Abstract
Background
We sought to establish a nonhuman primate model of vaginal Lactobacillus colonization suitable for evaluating live microbial microbicide candidates.
Methods
Vaginal and rectal microflora in Chinese rhesus macaques (Macaca mulatta) were analyzed, with cultivable bacteria identified by 16S rRNA gene sequencing. Live lactobacilli were intravaginally administered to evaluate bacterial colonization.
Results
Chinese rhesus macaques harbored abundant vaginal Lactobacillus, with Lactobacillus johnsonii as the predominant species. Like humans, most examined macaques harbored only one vaginal Lactobacillus species. Vaginal and rectal Lactobacillus isolates from the same animal exhibited different genetic and biochemical profiles. Vaginal Lactobacillus was cleared by a vaginal suppository of azithromycin, and endogenous L. johnsonii was subsequently restored by intravaginal inoculation. Importantly, prolonged colonization of a human vaginal Lactobacillus jensenii was established in these animals.
Conclusions
The Chinese rhesus macaque harbors vaginal Lactobacillus and is a potentially useful model to support the pre-clinical evaluation of Lactobacillus-based topical microbicides.
Keywords: bacterial colonization, Chinese rhesus macaques, Lactobacillus johnsonii, live microbial microbicide, vaginal microflora
Introduction
Human mucosal surfaces such as the non-keratinized epithelium of the vagina are colonized by commensal flora [1, 11, 21, 50]. The vagina, together with its microflora, constitutes a dynamic ecosystem with important host defense mechanisms that promote reproductive health in women. In healthy women of childbearing age, the vaginal flora is dominated by lactobacilli (107–109 CFU per gram of fluid) [41]. These facultative anaerobes metabolize glucose to lactic acid, contributing to the maintenance of a low vaginal pH (3.6–4.5) that accounts for a major part of the non-specific defense to reduce the risk of acquiring sexually transmitted diseases [5]. Depletion of vaginal lactobacilli is associated with establishment of opportunistic bacterial infections [14] and an increased risk of acquiring human immunodeficiency virus (HIV) and herpes simplex virus type 2 (HSV-2) [9, 10, 30, 40, 43]. Consequently, there has been considerable interest in exploring an ecologic approach to re-populate the vagina with lactobacilli, termed Lactobacillus replacement therapy. One unique biologic approach being pursued is the use of a ‘live’ recombinant human vaginal Lactobacillus as a self-renewable topical microbicide against vaginal HIV transmission [8, 27, 28].
Some smaller animals, including mice, rats, dogs, hamsters, and guinea pigs are reported to harbor low numbers of vaginal lactobacilli [34, 38]. Apparently, lactobacilli are not the dominant species in the vaginal flora as in the case of reproductive-age women. In addition, these smaller animals have an estrous cycle, so their reproductive physiology as well as anatomy is also very different from that of humans [34, 38]. Thus, selection of a relevant animal model that is closely related to humans and sensitive to mucosal HIV infection is crucial. We sought to establish a nonhuman primate model that could more accurately mimic the human female lower reproductive tract. This model would help to test colonization, persistence, efficacy, and safety of a ‘live’ topical microbicide against mucosal transmission of pathogens in the vagina.
Among nonhuman primates, the rhesus macaque (Macaca mulatta) reproductive system shares many similarities to the human reproductive system. They have been widely used in simian immunodeficiency virus and simian-human immunodeficiency virus vaginal challenge models [19, 29] to test various candidate microbicides [26, 45, 47–49]. In order to develop a rhesus macaque model for evaluating Lactobacillus-based microbicides, we analyzed the cultivable vaginal flora over one menstrual cycle of 13 Chinese rhesus macaques that were housed at two separate facilities. We recovered abundant endogenous vaginal Lactobacillus species in 12 of 13 animals and identified Lactobacillus johnsonii as the predominant species in these macaques. Moreover, vaginal colonization of a human vaginal Lactobacillus strain was established in these animals. This is the first report of persistent vaginal colonization of human Lactobacillus in the Chinese rhesus macaque. These findings demonstrate that the female Chinese rhesus macaque is a potentially useful model to test vaginal Lactobacillus replacement therapy for prevention of urogenital infections and to support the pre-clinical evaluation of Lactobacillus-based topical microbicides.
