Abstract
The field of regenerative medicine holds considerable promise for treating diseases that are currently intractable. Although many researchers are adopting the strategy of cell transplantation for tissue repair, an alternative approach to therapy is to manipulate the stem cell microenvironment, or niche, to facilitate repair by endogenous stem cells. The niche is highly dynamic, with multiple opportunities for intervention. These include administration of small molecules, biologics or biomaterials that target specific aspects of the niche, such as cell-cell and cell–extracellular matrix interactions, to stimulate expansion or differentiation of stem cells, or to cause reversion of differentiated cells to stem cells. Nevertheless, there are several challenges in targeting the niche therapeutically, not least that of achieving specificity of delivery and responses. We envisage that successful treatments in regenerative medicine will involve different combinations of factors to target stem cells and niche cells, applied at different times to effect recovery according to the dynamics of stem cell–niche interactions.
Regenerative medicine has been defined as the process of creating living, functional tissues to repair or replace tissue or organ function lost due to age, disease, damage or congenital defects (http://report.nih.gov/NIHfactsheets/ViewFactSheet.aspx?csid=62&key=R#R). Stem cells are the focus of many applications in regenerative medicine because of their extensive ability to self-renew and to generate differentiated progeny1. There are three broad categories of stem cells. Most adult tissues have resident stem cells that are responsible for maintaining that tissue; these cells have been best characterized in tissues that have a rapid rate of cell turnover, such as the blood, epidermis and intestine. Embryonic stem cells are derived in culture from pre-implantation embryos and are referred to as pluripotent because they have the ability to differentiate into all cell types in the body. Finally, pluripotent stem cells can be generated by reprogramming adult cells through the introduction of a small number of specific genes; these cells are known as induced pluripotent stem cells (iPSCs).
A central strategy in regenerative medicine is to treat patients by transplanting stem cells or their differentiated derivatives2. Transplantation of hematopoietic stem cells (HSCs) obtained from whole bone marrow, peripheral blood or umbilical cord blood provides a paradigm for other forms of cell therapy. HSCs donated by healthy individuals are matched as closely as possible to the recipients to minimize immune rejection. In this way, HSCs have been used for many therapeutic applications, including treatment of genetic blood disorders, such as thalassemia, immunodeficiencies or metabolic diseases, and restoration of the hematopoietic system of cancer patients after chemotherapy. Other validated cell therapies include transplantation of cultured sheets of autologous epidermal or corneal cells to repair burn injuries, and transplantation of ex vivo–expanded autologous chondrocytes to repair cartilage defects3,4. These examples involve cells from adult tissues. In addition, cells that have been differentiated from pluripotent stem cells are being tested in early-phase clinical trials for treatment of spinal cord injuries and various types of blindness5–7. Other experimental cell therapies include transplantation of autologous cells after genetic correction or modification (gene therapy) and the use of mesenchymal stem cells to modulate graft-versus-host disease, to augment HSC engraftment in allogeneic stem cell transplantation or to stimulate regenerative responses in heterogeneous tissues.
In principle, the future of regenerative medicine through cell transplantation is bright. Whereas previously it was only possible to transplant cells that could be harvested from accessible tissues, such as blood or skin, the ability to direct embryonic stem cells or iPSCs to differentiate into inaccessible or rare cell types means that potentially any cell type in the body can now be replaced. And with the advent of iPSC technology, patients can be treated with their own cells, avoiding the problems of immune rejection. Nevertheless, in practice, cell transplantation does have a number of limitations. Autologous treatments, whether with adult cells or iPSCs, are inherently more expensive and labor intensive than pharmaceutical interventions, as they require specialized facilities for cell collection, expansion, quality control and transplantation. In the case of iPSC-based treatments, there are still unaddressed concerns over safety, not least because of the capacity of iPSCs to generate teratomas8. Generation of banks of allogeneic cells can reduce the cost of scale-up and reduce batch-to-batch variation in cell quality, but the use of allogeneic cells comes with the need for immunosuppression, which can have undesirable effects in the long term. Regardless of cell source, survival of transplanted cells is often poor as a result of the cells being placed in a suboptimal environment, such as a wound or scar. Even in transplantation of autologous cells, the surgical intervention can provoke an innate immune reaction that hampers cell survival9.
One alternative or adjunct to cell transplantation is to manipulate stem cells in vivo, for example, by stimulating them to proliferate or to generate the requisite type of differentiated cells or by introducing gene sequences that correct a pathologic phenotype. This strategy has the advantage that the tissue would be regenerated by the patient’s own cells without the need for biopsy, ex vivo cell expansion and manipulation, and transplantation. Such an approach would avoid the costly manufacturing challenges associated with cell therapies, including characterization and quality control of a living therapeutic and scale-up of cell production to serve large numbers of patients. The question is thus how endogenous tissue repair could be achieved by administration of small mo lecules, biologics, genes, biomaterials or other agents that are less complex than cells.
In this Review we consider the strategy of targeting the stem cell microenvironment, or niche, to make it supportive of endogenous repair. We discuss the different components of the niche and the evidence that it directs cell behavior. We highlight the importance of cell-cell and cell–extracellular matrix interactions, and physical factors such as oxygen content. The picture that emerges is one of a highly dynamic cellular environment with multiple opportunities to intervene and optimize stem cell function.
The stem cell niche
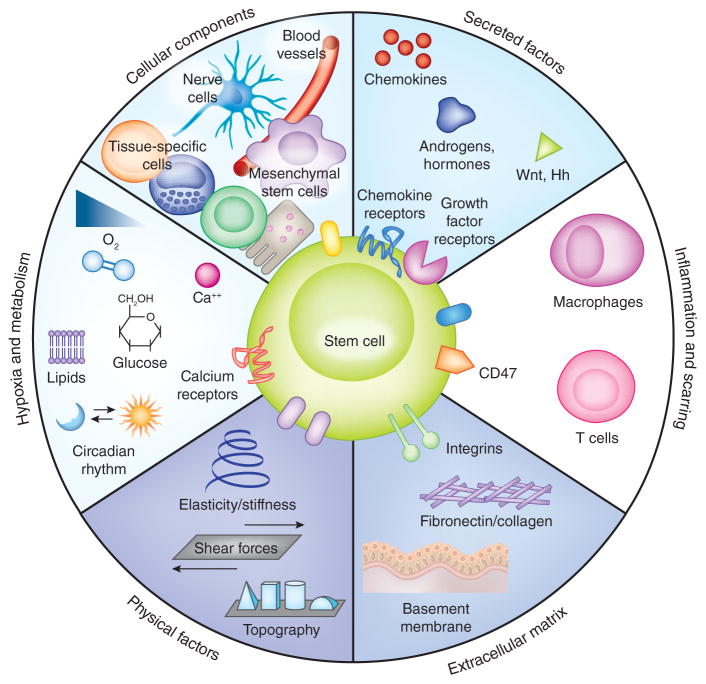
The term ‘niche’ was first used by Schofield in 1978 to explain the variation in the self-renewal ability of apparently pure populations of HSCs following transplantation in mice10. He hypothesized that the ability of stem cells to self-renew and retain their identity depends on the environment provided by neighboring, non-HSC cells. He further proposed that the progeny of a stem cell will undergo differentiation unless they can occupy a similar ‘niche’. In the decades since Schofield’s original article, this concept has been extended to encompass other aspects of the stem cell microenvironment11,12 (work by F.M.W. and colleagues). Key components of the niche include direct interactions between stem cells and neighboring cells, secreted factors, inflammation and scarring, extracellular matrix (ECM), physical parameters such as shear stress and tissue stiffness, and environmental signals such as hypoxia (Fig. 1). These different aspects of the niche are summarized in Box 1. We therefore consider targeting the stem cell niche to include any approach that modulates individual or multiple components of the niche to facilitate regeneration and tissue repair by activating or otherwise manipulating normal stem cell function.
