Abstract
Background
Lipid management is less aggressive in blacks than whites and women than men.
Purpose
To examine whether differences in lipid management for race–sex groups compared to white men (WM) are due to factors influencing health services utilization or physician prescribing patterns.
Methods
Because coronary heart disease (CHD) risk influences physician prescribing, Adult Treatment Panel III CHD risk categories were constructed using baseline data from REasons for Geographic And Racial Differences in Stroke study participants (recruited 2003–2007).
Prevalence, awareness, treatment, and control of hyperlipidemia were examined for race–sex groups across CHD risk categories. Multivariable models conducted in 2013 estimated prevalence ratios adjusted for predisposing, enabling, and need factors influencing health services utilization.
Results
The analytic sample included 7,809 WM, 7,712 white women (WW), 4,096 black men (BM), and 6,594 black women (BW). Except in the lowest risk group, BM were less aware of hyperlipidemia than others. A higher percentage of WM in the highest risk group was treated (83.2%) and controlled (72.8%) than others (treatment, 68.6%–72.1%; control, 52.2%–65.5%), with BW treated and controlled the least. These differences remained significant after adjustment for predisposing, enabling, and need factors. Stratified analyses demonstrated that treatment and control were lower for other race–sex groups relative to WM only in the highest risk category.
Conclusions
Hyperlipidemia was more aggressively treated and controlled among WM compared with WW, BM, and especially BW among those at highest risk for CHD. These differences were not attributable to factors influencing health services utilization.
Introduction
Coronary heart disease (CHD) mortality in the U.S. continues to be higher for blacks than for whites, largely attributable to greater risk factor burden among blacks.1–4 Statins reduce CHD risk, but blacks are less likely to take statins than whites. In the 1999–2004 National Health and Nutrition Examination Survey (NHANES), blacks were 39% less likely to be treated than whites.5 Among community-dwelling adults, blacks had lower odds of treatment and control compared to whites.6 Among veterans with diabetes in 1999–2000, blacks were 25% less likely to be treated with statins than whites, and among the treated, blacks had 25% lower odds for achieving low-density lipoprotein cholesterol (LDL-C) control.7
The reasons for these observations are poorly understood. Blacks are more likely to live in low socioeconomic circumstances and therefore face barriers to accessing health care, leading to under-treatment. Furthermore, CHD risk varies across race–sex groups, with white women (WW) having lower CHD risk than others; the role of variations in CHD risk in treatment differences has not been well defined.8 In fact, under-treatment of CHD by race and sex for individuals at similar risk has been reported, but the reasons for these differences are not clear.9 Few studies have been designed specifically to understand the role of factors that influence health services utilization in differences in awareness, treatment, and control of hyperlipidemia.
To fill these evidence gaps, data from the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort, a large national study of black men (BM), white men (WM), black women (BW) and WW, were analyzed. Investigators hypothesized that awareness, treatment, and control of hyperlipidemia would be lower for blacks and for women, regardless of CHD risk, but that these differences would be explained by factors influencing health services utilization.10
Methods
The REGARDS cohort study includes 30,239 individuals and was designed to examine regional and racial influences on stroke mortality. Details are described elsewhere11,12; briefly, participants were enrolled from 2003 to 2007 using commercially available lists for mail and telephone contacts to recruit English-speaking, community-dwelling black and white adults aged ≥45 years living in the continental U.S. Race and sex were balanced by design with oversampling from the Stroke Belt and Buckle in the southeastern U.S.; the final cohort included 58% women and 42% blacks. Race was self-reported. Baseline data included computer-assisted telephone surveys assessing medical history and health status; in-home exams by trained health professionals following standardized, quality-controlled protocols to collect fasting blood and urine samples; electrocardiograms (ECGs); blood pressure, height, and weight; and medications by pill bottle review. Blood and urine samples were centrally analyzed at the University of Vermont, and ECGs were centrally analyzed at Wake Forest University. IRBs at participating institutions approved the study protocol prior to data collection, and all participants provided written informed consent. Data analysis for the current study was conducted in 2013.
Hyperlipidemia was defined following the Adult Treatment Panel III (ATP III) of the National Cholesterol Education Program,13 based on whether individuals were treated or had LDL-C above the guideline-recommended goal for their level of CHD risk. Among participants with hyperlipidemia, awareness was defined as responding yes to the question: Have you ever been told by a doctor that you have high cholesterol or an abnormal level of fats in your blood? Treatment was defined as being on a medication for hyperlipidemia among those aware of their disease. Control was defined as being below the ATP III–defined goal for LDL-C among treated individuals.