Materials and methods
Experimental animals
Thirteen sexually mature, non-hormonal-treated female Chinese-origin rhesus macaques, ranging in age from 4 to 10 years, were individually housed at two separate AAALAC International accredited facilities in Maryland. Specifically, five monkeys were housed at Southern Research Institute and eight monkeys were housed at Advanced Bioscience Laboratories (ABL). The procedures were conducted under protocols approved by the Institutional Animal Care and Use Committee (protocol no. 348 at ABL, and protocol no. 05-05-047F at Southern Research Institute). Animals were housed, cared for and used humanely in accordance with the ‘Guide for the Care and Use of Laboratory Animals’ by the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. The animals housed at Southern Research Institute received a high protein monkey diet 5045 (Lab Diet, St. Louis, MO, USA), various fruits and vegetables, and nutritious supplements. The animals housed at ABL received a similar diet, including a high protein monkey chow (Ralston-Purina, St. Louis, MO, USA) supplemented with fruits and healthy treats.
Sampling
Prior to sample collections or intravaginal administration of vaginal suppository of azithromycin or lactobacilli, the animals were sedated with an injectable anesthetic, ketamine hydrochloride, at a dose of 10 mg/kg. Once sedated, the animals were removed from their cage and taken to a central procedure room. Vaginal and rectal cultures were obtained with polyester-tipped swabs (Becton-Dickinson, Cockeysville, MD, USA) and transported overnight to the Microbiology Core Laboratory at Osel Inc., CA in Port-A-Cul anaerobic transport tubes (Becton-Dickinson), which preserve the viability of anaerobic and aerobic bacterial specimens [4]. Approximately 130–150 mg of vaginal fluid (containing some sloughed-off vaginal epithelial cells) was obtained with the swabs. Vaginal fluid was smeared immediately onto pH-indicator strips (EMD Chemicals Inc., Darmstadt, Germany) to estimate vaginal pH values. They typically varied from pH 4 to 8 in Chinese rhesus macaques undergoing normal menstrual cycles.
Microbiology
The microflora was analyzed by standard culture-dependent assays, with modifications of previously described methods [35]. Briefly, the vaginal and rectal swabs were removed from the transport tubes and serially diluted in saline buffer in an anaerobic chamber, Bactron IV (Sheldon Manufacturing, Cornelius, OR, USA). Serial dilutions of the bacterial samples were then plated on different agar plates and incubated anaerobically at 37°C with 5% hydrogen, 5% CO2, and 90% nitrogen for 4–5 days, or aerobically at 37°C with 5% CO2 for 24–48 hours. The media used for anaerobic culture were Brucella blood agar, phenylethyl alcohol (PEA) agar with 5% sheep blood, laked blood with kanamycin and vancomycin agar, bacteroides bile esculin agar (Anaerobe Systems, Morgan Hill, CA, USA), and Mann Rogosa Sharpe agar (MRS) (EMD Chemicals Inc., Gibbstown, NJ, USA). The media used for aerobic culture were tryptic soy agar with 5% sheep blood, PEA with 5% sheep blood, MacConkey agar, Candida isolation agar (Hardy Diagnostics, Santa Maria, CA, USA), MRS agar and Rogosa agar (Becton, Dickinson and Co., Franklin Lakes, NJ, USA). Cultures on the primary plates were screened, semi-quantitated, and sub-cultured for isolation of pure cultures. They were then examined for pigment production and hemolysis of blood agar. Additional Gram stain and biochemical tests (e.g. catalase, oxidase, urease and indole production) were also performed for identification of the bacterial isolates.
Bacterial identification by 16S ribosomal RNA (rRNA) gene sequencing
Bacterial identification to the species level was performed using 16S rRNA gene sequencing. A single colony of bacteria was lysed in 100 μl of ‘PrepMan Ultra’ Sample Preparation Reagent (Applied Biosystems, Foster City, CA, USA) to prepare a DNA template for polymerase chain reaction (PCR) amplification. 16S rDNA fragments (~900 base pairs) were amplified with universal bacterial primers 8f (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 926r (5′-CCG TCA ATT CCT TTR AGT TT-3′, R = A/G) [11], purified with ExoSAP-IT (USB, Cleveland, OH, USA), and sequenced with a primer 519r (5′-GWA TTA CCG CGG CKG CTG-3′, W = A/T, K = G/T) [32]. Resulting sequences were subjected to nucleotide–nucleotide BLAST (blastn) in comparison to known 16S rRNA genes in the public databases to identify the species of Lactobacillus. A species was assigned to an isolate when it shared 98% or higher identity to known genes. For construction of a phylogenetic tree, full-length 16S rRNA genes of Lactobacillus were PCR amplified using primers 8f and 1492r (5′-TAC GGY TAC CTT GTT ACG ACT T-3′, Y = C/T) [21]. The resulting PCR products were cloned into pGEM-T Easy Vector System (Promega, Madison, WI, USA) and sequenced with universal primers SP6 and T7.
