Abstract
Stress response is well appreciated to induce the expression of heat shock proteins (Hsps) in the cell. Numerous studies have demonstrated that Hsps function as molecular chaperones in the stabilization of intracellular proteins, repairing damaged proteins, and assisting in protein translocation. Various kinds of stem cells (embryonic stem cells, adult stem cells, or induced pluripotent stem cells) have to maintain their stemness and, under certain circumstances, undergo stress. Therefore, Hsps should have an important influence on stem cells. Actually, numerous studies have indicated that some Hsps physically interact with a number of transcription factors as well as intrinsic and extrinsic signaling pathways. Importantly, alterations in Hsp expression have been demonstrated to affect stem cell behavior including self-renewal, differentiation, sensitivity to environmental stress, and aging. This chapter summarizes recent findings related to (1) the roles of Hsps in maintenance of stem cell dormancy, proliferation, and differentiation; (2) the expression signature of Hsps in embryonic/adult stem cells and differentiated stem cells; (3) the protective roles of Hsps in transplanted stem cells; and (4) the possible roles of Hsps in stem cell aging.
I. Introduction
Stem cells (SCs) are pluripotent cells that can self-renew and differentiate into various cell lineages.1–5 In mammals, there are two major types of SCs: (1) embryonic stem cells (ESCs), which exist in the inner cell mass of blastocytes and can differentiate into all the specialized cells of organs and (2) adult SCs, identified in most of the tissues, which are believed to act as a repair system to replenish worn out or damaged tissues.3–7 Indeed, SCs originating from bone marrow, adipose tissue, and blood have been successfully applied to the treatment of bone/blood-related cancers and cardiovascular disease.8–16 However, the quantity of pluripotent SCs in adult tissues is very small. In addition, a large number of transplanted SCs die within hours/days, which eventually limits the efficacy of cellular therapy.17–19 Therefore, there is an urgent need to expand our knowledge of SC behavior including SC renewal and differentiation, their survival and stress response, as well as their aging.
Heat shock response (HSR) was first described in 1962 by Ritossa20 who observed that heat stress caused chromosomal puffs in the salivary glands of the Drosophila larva. Since then, HSR (also referred to as stress response) and a vast array of heat shock proteins (Hsps) have been widely identified in various organisms ranging from prokaryotic Escherichia coli to eukaryotic mammalians. Actually, the HSR is an evolutionarily conserved and homeostatic genetic response to a multitude of physiological, pathological, chemical, and environmental stresses.21 This stress response is initiated by activation of the heat shock transcription factors (HSFs), leading to the enhanced transcription and translation of Hsps. To date, several members of the HSF family (HSFs 1–5) have been reported in vertebrates.22–25 HSF1 can be activated by heat shock (HS), metals, and ethanol, and, consequently, by upregulation of cellular Hsps (i.e., Hsp40, Hsp70, and Hsp90). In contrast, HSF2 is activated only during differentiation and development. HSF3, a heat-responsive transcription factor, is expressed only in avians, whereas HSF4 exhibits tissue-specific expression (i.e., human heart, brain, skeletal muscle, and pancreas) and functions to repress the expression of Hsps (i.e., Hsp27, Hsp70, and Hsp90).23–25 HSF5 is the most recently discovered HSF in human tissue26 and its function and characteristics remain unknown.
Based on the molecular weight and/or the stimuli, Hsps are usually categorized into three major groups.27–29 The first group consists of the high-molecular weight Hsps, including members of the Hsp110, Hsp90, Hsp70, and Hsp60 family. The second group includes the Hsps induced under conditions of glucose deprivation and are referred to as the “minor Hsps,” including glucose-regulated proteins (GRPs) 34, 47, 56, 75, 78, 94, and 174. The third group consists of the small Hsps (sHsps) and includes at least 10 members (HspB1–B10) whose molecular weights range from 12 to 30 kDa. It is well accepted that Hsps function as molecular chaperones in the modulation of cellular protein conformational state and protein translocation (i.e., shuttle proteins from one compartment to another inside the cell, and transport old proteins to “garbage disposals” inside the cell).21 While numerous intrinsic and extrinsic signals are involved in the tight regulation of SC self-renewal, differentiation, survival, and aging, increasing evidence indicates that Hsps play an essential role in the regulation of these behaviors of SCs.30–35 This chapter summarizes recent findings related to (1) the expression profiles of Hsps in embryonic and adult SCs as well as differentiated SCs; (2) the possible role of Hsps in the maintenance of SC dormancy, proliferation, and differentiation; (3) the protective effects of Hsps in transplanted SCs; and (4) the possible role of Hsps in SC aging.
II. Hsps in the Modulation of SC Self-Renewal
SC self-renewal is a complex regulatory process that is dependent on multiple factors including the transcription factors Nanog,36,37 Oct4,38 Sox2,39 and STAT3.40,41 While STAT3 appears to be critical for the self-renewal of the mouse, but not human, ESCs,40,41 the expression of Nanog, a key regulatory protein in the self-renewal of both human and mouse ESCs,36,37,42 is reported to be controlled by STAT3. Hence, these transcription factors may work in concert to regulate SC renewal. Interestingly, certain Hsps are highly expressed in ESCs, interacting with these transcription factors, and are essential for normal cell development and functioning.43–46 For instance, Hsp90 constitutes one of the most abundant cytosolic proteins commonly present in two major forms: the stress-induced Hsp90a43 and the constitutively expressed Hsp90β.44 Hsp90 functions to exclusively mediate the folding and maturation of transcription regulators and signal transducers; interactions have been mapped with approximately 300 client proteins. Recent studies further indicate that Hsp90 interacts with STAT3/Hop, and that the Hsp90/Hop complex is involved in the activation of STAT3 in mouse ESCs, suggesting that Hsp90 may modulate SC renewal. Indeed, Hsp90β knockout mouse embryos fail to develop past E9/9.5.47 Moreover, Baharvand et al.48 conducted an analysis for shared and unique chaperone expression profiles in ESC, mesenchymal stem cell (MSC), and neural stem cell (NSC). They found that all types of SCs shared high expression levels of Hsp70 protein 5 (HspA5), Hsp70 protein 8 (HspA8), and Hop (Stip1). ESCs showed a unique chaperone expression signature of Hsp70 protein 4 (HspA4), Hsp27 (HspB1), and Hsp90β (HspCb). These data indicate that high expression levels of chaperone and co-chaperones in SCs may exert a buffering effect against external and internal stressors, thereby maintaining their stemness.