Figure 1.
Composition of the niche. Stem cell niches are complex, heterotypic, dynamic structures, which include different cellular components, secreted factors, immunological control, ECM, physical parameters and metabolic control. These aspects of the niche are described in more detail in Box 1. The interactions between stem cells and their niches are bidirectional and reciprocal.
Box 1. Common features of different stem cell niches.
By their very nature, niches are unique and specific in their interactions with their cognate stem cell populations. However, it is important to recognize the many features that are shared between most, if not all, stem cell niches.
Heterologous cell-cell interactions are invariably present and often exhibit complex, bidirectional signaling that is dependent on tight regulation and often cell-cell contact. For example, both excess120 and deficient128 Wnt signaling within the endosteal niche can have deleterious consequences for HSCs. Stem cell niches contain both tissue-specific (e.g., osteoblastic30) and seemingly generic (e.g., endothelial31,129 or stromal32) cell populations that have specialized roles in each context.
Secreted and membrane-bound factors such as Wnt, SCF, Notch and chemokines directly bind surface receptors on stem cells to regulate cell fate, self-renewal and polarity17,33,37,56–58,130.
Immunological cells provide dynamic regulation of the niche during inflammation and tissue damage, and this is tightly regulated through the presence of “immune privilege” and evasion from this privilege36,78.
ECM proteins are critical for orientation and structural maintenance of the niche, but importantly provide instructive signals through ligand interaction with integrins expressed on stem cells and may also serve as reservoirs for soluble factors131 (work by D.A.W. and colleagues).
Physical parameters such as shape, stiffness (or elasticity) and blood flow direct stem cell maintenance and differentiation103,104,106,108.
Many stem cell niches have altered environmental characteristics, such as hypoxia, and require tight metabolic regulation to maintain the long-term quiescence and self-renewal of stem cell populations111,112,114,130.
Numerous studies have highlighted the importance of the niche in modulating stem cell behavior13, and since publication of Schofield’s hypothesis of an HSC niche10, stem cell niches have been described in a variety of adult tissues, including skin14, intestine15–18 and nervous system19,20. Figure 2 illustrates the main features of the stem cell niche in the bone marrow, skin and intestine; features common to all of them are shown in Figure 1 and Box 1.
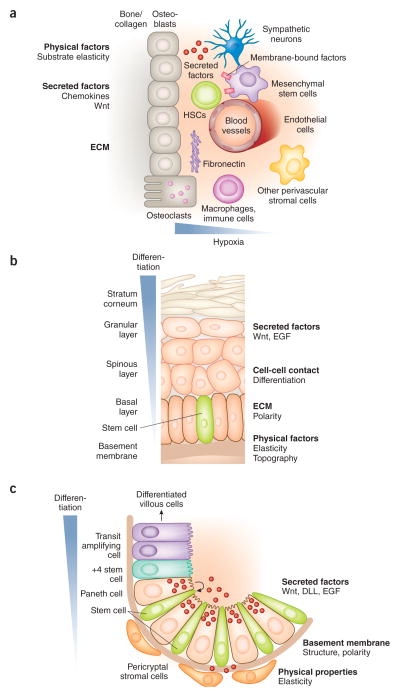
Figure 2.
Representative schema illustrating stem cell niches. (a–c) Discrete niches that support hematopoietic (a), epidermal (b) and intestinal stem cells (c). b adapted from ref. 14 with permission from Elsevier; c adapted from ref. 16, Nature Publishing Group.
The role of the niche is observed at several levels of resolution, which can be illustrated using the example of the epidermis. At the macro level, the importance of the epidermal niche was demonstrated by placing grafts of autologous cultured epidermis in direct contact with the muscle fascia in patients with extensive burns. Subsequently, engraftment of epidermal tissue was improved by placing it onto cadaveric stroma used to provide temporary coverage of the wound or by first culturing epidermal cells on an extracellular support made of fibrin rather than on tissue culture plastic21,22. This demonstrates that the nature of the extracellular matrix that epidermal cells attach to influences graft survival. At the level of individual stem cells, when different subpopulations of epidermal stem cells are disaggregated and used to reconstitute the skin, their differentiation potential is greater than when they are resident in the skin under homeostatic conditions23. In addition, the rate of proliferation of epidermal stem cells is dictated, at least in part, by signals such as growth factors and direct cell-cell contacts emanating from terminally differentiated epidermal cells overlying the stem cell compartment24 (work by F.M.W. and colleagues). The behavior of epidermal stem cells is also profoundly influenced by signals from cells within the dermis, which can occur over short range, as in the case of the dermal papilla at the base of each hair follicle25 (work by F.M.W. and colleagues), or over longer range, as in the case of skin adipocytes26,27. These three cell types can all be considered part of the epidermal stem cell niche. Furthermore, communication between stem cells and niche cells is reciprocal: signals from epidermal stem cells influence differentiation within the dermis, through both short- and long-range communication28,29 (work by F.M.W. and colleagues). One example of signaling at short range is that deposition of the ECM protein nephronectin by a subset of epidermal stem cells provides an adhesive substrate for adjacent mesenchymal cells that subsequently differentiate into smooth muscle cells28.
These studies of the skin highlight the ability of the niche to regulate stem cell self-renewal and generation of differentiated progeny. Niche signals can act at short or long range and at the level of individual cells or entire cell populations. A detailed discussion of the niche of every tissue is beyond the scope of this Review; rather, we will use examples to explore commonalities between different niches (Box 1) and to discuss specific precedents and opportunities for in vivo therapeutic intervention.