CHD risk was classified according to ATP III using the four following mutually exclusive strata: (1) CHD risk equivalent (CHD, diabetes, history of vascular disease); (2) 10-year Framingham CHD risk scores (FRS) >20% and no criteria for Stratum 1; (3) FRS of 10%–20%; and (4) FRS <10%. In this framework, those with a history of vascular disease and risk equivalents including diabetes were considered at highest risk with a treatment target of LDL-C <100 mg/dL. A history of vascular disease was detected by reporting a history of stroke, heart attack, aortic aneurysm, peripheral arterial disease, or coronary revascularization procedure; or for evidence of myocardial infarction on the baseline ECG. Diabetes was present if participants reported having been told by a doctor or other health professional that they had diabetes or were treated with diabetes medication or insulin. For those without diabetes or evidence of vascular disease, ATP III assigns points based on other risk factors, and points are summed to determine risk categories, with a point subtracted if high-density lipoprotein cholesterol (HDL-C) is ≥60 mg/dL. The risk group corresponding to FRS >20% had an LDL-C goal of <100 mg/dL. The FRS 10%–20% category had an LDL-C goal of <130 mg/dL. The FRS <10% category had an LDL-C goal of <160 mg/dL, or <130 mg/dL if two or more of the six following risk factors were present: (1) hypertension (>140/90 mmHg or on antihypertensive medications); (2) current smoking; (3) male sex; (4) age >45 years for men and >55 years for women; (5) family history of myocardial infarction at age <55 years in father or other male first-degree relative, or age <65 years in mother or other female first-degree relative; (6) or HDL-C <40 mg/dL. All participants were classified into one of these four strata with the appropriate LDL-C goal or treatment used to determine hyperlipidemia.
Aday and Andersen10 proposed that predisposing, enabling, and need factors influence health services utilization. Predisposing factors included race–sex group, age, annual household income (<$20,000, $20,000–$34,999, $35,000–$75,000, >$75,000, declined to report), educational attainment (less than high school education, high school education and above), and region of residence (Stroke Belt, Stroke Buckle, remainder of the continental U.S.). Enabling factors relate to healthcare access and included having health insurance, rural residence (versus non-rural or missing; rurality defined by Rural Urban Commuting Area Codes 4–1014), and the percentage of individuals in a census tract living below the federal poverty line. Need factors included perceived and observed need. Perceived need factors included medication adherence (assessed by Morisky's 4-item scale, with any no response classified as non-adherent15), awareness of hyperlipidemia, and current smoking. Observed need included CHD risk category; depressive symptoms as reflected in a Centers for Epidemiology Studies-Depression (CES-D) score ≥4 on the 4-item scale (because depression has been associated with CHD risk)16; HDL-C >60 mg/dL (because high HDL-C is associated with lower CHD risk); physical functioning as reflected in the Short Form 12 Physical Component Summary (PCS) score (because this score correlates well with illness burden)17; and BMI (because in some populations obesity is associated with higher risk).18
The population was first described across CHD risk categories and race–sex groups and then prevalence, awareness, treatment, and control were described within each race–sex group across CHD risk categories. Because outcomes were common, prevalence ratios (PR), rather than ORs, were estimated for hyperlipidemia prevalence, awareness, treatment, and control for each race– sex group compared with WM from multivariable log-binomial regression models. Models were constructed in stepwise fashion to observe how groups of covariates influenced the relationship of race–sex groups with the prevalence, awareness, treatment, and control of hyperlipidemia. The first model included only the race–sex group with WM as the reference category (Model 1). Then, investigators entered the remaining predisposing factors (age, income, education, REGARDS region) to the Model 1 covariates to construct Model 2. Enabling factors (health insurance, rural residence, percentage of individuals in the census tract of residence living below the federal poverty line) were added to the Model 2 covariates to construct Model 3. Need factors (CHD risk category, PCS score, BMI, CES-D ≥4, medication adherence, HDL-C ≥60 mg/dL) were added to the Model 3 covariates to construct Model 4. To examine whether the observations were consistent across CHD risk categories and across high-, moderate-, or low-poverty areas, interactions for race–sex group X CHD risk category as well as race–sex group X poverty tertile were examined. All analyses were carried out using SAS, version 9.3 (SAS Institute Inc., Cary NC).