On the other hand, cancer stem cells (CSCs) are characterized by enhanced expression of both oncogenes and Hsp genes.49–51 Importantly, CSCs exhibit self-renewal capacity to promote tumor regeneration and metastasis, and contribute to radioresistance and chemoresistance.52,53 Therefore, deciphering Hsp genes responsible for the maintenance of self-renewal and tumorigenicity in the CSCs is a crucial step in the development of new antitumor drugs. Wu et al.54 recently identified that the expression of GRP78 (also referred to as HspA5), a central mediator of endoplasmic reticulum homeostasis, was significantly increased in isolated head and neck cancer stem cells (HN-CSCs). These HN-CSCs display cancer-initiating properties in vitro and in vivo. Downregulation of GRP78 reduced self-renewal properties and inhibited tumorigenicity of HN-CSCs, whereas overexpression of GRP78 in HN-CSCs enhanced in vitro malignant potentials. Indeed, numerous studies have demonstrated that GRP78 is required for the survival of ESCs and is also highly expressed in hematopoietic stem cells (HSCs).55 Furthermore, Wu et al.54 found that knockdown of GRP78 increased the expression of PTEN, Bax, and Caspase-3 but reduced the expression of p-MAPK in HN-CSCs. Additionally, they observed that downregulation of GRP78 reduced the expression of Nanog in HN-CSCs. Overall, these studies suggest that GRP78 may significantly contribute to signaling and transcriptional regulatory networks in CSC renewal.
III. Expression Profiles of Hsp in Differentiated SCs
ESCs have the capacity to self-renew in an undifferentiated state but may also differentiate into cell types representing the three embryonic germ lineages (ectoderm, mesoderm, and endoderm), thereby demonstrating their pluripotent potential.1,5 Progress has been made toward the molecular understanding of the process of SC differentiation, using wide-scale transcriptional and translational profiling approaches. Especially, proteomics has emerged as a robust method for performing large-scale studies of proteins to complement high-throughput gene expression analyses at the RNA level. Accordingly, there have been an increasing number of publications comparing protein profiles in differentiated and undifferentiated SCs.56–61 Saretzki et al.58 reported that four Hsps, namely, HspB1 (Hsp27), HspA1a (Hsp70a1), HspA1b (Hsp70b1), and HspA9a (Hsp70a9, mortalin), were highly expressed in mESCs, which perhaps contributes to their remarkable resistance to potential genotoxic stress. However, these four Hsps were significantly decreased upon the differentiation of mESCs. Likewise, Baharvand et al.59 also observed that Hsp70, Hsp60, and co-chaperone Hop were downregulated in differentiated mESC cell lines Royan B1 and D3. Similarly, during the directed differentiation of mouse ESCs into neurogenic embryoid bodies (NEBs), a significant reduction of Hsp27 and mortalin (mtHsp70/mot-2/GRP75) was observed.60 Interestingly, during differentiation of human ESCs, the expression of HSPA1b decreased significantly whereas Hsp27 did not show any significant alteration.61 Together, these studies suggest that ESC differentiation may involve the restriction of protein expression rather than the addition of newly expressed genes, and that alterations in certain Hsps could serve as differentiation markers of ESCs. Nonetheless, it is important to note that downregulation of Hsp may occur before detectable expression of traditional differentiation markers, for example, Sox1. Furthermore, in human adipose-derived adult stem cells, differentiation enhances the expression of Hsp20 (HspB6), Hsp27 (HspB1), Hsp60, and αB-crystallin (HspB5).62 In contrast, the relative levels of Hsp47, Hsp70, Hsp90, and FK506-binding protein showed no change following adipogenesis.62 These results suggest that alterations of Hsp expression during adult SC differentiation may be different from ESC differentiation and may be organ-specific, as reviewed below.
IV. Roles of Hsps in Tissue Genesis
A. Hsps in Hematopoietic Differentiation
Hsps have also been recognized to be involved in differentiation events of hematopoietic progenitors toward erythroid,63 myeloid,64 and lymphoid65 lineages through a protective mechanism of peculiar transcription factors which are able to direct differentiation events in immature HSCs. For example, Ribeil et al.63 have demonstrated the role of Hsp70 in the prevention of the inactivation of transcription factor GATA-1 modulated by a caspase-mediated proteolysis, indicating that Hsp70 might indirectly trigger erythroid differentiation. HSF2 is rapidly downregulated during megakaryocytic differentiation, but activated during hemin-induced differentiation of human erythroleukemia cells. HSPA8, referred to as Hsc70, has been demonstrated to play a role in cytokine-mediated HSC survival and to negatively influence the stability of proapoptotic Bim mRNA, thus preventing apoptosis in hematopoiesis and leukemogenesis.66 Hsp27 and Hsp60 have been associated with myeloid commitment by functioning as cognate triggers for crucial monocyte–macrophage receptors, which induce activation and proliferation of these cells.64,67 Loss of HSPA9b (mortalin) recapitulates the ineffective hematopoiesis of myelodysplastic syndrome in zebrafish.68 Accordingly, Hsps could also be involved in differentiation events of umbilical cord blood-derived HSCs.69 Together, these studies suggest that Hsp might represent a key target either for future investigations or for approaches addressing mechanisms underlying the induction of SC proliferation and modulation of their differentiation events.
B. Essential Role of αB-crystallin in Muscle Differentiation
Myogenic differentiation is under the strict control of myogenic transcription factors such as MyoD, Myf5, myogenin, and MRF4 in coordination with a second class of transcription factors including MEF2A-2D.70–72 Mice lacking MyoD develop normally but are severely impaired in their ability to regenerate muscle after tissue injury.73 MyoD is found to be a master regulator of muscle differentiation and is involved in the determination of the muscle cell lineage.74 Importantly, the promoter of sHsps is predicted to contain at least one MyoD/myogenin binding site. Sugiyama et al.75 reported that sHsps form two different complexes during muscle differentiation, implying their importance in muscle development. Of these sHsps, αB-crystallin (HspB5) seems to play an essential role in muscle differentiation and its maintenance. During muscle differentiation, the level of αB-crystallin goes up 10-fold, and mice lacking αB-crystallin have lower muscle mass.76,77 A point mutation in αB-crystallin, namely, R120G, has been found to be associated with desmin-related myopathies, suggesting its importance in muscle tissue homeostasis.78,79 Recently, Singh et al.80 demonstrated that overexpression of αB-crystallin in C2C12 cells severely affected myotube formation. They further revealed that αB-crystallin modulates MyoD activity by the combined effect of its reduced synthesis and increased degradation, thus retarding muscle differentiation. αB-crystallin can also activate the NF-κB signaling pathway, which may cause a reduction of MyoD mRNA and augmentation of the cyclin D1, a growth stimulatory molecule.81 In addition, overexpression of αB-crystallin delayed p21 expression and prevented the activation of caspase-3, which might contribute to the delay in the muscle differentiation process.80 Taken together, these data suggest that αB-crystallin is essential for muscle differentiation because increased proliferation and/or degradation of MyoD by αB-crystallin are antagonistic to myogenesis.
