the systemic inflammatory response that accompanies acute appendicitis.
Table 3. Candidate urine marker proteins identified using relative enrichment ratio analysis.
| Protein | Accession Number* | U† | RER† |
|---|---|---|---|
| Adipocyte specific adhesion molecule | IPI00024929 | 1.0 | 18 |
| Leucine-rich α-2-glycoprotein | IPI00022417 | 1.0 | 9.5 |
| Zinc-α-2-glycoprotein | IPI00166729 | 1.0 | 7.3 |
| α-1-acid glycoprotein 2 | IPI00020091 | 1.0 | 5.8 |
| MLKL | IPI00180781 | 1.0 | 5.5 |
| α-1-acid glycoprotein 1 | IPI00022429 | 1.0 | 5.3 |
| Plasminogen | IPI00019580 | 1.0 | 5.1 |
| Carbonic anhydrase 1 | IPI00215983 | 0.8 | 15 |
| Angiotensin converting enzyme 2 | IPI00465187 | 0.8 | 12 |
| Nicastrin | IPI00021983 | 0.8 | 12 |
| Lipopolysaccharide binding protein | IPI00032311 | 0.8 | 11 |
| Vascular adhesion molecule 1 | IPI00018136 | 0.8 | 10 |
| PDZK1 interacting protein 1 | IPI00011858 | 0.8 | 7.5 |
| SLC9A3 | IPI00011184 | 0.8 | 7.5 |
| Lymphatic vessel endothelial hyaluronan receptor 1 | IPI00290856 | 0.8 | 6.9 |
| FXR2 | IPI00016250 | 0.7 | N/A |
| SORBS1 | IPI00002491 | 0.7 | N/A |
| SLC4A1 | IPI00022361 | 0.7 | 44 |
| PRIC285 | IPI00249305 | 0.7 | 14.9 |
| TGFbeta2R | IPI00383479 | 0.7 | 11.3 |
| SLC2A1 | IPI00220194 | 0.7 | 10.7 |
| Rcl | IPI00007926 | 0.7 | 9.7 |
| VA0D1 | IPI00034159 | 0.7 | 8.9 |
| SLC13A3 | IPI00103426 | 0.7 | 7.8 |
| TTYH3 | IPI00749429 | 0.7 | 7.3 |
| SPRX2 | IPI00004446 | 0.7 | 6.4 |
| BAZ1B | IPI00216695 | 0.7 | 6.1 |
| β-1,3 -galactosyltransferase | IPI00032034 | 0.7 | 6.1 |
| chromogranin A | IPI00383975 | 0.7 | 5.9 |
| Novel protein | IPI00550644 | 0.7 | 5.5 |
| SLC2A2 | IPI00003905 | 0.7 | 5.2 |
| FBLN7 | IPI00167710 | 0.7 | 5.1 |
International Protein Index (version 3.36, http://www.ebi.ac.uk/IPI).
Values of U = 1 indicate candidate markers detected in all appendicitis specimens, whereas values of RER = 1 indicate markers that exhibit no apparent enrichment in appendicitis as compared to non-appendicitis groups. N/A (not detected in non-appendicitis specimens).
The candidate markers also include a number of cell adhesion proteins such as adipocyte specific adhesion molecule, a component of the epithelial and endothelial tight junctions, leucine-rich α-2-glycoprotein (LRG), a marker of neutrophil differentiation involved in cell trafficking, vascular adhesion molecule 1, which mediates lymphocyte-endothelial adhesion, and lymphatic vessel endothelial hyaluronan acid receptor 1 involved in cell migration, consistent with earlier findings of leukocyte trafficking and infiltration into mucosal tissue that accompanies acute appendicitis.
Remaining top ranking candidate markers do not appear to share any known functional or structural similarities, though some of them such as β-1,3-galactosyltransferase and VA0D1 have been shown to function specifically in the colonic epithelium, and therefore, may include components of the local and systemic appendicitis response. Additional candidate markers were identified by using support vector machine (SVM) learning, as well as comparisons with tissue gene expression profiles of diseased appendices (Online Appendix, Tables S1 and S2). In total, 57 candidate markers were identified.