Results
The analytic sample included 7,809 WM, 7,712 WW, 4,096 BM, and 6,594 BW. The proportion of each race–sex group in the highest CHD risk category differed: 42.2% of WM, 28.4% of WW, 46.9% of BM, and 41.1% of BW (56.5% of participants in this category had diabetes [n=5,715]). The characteristics of the study sample by CHD risk category are shown in Table 1. For predisposing factors, compared with those at lowest risk, individuals in the highest risk category were older, and relatively fewer reported income >$75,000 and having at least a high school education. For enabling factors, compared with those at lowest risk, more individuals in the highest risk category had health insurance and lived in census tracts with higher poverty. For factors reflecting perceived need, compared with those at lowest risk, more individuals in the highest risk category were adherent to medications and currently smoking. For factors reflecting observed need, more individuals in the highest risk category had depressive symptoms and obesity than those at lowest risk, but fewer had HDL-C >60 mg/dL, and the mean PCS score was lower. The study sample by race–sex group is presented in Appendix Table 1.
Table 1.
REGARDS Study participant characteristics by CHD risk stratum
| Andersen Model Domain |
Category | Variable | CVD/risk equivalent (n=10,120) |
FRS >20% (n=761) |
FRS 10- 20% (n=5,300) |
FRS <10% (n=10,030) |
P- valuea |
|---|---|---|---|---|---|---|---|
| Predisposing Factors | Race-sex group, no. (%) | White Men | 3,295 (32.6) | 428 (56.2) | 2,989 (56.4) | 1,097 (10.9) | <0.001 |
| White Women | 2,194 (21.7) | 61 (8.0) | 467 (8.8) | 4,990 (49.8) | |||
| Black Men | 1,920 (19.0) | 214 (28.1) | 1,405 (26.5) | 557 (5.6) | |||
| Black Women | 2,711 (26.8) | 58 (7.6) | 439 (8.3) | 3,386 (33.8) | |||
| Age, mean (SD) | 66.7 (9.1) | 71.4 (9.3) | 67.2 (8.7) | 60.9 (8.6) | <0.001 | ||
| REGARDS Region, no. (% | Stroke Belt | 3,582 (35.4) | 256 (33.6) | 1,836 (34.6) | 3,452 (34.4) | <0.001 | |
| Stroke Buckle | 2,123 (21.0) | 127 (16.7) | 927 (17.5) | 2,163 (21.6) | |||
| Neither Belt/Buckle | 4,415 (43.6) | 378 (49.7) | 2,537 (47.9) | 4,415 (44.0) | |||
| Annual income, no. (%) | >$75,000 | 1,141 (11.3) | 92 (12.1) | 1,079 (20.4) | 1,974 (19.7) | <0.001 | |
| $35,000-$75,000 | 2,803 (27.7) | 230 (30.2) | 1,774 (33.5) | 3,162 (31.5) | |||
| $20,000-$34,999 | 2,705 (26.7) | 237 (31.1) | 1,249 (23.6) | 2,206 (22.0) | |||
| <$20,000 | 2,311 (22.8) | 132 (17.3) | 686 (12.9) | 1,449 (14.4) | |||
| Declined to report | 1,160 (11.5) | 70 (9.2) | 512 (9.7) | 1,239 (12.4) | |||
| Education, no. (%) | High school or more | 8,450 (83.5) | 635 (83.4) | 4,770 (90.0) | 9,266 (92.4) | <0.001 | |
| Less than high school | 1,670 (16.5) | 126 (16.6) | 530 (10.0) | 764 (7.6) | |||
| Enabling Factors | Health insurance, no. (%) | No | 565 (5.6) | 39 (5.1) | 270 (5.1) | 838 (8.4) | <0.001 |
| Living in zip code in lowest tertile of poverty, no. (%) | Lowest tertile | 2,918 (28.8) | 247 (32.5) | 2,033 (38.4) | 3,601 (35.9) | <0.001 | |
| Rural county residence, no. (%) | Not rural | 8,250 (81.5) | 631 (82.9) | 4,308 (81.3) | 8,568 (79.8) | 0.17 | |
| Rural | 959 (9.5) | 60 (7.9) | 516 (9.7) | 1,096 (10.2) | |||
| Missing | 911 (9.0) | 70 (9.2) | 476 (9.0) | 966 (9.6) | |||
| Perceived Need Factors | Morisky medication adherence, no. (%) | 6,636 (65.6) | 492 (64.7) | 3,386 (63.9) | 6,235 (62.2) | <0.001 | |
| Aware of hyperlipidemiab, no. (%) | 5,999 (69.3) | 336 (50.1) | 1,989 (65.1) | 2,234 (86.2) | <0.001 | ||
| Current smoking, no. (%) | 1,526 (15.1) | 252 (33.1) | 861 (16.2) | 1,154 (11.5) | <0.001 | ||
| Actual Need Factors | Depressive symptoms, no. (%) | CES-D score ≥4 | 1,379 (13.6) | 57 (7.5) | 347 (6.5) | 1,011 (10.1) | <0.001 |
| Elevated High Density Lipoprotein Cholesterol, no. (%) | HDL-C >60 mg/dL | 1,899 (18.8) | 16 (2.1) | 716 (13.5) | 4,118 (41.1) | <0.001 | |
| Obesity, no. (%) | BMI >30 kg/m2 | 4,559 (45.0) | 241 (31.7) | 1,612 (30.4) | 3,582 (35.7) | <0.001 | |
| Physical Component Summary Score, mean (SD) | 43.1 (11.2) | 47.5 (9.0) | 49.0 (8.9) | 48.7 (9.6) | <0.001 |
CES-D, Centers for Epidemiology Studies - Depression; CVD, Cardiovascular disease; FRS, Framingham risk score; HDL-C, High density lipoprotein cholesterol; REGARDS, REasons for Geographic And Racial Differences in Stroke.