Assay for detection of hydrogen peroxide production by Lactobacillus isolates
Hydrogen peroxide (H2O2) production by Lactobacillus strains was tested on MRS agar supplemented with tetramethylbenzidine and horseradish peroxidase (Sigma-Aldrich, St. Louis, MO, USA) [42]. Plates were incubated anaerobically at 37°C for 24 hours and then exposed to ambient air at room temperature. Colonies were observed for color development (from white to blue, indicating H2O2 production) for 30 min. H2O2 production was ranked as +++, ++, and +, if blue color developed in less than 10, 20, and 30 min, respectively. H2O2 production was ranked as negative if colonies remained white within 30 min.
Carbohydrate fermentation by Lactobacillus isolates
Phenotypic diversity among Lactobacillus isolates to ferment carbohydrates was evaluated using API 50 CH carbohydrate fermentation strips (bioMerieux, Inc., Marcy-I’Etoile, France) [6]. Pure Lactobacillus colonies that were grown anaerobically on MRS agar plates for 24 hours were harvested with a sterile swab, and resuspended in an API 50 CHL medium to turbidity equal to or greater than 0.5 McFarland. Strips were incubated anaerobically for 24–48 hours and results were recorded.
Repetitive extragenic palindromic (Rep)-PCR
Chromosomal DNA of Lactobacillus culture at early stationary phase was isolated by using the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA). An aliquot of chromosomal DNA was used for Rep-PCR using primers Rep-R (5′-III NCG NCG NCA TCN GGC-3′, I = inosine, N = C/G/A/T) and Rep-L (5′-NCG NCT TAT CNG GCC TAC-3′, N = C/G/A/T) in the PCR mixtures [2]. PCR was performed with a PTC-200 model thermal cycler (MJ Research, Waltham, MA, USA). The PCR reaction was initiated by incubating the reaction mixture at 95°C for 7 min to activate the Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA), followed by 35 cycles of 90°C for 30 s, 40°C for 1 min, and 65°C for 8 min. The reaction was terminated with an extension step of 65°C for 16 min. An aliquot of each reaction mixture was resolved on a 1% agarose gel at 30 V for 18 hours.
In vitro testing of antibiotic susceptibility of vaginal Lactobacillus isolates
A broth microdilution method [33] was used to test the minimum inhibitory concentrations (MICs) of antibiotics for vaginal Lactobacillus isolates.
Clearance of vaginal Lactobacillus by vaginal suppository of azithromycin
Fatty acid-based suppositories containing 200 mg of azithromycin were compounded by Foer’s Pharmacy (Bethesda, MD, USA) and administered intravaginally once a day for five consecutive days by digital manipulation to sedated animals.
Intravaginal administration of L. johnsonii and human vaginal isolate of L. jensenii to Chinese rhesus macaques
Endogenous vaginal isolates of L. johnsonii isolated from two Chinese rhesus macaques and a human vaginal isolate of L. jensenii 1153 [28] were grown overnight to late log/early stationary phase in MRS broth. The bacterial cells were centrifuged and washed with PBS (pH 7.0). Approximately 109 CFU of Lactobacillus cells were mixed in 1 ml of fresh MRS medium and 2 ml of 2.7% hydroxyethyl cellulose (HEC) solution [44], and inoculated intravaginally into the sedated macaques for 7 consecutive days for L. jensenii 1153 [28] or every other day for three times for L. johnsonii recovered from rhesus macaques. Sedated animals were closely monitored by veterinary staff.
Results
Lactobacillus was isolated among endogenous vaginal microflora of Chinese rhesus macaques
The vaginal microflora of nine naïve Chinese rhesus macaques that have not been treated with antibiotics was analyzed from samples collected at multiple time points over one menstrual cycle (36–46 days). Cultivable bacterial species isolated from these animals over the course of the studies are listed in Table 1. The most common bacterial species isolated were Enterococcus and Staphylococcus species (100%). Surprisingly, Lactobacillus was the next most common bacterial species isolated from these animals (89%). Six out of nine animals harbored H2O2-producing Lactobacillus species, while four out of nine animals harbored non-H2O2-producing Lactobacillus species. Among the anaerobes, Gram-positive cocci Peptoniphilus sp., Peptostreptococcus sp., and Anaerococcus sp., and Gram-negative rods Bacteroides sp. and Porphyromonas sp. were the most frequently and the most prevalent ones isolated. Yeast was not isolated from any of the animals.
Table 1.