C. Altered HSR in the Differentiated Neural Cells
The HSR (stress response) is a primary, evolutionarily conserved, and homeostatic genetic response to many stressors. Activation of HSR causes the induction of Hsps that function as molecular chaperones to facilitate the folding and refolding of non-native proteins and other proteins essential for recovery from cell damage.21,82–84 Numerous studies have shown that HSR and the ability to induce Hsp expression are attenuated in various brain and spinal cord neurons in vivo and in vitro.84,85 A recent study by Yang et al.86 reported that differentiation of neural progenitor cells was associated with an attenuated HSR. In consistence with this, Battersby et al.60 performed proteomic analysis in mESC-derived NEBs. They observed that, following mESC differentiation, expression of Hsp25 became excluded from neural precursors as well as other differentiating cells. The reduced HSR in the differentiated neural cells is likely to contribute to their vulnerability to stress-induced pathologies and death. As shown by Yang et al.86, the differentiated NG108-15 cells (mouse neuroblastoma–glioma hybrid cell line used as the prototype neural progenitor cells) were more sensitive to the cytotoxic effect of an oxidizer, namely, sodium arsenite, than the undifferentiated cells. Furthermore, they observed that pre-conditioning with HS or forced expression of Hsp70 conferred cytoprotection when the differentiated cells were challenged. These data provide evidence of an attenuated HSR as part of the neural differentiation program. Hence, development of a treatment regimen or a pharmacological agent to rectify the defective HSR in the differentiated neuron would be a promising approach to mitigate the dire consequences of protein misfolding and boost neuron survival under stress.
On the other hand, NSCs, present in the developing mammalian CNS, are immature cells that are capable of self-renewal and can differentiate into neurons, astrocytes, or oligodendrocytes. NSCs play a pivotal role in the development of the CNS.87 However, it has been reported that the exposure of fetuses or neonates to alcohol during the critical periods of brain development can result in fetal alcohol syndrome (FAS).88 While numerous studies have been conducted to investigate the effects of ethanol on the brain tissue, the genes regulated by alcohol and their associated biological pathways remain obscure. Recently, Choi et al.89 performed a genome-wide transcriptional analysis in differentiated NSCs derived from the forebrain of E15 mouse embryos with/out ethanol exposure. Interestingly, they observed that genes of the sHsp family including HspB2, HspB7, and HspB8, as well as Hsp40 (Hsp40 is responsible for Hsp70 recruitment and stimulates Hsp70 ATPase activity), were all upregulated in NSCs during short-term differentiation without ethanol exposure, and were more pronounced in the presence of ethanol, compared to undifferentiated NSCs. In contrast, HspB1 was downregulated during NSC differentiation regardless of ethanol exposure. Furthermore, they examined which HSF genes were expressed during the short-term differentiation of NSCs in the presence of ethanol, and observed that the gene expression patterns of HSF1 and HSF4 were not modulated by differentiation or by the presence of ethanol. Conversely, the gene expression levels of HSF2 and HSF5 increased during differentiation, and were further increased following treatment with ethanol. Interestingly, other studies have reported that HSF2 is not activated in response to classical stress but rather during tissue development.90,91 Therefore, the results of Choi et al.89 suggest that exposure to ethanol might activate HSF2 in the CNS, resulting in its translocation to the nucleus and the subsequent transcriptional regulation of various sHsp target genes. Overall, these data implicate the possibility that the upregulation of HspB2/7/8 and HSF2/5 in response to ethanol may function to reduce cell damage and may be a good candidate for the diagnosis of FAS.
D. Hsps in Mouse Trophoblast SC Differentiation
Increasing evidence has shown that Hsps are necessary for placental development. For example, targeted deletion of Hsp90 results in embryonic lethality due to placental defects as a result of a failure to form a placental labyrinth layer.92 HSF1 is the major HSF controlling rapid Hsp induction. Mice mutant for Hsf1 has a common phenotype that produces a thinning of the spongiotrophoblast layer, leading to embryonic lethality.93 In the rat, HspB1 was strongly expressed in trophoblast giant cells beginning at Day 11 of gestation.94 In human pregnancies, HspB1 can be detected in the intermediate trophoblast and syncytiotrophoblast cells during the first two trimesters.95 In addition, HspB1 is more highly expressed in placenta from patients with severe preeclampsia compared with that from normal-term gestations.96 A study by Winger et al.97 demonstrated that expression levels of HspB1 significantly increased by the second day of trophoblast stem cell (TSC) differentiation and greater at Days 4 and 6 of differentiation. Meanwhile, levels of phosphorylated HspB1 and MAPKAPK2 protein were also increased during differentiation, and this increase could be inhibited when cells were differentiated in the presence of an inhibitor of MAPK14. These results indicate that the MAPK14–HspB1 axis may be playing an important role during TSC differentiation.
E. HS Enhances Inducible Differentiation of MSC/ESC
MSCs can differentiate into a variety of cell types including osteoblasts, adipocytes, chondrocytes, cardiomyocytes, and endothelial-like cells.2–7 In order to improve the therapeutic use of SCs, it is important to find ways to enhance the qualitative and quantitative extent of differentiation, and thereby increase the chances of the cells to grow into the right kind of tissue or structure. Given that Hsps have been recognized to maintain cellular homoeostasis during changes in the microenvironment, Norgaard et al.98 examined the effects of HS on the differentiation of human MSCs as measured by the synthesis of an osteoblastic marker, alkaline phosphatase, and cell matrix mineralization. They used a telomerase-immortalized human MSC line designated hMSC-TERT, which was treated with HS 3 h prior to adding the medium, promoting osteoblast differentiation and mineralized matrix formation, respectively. They observed that mild heat stress induced the levels of Hsps and enhanced the responsiveness of SCs to differentiate, and concluded that a 1-h HS of 42.5 °C is the most beneficial pretreatment to hMSC-TERT cells when inducing them to differentiate into osteoblasts. Afzal et al.30 also determined the effects of HS on the neural differentiation of mouse embryonal carcinoma SCs (P19). They induced neural differentiation from pre-HS treated EBs (embryoid bodies) in the presense of 1 mM RA (retinoic acid) and 5% FBS (fetal bovine serum). Interestingly, they observed that a HS 12 h before the initiation of differentiation did not affect the expression of neuroectodermal and neural markers nestin and β-tubulin III, respectively. However, both markers increased remarkably when heat stress was induced after treatment and when EBs were formed. Taken together, these data suggest that Hsps could regulate inducible differentiation of SCs.
V. Protective Effects of Hsps in Transplanted SCs
SC therapy is quickly emerging as an attractive strategy in the treatment of neurodegenerative diseases, stroke, some striated muscle pathologies, and ischemic heart diseases. The promising therapeutic effect(s) of SCs is dependent on their capacity to survive and engraft in the target tissue. However, poor viability of transplanted SCs was observed, which yields only marginal beneficial effects. The Yacoub group showed that 7% of skeletal myoblasts survive 3 days after grafting into the infarcted mouse heart,99 and Muller-Ehmsen et al.100 found that 28% of implanted neonatal cardiomyocytes survived 1 week after grafting into normal rat hearts. Similarly, Freyman et al.101 observed that ~5% of MSCs survived after transplanting into the infarcted porcine heart. Toma et al.102 reported that less than 0.44% MSCs survive till day 4 after engraftment in an immunodeficient mouse heart model. Clearly, it is imperative to reinforce the transplanted cells to withstand the rigors of the microenvironment of the damaged tissue incurred from ischemia, inflammatory responses, and proapoptotic factors in order to acquire an effective therapeutic modality.