Note: Boldface indicates statistical significance
P-values were calculated using chi-square tests for categorical variables and ANOVA for continuous variables.
Awareness of hyperlipidemia was calculated among participants with hyperlipidemia.
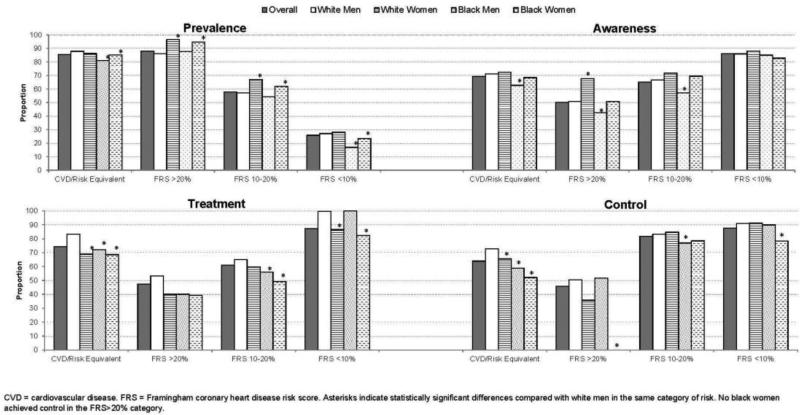
The prevalence, awareness, treatment, and control of hyperlipidemia are presented across risk categories and by race–sex group in Figure 1. The prevalence of hyperlipidemia varied 7%–13% across risk categories, with 81%–88% prevalence among those at highest risk, 86%–97% in the FRS >20% category, 54%–67% in the FRS 10%–20% category, and 17%–28% in the FRS <10% category. WM had the highest prevalence in only the highest risk category, in which prevalence was also most similar across race–sex groups. Awareness of hyperlipidemia was more prevalent in WW across all risk categories, but statistically significantly different from WM only for FRS >20%. On the other hand, BM had the lowest awareness, except in the lowest risk category, and these differences were statistically significantly lower than WM's awareness for all but the lowest risk category.
Figure 1.
Prevalence, awareness, treatment and control of hyperlipidemia, by race-sex group and coronary heart disease risk category
Note: Asterisks indicate statistically significant differences compared with white men in the same category of risk. No black women achieved control in the FRS>20% category.
CVD, cardiovascular disease; FRS, Framingham coronary heart disease risk score
As also shown in Figure 1, more WM (83.7%) than other race–sex groups (68.9% of WW, 72.1% of BM, and 68.6% of BW) were treated in the highest risk category, the FRS >20% category (53.2% of WM, 40.0% of WW, 40.0% of BM, 39.3% of BW), and the FRS 10%–20% category (64.9% of WM, 59.7% of WW, 56.1% of BM, 49.2% of BW) but not the lowest risk category (99.6% of WM, 86.6 % of WW, 100% of BM, 82.4% of BW). BW were the least treated in all risk categories, and were treated 14%–17% less than WM, depending on the category; these differences were significantly different from WM in all but the FRS >20% category. Achieving control were 72.8% of WM, 65.5% of WW, 58.9% of BM, and 52.2% of BW in the highest risk category; 50.5% of WM, 35.7% of WW, 51.7% of BM, and no BW in the FRS >20% category; 83.3% of WM, 84.7% of WW, 76.9% of BM, and 78.5% of BW in the FRS 10%–20% category; and 90.9% of WM, 91.4% of WW, 90.0% of BM, and 78.3% of BW in the FRS <10% category. Fewer BW achieved control than other race–sex groups, which were significantly different in comparison with WM, except for the FRS 10%–20% category.