Vaginal microflora of Chinese rhesus macaques
| Organism | Number of macaques harboring the organism (n = 9) |
|---|---|
| Aerobic/facultative anaerobic | |
| Lactobacillus sp. | 8 |
| H2O2+ | 6 |
| H2O2− | 4 |
| Enterococcus sp. | 9 |
| Staphylococcus sp. | 9 |
| Streptococcus sp. | 6 |
| Micrococcus sp. | 7 |
| Escherichia coli | 5 |
| Proteus sp. | 5 |
| Aerococcus sp. | 3 |
| Aerosphaera sp. | 1 |
| Corynebacterium sp. | 1 |
| Citrobacter sp. | 1 |
| Facklamia sp. | 1 |
| Klebsiella sp. | 1 |
| Psychrobacter sp. | 1 |
| Vagococcus sp. | 1 |
| Weissella sp. | 1 |
| Anaerobic | |
| Peptoniphilus sp. | 5 |
| Peptostreptococcus sp. | 3 |
| Anaerococcus sp. | 2 |
| Finegoldia sp. | 1 |
| Micromonas micros | 1 |
| Propionibacterium sp. | 2 |
| Bifidobacterium sp. | 1 |
| Bacteroides sp. | 4 |
| Porphyromonas sp. | 4 |
| Prevotella sp. | 1 |
| Bilophila wadsworthia | 1 |
| Veillonella sp. | 2 |
Identification and characterization of endogenous vaginal Lactobacillus in Chinese rhesus macaques
Lactobacillus isolated from a total of 13 Chinese rhesus macaques housed at two separate locations was analyzed. Analysis of 16S rRNA gene sequences determined that the predominant Lactobacillus species recovered from the vagina of 12 of 13 macaques is L. johnsonii (Table 2). Endogenous vaginal Lactobacillus was not recovered from one of the animals (ABL-5) over the course of the study. A majority of the macaques only harbored one vaginal Lactobacillus species, but a few of them harbored more than one. The level of cultivable lactobacilli ranged from 102 to 108 CFU per vaginal swab. Two isolates of L. johnsonii that had different colony morphology on the MRS agar plates were recovered from one macaque, SRI-3. When carbohydrate fermentation profiles were compared between these two vaginal L. johnsonii isolates, differences were noted in the fermentation of D-lactose, D-raffinose and D-tagatose, demonstrating that they are different strains (Table 2). Other vaginal Lactobacillus species were also recovered from three macaques which harbored L. johnsonii. Lactobacillus murinus was isolated from ABL-6, L. amylovorous was isolated from ABL-8, and L. acidophilus and L. amylovorous were recovered from ABL-7 (Table 2). However, these other Lactobacillus species constituted the minority, as they were recovered less consistently (at one time point only) than L. johnsonii. Most of the endogenous lactobacilli recovered from Chinese rhesus macaques are strong H2O2 producers (Table 2). Nine of 13 strains of L. johnsonii are strong H2O2 producers. For the other vaginal Lactobacillus species, only L. amylovorous from ABL-7 (ABL-V7C) is a H2O2 producer.
Table 2.
Characterization of endogenous vaginal and rectal Lactobacillus isolates recovered from Chinese rhesus macaques
| Animal | Isolate | Origin of isolation |
Lactobacillus species1 |
Carbohydrate fermentation by API 50 CH tests2 | H2O23 production |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LARA | RIB | GAL | GLU | FRU | MNE | DUL | MAN | SOR | MDG | NAG | AMY | ARB | ESC | SAL | CEL | MAL | LAC | MEL | SAC | TRE | RAF | AMD | GEN | TUR | TAG | |||||
| ABL-1 | ABL-V1 | Vagina | L. johnsonii | − | ||||||||||||||||||||||||||
| ABL-2 | ABL-V2 | Vagina | L. johnsonii | +++ | ||||||||||||||||||||||||||
| ABL-3 | ABL-V3 | Vagina | L. johnsonii | +++ | ||||||||||||||||||||||||||
| ABL-4 | ABL-V4 | Vagina | L. johnsonii | − | ||||||||||||||||||||||||||
| ABL-6 | ABL-V6A | Vagina | L. johnsonii | − | ||||||||||||||||||||||||||
| ABL-R6A | Rectum | L. johnsonii | − | |||||||||||||||||||||||||||
| ABL-R6B | Rectum | L. johnsonii | +++ | |||||||||||||||||||||||||||
| ABL-V6B | Vagina | L. murinus | − | |||||||||||||||||||||||||||
| ABL-R6C | Rectum | L. murinus | − | |||||||||||||||||||||||||||
| ABL-7 | ABL-V7A | Vagina | L. johnsonii | +++ | ||||||||||||||||||||||||||
| ABL-R7A | Rectum | L. johnsonii | − | |||||||||||||||||||||||||||