Hsps are well appreciated to provide protection against various stress stimuli such as high temperature, oxidative stress, pressure overload, and hypoxia/ischemia.21 A number of protective phenotypes have been attributed to Hsps, including ion channel repair, redox balance restoration, inhibition of proinflammatory cytokines, and activation of survival signaling or inhibition of apoptotic pathways.103,104 Therefore, increased Hsp levels would be expected to improve the survival of transplanted SCs and, consequently, their therapeutic efficacy. Indeed, myriad studies have utilized multiple approaches to enhance Hsp expression in SCs, which significantly augments SC therapeutic effectiveness, as reviewed below.
Over the past decade, many groups have consistently demonstrated that exposure of myoblasts to short-term HS resulted in the upregulation of Hsps including Hsp70/72 and HO-1 (Hsp32) without loss of their myogenic characteristics and improved the survival rate after autotransplantation in intact skeletal muscle.105–109 For example, Laumonier et al.109 demonstrated that lentivirus-mediated HO-1 gene transfer enhanced survival of porcine myogenic precursor cells after autologous transplantation. Likewise, exposure of rat MSCs to the chemical inducer 2 mM β-mercaptoethanol for 1–3 h followed by 24-h incubation led to Hsp72 synthesis and exhibited higher resistance to oxidative stress.110 Preconditioning rat MSCs with recombinant human Hsp90a for 24 h was shown to protect against hypoxia and serum-deprivation-triggered cell death via activation of the Akt and ERK1/2 pathway.111 Similarly, Chang et al.112 directly delivered Hsp70 into rat MSCs, using the Hph-1 protein transduction domain ex vivo, and found that Hph-1-Hsp70-MSCs displayed higher viability and antiapoptotic properties upon hypoxic stress, evidenced by the upregulation of Bcl-2 expression, reduction of Bax, phosphorylation of JNK, and activity of caspase-3. In addition, Hph-1-Hsp70-MSCs retained function in the infarcted region of the myocardium and promoted the expression of cardiac-specific markers on MSCs. Transplantation of Hph-1-Hsp70-MSCs into infarcted hearts significantly improved left ventricular (LV) function and limited LV remodeling. Notably, neither cytotoxic effects on MSCs with significantly higher concentrations of Hph-1-Hsp70 nor tumorigenesis of Hph-1-Hsp70-treated MSCs were detected. Together, these data suggest that enhancing the viability and integration potential of MSCs by Hph-1-Hsp70 treatment before transplantation may provide a novel therapeutic opportunity for the treatment of myocardial infarction and end-stage cardiac failure. In consistence with this, Wang et al.113 modified rat MSCs with the Hsp20 gene (Hsp20-MSCs), and observed that adenovirus-mediated Hsp20 delivery protected MSCs against H2O2-triggered cell death via activation of Akt and enhanced release of growth factors (VEGF, FGF-2, and IGF). Importantly, manipulation of MSCs with the Hsp20 gene augmented the paracrine effects of MSCs on infarcted hearts, leading to a significantly increased number of new blood vessels. In addition, in vivo transplantation of Hsp20-MSCs enhanced the survival rate of MSCs in the infarcted heart, which resulted in the attenuation of postinfarction LV remodeling and functional improvement.
Similar protective effects were also observed in Hsp27-overexpressing mouse ESCs, which correlates with resistance to the toxicity of cadmium chloride, mercuric chloride, cis-platinum (II)-diammine dichloride, and sodium arsenite, as well as in Hsp27-transfected hippocampal progenitor cells against glucocorticoid-evoked apoptotic cell death.114,115
In summary, increased levels of Hsps in SCs by HS or pharmacological preconditioning, direct delivery, or genetic modification has been validated as an effective approach for improvement of in vivo cell therapy efficacy, because most of the Hsps identified so far (i.e., Hsp70, Hsp20, Hsp27, HO-1) have displayed the features instrumental for antiapoptosis, antioxidative stress, anti-inflammation, enhanced paracrine effects, and neo-angiogenesis (Fig. 1). Therefore, Hsp-engineered SCs could become a promising means for the better survival of clinically transplanted stem cells.
FIG. 1.
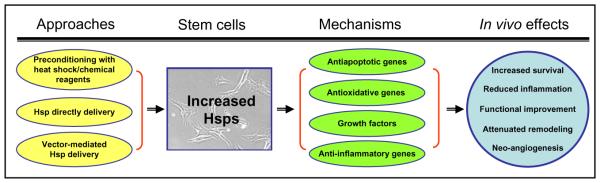
A scheme for the approaches to increasing Hsp levels in stem cells and the beneficial consequences of in vivo transplantation.
VI. Roles of Hsps in SC Aging
As reviewed above, mild heat stress or preconditioning enhances the defense capacity of SCs to adapt to environmental changes and increases implanted cell survival. However, this is not the case in aged cells/tissues. For example, McArdle et al.116 demonstrated that the maximum force generated by muscles of young mice following a period of damaging lengthening contractions in vivo was severely diminished but then slowly recovered to reach pre-exercise values by approximately 28 days following the damaging contractions. In contrast, muscles of old mice maintained a significant deficit in maximum force generation at this time point, and this deficit persisted for at least 2 months. This inability to recover is found to be associated with the attenuated ability of muscles of old mice to generate Hsps in response to stress because muscles of old mice overexpressing Hsp70 recover successfully following damage. Another study by McArdle et al.117 observed that the levels of HSF1 and HSF2 were temporally altered during muscle maturation. The HSF2 content of myotubes was significantly increased during the early stages of skeletal muscle regeneration. In contrast, the HSF1 content of myotubes remained relatively low until late during regeneration. These data suggest that abnormal activation of HSF1 may play a role in the defective regeneration seen in muscles of old mice.
Likewise, Stolzing et al.33 showed that, in MSCs from young (6-week) rats, cold temperature (32 °C) significantly upregulated the expression of Hsp27, Hsp70, and Hsp90, but downregulated the Hsp60 expression. In contrast, aged MSCs from 56-week-old rats did not display or attenuate these alterations in Hsp levels. Indeed, Yu et al.118 recently compared the ability of stress response in MSCs from rhesus monkeys among three age groups: young (< 5 years), middle (8–10 years), and old (> 12 years). They observed that MSCs exhibited decreased capacities for proliferation and differentiation with aging. While the levels of HSF-2, Hsp27, Hsp47, Hsp60, Hsp90A, and Hsp90B did not alter, there was a twofold decrease in the expression levels of HSF-1 and a fourfold reduction in mRNA levels of Hsp70 in middle-age and old MSCs compared to young MSCs. Together, these results indicate that SCs derived from aged tissue may down-regulate the Hsp expression and impair the capacity of stress response.