Cellular components of the niche
Resident niche cells
In many adult tissues, the stem cell niche contains a variety of cell types, each with a distinct function. This is clearly illustrated in the case of the hematopoietic microenvironment localized in the marrow space in adult bone and comprising a range of different cell types. Osteoblastic30, vascular31,32 and neural cells33, megakaryocytes34, macrophages35 and immune cells36 each have important roles and can be considered to define distinct HSC niches. Currently, controversy surrounds the differential roles of the osteoblastic and perivascular niches and, in particular, whether they have distinct, specialized roles or whether there is coordinated regulation of HSCs and therefore functional overlap13. For example, NG2+ peri-arteriolar cells regulate quiescence within long-term HSCs, and this quiescence appears essential for HSC function32. Other cells, such as endosteal macrophages, retain HSCs within the niche, and loss of these cells causes mobilization of HSCs out of their supportive microenvironment35.
In the case of stem cells in the colon and intestine, key niche cell types include the differentiated progeny of the stem cells. In the small intestine, Paneth cells physically co-localize with, and in turn support, intestinal stem cells through secretion of Wnt3a, Notch and epidermal growth factor16,18,37. In the colon, stem cells co-localize with their differentiated progeny, the goblet cells, which express c-kit, notch ligands and epidermal growth factor38. Thus, discrete niches exist in different parts of the gastrointestinal tract and contribute to tissue homeostasis.
A further concept that could be of practical importance is that experimental ablation of stem cells can result in neighboring cells dedifferentiating to replace them. When germline stem cells are ablated in Drosophila ovarioles, neighboring stromal cap cells (niche cells) persist and support the entry of somatic cells into the empty niche where they subsequently proliferate39. In mouse skin, laser ablation of hair follicle stem cells leads to repopulation of the niche by neighboring epithelial cells that are able to sustain hair regeneration40. In the liver, activation of Notch signaling reprograms hepatocytes to become biliary epithelial cells41. The presence of reserve stem cell populations42 and the reversion of differentiated cells to stem cells regulated in part by the niche17 have clear therapeutic implications for degenerative diseases. However, at present it is an open question as to whether the frequency of dedifferentiation could ever be sufficiently high to be of practical importance in tissue regeneration.
Direct cell contact
Communication between stem cells and niche cells is either direct, through physical interactions, or indirect, through secreted factors that mediate communication between cells that are not in direct contact. Direct contact can be mediated by a range of receptors, including bona fide cell-cell adhesion molecules and receptors with membrane-bound ligands. In the latter category, the Notch pathway stands out as being important in regulating stem cell function in many tissues. In the skin, it is well established that Notch signaling mediates distinct outcomes according to the level of pathway activation and acts both cell autonomously and non–cell autonomously by means of signaling between epidermal cells, fibroblasts and bone marrow–derived cells43,44 (work by F.M.W. and colleagues). In bone marrow, Notch ligands expressed by sinusoidal cells are essential for HSC self-renewal during recovery from myeloablative injury45.
In addition to Notch-receptor interactions, a number of other proteins that mediate intercellular communication through direct cell-cell contact are important in the niche. In Drosophila testis, the receptor tyrosine phosphatase Lar regulates adhesion between germline stem cells and niche cells46. In bone marrow, the cell adhesion molecule E-selectin is expressed by endothelial cells and promotes HSC proliferation47. HSC quiescence can be induced by administration of an E-selectin antagonist, which enhances HSC survival following treatment with chemotherapeutic agents or irradiation. Another interesting example is SCF, the ligand for the receptor tyrosine kinase c-kit. SCF is expressed in both soluble and membrane-bound isoforms, and experimental and genetic data suggest that stem cells expressing c-kit (including HSCs, melanocyte precursors and germ cells) have a specific requirement for membrane-bound SCF expressed on marrow stromal cells for their lodgement into the niche48,49 (work by D.A.W. and colleagues). This pathway may be modulated through a variety of small molecules, such as tyrosine kinase inhibitors50, and by neutralizing antibodies to c-kit51.
Secreted factors
Indirect communication between stem cells and niche cells is mediated by secreted factors. In the hematological system, this phenomenon is routinely exploited in clinical practice to modulate the HSC niche in vivo (Fig. 3). Mobilization of HSCs from their niche, for example, by using cytokines such as granulocyte colony-stimulating factor (G-CSF) or granulocyte-macrophage colony-stimulating factor (GM-CSF), is widely used to support treatment of hematological malignancy, bone marrow failure and rare genetic disorders (reviewed in To et al.52). These factors act in a variety of ways, including promoting expansion of HSCs and release of HSC-niche adhesion. The utility of targeting the niche with soluble factors is further illustrated by the finding that activation of the parathyroid hormone (PTH) receptor on osteoblasts by PTH increases HSC number53. HSCs do not express the PTH receptor; instead, stimulation of osteoblasts by PTH activates Notch signaling in HSCs53. PTH treatment is therapeutically beneficial in several different experimental, clinically relevant mouse models: it increases the number of HSCs mobilized into the peripheral blood, protects stem cells from cytotoxic drugs used in chemotherapy and expands stem cells in transplant recipients54. The potentially beneficial effect of PTH does not appear to be due to osteoblastic proliferation, as strontium also expands osteoblastic cells but does not alter HSC function55.
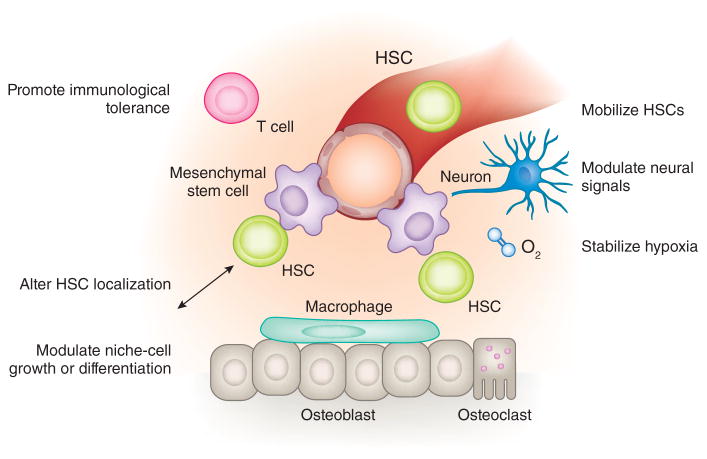
Figure 3.
Manipulation of the hematopoietic stem cell niche in vivo. In vivo manipulation of HSCs may be achieved by altering constituent niche cells, by administering drugs to alter cellular localization, by disrupting adhesive interactions or by stabilization of nutritional support (e.g., promoting hypoxia). Immune regulation of the HSC niche may be targeted through immunosuppressive medications or in allogeneic transplantation. HSC mobilization is regulated in part by the HSC niche and can be achieved with cytokine growth factors or by blocking adhesion molecules.