| ABL-V7B | Vagina | L. acidophilus | − | |||||||||||||||||||||||||||
| ABL-R7B | Rectum | L. acidophilus | +++ | |||||||||||||||||||||||||||
| ABL-V7C | Vagina | L. amylovorous | +++ | |||||||||||||||||||||||||||
| ABL-8 | ABL-V8A | Vagina | L. johnsonii | +++ | ||||||||||||||||||||||||||
| ABL-R8A | Rectum | L. johnsonii | − | |||||||||||||||||||||||||||
| ABL-V8B | Vagina | L. amylovorous | − | |||||||||||||||||||||||||||
| SRI-1 | SRI-V1 | Vagina | L. johnsonii | +++ | ||||||||||||||||||||||||||
| SRI-R1 | Rectum | L. johnsonii | +++ | |||||||||||||||||||||||||||
| SRI-2 | SRI-V2 | Vagina | L. johnsonii | − | ||||||||||||||||||||||||||
| SRI-3 | SRI-V3A | Vagina | L. johnsonii | +++ | ||||||||||||||||||||||||||
| SRI-V3B | Vagina | L. johnsonii | +++ | |||||||||||||||||||||||||||
| SRI-R3 | Rectum | L. johnsonii | +++ | |||||||||||||||||||||||||||
| SRI-4 | SRI-V4 | Vagina | L. johnsonii | +++ | ||||||||||||||||||||||||||
| SRI-R4 | Rectum | L. johnsonii | +++ | |||||||||||||||||||||||||||
| SRI-5 | SRI-V5 | Vagina | L. johnsonii | +++ | ||||||||||||||||||||||||||
| SRI-R5 | Rectum | L. johnsonii | +++ | |||||||||||||||||||||||||||
Species of Lactobacillus isolates was determined by 16S rRNA gene sequencing as described in Materials and Methods.
White box: positive fermentation; grey box: negative fermentation. LARA, L-arabinose; RIB, D-ribose; GAL, D-galactose; GLU, D-glucose; FRU, D-fructose; MNE, D-mannose; DUL, dulcitol; MAN, D-mannitol; SOR, D-sorbitol; MDG, methyl-αD-glucopyranoside; NAG, N-acetylglucosamine; AMY, amygdalin; ARB, arbutin; ESC, esculin ferric citrate; SAL, salicin; CEL, D-cellobiose; MAL, D-maltose; LAC, D-lactose; MEL, D-melibiose; SAC, D-saccharose; TRE, D-trehalose; RAF, D-raffinose; AMD, amidon (starch); GEN, gentiobiose; TUR, D-turanose; TAG, D-tagatose.
+++: strong H2O2 production; −: no H2O2 production in 30 min.
Rectal and vaginal Lactobacillus isolates recovered from the same macaque are uniquely different from each other
Rectal swabs were also collected from the above-mentioned nine naïve macaques (ABL 5–8 and SRI 1–5), and endogenous lactobacilli were recovered from all samples analyzed, including ABL-5 that did not harbor vaginal lactobacilli at any sampling times. Lactobacillus johnsonii was found in seven of the nine animals, but it is not the predominant Lactobacillus species in the rectum as in the case of the vagina. In the rectum, many different Lactobacillus species were recovered, including Lactobacillus reuteri, Lactobacillus animalis, Lactobacillus ingluviei, Lactobacillus salivarius, Lactobacillus sobrius and Lactobacillus mucosae. In most instances, more than one species of rectal lactobacilli was recovered from each individual macaque. Seven of the nine macaques harbored the same Lactobacillus species in both the vagina and the rectum (Table 2), but it was subsequently shown that they have different biochemical and genetic profiles.
Additional biochemical tests and chromosomal DNA fingerprinting conclusively determined that the rectal and vaginal Lactobacillus isolates from the same animal are uniquely different from each other (Table 2 and Fig. 1). All L. johnsonii, of both vaginal and rectal origins, could ferment D-glucose, D-fructose, D-maltose, and sucrose. However, a few rectal L. johnsonii isolates (ABL-R6A, ABL-R6B and SRI-R3) could not utilize several sugars (D-galactose, N-acetylglucosamine, amygdalin, arbutin, esculin ferric citrate, salicin, D-cellobiose, D-trehalose, and gentiobiose) that were fermented by all of the vaginal isolates. D-ribose, dulcitol, D-mannitol, D-sorbitol, and methyl-αD-glucopyranoside were used by some of the rectal but none of the vaginal L. johnsonii isolates. L-arabinose was fermented only by L. murinus isolated from the rectum of animal ABL-6 (ABL-R6C), and D-turanose was utilized only by the L. acidophilus isolates, of both vaginal and rectal origins, from ABL-7 (ABL-V7B and ABL-R7B) (Table 2).