VII. Conclusions
A multitude of physiologic stresses and diseases result in cellular protein damage and misfolded protein structure, leading to the alteration of cell behavior/phenotype and dysfunction. The cell has evolved a number of protective means to maintain homeostatsis and promote survival during these periods of environmental stress and disease. One of the most highly conserved mechanisms of cellular protection involves the expression of a class of heat shock or stress proteins (Hsps). Hsps are molecular chaperones whose functions include assistance with native protein folding, maintenance of multiprotein complexes, intraorganellar protein shuttling, and degradation of senescent proteins. In general, various kinds of SCs (ESCs, adult SCs, induced pluripotent SCs) have to maintain their stemness and, under certain circumstances, undergo stress. Therefore, Hsps should be playing an important role in SCs. As reviewed above, ESCs contain abundant Hsps and display resistance to stress. Furthermore, Hsps interact with transcript factors (i.e., STAT3, Nanog, Oct4) and signaling pathways, which may modulate SC differentiation and proliferation. Importantly, SCs originating from aged tissues/organs exhibit impaired stress response and reduced levels of Hsps, which consequently affects SC behavior. Finally, considering the poor survival rate of transplanted SCs, upregulation of cellular Hsp levels should be a promising approach to the improvement of SC therapy.
References
- 1.Denker HW. Potentiality of embryonic stem cells: an ethical problem even with alternative stem cell sources. J Med Ethics. 2006;32(11):665–71. doi: 10.1136/jme.2005.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ratajczak MZ, Zuba-Surma EK, Wysoczynski M, Wan W, Ratajczak J, Wojakowski W, et al. Hunt for pluripotent stem cell—regenerative medicine search for almighty cell. J Autoimmun. 2008;30(3):151–62. doi: 10.1016/j.jaut.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kucia M, Ratajczak MZ. Stem cells as a two edged sword—from regeneration to tumor formation. J Physiol Pharmacol. 2006;57(Suppl. 7):5–16. [PubMed] [Google Scholar]
- 4.Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22(15):1987–97. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condic ML, Rao M. Alternative sources of pluripotent stem cells: ethical and scientific issues revisited. Stem Cells Dev. 2010;19(8):1121–9. doi: 10.1089/scd.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motaln H, Schichor C, Lah TT. Human mesenchymal stem cells and their use in cell-based therapies. Cancer. 2010;116(11):2519–30. doi: 10.1002/cncr.25056. [DOI] [PubMed] [Google Scholar]
- 7.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28(3):585–96. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bladé J, Rosiñol L, Cibeira MT, Rovira M, Carreras E. Hematopoietic stem cell transplantation for multiple myeloma beyond 2010. Blood. 2010;115(18):3655–63. doi: 10.1182/blood-2009-08-238196. [DOI] [PubMed] [Google Scholar]
- 10.Madonna R, Geng YJ, De Caterina R. Adipose tissue-derived stem cells: characterization and potential for cardiovascular repair. Arterioscler Thromb Vasc Biol. 2009;29(11):1723–9. doi: 10.1161/ATVBAHA.109.187179. [DOI] [PubMed] [Google Scholar]
- 11.Burt RK, Loh Y, Pearce W, Beohar N, Barr WG, Craig R, et al. Clinical applications of blood-derived and marrow-derived stem cells for nonmalignant diseases. JAMA. 2008;299(8):925–36. doi: 10.1001/jama.299.8.925. [DOI] [PubMed] [Google Scholar]
- 12.Velazquez OC. Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J Vasc Surg. 2007;45(Suppl. A):A39–47. doi: 10.1016/j.jvs.2007.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tendera M, Wojakowski W. Clinical trials using autologous bone marrow and peripheral blood-derived progenitor cells in patients with acute myocardial infarction. Folia Histochem Cytobiol. 2005;43(4):233–5. [PubMed] [Google Scholar]
- 14.Steward CG, Jarisch A. Haemopoietic stem cell transplantation for genetic disorders. Arch Dis Child. 2005;90(12):1259–63. doi: 10.1136/adc.2005.074278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee N, Thorne T, Losordo DW, Yoon YS. Repair of ischemic heart disease with novel bone marrow-derived multipotent stem cells. Cell Cycle. 2005;4(7):861–4. doi: 10.4161/cc.4.7.1799. [DOI] [PubMed] [Google Scholar]
- 16.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46(1):7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 17.Haider HKh, Ashraf M. Bone marrow cell transplantation in clinical perspective. J Mol Cell Cardiol. 2005;38(2):225–35. doi: 10.1016/j.yjmcc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77(1):134–42. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 19.Haider HKh, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45(4):554–66. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ritossa FM. Behavior of RNA and DNA synthesis at the puff level in salivary gland chromosomes of Drosophila. Exp Cell Res. 1964;36:515–23. doi: 10.1016/0014-4827(64)90308-8. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin IJ, McMillan DR. Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res. 1998;83:117–32. doi: 10.1161/01.res.83.2.117. [DOI] [PubMed] [Google Scholar]
- 22.Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–69. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- 23.Pirkkala L, Nykanen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–31. doi: 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto N, Takemori Y, Sakurai M, Sugiyama K. Differential recognition of heat shock elements by members of the heat shock transcription factor family. FEBS J. 2009;276:1962–74. doi: 10.1111/j.1742-4658.2009.06923.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakai A, Tanabe M, Kawazoe Y, Inazawa J, Morimoto RI, Nagata K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol Cell Biol. 1997;17:469–81. doi: 10.1128/mcb.17.1.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ota T, Suzuki Y, Nishikawa T, Otsuki T, Sugiyama T, Irie R, et al. Complete sequencing and characterization of 21, 243 full-length human cDNAs. Nat Genet. 2004;36:40–5. doi: 10.1038/ng1285. [DOI] [PubMed] [Google Scholar]
- 27.Sciandra JJ, Subjeck JR. The effects of glucose on protein synthesis and thermosensitivity in Chinese hamster ovary cells. J Biol Chem. 1983;258:12091–3. [PubMed] [Google Scholar]