Although the studies with PTH demonstrate that the niche can be targeted with soluble factors, more recent studies show that the effects of a single niche factor differ according to the niche cell that expresses it. Deletion of Cxcl12 in different HSC niche cells has different outcomes56,57. Its deletion from perivascular stromal cells depletes HSCs and mobilizes them into the circulation, whereas deletion from osteoblasts depletes early lymphoid progenitors but not HSCs and does not lead to HSC mobilization. Deletion of Cxcl12 from endothelial cells has relatively little effect on the HSC compartment. These studies show that modulating a single secreted niche factor has different outcomes depending on which niche cell is producing it and highlight the potential difficulty of achieving therapeutic benefit by targeting a single component of the niche.
A signaling pathway that is involved in the regulation of almost all stem cell populations is the Wnt pathway58. Modulation of Wnt activity in the stem cell compartment has intrinsic effects both on those cells and on neighboring cells. For example, activation of the Wnt pathway in epidermal stem cells not only expands the stem cell compartment and promotes hair follicle differentiation but also stimulates melanocyte differentiation and reprograms adult dermis to acquire characteristics of neonatal dermis29,59–61 (work by F.M.W. and colleagues). Different levels of Wnt pathway activation have different effects, both within the epidermis and in the underlying dermis59,62.
The Wnt pathway is inappropriately activated in a wide range of cancers, and considerable progress has been made in developing drugs that inhibit different parts of the pathway63. However, methods for activating the pathway using recombinant Wnt proteins are challenging because the proteins are hydrophobic and difficult to produce in biologically active form64. Furthermore, given that Wnt proteins act both on stem cells and niche cells within the same tissue59,61, localized delivery could be a major issue that is irrelevant in other contexts, such as PTH in the HSC niche. One elegant way to overcome this is to immobilize biologically active Wnt on beads or other inert scaffolds65. This enables the application of Wnt protein to modulate juxtacrine signaling, as occurs during normal development66.
Other self-renewal pathways, such as Hedgehog signaling, are also important in the normal67 or cancer-stem-cell niche68, and novel Hedgehog inhibitors have reached early-phase clinical trials, with promising results in the treatment of medulloblastoma and basal cell carcinoma69. Basal cell carcinoma is believed to develop from epidermal stem cells and, not surprisingly, a side effect of inhibiting Hedgehog signaling in this disease is hair loss70. Therefore, as previously noted for Cxcl12, the challenge of targeting the niche therapeutically by secreted factors is how to achieve specificity in terms of which cells respond.
Metastatic malignancy provides a compelling argument that manipulation of the niche for therapeutic ends is feasible71. Endogenous soluble factors, such as transforming growth factor, matrix metalloproteinase, tumor necrosis factor or receptor activator of nuclear factor kappa-B ligand, derived from circulating bone marrow cells create a pre-metastatic niche at distal sites (e.g., the lungs) that supports the engraftment and metastasis of cancer stem cells72. Additionally, in some hematological cancers such as leukemia or multiple myeloma, cancer stem cells secrete CCL3 or other paracrine factors that lead to remodeling of normal niches through bone loss and increased osteoclastic activity73.
The secreted factors discussed so far are proteins that are expressed during normal tissue development, homeostasis and repair. A complementary approach, which is potentially easier to scale up and more cost effective, is to screen compound libraries for small molecules that target the niche. Screens for compounds that target stem cells are a very active area of research74 and, provided that the right assays to determine effects on stem cell–niche interactions are used, there is no reason why similar niche-regulator screens could not be designed75. Although we view high-throughput screening approaches with optimism, clinical translation of small molecules identified in this manner remains to be fulfilled, in part explained by the inherent limitations of taking a reductionist, ex vivo approach to niche interactions rather than faithfully modeling what is certainly a more complex in vivo microenvironment.
In summary, clinical practice in hematology and studies in animal models for tissues other than the blood suggest that new regenerative strategies that involve modifying the cellular components of the stem cell niche could be developed to expand or recreate the stem cell compartment or to change the fate of stem cells and their progeny. A number of strategies can be envisaged, from modulating the factors secreted by niche cells to interfering with direct cell-cell contact or altering the number and type of niche cells (Fig. 3).
The dynamic niche: inflammation and scarring
Although every stem cell niche is dynamic and exhibits cell turnover, it is useful to distinguish between niche cells that are ‘permanent residents’ and cells that occupy the niche in a transient fashion. Permanent residents would include endothelial cells, nerve cells and connective-tissue fibroblasts. The ‘visitors’ would include immune cells and cells that respond to tissue damage, for example, to protect against pathogens or to promote healing.
In contrast to resident niche cells, many cells of the innate and adaptive immune system migrate into and out of tissues. The function of immune cells can be modulated to promote stem cell function. For example, HSCs can be genetically modified to drive tolerogenic expression of antigens, thereby improving the long-term efficacy of HSC transplants76. Severe aplastic anemia, a condition in which bone marrow failure is caused by an immune attack on endogenous HSCs, can be effectively treated with anti-thymocyte globulin and immunosuppressive medications77.
Regulatory T lymphocytes provide immune privilege to the HSC niche36, and this finding is being exploited in clinical trials to prevent rejection of transplanted organs. Interestingly, mobilized HSCs upregulate surface CD47 expression, which acts to prevent phagocytic clearance of these cells78. Anti-CD47 antibody–mediated phagocytosis of tumor cells by macrophages is being evaluated as an anti-cancer therapy, and one could envisage that a similar strategy could be used to promote macrophage-mediated clearance of cells that are hindering endogenous tissue repair. Acute brain injury not only causes neuronal cell death but also causes damage to, and death of, niche-resident endothelial cells and macrophages, with resulting generation of reactive oxygen species (ROS). Accordingly, administration of the ROS scavenger, glutathione, promotes meningeal macrophage survival, reduces inflammation and ameliorates brain injury79.
Tissue injury and scarring represent other aspects of transient stem cell–niche interactions that can be targeted for therapeutic benefit. Fibrosis is an undesirable consequence of repeated injury and repair in a variety of tissues. In the skin, the existence of two different fibroblast lineages has recently been reported29. The lineage that mediates the initial wave of wound repair is unable to support hair follicle formation. But both subsets of dermal fibroblasts can be modulated by Wnt signaling, offering a potential route to changing the composition of the niche29. In genetically modified mice, Wnt-induced expansion of the fibroblast lineage that is required for hair follicle formation leads to the formation of new hair follicles in skin wounds. In wounded skin, gamma delta (γ/δ) T cells secrete fibroblast growth factor 9, which in turn triggers Wnt expression in fibroblasts and promotes hair follicle regeneration80. It remains to be determined whether γ/δT cells communicate selectively with the fibroblast lineage that is required for new hair follicle formation, or whether the T cells are able to confer hair follicle induction ability on other fibroblast populations.