Validation of candidate urine protein markers by using targeted mass spectrometry
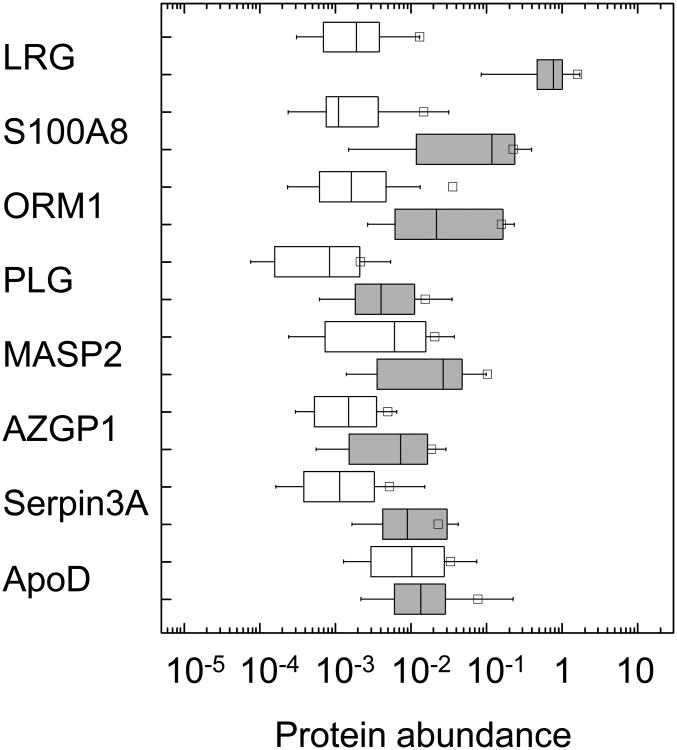
In order to assess their diagnostic performance, we determined their relative concentrations in urine of all enrolled patients in a prospective fashion, with experimental measurements blinded to the patients' outcomes. Candidate proteins detected with sufficient uniformity among the 67 specimens examined are listed in Table 4. The remaining candidate proteins were detected in less than half of specimens, likely as a result of differences in processing of the discovery and validation specimens (Online Appendix). Comparison of differences in urinary concentration between the appendicitis and non-appendicitis patient groups revealed LRG, S100-A8, and α-1-acid glycoprotein 1 (orosomucoid, ORM1) as exhibiting substantial apparent enrichment in the urines of patients with appendicitis (Figure 2).
Table 4. Urine marker proteins validated by targeted mass spectrometry.
| Protein | ROC AUC | AUC 95 % confidence interval |
|---|---|---|
| Leucine-rich α-2-glycoprotein | 0.97 | 0.93-1.0 |
| S100-A8 | 0.84 | 0.72-0.95 |
| α-1-acid glycoprotein 1 | 0.84 | 0.72-0.95 |
| Plasminogen | 0.79 | 0.67-0.91 |
| Mannan-binding lectin serine protease 2 | 0.74 | 0.61-0.88 |
| Zinc-α-2-glycoprotein | 0.74 | 0.60-0.88 |
| α-1-antichymotrypsin | 0.84 | 0.73-0.94 |
| Apolipoprotein D | 0.53 | 0.38-0.69 |
ROC (receiver operating characteristic), AUC (area under the curve).
Figure 2.
Boxplots of the relative urine protein abundance (logarithm normalized ion current units) of the validated candidate diagnostic markers for the non-appendicitis (open) and appendicitis (gray) patient groups for leucine-rich α-2-glycoprotein (LRG), calgranulin A (S100-A8), α-1-acid glycoprotein 1 (ORM1), plasminogen (PLG), mannan-binding lectin serine protease 2 (MASP2), zinc-α-2-glycoprotein (AZGP1), α-1-antichymotrypsin (serpin3A), and apolipoprotein D (ApoD). Normalized value of 1 corresponds to the apparent abundance of internal reference standard (Online Appendix). Boxes contain the 25-75 % interquartile range, with the dividing bars representing medians, and whiskers representing 10-90 % range. Square symbols represent means. Abundance of LRG in patients with pyelonephritis (solid dot, ●) and those who underwent appendectomies with findings of histologically normal appendices (open dot, ○)
Indeed, receiver operating characteristic (ROC) curves for these markers exhibited excellent performance, with LRG having area under the curve (AUC) value of 0.97 (Figure 3, Table 4). Other prospectively validated markers with apparently good performance included S100-A8, orosomucoid 1, and α-1-antichymotrypsin (serpin A3); plasminogen, mannan-binding lectin serine protease 2 (MASP2), zinc-α-2-glycoprotein (AZGP) exhibited intermediate performance, and apolipoprotein D exhibited poor performance. These findings are consistent with most of these proteins being components of the general acute phase response, during which they may be upregulated by a variety of infectious and inflammatory conditions, including some that are represented in the non-appendicitis group (Table 2).