Table 2 presents the crude and sequentially adjusted PRs for the prevalence, awareness, treatment, and control of hyperlipidemia for each race–sex group compared with WM. Each race–sex group was less likely than WM to have hyperlipidemia in the crude analyses. After adjustment for need factors, WW and BW had similar prevalence, but BM were 5% less likely to have hyperlipidemia than WM. Compared with WM, WW were more likely and BM less likely to be aware of having hyperlipidemia in both crude and fully adjusted analyses. WW, BM, and BW were 12%, 13%, and 19% less likely to be treated than WM, respectively, after adjusting for all factors that influence health services utilization; all of these differences were statistically significant. Similarly, WW, BM, and BW were 4%, 6%, and 11% less likely than WM to have achieved control after full adjustment.
Table 2.
Incrementally adjusted prevalence ratios for prevalence, awareness, treatment and control of hyperlipidemia for race-sex groups
| Model | White Women vs. White Men PR (95% CI) | Black Men vs. White Men PR (95% CI) | Black Women vs. White Men PR (95% CI) |
|---|---|---|---|
| Prevalence of hyperlipidemia vs. not prevalent (n=26,211) | |||
| Model 1a | 0.71 (0.69, 0.73) | 0.94 (0.92, 0.97) | 0.77 (0.75, 0.79) |
| Model 2b | 0.72 (0.70, 0.74) | 0.94 (0.91, 0.96) | 0.78 (0.76, 0.80) |
| Model 3c | 0.72 (0.70, 0.74) | 0.94 (0.91, 0.96) | 0.78 (0.75, 0.80) |
| Model 4d | 1.02 (1.00, 1.04) | 0.95 (0.93, 0.97) | 1.00 (0.98, 1.02) |
| Awareness of hyperlipidemia vs. unawareness among those with hyperlipidemia (n=14,982) | |||
| Model 1a | 1.10 (1.07, 1.13) | 0.88 (0.85, 0.91) | 1.00 (0.97, 1.03) |
| Model 2b | 1.11 (1.08, 1.14) | 0.89 (0.86, 0.92) | 1.02 (0.99, 1.05) |
| Model 3c | 1.10 (1.08, 1.13) | 0.90 (0.87, 0.93) | 1.03 (1.00, 1.06) |
| Model 4d | 1.07 (1.04, 1.10) | 0.89 (0.86, 0.93) | 0.98 (0.95, 1.02) |
| Lipid treatment vs. not treated among those who are aware of hyperlipidemia (n=10,558) | |||
| Model 1a | 0.90 (0.87, 0.93) | 0.87 (0.83, 0.90) | 0.83 (0.81, 0.86) |
| Model 2b | 0.90 (0.87, 0.93) | 0.88 (0.85, 0.92) | 0.85 (0.82, 0.88) |
| Model 3c | 0.90 (0.87, 0.93) | 0.88 (0.85, 0.92) | 0.86 (0.82, 0.89) |
| Model 4d | 0.88 (0.85, 0.91) | 0.87 (0.84, 0.91) | 0.81 (0.78, 0.84) |
| Achievement of LDL-C goal vs. not achieving goal among those treated with lipid lowering medication (n=7,649) | |||
| Model 1a | 1.04 (1.01, 1.07) | 0.85 (0.81, 0.89) | 0.81 (0.77, 0.84) |
| Model 2b | 1.06 (1.03, 1.09) | 0.87 (0.83, 0.91) | 0.84 (0.81, 0.88) |
| Model 3c | 1.06 (1.03, 1.09) | 0.87 (0.83, 0.92) | 0.85 (0.81, 0.89) |
| Model 4d | 0.96 (0.94, 0.99) | 0.94 (0.91, 0.97) | 0.89 (0.86, 0.91) |
PR, prevalence ratio; CI, confidence interval; LDL-C, low density lipoprotein cholesterol
Race-sex group (main exposure)
Model 1 + age, income, education, region (predisposing factors)
Model 2 + insurance, rural residence, % of residents of living below the poverty line (enabling factors)
Model 3 + Coronary heart disease risk category, diabetes, physical component summary score, obesity, depressive symptoms, high density lipoprotein cholesterol >60 mg/dL, current smoking, medication adherence (need factors)
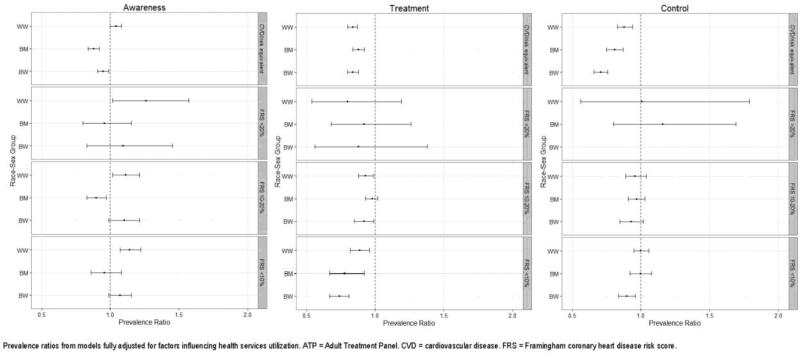