Reducing fibrotic scar formation is a goal in many regenerative strategies, but in some cases, such as in the injured spinal cord, it may actually inhibit repair. Scar tissue is an inappropriate environment for repair over the long term, but immediately after injury it can limit damage. After spinal cord injury, scarring by astrocytes may restrict enlargement of the lesion and axonal loss81. In addition, neural stem cell progeny secrete a range of neurotrophic factors that promote neuronal survival81. As the example of the spinal cord shows, therapies to increase regeneration by inhibiting the scar niche require further investigation as they could have undesirable effects.
Extracellular matrix
The ECM is a key component of the stem cell niche in almost all tissues, although its composition and the nature of its contact with stem cells vary considerably82 (work by F.M.W. and colleagues). It has been appreciated for many decades that the ECM not only anchors stem cells but also directs their fate11. Many of the intracellular signaling pathways involved in ECM–stem cell interactions have been elucidated82. In some cases, the ECM also anchors soluble growth factors, increasing the local concentration of agonists to which target populations in the niche are exposed83. For example, adhesion molecules regulate interactions between stem cells, ECM and resident niche cells, and the expression of these molecules may be regulated by secreted factors84. The major ECM receptors are integrins, and their functions can be modulated with biologics, such as antibodies, or with small-molecule drugs. Just as with the Wnt pathway, abnormal integrin signaling is linked to cancer and other pathologies, including thrombotic diseases and inflammation, and pharmacological inhibitors of integrins are in the clinic85. Conversely, activating integrin antibodies are available to promote interactions between the ECM and stem cells86. In the case of the epidermis, such activating antibodies can decrease differentiation of stem cells87 (work by F.M.W. and colleagues).
The interaction of the ECM with stem cells depends not only on its protein composition but also on its physical properties. There is strong evidence that ECM surface topography and bulk stiffness can profoundly influence stem cell behavior82,88. These findings are increasingly informing the design of appropriate scaffolds for tissue repair89. Considerable progress has been made in the design of porous bioactive scaffolds that support bone regeneration and are resorbable90. High-throughput niche screens have demonstrated the synergistic effects of combinations of ECM and soluble factors91. Scaffolds can incorporate ECM protein motifs and/or growth factors. They can be used to localize stem cells and soluble molecules, for controlled release of soluble factors and for delivery of niche cells92,93. Several examples of the use of artificial scaffolds are shown in Table 1.
Table 1.
Examples of in vivo, niche-directed regenerative therapies in current clinical use or in clinical trials
| Disease indication | Niche target | Therapeutic approach |
|---|---|---|
| Hematopoietic regeneration post-transplantation | Osteoblastic cells | Parathyroid hormone to stimulate osteoblasts (N.B., efficacy was not demonstrated)121 |
| Bone marrow failure (severe aplastic anemia) | Secreted growth factors | Thrombopoietin mimetics122 |
| Immune cells | Anti-thymocyte globulin*77 | |
| HSC mobilization | Niche cells and secreted factors | G-CSF* or GM-CSF*52 |
| AMD3100* (ref. 52) | ||
| Spinal cord injury | Hypoxia | Daily, intermittent hypoxia exposure123 |
| Bone fracture or excision | ECM, mesenchymal cells, secreted factors | 3-dimensional bioengineered scaffolds, mesenchymal stem cells and bone morphogenic protein 2 (NCT01958502, clinicaltrials.gov) |
| Physical forces | Low-magnitude mechanical stimulation (NCT019215517, clinicaltrials.gov) | |
| Scaffolds and secreted growth factors | Scaffolds linked to BMP-2* (ref. 124) or platelet-derived growth factor125 | |
| Skin damage (e.g., burns, diabetic ulcers, wound excisions) | ECM and scaffolds | Dermal replacement scaffold (NCT02059252, clinicaltrials.gov) |
| ECM and growth factors | Platelet-derived growth factor in carboxymethylcellulose gel*126 | |
| Vascular niche cells | GM-CSF127 |
Approved therapies are indicated with an asterisk.
One category of disease that is readily attributable to defective ECM–stem cell interactions is epidermolysis bullosa (EB), a family of rare genetic skin blistering disorders94. Mutations responsible for different types of EB have been identified, including recessive dystrophic junctional EB (RDEB), which results from a failure to deposit type VII collagen in the basement membrane, and junctional EB, which is characterized by defective production of laminin 5. In one clinical study, junctional EB was corrected by culturing epidermal stem cells from the patient, transducing them with a retroviral vector encoding the missing laminin gene and grafting the gene-corrected cells onto the patient95.
Although such an approach is potentially feasible for RDEB, studies in mice have suggested a different strategy: transplantation of allogeneic fibroblasts96 or bone marrow from unaffected individuals. In a clinical trial of whole bone marrow transplantation, there was correction of the basement membrane defect in some patients97,98. In another study, a single injection of fibroblasts led to type VII collagen expression that was sustained for several months, with the newly deposited type VII collagen derived from the injected fibroblasts96. This illustrates the challenges to identify the most effective mechanism to repair the ECM in vivo and to further elucidate the signals from the damaged epidermis that stimulate pathological niche remodeling99.
One further intriguing feature of EB is the phenomenon of revertant mosaicism, whereby patches of epidermis spontaneously recover and produce the wild-type gene product, leading to healthy, non-blistered epidermis100,101. This phenomenon is important for several reasons. One is that if the underlying mechanism can be discovered and stimulated it would lead to new therapies94. Another is that iPSCs can be generated from the revertant areas, differentiated into epidermis and then used for grafting the affected regions102. Finally, clinical observations indicate that many of the revertant regions, although stable, do not expand over time. If expansion could be stimulated, presumably by modulating the niche, this would offer another avenue for tissue repair.
Physical factors
Stem cells rely on cues from their physical surroundings—substrate elasticity or stiffness, physical shape and shear forces. These processes have been applied both to improve in vitro culture and in an attempt to expand stem cell populations, such as HSCs and skeletal muscle stem cells103,104, and to various therapeutic contexts. Drugs that alter the balance between physical parameters, such as rigid (e.g., bone) or elastic (e.g., arteriolar, dermal connective tissue), already exist and are in clinical use for conditions such as osteoporosis and metastatic bone disease. Potassium channel openers, such as minoxidil, may act to increase elastic fiber content, thus maintaining niche elasticity in vivo105. Shear forces and drugs that promote blood flow accelerate the development of zebrafish embryonic HSCs in vivo106, whereas zebrafish and murine mutants that lack blood circulation exhibit reduced hemo-poiesis. At the single-cell level, promotion of contractility by nonmuscle myosin-II in HSCs is required for engraftment and niche sensing107. Finally, distinct niche topographies induce cytoskeletal deformation on stem cells, and this in turn activates specific downstream signaling pathways and directs differentiation108,109. These pathways could be modulated in vivo by inhibitors of RHO-GTPase signaling or indirectly through chromatin modifiers such as trichostatin A110.