Figure 3.
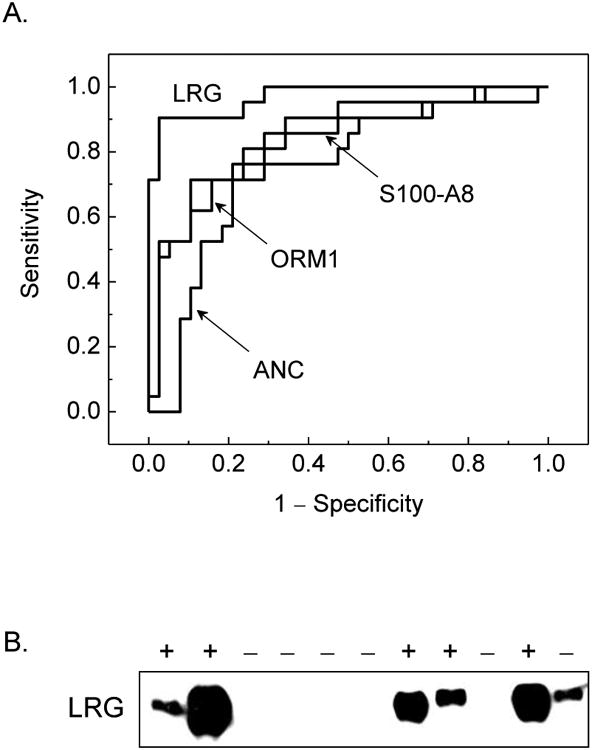
A. Receiver operating characteristics of urine protein markers validated by targeted mass spectrometry, demonstrating the relative diagnostic performance of leucine-rich α-2-glycoprotein (LRG), calgranulin A (S100-A8), α-1-acid glycoprotein 1 (ORM1), and peripheral blood absolute neutrophil count (ANC). The listed confidence intervals were computed for single comparisons, and do not include possible correction for multiple testing, which is expected to broaden them in proportion to the correlation and number of simultaneous tests. B. Enrichment of LRG in a random sample of urine of patients with histologically proven appendicitis (+) as compared to those without (−) by using Western immunoblotting. LRG signal was observed in 5/5 patients with appendicitis and no signal was observed in 5/6 patients without appendicitis. Development of quantitative LRG urine immunoblotting and assessment of its diagnostic performance in interventional studies are important directions of future work.
We assessed the relationship between apparent urine protein abundance of markers and the apparent severity of appendicitis by classifying appendectomy specimens with respect to the degree of neutrophil infiltration.18 As can be seen from Figure 4, LRG appears to be a marker of focal appendicitis, whereas S100-A8 appears to be a marker of progressive disease, reaching a peak level with moderate appendicitis. In addition to exhibiting excellent diagnostic performance, LRG was detected strongly in diseased as compared to normal appendices by using tissue immunohistochemistry (Figure 4), consistent with its biological function and proposed role in appendicitis (see below). Its enrichment in urine of patients with appendicitis relative to those with other conditions was confirmed by using Western immunoblotting (Figure 3B), suggesting that clinical diagnostic immunoassays may be devised.
Figure 4.
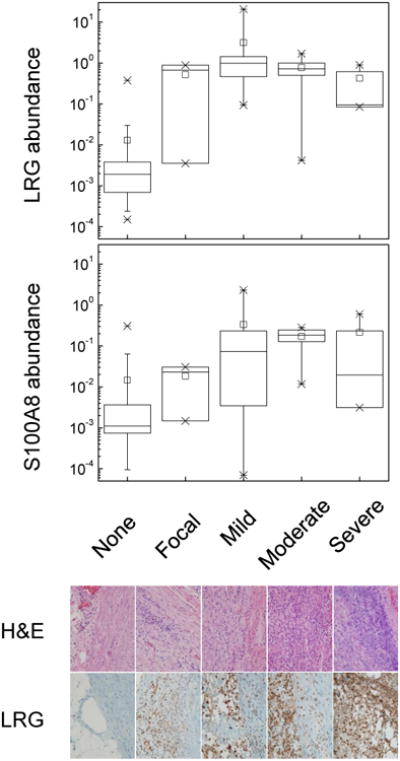
Top panel. Boxplots of the relative urine protein abundance (normalized ion current units) of leucine-rich α-2-glycoprotein (LRG) and calgranulin A (S100-A8) as a function of appendicitis severity, as assessed using histologic classification. Crosses represent 1-99 % range. Note that the group with histologically normal appendices includes both patients who underwent appendectomies and patients without clinical diagnosis of appendicitis. Bottom panel. Representative micrographs of appendectomy specimens and immunohistochemistry staining against LRG.