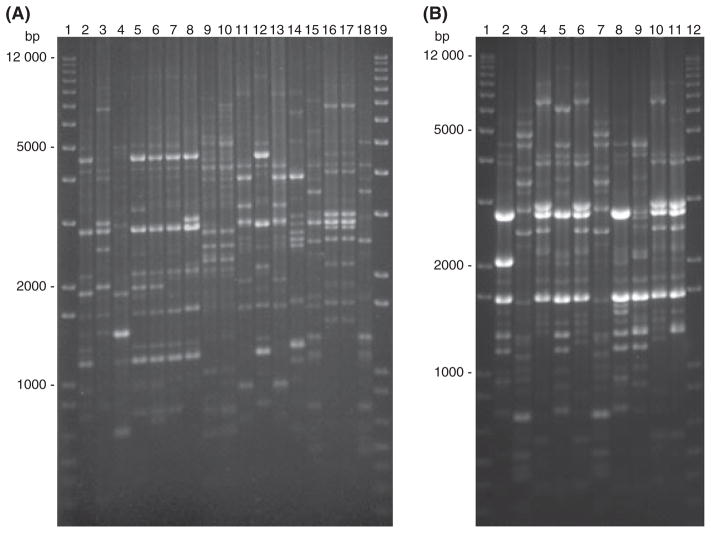
Fig. 1.
Rep-PCR DNA fingerprint patterns of vaginal and rectal Lactobacillus strains isolated from Chinese rhesus macaques housed at (A) ABL and (B) Southern Research Institute. (A) Lanes 1 and 19, a 1-kb plus DNA ladder; lane 2, L. johnsonii ABL-V1; lane 3, L. johnsonii ABLV2; lane 4, L. johnsonii ABL-V3; lane 5, L. johnsonii ABL-V4; lane 6, L. johnsonii ABL-V6A; lane 7, L. johnsonii ABL-R6A; lane 8, L. johnsonii ABL-R6B; lane 9, L. murinus ABL-V6B; lane 10, L. murinus ABL-R6C; lane 11, L. johnsonii ABL-V7A; lane 12, L. johnsonii ABL-R7A; lane 13, L. acidophilus ABL-V7B; lane 14, L. acidophilus ABL-R7B; lane 15, L. amylovorous ABL-V7C; lane 16, L. johnsonii ABL-V8A; lane 17, L. johnsonii ABL-R8A; lane 18, L. amylovorous ABL-V8B. (B) lanes 1 and 12, a 1-kb plus DNA ladder; lanes 2–11, all L. johnsonii isolates: lane 2, SRI-V1; lane 3, SRI-R1; lane 4, SRI-V2; lane 5, SRI-V3A; lane 6, SRI-V3B; lane 7, SRI-R3; lane 8, SRI-V4; lane 9, SRI-R4, lane 10, SRI-V5; lane 11, SRI-R5. bp: base pair of DNA fragments.
Rep-PCR was also performed to determine the genomic fingerprint patterns of the Lactobacillus isolates. The fingerprint patterns were consistent in three independent rep-PCR runs, and a representative figure is shown in Fig. 1. As shown in Fig. 1, fingerprints of the vaginal isolates of L. johnsonii from animals ABL 1–4 are each different from one another, indicating that they are different isolates (Fig. 1A, lanes 2–5). When fingerprints of L. johnsonii (Fig. 1A, lanes 6–8) isolated from the same animal ABL-6 were analyzed, the band patterns were similar, but not identical, indicating again that the vaginal and rectal Lactobacillus isolates from the same animal are uniquely different from each other. Similar results were observed for the vaginal and rectal lactobacilli isolates from other animals: L. johnsonii isolates from ABL-7 (Fig. 1A, lanes 11 and 12), L. acidophilus isolates from ABL-7 (Fig. 1A, lanes 13 and 14), L. johnsonii from SRI-1 (Fig. 1B, lanes 2 and 3), L. johnsonii from SRI-3 (Fig. 1B, lanes 5–7), L. johnsonii from SRI-4 (Fig. 1B, lanes 8 and 9), and L. johnsonii from SRI-5 (Fig. 1B, lanes 10 and 11). Although the fingerprints of the vaginal and rectal L. johnsonii isolates from animal ABL-8 seemed almost identical (Fig. 1A, lanes 16 and 17), carbohydrate fermentation profiles revealed differences in the fermentation of methyl-αD-glucopyranoside and D-lactose, and they also differed in terms of H2O2 production (Table 2). Similarly, vaginal and rectal L. murinus (Fig. 1A, lanes 9 and 10) from ABL-6 also have very similar fingerprints, but they differed in the fermentation of multiple carbohydrates (Table 2). Together, carbohydrate fermentation profiles, H2O2 production, and genomic fingerprints revealed that the Lactobacillus isolates of the same species from the same animals are indeed different (Table 2 and Fig. 1).