- 28.Kappé G, Franck E, Verschuure P, Boelens WC, Leunissen JA, de Jong WW. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones. 2003;8:53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan GC, Kranias EG. Small heat shock protein 20 (HspB6) in cardiac hypertrophyand failure. J Mol Cell Cardiol. 2011;51:574–7. doi: 10.1016/j.yjmcc.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Afzal E, Ebrahimi M, Najafi SM, Daryadel A, Baharvand H. Potential role of heat shock proteins in neural differentiation of murine embryonal carcinoma stem cells (P19) Cell Biol Int. 2011;35(7):713–20. doi: 10.1042/CBI20100457. [DOI] [PubMed] [Google Scholar]
- 31.Chen TH, Kambal A, Krysiak K, Walshauser MA, Raju G, Tibbitts JF, et al. Knockdown of Hspa9, a del(5q31.2) gene, results in a decrease in hematopoietic progenitors in mice. Blood. 2011;117(5):1530–9. doi: 10.1182/blood-2010-06-293167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turturici G, Geraci F, Candela ME, Cossu G, Giudice G, Sconzo G. Hsp70 is required for optimal cell proliferation in mouse A6 mesoangioblast stem cells. Biochem J. 2009;421(2):193–200. doi: 10.1042/BJ20082309. [DOI] [PubMed] [Google Scholar]
- 33.Stolzing A, Sethe S, Scutt AM. Stressed stem cells: temperature response in aged mesenchymal stem cells. Stem Cells Dev. 2006;15(4):478–87. doi: 10.1089/scd.2006.15.478. [DOI] [PubMed] [Google Scholar]
- 34.Patterson ST, Li J, Kang JA, Wickrema A, Williams DB, Reithmeier RA. Loss of specific chaperones involved in membrane glycoprotein biosynthesis during the maturation of human erythroid progenitor cells. J Biol Chem. 2009;284(21):14547–57. doi: 10.1074/jbc.M809076200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sauvageot CM, Weatherbee JL, Kesari S, Winters SE, Barnes J, Dellagatta J, et al. Efficacy of the HSP90 inhibitor 17-AAG in human glioma cell lines and tumorigenic glioma stem cells. Neuro Oncol. 2009;11(2):109–21. doi: 10.1215/15228517-2008-060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, et al. The homeoprotein nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–42. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 37.Chambers I, Colby D, Robertson M, Nichols J, Lee S, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–55. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 38.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–91. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 39.Botquin V, Hess H, Fuhrmann G, Anastassiadis C, Gross MK, et al. New POU dimmer configuration mediates antagonistic control of an osteopontin preimplantation enhancer by Oct-4 and Sox-2. Genes Dev. 1998;12:2073–90. doi: 10.1101/gad.12.13.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–60. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raz R, Lee CK, Cannizaro LA, D’eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci USA. 1999;96:2846–51. doi: 10.1073/pnas.96.6.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyslop L, Stojkovic M, Armstrong L, Walter T, Stojkovic P, et al. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035–43. doi: 10.1634/stemcells.2005-0080. [DOI] [PubMed] [Google Scholar]
- 43.Sreedhar AS, Kalmar E, Csermely P, Shen YF. Hsp90 isoforms: functions, expression and clinical importance. FEBS Lett. 2004;562:11–5. doi: 10.1016/s0014-5793(04)00229-7. [DOI] [PubMed] [Google Scholar]
- 44.Lai BT, Chin NW, Stanek AE, Keh W, Lanks KW. Quantitation and intracellular localization of the 85 K heat shock protein by using monoclonal and polyclonal antibodies. Mol Cell Biol. 1984;4:2802–10. doi: 10.1128/mcb.4.12.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol. 2001;188:281–90. doi: 10.1002/jcp.1131. [DOI] [PubMed] [Google Scholar]
- 46.Setati MM, Prinsloo E, Longshaw VM, Murray PA, Edgar DH, Blatch GL. Leukemia inhibitory factor promotes Hsp90 association with STAT3 in mouse embryonic stem cells. IUBMB Life. 2010;62(1):61–6. doi: 10.1002/iub.283. [DOI] [PubMed] [Google Scholar]
- 47.Voss AK, Thomas T, Gruss P. Mice lacking Hsp90b fail to develop a placental labyrinth. Development. 2000;127:1–11. doi: 10.1242/dev.127.1.1. [DOI] [PubMed] [Google Scholar]
- 48.Baharvand H, Fathi A, Van Hoof D, Salekdeh GH. Concise review: trends in stem cell proteomics. Stem Cells. 2007;25:1888–903. doi: 10.1634/stemcells.2007-0107. [DOI] [PubMed] [Google Scholar]
- 49.Bensaude O, Morange M. Spontaneous high expression of heat-shock proteins in mouse embryonal carcinoma cells and ectoderm from day 8 mouse embryo. EMBO J. 1983;2(2):173–7. doi: 10.1002/j.1460-2075.1983.tb01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Creagh EM, Sheehan D, Cotter TG. Heat shock proteins—modulators of apoptosis in tumour cells. Leukemia. 2000;14(7):1161–73. doi: 10.1038/sj.leu.2401841. [DOI] [PubMed] [Google Scholar]
- 51.Setroikromo R, Wierenga PK, van Waarde MA, Brunsting JF, Vellenga E, Kampinga HH. Heat shock proteins and Bcl-2 expression and function in relation to the differential hyperthermic sensitivity between leukemic and normal hematopoietic cells. Cell Stress Chaperones. 2007;12(4):320–30. doi: 10.1379/CSC-279.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lathia JD, Venere M, Rao MS, Rich JN. Seeing is believing: are cancer stem cells the Loch Ness monster of tumor biology? Stem Cell Rev. 2011;7(2):227–37. doi: 10.1007/s12015-010-9194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gil J, Stembalska A, Pesz KA, Sasiadek MM. Cancer stem cells: the theory and perspectives in cancer therapy. J Appl Genet. 2008;49(2):193–9. doi: 10.1007/BF03195612. [DOI] [PubMed] [Google Scholar]
- 54.Wu MJ, Jan CI, Tsay YG, Yu YH, Huang CY, Lin SC, et al. Elimination of head and neck cancer initiating cells through targeting glucose regulated protein78 signaling. Mol Cancer. 2010;9:283. doi: 10.1186/1476-4598-9-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo S, Mao C, Lee B, Lee AS. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol. 2006;26:5688–97. doi: 10.1128/MCB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo X, Ying W, Wan J, Hu Z, Qian X, Zhang H, et al. Proteomic characterization of early-stage differentiation of mouse embryonic stem cells into neural cells induced by all-trans retinoic acid in vitro. Electrophoresis. 2001;22(14):3067–75. doi: 10.1002/1522-2683(200108)22:14<3067::AID-ELPS3067>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 57.Wang D, Gao L. Proteomic analysis of neural differentiation of mouse embryonic stem cells. Proteomics. 2005;5(17):4414–26. doi: 10.1002/pmic.200401304. [DOI] [PubMed] [Google Scholar]
- 58.Saretzki G, Armstrong L, Leake A, Lako M, von Zglinicki T. Stress defense in murine embryonic stem cells is superior to that of various differentiated murine cells. Stem Cells. 2004;22(6):962–71. doi: 10.1634/stemcells.22-6-962. [DOI] [PubMed] [Google Scholar]
- 59.Baharvand H, Fathi A, Gourabi H, Mollamohammadi S, Salekdeh GH. Identification of mouse embryonic stem cell-associated proteins. J Proteome Res. 2008;7:412–23. doi: 10.1021/pr700560t. [DOI] [PubMed] [Google Scholar]