Limitations
We have not tested urine protein markers of acute appendicitis in patients evaluated in settings other than the emergency department, as well as in older adult patients, who may include other causes of abdominal pain from those observed in our cohort. Though our cohort included patients with short duration of symptoms of less than one day, the median duration of symptoms was two days, and the diagnostic performance of identified markers may be different earlier in the disease course. Likewise, urine protein markers identified by our study will require further study in individuals with underlying renal or urologic disease, as well as in patients with extreme dehydration. Though our mass spectrometry measurements included internal correction for variable urine concentration by incorporating albumin normalization, clinical LRG testing using immunoassays such as analytical or dipstick ELISAs may require assessments and/or corrections for variable or age dependent urine concentration.
Discussion
The use of high resolution CT and US has led to improvements in the diagnosis of acute appendicitis, with respect to both the rates of complications and unnecessary appendectomies. 5-10 However, significant diagnostic challenges remain, largely because of the non-specific nature of signs and symptoms of many conditions that can mimic acute appendicitis. Similarly, CT and US findings can often be indeterminate or equivocal.32
Numerous studies have sought to identify biomarkers to aid the diagnosis of appendicitis, with the total peripheral blood white cell count, absolute neutrophil count, and serum C-reactive protein levels being most useful, but still limited with respect to their sensitivity and specificity.33-36 Recent attempts to identify new and improved diagnostic markers, such as CD44, interleukin-6, interleukin-8, and 5-hydroxy indole acetate, produced limited improvements as compared to the existing ones,12-17 likely as a result of being closely correlated with the existing markers of the general acute phase response, or not specific for the distinct immune mechanisms that characterize acute appendicitis.
By taking advantage of the latest generation of mass spectrometers that combine high accuracy with high sensitivity, and carrying out exhaustive protein capture and fractionation of routinely collected urine specimens, we developed a method that enables discovery and validation of multiple diagnostic markers, thereby overcoming the limitations of conventional approaches based on single hypothesis testing. Because of the depth of discovery achieved, identifying more than 2,000 unique proteins in total, urine proteomic profiling, like gene expression profiling, may be susceptible to noise and selection bias. In order to minimize these potential problems, 12 discovery urine proteomes were compared not only between patients with histologically proven appendicitis and those without, but also with the same patients after they recovered from appendectomies (Online Appendix), thereby minimizing individual differences due to age, gender, physiologic state or genetic variation. High stringency identification criteria were used, essentially eliminating false protein identifications. The discriminatory power of candidate diagnostic markers was assessed by examining the level and uniformity of their enrichment in patients with appendicitis (Table 3), by using pattern recognition class prediction learning algorithms (Table S1), and by comparing discovered urine protein markers with tissue gene expression profiles of diseased appendices (Table S2).30
As a result, the 57 discovered candidate urinary markers constitute an extensive characterization of the molecular response that accompanies acute appendicitis, including both systemically and locally produced molecules that participate in the systemic inflammatory response and/or its localization to the appendiceal tissue. Seven of these candidate markers were validated successfully, including LRG in particular (Figure 3, Table 4). LRG is expressed by differentiating neutrophils, liver, and high endothelial venules of the mesentery, including the meso-appendix, functioning in leukocyte activation and chemotaxis, respectively.37, 38 Its enrichment in the urine of patients with acute appendicitis suggests that it may be shed by locally activated neutrophils and/or local inflammatory sites such as the meso-appendix through which they likely traffic (Figure 4, Online Appendix). As such, it is likely a specific marker of local inflammatory processes such as those that specifically characterize acute appendicitis, as opposed to general markers of systemic response such as the acute phase reactants, and macroscopic markers of local inflammation such as those observed using US and CT imaging.