Hypoxia and metabolism
Many cell populations, including HSCs and cardiac progenitors, reside in a low oxygen–tension (hypoxic) microenvironment111, which contributes to their survival and maintenance. Cells in such an environment carry out glycolysis rather than mitochondrial oxidative phosphorylation, and express high levels of hypoxia inducible factor 1α (HIF-1α). Growing a range of different mammalian cell types in culture under hypoxic conditions is beneficial for promoting survival, proliferation and function after engraftment112. In the hemopoietic system, the hypoxic environment is required for HSC quiescence and self-renewal, and stabilization of HIF-1α, either through the administration of dimethyl prostaglandin E2 (dmPGE2)113 or with dimethyloxalyl glycine (DMOG) or FG-4497, improves HSC quiescence and long-term HSC function114.
Cellular metabolism plays a pivotal role in determining whether a cell proliferates, differentiates or remains quiescent. There is a shift in the balance between glycolysis, mitochondrial oxidative phosphorylation and oxidative stress during the maturation of adult stem cells and during reprogramming of cells to a pluripotent state. This opens the way for novel metabolic or pharmacological therapies to enhance regeneration115,116. At present the most tractable applications of the recent insights into stem cell metabolism are to improve culture conditions for ex vivo cell expansion and differentiation. Nevertheless, one could envisage the development of drugs that target relevant metabolic enzymes and new technologies to track changes in cell metabolism in vivo.
At the level of the whole body, stem cell behavior is affected by factors such as nutritional status, aging117 and circadian rhythms8,118. It remains to be determined whether modulating the effects of these processes on the stem cell niche could have regenerative effects in specific target organs.
Conclusion
The past 10 to 15 years have witnessed an explosion in our understanding of the way that stem cells interact with their supporting niche, defined as the totality of the stem-cell microenvironment. More recently, tantalizing evidence has emerged in human and animal studies that modulating the stem cell niche can modulate the function of stem cell populations. Table 1 lists examples of this approach that are already approved or are in clinical trials. Niche-directed therapies may eventually be used more broadly in regenerative medicine for chronic degenerative diseases as well as in transplantation medicine and oncology. There are many hurdles on the path to achieving this vision. Efficacy and safety have been demonstrated in humans for restoration of the hematopoietic system, but progress has been slower in other tissues and organs. Challenges include assuring the tissue specificity of any intervention, guaranteeing the quality of repair over the long term and avoiding side effects of treatment such as carcinogenesis. Any therapeutic intervention that modulates critical developmental pathways, such as Wnt, Hedgehog or Notch signaling, may have teratogenic119 or carcinogenic120 effects. Although a more liberal ‘therapeutic window’ may be justified in the case of life-threatening conditions such as cancer, the potential for detrimental effects requires particularly careful attention in the context of regenerative therapies for conditions that are less serious or for which alternative therapies are available.
In practice, the most successful regenerative medicine treatments involving endogenous repair will probably be combination therapies. Targeting the niche is complementary to approaches that target stem cells directly, providing substantial opportunities for synergy. One could envisage treatments that involve not only different combinations of factors to target stem cells and niche cells but also applying such factors at different times to effect recovery according to the dynamics of stem cell–niche interactions.
Acknowledgments
We apologize for any omissions due to space constraints. S.W.L. is supported by research funding from the National Health and Medical Research Council, the Leukaemia Foundation of Australia and the Rhys Pengelly Fellowship in Leukaemia Research. D.A.W. is supported by National Institutes of Health DK062757. F.M.W. gratefully acknowledges the financial support of the Medical Research Council and the Wellcome Trust.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Watt FM, Driskell RR. The therapeutic potential of stem cells. Phil Trans R Soc Lond B. 2010;365:155–163. doi: 10.1098/rstb.2009.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daley GQ. The promise and perils of stem cell therapeutics. Cell Stem Cell. 2012;10:740–749. doi: 10.1016/j.stem.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pellegrini G, Rama P, Di Rocco A, Panaras A, De Luca M. Concise review: hurdles in a successful example of limbal stem cell-based regenerative medicine. Stem Cells. 2014;32:26–34. doi: 10.1002/stem.1517. [DOI] [PubMed] [Google Scholar]
- 4.Oldershaw RA. Cell sources for the regeneration of articular cartilage: the past, the horizon and the future. Int J Exp Pathol. 2012;93:389–400. doi: 10.1111/j.1365-2613.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bretzner F, Gilbert F, Baylis F, Brownstone RM. Target populations for first-inhuman embryonic stem cell research in spinal cord injury. Cell Stem Cell. 2011;8:468–475. doi: 10.1016/j.stem.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Wirth E, III, Lebkowski JS, Lebacqz K. Response to Frederic Bretzner et al Target populations for first-in-human embryonic stem cell research in spinal cord injury. Cell Stem Cell. 2011;8:476–478. doi: 10.1016/j.stem.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Ramsden CM, et al. Stem cells in retinal regeneration: past, present and future. Development. 2013;140:2576–2585. doi: 10.1242/dev.092270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abad M, et al. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature. 2013;502:340–345. doi: 10.1038/nature12586. [DOI] [PubMed] [Google Scholar]
- 9.Farrar CA, Kupiec-Weglinski JW, Sacks SH. The innate immune system and transplantation. Cold Spring Harb Perspect Med. 2013;3:a015479. doi: 10.1101/cshperspect.a015479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 11.Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- 12.Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 13.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505:327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuchs E. Finding one’s niche in the skin. Cell Stem Cell. 2009;4:499–502. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan DW, Barker N. Intestinal stem cells and their defining niche. Curr Top Dev Biol. 2014;107:77–107. doi: 10.1016/B978-0-12-416022-4.00003-2. [DOI] [PubMed] [Google Scholar]
- 16.Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol. 2014;15:19–33. doi: 10.1038/nrm3721. [DOI] [PubMed] [Google Scholar]
- 17.van Es JH, et al. Dll1+ secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol. 2012;14:1099–1104. doi: 10.1038/ncb2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conover JC, Notti RQ. The neural stem cell niche. Cell Tissue Res. 2008;331:211–224. doi: 10.1007/s00441-007-0503-6. [DOI] [PubMed] [Google Scholar]
- 20.Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7:733–736. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 21.Simman R, Priebe CJ, Jr, Simon M. Reconstruction of aplasia cutis congenita of the trunk in a newborn infant using acellular allogenic dermal graft and cultured epithelial autografts. Ann Plast Surg. 2000;44:451–454. doi: 10.1097/00000637-200044040-00019. [DOI] [PubMed] [Google Scholar]
- 22.Green H. The birth of therapy with cultured cells. BioEssays. 2008;30:897–903. doi: 10.1002/bies.20797. [DOI] [PubMed] [Google Scholar]