Lactobacillus johnsonii isolated from Chinese rhesus macaques are closely related to Lactobacillus gasseri
Sequence data of the 1537-nucleotide region of the 16S rRNA gene of the vaginal L. johnsonii isolates from Chinese rhesus macaques were aligned using Clustalw and a phylogenetic tree was constructed showing the relationship of L. johnsonii and a few representative human vaginal isolates. As shown in Fig. 2, all vaginal L. johnsonii isolates from the 12 macaques clustered together. They also have a very close phylogenetic relationship to a human vaginal L. johnsonii isolate from our collection (L. johnsonii 135-1). The 16S rRNA gene-based phylogenetic tree also showed that L. johnsonii has a closer relationship to human vaginal strains of L. gasseri than to Lactobacillus crispatus or L. jensenii.
Fig. 2.
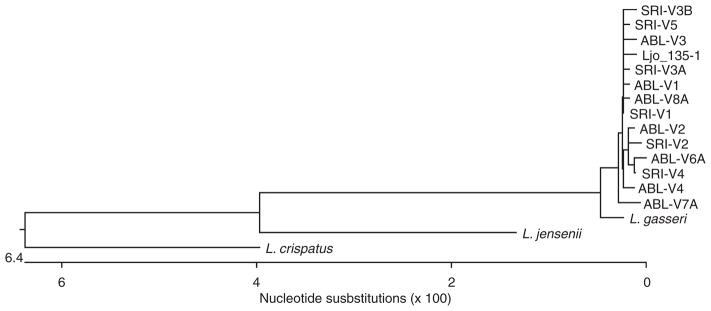
Phylogenetic tree showing the relationship based on the analysis of a 1537-nucleotide region of 16S rRNA genes of vaginal Lactobacillus johnsonii from Chinese rhesus macaques to some representative human vaginal Lactobacillus species. The tree was constructed using the Clustalw program. Ljo_135-1 is a human vaginal isolate of L. johnsonii.
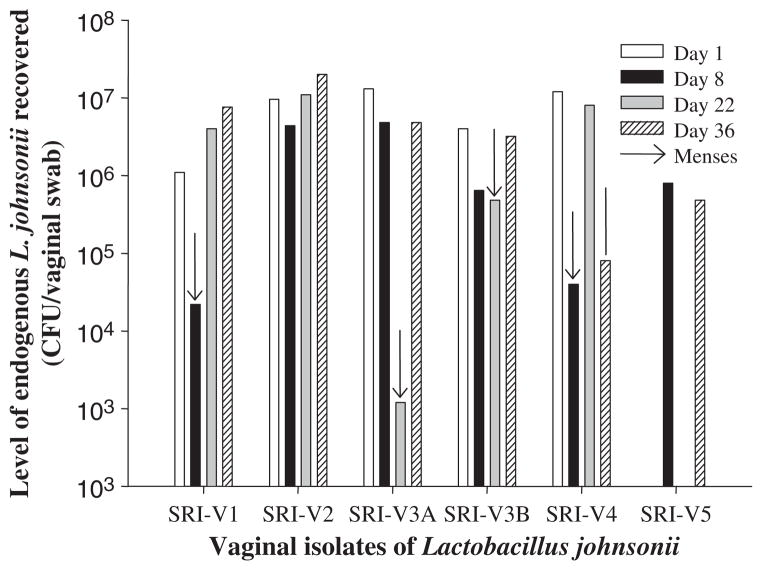
Dynamic nature of endogenous vaginal L. johnsonii in Chinese rhesus macaques
In order to understand the dynamic nature of vaginal Lactobacillus flora over menstrual cycles, the level of endogenous vaginal lactobacilli was monitored at four time points over a period of 36 days in five Chinese rhesus macaques housed at Southern Research Institute. The macaques did not receive hormonal treatment, so their menstrual cycles were not synchronized. On average, between 105 and 107 CFU per vaginal swab of L. johnsonii was recovered in all macaques. For animal SRI-5, lactobacilli were not recovered at two time points (days 1 and 22). This may be because the amount of lactobacilli present at those time points was below the detection level of our microbiologic techniques. It was also noted that the level of endogenous lactobacilli dropped precipitously (to ~104) during or immediately after menses (Fig. 3). For other non-lactobacilli endogenous bacteria, a similar pattern of changes was also observed. The average level of other endogenous microflora recovered was also about 106 to 107 CFU per vaginal swab, and less variety and lower quantity of each bacterial species was recovered during or immediately after menses (data not shown).