LRG appears to be enriched in the urine of patients with appendicitis in the absence of macroscopic inflammatory changes, as evidenced by its accurate diagnosis of appendicitis of 2 patients who exhibited normal imaging findings but had evidence of acute appendicitis on histologic examination, as well as its accurate diagnosis of the absence of appendicitis in a patient without histologic evidence of appendicitis, but who underwent appendectomy as a result of findings of appendiceal enlargement on CT. Lastly, LRG appears to be enriched in the urine of patients with pyelonephritis, consistent with its proposed role in local inflammatory processes. Consequently, its diagnostic performance of acute appendicitis will likely depend on accurate ability to rule out other local tissue infections, such as pyelonephritis, abscesses, and pelvic inflammatory disease, consistent with early studies.39 Importantly, LRG appears to be strongly expressed in diseased appendices, suggesting that it may underlie a principal pathway of appendiceal inflammation by localizing or sustaining the local neutrophilic infiltration that specifically characterizes acute appendicitis.18, 19, 30 The mechanism(s) by which LRG and other local cytokines accumulate in urine, as well as their relationship to the pathophysiology of acute appendicitis are important directions of future work.
Though the availability of clinical mass spectrometry is expanding rapidly, it is currently limited to large academic centers. However, detection of LRG in urine of patients with appendicitis by using Western immunoblotting suggests that widely available clinical diagnostic immunoassays may be devised (Figure 3B). Indeed, measurement of serum concentrations of LRG was recently demonstrated by using enzyme-linked immunosorbent assay (ELISA).40 This can be developed into analytical clinical laboratory urine tests or a dipstick format for rapid point of care testing. We were able to detect LRG using small, one ml volumes of urine (Figure 3B), which would be readily obtainable from patients of all ages.
Testing of these markers in multi-institutional, interventional studies is an important direction of future work. In all, this work promises to establish a paradigm for the identification of clinically useful markers of human disease.
Supplementary Material
Figure S1. Relative enrichment of candidate urine protein markers as a function of appendicitis tissue overexpression of the corresponding genes, demonstrating that more than 50 % of candidate markers with tissue overexpression exhibit urine enrichment (□), but that only 3 of these (■) were identified as candidate markers by urine proteome profiling.
Table S1: Candidate urine marker proteins identified using SVM analysis
Table S2: Candidate urine marker proteins identified by comparisons with corresponding tissue gene overexpression
Acknowledgments
We are grateful to the staff of the Children's Hospital Boston's Division of Emergency Medicine and Department of Surgery for help with specimen collection. We thank Samuel Lux for critical discussions, and Zachary Waldon and Bernhard Renard for technical assistance. Funded in part by the Frederick Lovejoy, Jr, MD Housestaff Research and Education grant, and by Children's Hospital Boston Houseofficer Development Award.
References
- 1.Addiss DG, Shaffer N, Fowler BS, et al. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990 Nov;132(5):910–925. doi: 10.1093/oxfordjournals.aje.a115734. [DOI] [PubMed] [Google Scholar]
- 2.Henderson J, Goldacre MJ, Fairweather JM, et al. Conditions accounting for substantial time spent in hospital in children aged 1-14 years. Arch Dis Child. 1992 Jan;67(1):83–86. doi: 10.1136/adc.67.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rappaport WD, Peterson M, Stanton C. Factors responsible for the high perforation rate seen in early childhood appendicitis. Am Surg. 1989 Oct;55(10):602–605. [PubMed] [Google Scholar]
- 4.Williams N, Bello M. Perforation rate relates to delayed presentation in childhood acute appendicitis. J R Coll Surg Edinb. 1998 Apr;43(2):101–102. [PubMed] [Google Scholar]
- 5.Rao PM, Rhea JT, Novelline RA, et al. Effect of computed tomography of the appendix on treatment of patients and use of hospital resources. N Engl J Med. 1998 Jan 15;338(3):141–146. doi: 10.1056/NEJM199801153380301. [DOI] [PubMed] [Google Scholar]