- 23.Watt FM, Jensen KB. Epidermal stem cell diversity and quiescence. EMBO Mol Med. 2009;1:260–267. doi: 10.1002/emmm.200900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arwert EN, et al. Tumor formation initiated by nondividing epidermal cells via an inflammatory infiltrate. Proc Natl Acad Sci USA. 2010;107:19903–19908. doi: 10.1073/pnas.1007404107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. J Cell Sci. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plikus MV, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451:340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujiwara H, et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. 2011;144:577–589. doi: 10.1016/j.cell.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo Celso C, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 32.Kunisaki Y, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katayama Y, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 34.Olson TS, et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood. 2013;121:5238–5249. doi: 10.1182/blood-2012-10-463414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winkler IG, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 36.Fujisaki J, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474:216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 38.Rothenberg ME, et al. Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology. 2012;142:1195–1205. doi: 10.1053/j.gastro.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kai T, Spradling A. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci USA. 2003;100:4633–4638. doi: 10.1073/pnas.0830856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rompolas P, Mesa KR, Greco V. Spatial organization within a niche as a determinant of stem-cell fate. Nature. 2013;502:513–518. doi: 10.1038/nature12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanger K, et al. Robust cellular reprogramming occurs spontaneously during liver regeneration. Genes Dev. 2013;27:719–724. doi: 10.1101/gad.207803.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tian H, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watt FM, Estrach S, Ambler CA. Epidermal Notch signalling: differentiation, cancer and adhesion. Curr Opin Cell Biol. 2008;20:171–179. doi: 10.1016/j.ceb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambler CA, Watt FM. Adult epidermal Notch activity induces dermal accumulation of T cells and neural crest derivatives through upregulation of jagged 1. Development. 2010;137:3569–3579. doi: 10.1242/dev.050310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler JM, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srinivasan S, Mahowald AP, Fuller MT. The receptor tyrosine phosphatase Lar regulates adhesion between Drosophila male germline stem cells and the niche. Development. 2012;139:1381–1390. doi: 10.1242/dev.070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winkler IG, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 48.Driessen RL, Johnston HM, Nilsson SK. Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Exp Hematol. 2003;31:1284–1291. doi: 10.1016/j.exphem.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 49.Toksoz D, et al. Support of human hematopoiesis in long-term bone marrow cultures by murine stromal cells selectively expressing the membrane-bound and secreted forms of the human homolog of the steel gene product, stem cell factor. Proc Natl Acad Sci USA. 1992;89:7350–7354. doi: 10.1073/pnas.89.16.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schittenhelm MM, et al. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 2006;66:473–481. doi: 10.1158/0008-5472.CAN-05-2050. [DOI] [PubMed] [Google Scholar]
- 51.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.To LB, Levesque JP, Herbert KE. How I treat patients who mobilize hematopoi-etic stem cells poorly. Blood. 2011;118:4530–4540. doi: 10.1182/blood-2011-06-318220. [DOI] [PubMed] [Google Scholar]
- 53.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 54.Adams GB, et al. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–243. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 55.Lymperi S, et al. Strontium can increase some osteoblasts without increasing hema-topoietic stem cells. Blood. 2008;111:1173–1181. doi: 10.1182/blood-2007-03-082800. [DOI] [PubMed] [Google Scholar]
- 56.Greenbaum A, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495:231–235. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 59.Silva-Vargas V, et al. Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell. 2005;9:121–131. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 60.Tanimura S, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 61.Collins CA, Kretzschmar K, Watt FM. Reprogramming adult dermis to a neonatal state through epidermal activation of beta-catenin. Development. 2011;138:5189–5199. doi: 10.1242/dev.064592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito M, et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- 63.Garber K. Drugging the Wnt pathway: problems and progress. J Natl Cancer Inst. 2009;101:548–550. doi: 10.1093/jnci/djp084. [DOI] [PubMed] [Google Scholar]
- 64.Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. 2012;4:a007864. doi: 10.1101/cshperspect.a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Habib SJ, et al. A localized Wnt signal orients asymmetric stem cell division in vitro. Science. 2013;339:1445–1448. doi: 10.1126/science.1231077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alexandre C, Baena-Lopez A, Vincent JP. Patterning and growth control by membrane-tethered Wingless. Nature. 2014;505:180–185. doi: 10.1038/nature12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siggins SL, et al. The Hedgehog receptor Patched1 regulates myeloid and lymphoid progenitors by distinct cell-extrinsic mechanisms. Blood. 2009;114:995–1004. doi: 10.1182/blood-2009-03-208330. [DOI] [PubMed] [Google Scholar]
- 68.Morris JPt, Wang SC, Hebrok M. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rudin CM, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang JY, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114:1150–1157. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frisch BJ, et al. Functional inhibition of osteoblastic cells in an in vivo mouse model of myeloid leukemia. Blood. 2012;119:540–550. doi: 10.1182/blood-2011-04-348151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li W, Li K, Wei W, Ding S. Chemical approaches to stem cell biology and therapeutics. Cell Stem Cell. 2013;13:270–283. doi: 10.1016/j.stem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hartwell KA, et al. Niche-based screening identifies small-molecule inhibitors of leukemia stem cells. Nat Chem Biol. 2013;9:840–848. doi: 10.1038/nchembio.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coleman MA, et al. Tolerance induction with gene-modified stem cells and immune-preserving conditioning in primed mice: restricting antigen to differentiated antigen-presenting cells permits efficacy. Blood. 2013;121:1049–1058. doi: 10.1182/blood-2012-06-434100. [DOI] [PubMed] [Google Scholar]
- 77.Rosenfeld SJ, Kimball J, Vining D, Young NS. Intensive immunosuppression with antithymocyte globulin and cyclosporine as treatment for severe acquired aplastic anemia. Blood. 1995;85:3058–3065. [PubMed] [Google Scholar]
- 78.Jaiswal S, et al. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roth TL, et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gay D, et al. Fgf9 from dermal gammadelta T cells induces hair follicle neogenesis after wounding. Nat Med. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sabelström H, et al. Resident neural stem cells restrict tissue damage and neuronal loss after spinal cord injury in mice. Science. 2013;342:637–640. doi: 10.1126/science.1242576. [DOI] [PubMed] [Google Scholar]
- 82.Watt FM, Huck WT. Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol. 2013;14:467–473. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- 83.Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol. 2012;24:645–651. doi: 10.1016/j.ceb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 84.Williams CK, et al. Regulation of CXCR4 by the Notch ligand delta-like 4 in endothelial cells. Cancer Res. 2008;68:1889–1895. doi: 10.1158/0008-5472.CAN-07-2181. [DOI] [PubMed] [Google Scholar]