The interactions for race–sex group X poverty tertile were not significant in any models (prevalence, p=0.43; awareness, p=0.35; treatment, p=0.16; control, p=0.52), indicating that these patterns did not differ by the poverty of the participant's census tract. However, the p-values for race–sex group X CHD risk category were significant for the awareness (p=0.04), treatment (p<0.001), and control models (p<0.001), but not the prevalence model (p=1.00). Figure 2 shows the results of the fully adjusted analyses stratified by CHD risk category for awareness, treatment, and control of hyperlipidemia. Compared with WM, WW were significantly more likely to be aware of hyperlipidemia regardless of CHD risk category; BM were less likely to be aware, but this lower awareness was statistically significant only for the highest risk and FRS 10%–20% categories; and BW were significantly less likely to be aware in the highest risk category, but more likely to be aware in the other categories, albeit not statistically significantly. All race–sex groups in the highest and lowest risk categories were less likely to be treated than WM, and BW but not others in the FRS 10%–20% category were less likely to be treated than WM. Although all race–sex groups in the FRS >20% category were less likely to be treated than WM, these findings were not statistically significant. WW in the highest risk category were 12% less likely to achieve control than WM, BM were 19% less likely, and BW were 29% less likely. These differences were all statistically significant. These differences were not observed in the other risk categories, with the exception of BW at lowest risk, who were 10% less likely to achieve control compared with WM, a significant difference.
Figure 2.
Prevalence ratios and 95% CIs for each race-sex group relative to white men, stratified by ATP III risk group
Note: Prevalence ratios from models fully adjusted for factors influencing health services utilization.
ATP, Adult Treatment Panel; CVD, cardiovascular disease; FRS, Framingham coronary heart disease risk score
Discussion
This study described several potentially actionable findings that likely contribute to racial disparities in acute CHD. BM were less likely to be aware of hyperlipidemia compared with other race–sex groups, supporting the need for interventions to improve their awareness. This study also found that among those at highest CHD risk, WM were significantly more likely to be treated and controlled, and blacks in general but especially BW were less likely to be treated or controlled; factors influencing health services utilization did not explain these results.
This study's findings confirm prior reports of lower treatment and control of hyperlipidemia among women and blacks. A study using NHANES data reported improvements in lipid testing, treatment, and control from 1999 to 2006, but less so for women and minorities, without report of findings by race–sex group.19 In Massachusetts, a state with relatively low access barriers to health care, in 1999–2000, cholesterol management occurred in 7.3% fewer ambulatory encounters for women than for men, but 5.5% more encounters for blacks than for whites; LDLC targets were achieved for 20% fewer women than men and 19% fewer blacks than whites, but no race–sex findings were reported.20 A 2005 National Ambulatory Medical Care Survey study reported less-aggressive lipid screening and management for blacks than whites.21 This study builds on these prior observations by reporting findings by race–sex group across CHD risk categories, revealing that WM were likely driving previous findings.