- 6.Peck J, Peck A, Peck C. The clinical role of noncontrast helical computed tomography in the diagnosis of acute appendicitis. Am J Surg. 2000 Aug;180(2):133–136. doi: 10.1016/s0002-9610(00)00435-9. [DOI] [PubMed] [Google Scholar]
- 7.Partrick DA, Janik JE, Janik JS, et al. Increased CT scan utilization does not improve the diagnostic accuracy of appendicitis in children. J Pediatr Surg. 2003 May;38(5):659–662. doi: 10.1016/jpsu.2003.5017. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez DP, Vargas S, Callahan MJ, et al. Appendicitis in young children: imaging experience and clinical outcomes. AJR Am J Roentgenol. 2006 Apr;186(4):1158–1164. doi: 10.2214/AJR.05.0055. [DOI] [PubMed] [Google Scholar]
- 9.Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for Diagnosis of Appendicitis in Children and Adults? A Meta-Analysis. Radiology. 2006 Oct;241(1):83–94. doi: 10.1148/radiol.2411050913. [DOI] [PubMed] [Google Scholar]
- 10.Pena BM, Taylor GA, Lund DP, et al. Effect of computed tomography on patient management and costs in children with suspected appendicitis. Pediatrics. 1999 Sep;104(3 Pt 1):440–446. doi: 10.1542/peds.104.3.440. [DOI] [PubMed] [Google Scholar]
- 11.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007 Nov 29;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 12.Taha AS, Grant V, Kelly RW. Urinalysis for interleukin-8 in the non-invasive diagnosis of acute and chronic inflammatory diseases. Postgrad Med J. 2003 Mar;79(929):159–163. doi: 10.1136/pmj.79.929.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolandparvaz S, Vasei M, Owji AA, et al. Urinary 5-hydroxy indole acetic acid as a test for early diagnosis of acute appendicitis. Clin Biochem. 2004 Nov;37(11):985–989. doi: 10.1016/j.clinbiochem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Apak S, Kazez A, Ozel SK, et al. Spot urine 5-hydroxyindoleacetic acid levels in the early diagnosis of acute appendicitis. J Pediatr Surg. 2005 Sep;40(9):1436–1439. doi: 10.1016/j.jpedsurg.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 15.Rivera-Chavez FA, Peters-Hybki DL, Barber RC, et al. Innate immunity genes influence the severity of acute appendicitis. Ann Surg. 2004 Aug;240(2):269–277. doi: 10.1097/01.sla.0000133184.10676.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paajanen H, Mansikka A, Laato M, et al. Novel serum inflammatory markers in acute appendicitis. Scand J Clin Lab Invest. 2002;62(8):579–584. doi: 10.1080/003655102764654312. [DOI] [PubMed] [Google Scholar]
- 17.Kafetzis DA, Velissariou IM, Nikolaides P, et al. Procalcitonin as a predictor of severe appendicitis in children. Eur J Clin Microbiol Infect Dis. 2005 Jul;24(7):484–487. doi: 10.1007/s10096-005-1360-4. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji M, Puri P, Reen DJ. Characterisation of the local inflammatory response in appendicitis. J Pediatr Gastroenterol Nutr. 1993 Jan;16(1):43–48. doi: 10.1097/00005176-199301000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Mazzucchelli L, Hauser C, Zgraggen K, et al. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol. 1994 May;144(5):997–1007. [PMC free article] [PubMed] [Google Scholar]
- 20.Rai AJ, Stemmer PM, Zhang Z, et al. Analysis of Human Proteome Organization Plasma Proteome Project (HUPO PPP) reference specimens using surface enhanced laser desorption/ionization-time of flight (SELDI-TOF) mass spectrometry: multi-institution correlation of spectra and identification of biomarkers. Proteomics. 2005 Aug;5(13):3467–3474. doi: 10.1002/pmic.200401320. [DOI] [PubMed] [Google Scholar]
- 21.Pisitkun T, Johnstone R, Knepper MA. Discovery of urinary biomarkers. Mol Cell Proteomics. 2006 Jul 12; doi: 10.1074/mcp.R600004-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Adachi J, Kumar C, Zhang Y, et al. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol. 2006;7(9):R80. doi: 10.1186/gb-2006-7-9-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woroniecki RP, Orlova TN, Mendelev N, et al. Urinary proteome of steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome of childhood. Am J Nephrol. 2006;26(3):258–267. doi: 10.1159/000093814. [DOI] [PubMed] [Google Scholar]
- 24.Oetting WS, Rogers TB, Krick TP, et al. Urinary beta2-microglobulin is associated with acute renal allograft rejection. Am J Kidney Dis. 2006 May;47(5):898–904. doi: 10.1053/j.ajkd.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Berger RP, Kochanek PM. Urinary S100B concentrations are increased after brain injury in children: A preliminary study. Pediatr Crit Care Med. 2006 Nov;7(6):557–561. doi: 10.1097/01.PCC.0000244426.37793.23. [DOI] [PubMed] [Google Scholar]