- 85.Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol Sci. 2012;33:405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 86.Byron A, et al. Anti-integrin monoclonal antibodies. J Cell Sci. 2009;122:4009–4011. doi: 10.1242/jcs.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Evans RD, et al. A tumor-associated beta 1 integrin mutation that abrogates epithelial differentiation control. J Cell Biol. 2003;160:589–596. doi: 10.1083/jcb.200209016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Swift J, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lu B, Atala A. Small molecules and small molecule drugs in regenerative medicine. Drug Discov Today. 2013;19:801–808. doi: 10.1016/j.drudis.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 90.Ren J, et al. Melt-electrospun polycaprolactone strontium-substituted bioactive glass scaffolds for bone regeneration. J Biomed Mater Res A. 2013 Oct 7; doi: 10.1002/jbma.34985. [DOI] [Google Scholar]
- 91.Gobaa S, et al. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat Methods. 2011;8:949–955. doi: 10.1038/nmeth.1732. [DOI] [PubMed] [Google Scholar]
- 92.Ko IK, Lee SJ, Atala A, Yoo JJ. In situ tissue regeneration through host stem cell recruitment. Exp Mol Med. 2013;45:e57. doi: 10.1038/emm.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lampe KJ, Heilshorn SC. Building stem cell niches from the molecule up through engineered peptide materials. Neurosci Lett. 2012;519:138–146. doi: 10.1016/j.neulet.2012.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uitto J, Christiano AM, McLean WH, McGrath JA. Novel molecular therapies for heritable skin disorders. J Invest Dermatol. 2012;132:820–828. doi: 10.1038/jid.2011.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mavilio F, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12:1397–1402. doi: 10.1038/nm1504. [DOI] [PubMed] [Google Scholar]
- 96.Wong T, et al. Potential of fibroblast cell therapy for recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2008;128:2179–2189. doi: 10.1038/jid.2008.78. [DOI] [PubMed] [Google Scholar]
- 97.Wagner JE, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med. 2010;363:629–639. doi: 10.1056/NEJMoa0910501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Abdul-Wahab A, Petrof G, McGrath JA. Bone marrow transplantation in epidermolysis bullosa. Immunotherapy. 2012;4:1859–1867. doi: 10.2217/imt.12.120. [DOI] [PubMed] [Google Scholar]
- 99.Tamai K, et al. PDGFRalpha-positive cells in bone marrow are mobilized by high mobility group box 1 (HMGB1) to regenerate injured epithelia. Proc Natl Acad Sci USA. 2011;108:6609–6614. doi: 10.1073/pnas.1016753108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pasmooij AM, Jonkman MF, Uitto J. Revertant mosaicism in heritable skin diseases: mechanisms of natural gene therapy. Discov Med. 2012;14:167–179. [PubMed] [Google Scholar]
- 101.Lai-Cheong JE, McGrath JA, Uitto J. Revertant mosaicism in skin: natural gene therapy. Trends Mol Med. 2011;17:140–148. doi: 10.1016/j.molmed.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tolar J, et al. Patient-specific naturally gene-reverted induced pluripotent stem cells in recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2013;134:1246–1254. doi: 10.1038/jid.2013.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holst J, et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol. 2010;28:1123–1128. doi: 10.1038/nbt.1687. [DOI] [PubMed] [Google Scholar]
- 104.Gilbert PM, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Slove S, et al. Potassium channel openers increase aortic elastic fiber formation and reverse the genetically determined elastin deficit in the BN rat. Hypertension. 2013;62:794–801. doi: 10.1161/HYPERTENSIONAHA.113.01379. [DOI] [PubMed] [Google Scholar]
- 106.North TE, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shin JW, et al. Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell Stem Cell. 2014;14:81–93. doi: 10.1016/j.stem.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 109.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci USA. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Connelly JT, Mishra A, Gautrot JE, Watt FM. Shape-induced terminal differentiation of human epidermal stem cells requires p38 and is regulated by histone acetylation. PLoS ONE. 2011;6:e27259. doi: 10.1371/journal.pone.0027259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kimura W, Sadek HA. The cardiac hypoxic niche: emerging role of hypoxic microenvironment in cardiac progenitors. Cardiovasc Diagn Ther. 2012;2:278–289. doi: 10.3978/j.issn.2223-3652.2012.12.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Muscari C, et al. Priming adult stem cells by hypoxic pretreatments for applications in regenerative medicine. J Biomed Sci. 2013;20:63. doi: 10.1186/1423-0127-20-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Speth JM, Hoggatt J, Singh P, Pelus LM. Pharmacologic increase in HIF1alpha enhances hematopoietic stem and progenitor homing and engraftment. Blood. 2014;123:203–207. doi: 10.1182/blood-2013-07-516336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Forristal CE, et al. Pharmacologic stabilization of HIF-1alpha increases hematopoietic stem cell quiescence in vivo and accelerates blood recovery after severe irradiation. Blood. 2013;121:759–769. doi: 10.1182/blood-2012-02-408419. [DOI] [PubMed] [Google Scholar]
- 115.Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Spéder P, Liu J, Brand AH. Nutrient control of neural stem cells. Curr Opin Cell Biol. 2011;23:724–729. doi: 10.1016/j.ceb.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 117.Signer RA, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12:152–165. doi: 10.1016/j.stem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Janich P, et al. Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem Cell. 2013;13:745–753. doi: 10.1016/j.stem.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 119.Belloni E, et al. Identification of Sonic hedgehog as a candidate gene responsible for holoprosencephaly. Nat Genet. 1996;14:353–356. doi: 10.1038/ng1196-353. [DOI] [PubMed] [Google Scholar]
- 120.Kode A, et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature. 2014;506:240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ballen K, et al. Phase II trial of parathyroid hormone after double umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2012;18:1851–1858. doi: 10.1016/j.bbmt.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Olnes MJ, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11–19. doi: 10.1056/NEJMoa1200931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hayes HB, et al. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology. 2014;82:104–113. doi: 10.1212/01.WNL.0000437416.34298.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft) Int Orthop. 2007;31:729–734. doi: 10.1007/s00264-007-0418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Solchaga LA, Hee CK, Roach S, Snel LB. Safety of recombinant human platelet-derived growth factor-BB in Augment((R)) Bone Graft. J Tissue Eng. 2012;3:2041731412442668. doi: 10.1177/2041731412442668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care. 1998;21:822–827. doi: 10.2337/diacare.21.5.822. [DOI] [PubMed] [Google Scholar]
- 127.Cianfarani F, et al. Granulocyte/macrophage colony-stimulating factor treatment of human chronic ulcers promotes angiogenesis associated with de novo vascular endothelial growth factor transcription in the ulcer bed. Br J Dermatol. 2006;154:34–41. doi: 10.1111/j.1365-2133.2005.06925.x. [DOI] [PubMed] [Google Scholar]
- 128.Fleming HE, et al. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2:274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Méndez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 131.Williams DA, Rios M, Stephens C, Patel VP. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature. 1991;352:438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]