- 60.Battersby A, Jones RD, Lilley KS, McFarlane RJ, Braig HR, et al. Comparative proteomic analysis reveals differential expression of Hsp25 following the directed differentiation of mouse embryonic stem cells. Biochim Biophys Acta. 2007;1773:147–56. doi: 10.1016/j.bbamcr.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 61.Saretzki G, Walter T, Atkinson S, Passos JF, Bareth B, Keith WN, et al. Downregulation of multiple stress defense mechanisms during differentiation of human embryonic stem cells. Stem Cells. 2008;26(2):455–64. doi: 10.1634/stemcells.2007-0628. [DOI] [PubMed] [Google Scholar]
- 62.DeLany JP, Floyd ZE, Zvonic S, Smith A, Gravois A, Reiners E, et al. Proteomic analysis of primary cultures of human adipose-derived stem cells: modulation by Adipogenesis. Mol Cell Proteomics. 2005;4(6):731–40. doi: 10.1074/mcp.M400198-MCP200. [DOI] [PubMed] [Google Scholar]
- 63.Ribeil JA, Zermati Y, Vandekerckhove J, et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature. 2006;445:102–5. doi: 10.1038/nature05378. [DOI] [PubMed] [Google Scholar]
- 64.Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD15 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–7. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- 65.Ripley BJ, Stephanou A, Isenberg DA, Latchman DS. Interleukin-10 activates heat-shock protein 90beta gene expression. Immunology. 1999;97:226–31. doi: 10.1046/j.1365-2567.1999.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsui H, Asou H, Inaba T. Cytokines direct the regulation of Bim mRNA stability by heat-shock cognate protein 70. Mol Cell. 2007;25:99–112. doi: 10.1016/j.molcel.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Bermejo L, Vilaboa NC, Perez C, deBlas E, Calle C, Aller P. Modulation of hsp70 and hsp27 gene expression by the differentiation inducer sodium butyrate in U-937 human promonocytic leukemia cells. Leuk Res. 1995;19:713–8. doi: 10.1016/0145-2126(95)00045-p. [DOI] [PubMed] [Google Scholar]
- 68.Craven SE, French D, Ye W, de Sauvage F, Rosenthal A. Loss of Hspa9b in zebrafish recapitulates the ineffective hematopoiesis of the myelodysplastic syndrome. Blood. 2005;105:3528–34. doi: 10.1182/blood-2004-03-1089. [DOI] [PubMed] [Google Scholar]
- 69.D’Alessandro A, Grazzini G, Giardina B, Zolla L. In silico analyses of proteomic data suggest a role for heat shock proteins in umbilical cord blood hematopoietic stem cells. Stem Cell Rev. 2010;6(4):532–47. doi: 10.1007/s12015-010-9180-z. [DOI] [PubMed] [Google Scholar]
- 70.Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993;75:1241–4. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- 71.Walsh K, Perlman H. Cell cycle exit upon myogenic differentiation. Curr Opin Genet Dev. 1997;7:597–602. doi: 10.1016/s0959-437x(97)80005-6. [DOI] [PubMed] [Google Scholar]
- 72.Maione R, Amati P. Interdependence between muscle differentiation and cell-cycle control. Biochim Biophys Acta, Rev Cancer. 1997;332:M19–30. doi: 10.1016/s0304-419x(96)00036-4. [DOI] [PubMed] [Google Scholar]
- 73.Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol. 1999;210:440–55. doi: 10.1006/dbio.1999.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weintraub H, Tapscott SJ, Davis RL, Thayer MJ, Adam MA, Lassar AB, et al. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci USA. 1989;86:5434–8. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sugiyama Y, Suzuki A, Kishikawa M, Akutsu R, Hirose T, Waye MM, et al. Muscle develops a specific form of small heat shock protein complex composed of MKBP/HSPB2 and HSPB3 during myogenic differentiation. J Biol Chem. 2000;275:1095–104. doi: 10.1074/jbc.275.2.1095. [DOI] [PubMed] [Google Scholar]
- 76.Ito H, Kamei K, Iwamoto I, Inaguma Y, Kato K. Regulation of the levels of small heat-shock proteins during differentiation of C2C12 cells. Exp Cell Res. 2001;266:213–21. doi: 10.1006/excr.2001.5220. [DOI] [PubMed] [Google Scholar]
- 77.Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42:2924–34. [PubMed] [Google Scholar]
- 78.Vicart P, Caron A, Guicheney P, Li Z, Prévost MC, Faure A, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–5. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 79.Sanbe A, Osinska H, Saffitz JE, Glabe CG, Kayed R, Maloyan A, et al. Desmin-related cardiomyopathy in transgenic mice: a cardiac amyloidosis. Proc Natl Acad Sci USA. 2004;101:10132–6. doi: 10.1073/pnas.0401900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh BN, Rao KS, Rao ChM. Ubiquitin-proteasome-mediated degradation and synthesis of MyoD is modulated by alphaB-crystallin, a small heat shock protein, during muscle differentiation. Biochim Biophys Acta. 2010;1803(2):288–99. doi: 10.1016/j.bbamcr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 81.Adhikari AS, Singh BN, Rao KS, Rao ChM. aB-crystallin, a small heat shock protein, modulates NF-kB activity in a phosphorylation-dependent manner and protects muscle myoblasts from TNF-a induced cytotoxicity. Biochim Biophys Acta. 2011;1813(8):1532–42. doi: 10.1016/j.bbamcr.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 82.Morimoto RI, Tissieres A, Georgopoulos C. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press; New York: 1994. p. 610. [Google Scholar]
- 83.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:378–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 84.Morimoto RI. Stress, aging, and neurodegenerative disease. N Engl J Med. 2006;355:2254–5. doi: 10.1056/NEJMcibr065573. [DOI] [PubMed] [Google Scholar]
- 85.Forman MS, Trojanowski JQ, Lee VM-Y. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–63. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 86.Yang J, Oza J, Bridges K, Chen KY, Liu AY. Neural differentiation and the attenuated heat shock response. Brain Res. 2008;1203:39–50. doi: 10.1016/j.brainres.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 87.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–8. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 88.Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–58. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- 89.Choi MR, Jung KH, Park JH, Das ND, Chung MK, Choi IG, et al. Ethanol-induced small heat shock protein genes in the differentiation of mouse embryonic neural stem cells. Arch Toxicol. 2011;85(4):293–304. doi: 10.1007/s00204-010-0591-z. [DOI] [PubMed] [Google Scholar]