This study's finding of more aggressive treatment and control of hyperlipidemia in WM relative to other race–sex groups is consistent with a 1999 study of CHD clinical management conducted by Shulman et al.9 In that study, multivariable results revealed that only BW were significantly less likely to be referred than others despite having similar symptoms and risk profiles, but blacks and women were referred for further evaluation 6% less often than whites and men. These differences are smaller than the 11%–15% lower treatment of hyperlipidemia and 7%–21% less control among WW, BM, and BW compared with WM in the highest risk category observed in this study. Two findings could help explain the more aggressive treatment of WM that were observed. First, a survey by Mosca and colleagues22 revealed that although primary care physicians widely endorsed the ATP III guidelines and CHD risk stratification, fewer than half reported actually calculating CHD risk in routine clinical practice; estimating CHD risk without using a tool resulted in consistent underestimation, greatest among women. Another study of primary care physicians also reported risk underestimation based on risk factor information alone.23 Second, because most acute CHD cases are WM,18,24 physicians’ personal clinical experience may reinforce their impression of high risk in this group, and without readily available risk prediction tools at the point of care, more aggressive treatment in WM relative to other race–sex groups may ensue. Recently announced guidelines for the management of hyperlipidemia continue to require calculations to risk stratify.25 If this study's findings are confirmed in other studies, interventions designed to improve objective risk prediction at the time of clinical decision making could help to decrease racial disparities in CHD.
Study strengths include its national reach, large numbers of blacks and women, and large number of available covariates including rigorously collected physiologic measures and validated self-reported variables like health status and medication adherence. Limitations include its observational study with attendant caution in drawing causal inferences. Some covariates were self-reported with known limitations. The cross-sectional design does not capture how management might have changed over time, but prior temporally contiguous longitudinal studies reached similar conclusions.26 Some people may have been treated for reasons other than hyperlipidemia, but information about the indication for treatment was not available. Although many covariates in Aday and Andersen's model were operationalized, domains including health beliefs, trust in physicians, family history of hyperlipidemia, and social support were not available.
In conclusion, this study describes a marked gap in treatment and control of hyperlipidemia for WW, BM, and BW compared with WM, especially among those at highest CHD risk. Findings were not attributable to numerous factors that influence health services utilization.
Supplementary Material
Acknowledgments
This research project is supported by a cooperative agreement UO1NS041588 from the National Institute of Neurological Disorders and Stroke, NIH (Howard, Safford), K24HL111154 from the National Heart, Lung, and Blood Institute (Safford, Gamboa), and R01HL080477 from the National Heart, Lung, and Blood Institute (Safford, Brown, Durant, Shikany, Glasser, Muntner, Gamboa). The study contents are solely the responsibility of the authors and do not necessarily represent the official vies of NIH, the National Institute of Neurological Disorders and Stroke, or the National Heart, Lung, and Blood Institute. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. Amgen did not have any role in the design and conduct of the study, the collection, management, data analysis, or interpretation of the data, or the preparation or approval of the manuscript. Drs. Safford, Muntner, and Brown reported receiving research support from the Amgen Corporation. Monika M. Safford has received consulting fees from diaDexus, Inc., a medical diagnostics company. In addition, Dr. Safford receives support from an investigator-initiated research grant sponsored by Amgen. Paul M. Muntner receives support from an investigator-initiated research grant sponsored by Amgen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No other financial disclosures were reported by the other authors of this paper.
References
- 1.National Heart, Lung, and Blood Institute . Morbidity & Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Diseases. NIH; Bethesda, MD: 2012. [Google Scholar]
- 2.Safford MM, Brown TM, Muntner PM, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308(17):1768–74. doi: 10.1001/jama.2012.14306. http://dx.doi.org/10.1001/jama.2012.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams JE, Massing M, Rosamond WD, Sorlie PD, Tyroler HA. Racial disparities in CHD mortality from 1968-1992 in the state economic areas surrounding the ARIC study communities. Atherosclerosis Risk in Communities. Ann Epidemiol. 1999;9(8):472–480. doi: 10.1016/s1047-2797(99)00029-0. http://dx.doi.org/10.1016/S1047-2797(99)00029-0. [DOI] [PubMed] [Google Scholar]