- 26.Propst A, Propst T, Herold M, et al. Interleukin-1 receptor antagonist in differential diagnosis of inflammatory bowel diseases. Eur J Gastroenterol Hepatol. 1995 Nov;7(11):1031–1036. doi: 10.1097/00042737-199511000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Campbell MJ. Estimating sample sizes for binary, ordered categorical, and continuous outcomes in two group comparisons. British Medical Journal. 1995;311:1145–1148. doi: 10.1136/bmj.311.7013.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beltran MA, Almonacid J, Vicencio A, et al. Predictive value of white blood cell count and C-reactive protein in children with appendicitis. J Pediatr Surg. 2007 Jul;42(7):1208–1214. doi: 10.1016/j.jpedsurg.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho PC, Hewel J, Barbosa VC, et al. Identifying differences in protein expression levels by spectral counting and feature selection. Genet Mol Res. 2008;7(2):342–356. doi: 10.4238/vol7-2gmr426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy CG, Glickman JN, Tomczak K, et al. Acute Appendicitis is Characterized by a Uniform and Highly Selective Pattern of Inflammatory Gene Expression. Mucosal Immunol. 2008;1:297–308. doi: 10.1038/mi.2008.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kentsis A, Topisirovic I, Culjkovic B, et al. Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci U S A. 2004 Dec 28;101(52):18105–18110. doi: 10.1073/pnas.0406927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kharbanda AB, Taylor GA, Bachur RG. Suspected appendicitis in children: rectal and intravenous contrast-enhanced versus intravenous contrast-enhanced CT. Radiology. 2007 May;243(2):520–526. doi: 10.1148/radiol.2432060181. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto T, Sano K, Ogasahara K. Receiver-operating characteristic analysis of leukocyte counts and serum C-reactive protein levels in children with advanced appendicitis. Surg Today. 2006;36(6):515–518. doi: 10.1007/s00595-006-3189-6. [DOI] [PubMed] [Google Scholar]
- 34.Bundy DG, Byerley JS, Liles EA, et al. Does this child have appendicitis? Jama. 2007 Jul 25;298(4):438–451. doi: 10.1001/jama.298.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kharbanda AB, Taylor GA, Fishman SJ, et al. A clinical decision rule to identify children at low risk for appendicitis. Pediatrics. 2005 Sep;116(3):709–716. doi: 10.1542/peds.2005-0094. [DOI] [PubMed] [Google Scholar]
- 36.Murphy CG, Glickman JN, Tomczak K, et al. Acute Appendicitis is Characterized by a Uniform and Highly Selective Pattern of Inflammatory Gene Expression. Submitted. 2007 doi: 10.1038/mi.2008.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Donnell LC, Druhan LJ, Avalos BR. Molecular characterization and expression analysis of leucine-rich alpha2-glycoprotein, a novel marker of granulocytic differentiation. J Leukoc Biol. 2002 Sep;72(3):478–485. [PubMed] [Google Scholar]
- 38.Saito K, Tanaka T, Kanda H, et al. Gene expression profiling of mucosal addressin cell adhesion molecule-1+ high endothelial venule cells (HEV) and identification of a leucine-rich HEV glycoprotein as a HEV marker. J Immunol. 2002 Feb 1;168(3):1050–1059. doi: 10.4049/jimmunol.168.3.1050. [DOI] [PubMed] [Google Scholar]
- 39.Bini L, Magi B, Marzocchi B, et al. Two-dimensional electrophoretic patterns of acute-phase human serum proteins in the course of bacterial and viral diseases. Electrophoresis. 1996 Mar;17(3):612–616. doi: 10.1002/elps.1150170333. [DOI] [PubMed] [Google Scholar]
- 40.Weivoda S, Andersen JD, Skogen A, et al. ELISA for human serum leucine-rich alpha-2-glycoprotein-1 employing cytochrome c as the capturing ligand. J Immunol Methods. 2008 Jul 20;336(1):22–29. doi: 10.1016/j.jim.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Relative enrichment of candidate urine protein markers as a function of appendicitis tissue overexpression of the corresponding genes, demonstrating that more than 50 % of candidate markers with tissue overexpression exhibit urine enrichment (□), but that only 3 of these (■) were identified as candidate markers by urine proteome profiling.
Table S1: Candidate urine marker proteins identified using SVM analysis
Table S2: Candidate urine marker proteins identified by comparisons with corresponding tissue gene overexpression