- 90.Rallu M, Loones MT, Lallemand Y, Morimoto RI, Morange M, Mezger V. Function and regulation of heat shock factor 2 during mouse embryogenesis. Proc Natl Acad Sci USA. 1997;94:2392–7. doi: 10.1073/pnas.94.6.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mandrekar P, Catalano D, Jeliazkova V, Kodys K. Alcohol exposure regulates heat shock transcription factor binding and heat shock proteins 70 and 90 in monocytes and macrophages: implication for TNF-alpha regulation. J Leukoc Biol. 2008;84:1335–45. doi: 10.1189/jlb.0407256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Voss AK, Thomas T, Gruss P. Mice lacking HSP90beta fail to develop a placental labyrinth. Development. 2000;127:1–11. doi: 10.1242/dev.127.1.1. [DOI] [PubMed] [Google Scholar]
- 93.Xiao X, Zuo X, Davis AA, McMillan DR, Curry BB, Richardson JA, et al. HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J. 1999;18:5943–52. doi: 10.1093/emboj/18.21.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ciocca DR, Stati AO, Fanelli MA, Gaestel M. Expression of heat shock protein 25,000 in rat uterus during pregnancy and pseudopregnancy. Biol Reprod. 1996;54:1326–35. doi: 10.1095/biolreprod54.6.1326. [DOI] [PubMed] [Google Scholar]
- 95.Shah M, Stanek J, Handwerger S. Differential localization of heat shock proteins 90, 70, 60 and 27 in human decidua and placenta during pregnancy. Histochem J. 1998;30:509–18. doi: 10.1023/a:1003259907014. [DOI] [PubMed] [Google Scholar]
- 96.Geisler JP, Manahan KJ, Geisler HE, Tammela JE, Rose SL, Hiett AK, et al. Heat shock protein 27 in the placentas of women with and without severe preeclampsia. Clin Exp Obstet Gynecol. 2004;31:12–4. [PubMed] [Google Scholar]
- 97.Winger QA, Guttormsen J, Gavin H, Bhushan F. Heat shock protein 1 and the mitogen-activated protein kinase 14 pathway are important for mouse trophoblast stem cell differentiation. Biol Reprod. 2007;76(5):884–91. doi: 10.1095/biolreprod.106.056820. [DOI] [PubMed] [Google Scholar]
- 98.Nørgaard R, Kassem M, Rattan SI. Heat shock-induced enhancement of osteoblastic differentiation of hTERT-immortalized mesenchymal stem cells. Ann N Y Acad Sci. 2006;1067:443–7. doi: 10.1196/annals.1354.063. [DOI] [PubMed] [Google Scholar]
- 99.Suzuki K, Murtuza B, Beauchamp JR, Brand NJ, Barton PJ, Varela-Carver A, et al. Role of interleukin-1beta in acute inflammation and graft death after cell transplantation to the heart. Circulation. 2004;110(11 Suppl. 1):II219–24. doi: 10.1161/01.CIR.0000138388.55416.06. [DOI] [PubMed] [Google Scholar]
- 100.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, et al. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34(2):107–16. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 101.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27(9):1114–22. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 102.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 103.Jäättelä M. Heat shock proteins as cellular lifeguards. Ann Med. 1999;31:261–71. doi: 10.3109/07853899908995889. [DOI] [PubMed] [Google Scholar]
- 104.Latchman DS. Heat shock proteins and cardiac protection. Cardiovasc Res. 2001;51:637–46. doi: 10.1016/s0008-6363(01)00354-6. [DOI] [PubMed] [Google Scholar]
- 105.Bouchentouf M, Benabdallah BF, Tremblay JP. Myoblast survival enhancement and transplantation success improvement by heat-shock treatment in mdx mice. Transplantation. 2004;77(9):1349–56. doi: 10.1097/01.tp.0000121503.01535.f5. [DOI] [PubMed] [Google Scholar]
- 106.Riederer I, Negroni E, Bigot A, Bencze M, Di Santo J, Aamiri A, et al. Heat shock treatment increases engraftment of transplanted human myoblasts into immunodeficient mice. Transplant Proc. 2008;40(2):624–30. doi: 10.1016/j.transproceed.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki K, Smolenski RT, Jayakumar J, Murtuza B, Brand NJ, Yacoub MH. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102(19 Suppl. 3):III216–21. doi: 10.1161/01.cir.102.suppl_3.iii-216. [DOI] [PubMed] [Google Scholar]
- 108.Maurel A, Azarnoush K, Sabbah L, Vignier N, Maurel A, Azarnoush K, et al. Can cold or heat shock improve skeletal myoblast engraftment in infarcted myocardium? Transplantation. 2005;80(5):660–5. doi: 10.1097/01.tp.0000172178.35488.31. [DOI] [PubMed] [Google Scholar]
- 109.Laumonier T, Yang S, Konig S, Chauveau C, Anegon I, Hoffmeyer P, et al. Lentivirus mediated HO-1 gene transfer enhances myogenic precursor cell survival after autologous transplantation in pig. Mol Ther. 2008;16(2):404–10. doi: 10.1038/sj.mt.6300354. [DOI] [PubMed] [Google Scholar]
- 110.Cízková D, Rosocha J, Vanický I, Radonák J, Gálik J, Cízek M. Induction of mesenchymal stem cells leads to HSP72 synthesis and higher resistance to oxidative stress. Neurochem Res. 2006;31(8):1011–20. doi: 10.1007/s11064-006-9107-x. [DOI] [PubMed] [Google Scholar]
- 111.Gao F, Hu XY, Xie XJ, Xu QY, Wang YP, Liu XB, et al. Heat shock protein 90 protects rat mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis via the PI3K/Akt and ERK1/2 pathways. J Zhejiang Univ Sci B. 2010;11(8):608–17. doi: 10.1631/jzus.B1001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chang W, Song BW, Lim S, Song H, Shim CY, Cha MJ, et al. Mesenchymal stem cells pretreated with delivered Hph-1-Hsp70 protein are protected from hypoxia-mediated cell death and rescue heart functions from myocardial injury. Stem Cells. 2009;27(9):2283–92. doi: 10.1002/stem.153. [DOI] [PubMed] [Google Scholar]
- 113.Wang X, Zhao T, Huang W, Wang T, Qian J, Xu M, et al. Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells. 2009;27(12):3021–31. doi: 10.1002/stem.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu W, Welsh MJ. Expression of the 25-kDa heat-shock protein (HSP27) correlates with resistance to the toxicity of cadmium chloride, mercuric chloride, cis-platinum(II)-diammine dichloride, or sodium arsenite in mouse embryonic stem cells transfected with sense or antisense HSP27 cDNA. Toxicol Appl Pharmacol. 1996;141(1):330–9. doi: 10.1006/taap.1996.0290. [DOI] [PubMed] [Google Scholar]
- 115.Son GH, Geum D, Chung S, Park E, Lee KH, Choi S, et al. A protective role of 27-kDa heat shock protein in glucocorticoid-evoked apoptotic cell death of hippocampal progenitor cells. Biochem Biophys Res Commun. 2005;338(4):1751–8. doi: 10.1016/j.bbrc.2005.10.152. [DOI] [PubMed] [Google Scholar]
- 116.McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J. 2004;18(2):355–7. doi: 10.1096/fj.03-0395fje. [DOI] [PubMed] [Google Scholar]
- 117.McArdle A, Broome CS, Kayani AC, Tully MD, Close GL, Vasilaki A, et al. HSF expression in skeletal muscle during myogenesis: implications for failed regeneration in old mice. Exp Gerontol. 2006;41(5):497–500. doi: 10.1016/j.exger.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 118.Yu JM, Wu X, Gimble JM, Guan X, Freitas MA, Bunnell BA. Age-related changes in mesenchymal stem cells derived from rhesus macaque bone marrow. Aging Cell. 2011;10(1):66–79. doi: 10.1111/j.1474-9726.2010.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