- 4.Jones DW, Chambless LE, Folsom AR, et al. Risk factors for coronary heart disease in African Americans: the atherosclerosis risk in communities study, 1987-1997. Arch Intern Med. 2002;162(22):2565–71. doi: 10.1001/archinte.162.22.2565. http://dx.doi.org/10.1001/archinte.162.22.2565. [DOI] [PubMed] [Google Scholar]
- 5.Mann D, Reynolds K, Smith D, Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. Ann Pharmacother. 2008;42(9):1208–15. doi: 10.1345/aph.1L181. http://dx.doi.org/10.1345/aph.1L181. [DOI] [PubMed] [Google Scholar]
- 6.Zweifler RM, McClure LA, Howard VJ, et al. Racial and geographic differences in prevalence, awareness, treatment and control of dyslipidemia: the reasons for geographic and racial differences in stroke (REGARDS) study. Neuroepidemiology. 2011;37(1):39–44. doi: 10.1159/000328258. http://dx.doi.org/10.1159/000328258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safford M, Eaton L, Hawley G, et al. Disparities in use of lipid-lowering medications among people with type 2 diabetes mellitus. Arch Intern Med. 2003;163(8):922–928. doi: 10.1001/archinte.163.8.922. http://dx.doi.org/10.1001/archinte.163.8.922. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 9.Schulman KA, Berlin JA, Harless W, et al. The effect of race and sex on physicians’ recommendations for cardiac catheterization. N Engl J Med. 1999;340(8):618–26. doi: 10.1056/NEJM199902253400806. http://dx.doi.org/10.1056/NEJM199902253400806. [DOI] [PubMed] [Google Scholar]
- 10.Aday LA, Andersen R. A framework for the study of access to medical care. Health Serv Res. 1974;9(3):208–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Howard VJCM, Pulley L, Gomez CR, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25(3):135–43. doi: 10.1159/000086678. http://dx.doi.org/10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 12.Howard VJ, Woolson RF, Egan BM, et al. Prevalence of hypertension by duration and age at exposure to the stroke belt. J Am Soc Hypertens. 2010;4(1):32–41. doi: 10.1016/j.jash.2010.02.001. http://dx.doi.org/10.1016/j.jash.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Expert Panel on Detection E, Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. http://dx.doi.org/10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.Larson E, Skillman S. RUCA Data Code Definitions: Version 2.0. 2013 http://depts.washington.edu/uwruca/ruca-codes.php.
- 15.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. http://dx.doi.org/10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Melchior L, Huba G, Brown V, Reback C. A short depression index for women. Educational and Psychological Measurement. 1993;53(4):1117–1125. http://dx.doi.org/10.1177/0013164493053004024. [Google Scholar]
- 17.Quality Metric The SF-12®: An Even Shorter Health Survey. 2007 www.sf-36.org/tools/sf12.shtml.
- 18.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. http://dx.doi.org/10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford ES, Li C, Pearson WS, Zhao G, Mokdad AH. Trends in hypercholesterolemia, treatment and control among United States adults. Int J Cardiol. 2010;140(2):226–35. doi: 10.1016/j.ijcard.2008.11.033. http://dx.doi.org/10.1016/j.ijcard.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 20.Persell SD, Maviglia SM, Bates DW, Ayanian JZ. Ambulatory hypercholesterolemia management in patients with atherosclerosis. Gender and race differences in processes and outcomes. J Gen Intern Med. 2005;20(2):123–30. doi: 10.1111/j.1525-1497.2005.40155.x. http://dx.doi.org/10.1111/j.1525-1497.2005.40155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willson MN, Neumiller JJ, Sclar DA, Robison LM, Skaer TL. Ethnicity/race, use of pharmacotherapy, scope of physician-ordered cholesterol screening, and provision of diet/nutrition or exercise counseling during US office-based visits by patients with hyperlipidemia. Am J Cardiovasc Drugs. 2010;10(2):105–8. doi: 10.2165/11532820-000000000-00000. http://dx.doi.org/10.2165/11532820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Mosca L, Linfante AH, Benjamin EJ, et al. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. 2005;111(4):499–510. doi: 10.1161/01.CIR.0000154568.43333.82. http://dx.doi.org/10.1161/01.CIR.0000154568.43333.82. [DOI] [PubMed] [Google Scholar]
- 23.Persell SD, Zei C, Cameron KA, Zielinski M, Lloyd-Jones DM. Potential use of 10-year and lifetime coronary risk information for preventive cardiology prescribing decisions: a primary care physician survey. Arch Intern Med. 2010;170(5):470–7. doi: 10.1001/archinternmed.2009.525. http://dx.doi.org/10.1001/archinternmed.2009.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizk DV, Gutierrez O, Levitan EB, et al. Prevalence and prognosis of unrecognized myocardial infarctions in chronic kidney disease. Nephrol Dial Transplant. 2012;27(9):3482–8. doi: 10.1093/ndt/gfr684. http://dx.doi.org/10.1093/ndt/gfr684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 [Google Scholar]
- 26.Turner BJ, Hollenbeak CS, Weiner M, Tang SS. A retrospective cohort study of the potency of lipid-lowering therapy and race-gender differences in LDL cholesterol control. BMC Cardiovasc Disord. 2011;11:58. doi: 10.1186/1471-2261-11-58. http://dx.doi.org/10.1186/1471-2261-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.