Fig. 3.
Dynamic nature of endogenous vaginal Lactobacillus johnsonii in Chinese rhesus macaques housed at Southern Research Institute. Vaginal swabs were collected at multiple time points over one menstrual cycle, and the level of endogenous L. johnsonii recovered from each animal was studied. Levels of endogenous lactobacilli varied from 103 to 107 per swab during the cycle. Arrows indicated sample collection immediately (one day) after menses (animal SRI-1) or during menses (animals SRI-3 and SRI-4). Macaques were not synchronized for their menstrual cycles.
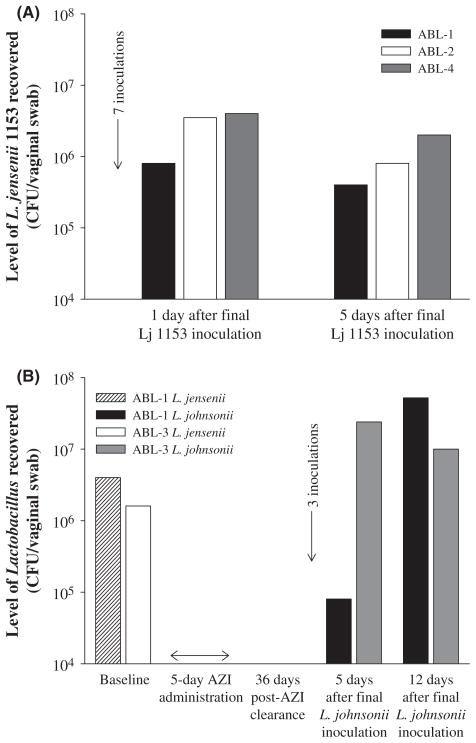
Colonization of a human vaginal isolate of L. jensenii 1153 in Chinese rhesus macaques
To test the potential use of Chinese rhesus macaques as a model for studying colonization of human vaginal Lactobacillus, the macaques were inoculated with a human vaginal isolate of L. jensenii 1153 for seven consecutive days. Bacterial concentrations of 106 and 105 CFU per vaginal swab were recovered 1 and 5 days after final bacterial inoculation, respectively (Fig. 4A). Lactobacillus jensenii 1153 forms small smooth colonies on the MRS agar plates, which can be differentiated from the large rough colonies of the endogenous L. johnsonii. L. jensenii isolates were further confirmed by 16S rRNA gene sequencing. In one of the macaques, 9 × 106 CFU per vaginal swab of L. jensenii was recovered 26 days after the final bacterial inoculation (data not shown). Apparently, the introduction of the human vaginal Lactobacillus isolate has no significant impact on the diversity or quantity of the endogenous vaginal flora of the macaques (data not shown).
Fig. 4.
Vaginal colonization of Lactobacillus in Chinese rhesus macaques. (A) 7-day inoculation of a human vaginal isolate of L. jensenii 1153 in three Chinese rhesus macaques (ABL-1, ABL-2 and ABL-4). Level of Lactobacillus jensenii recovered was analyzed for vaginal swabs collected at 1 and 5 days after the final bacterial inoculation. Lj 1153, L. jensenii 1153; 7 inoculations, 7-day inoculation of L. jensenii 1153. (B) Clearance of vaginal Lactobacillus by a 5-day administration of vaginal suppository of azithromycin, followed by restoration of L. johnsonii in two Chinese rhesus macaques (ABL-1 and ABL-3). Baseline level of vaginal Lactobacillus was analyzed before azithromycin administration. Level of Lactobacillus was monitored for 36 days after the final azithromycin administration. Lactobacillus johnsonii previously isolated from individual macaques was reintroduced intravaginally every other day for three times into each of the two macaques. Level of L. johnsonii was analyzed for vaginal swabs collected at 5 and 12 days after the final bacterial inoculation. AZI, vaginal suppository of azithromycin; 3 inoculations, 3 inoculations (every other day) of L. johnsonii.
Clearance of vaginal Lactobacillus and restoration of L. johnsonii in Chinese rhesus macaques
A human vaginal isolate of L. jensenii 1153 strain and the Lactobacillus isolates recovered from Chinese rhesus macaques were sensitive to erythromycin and azithromycin, an erythromycin derivative (data not shown). We chose to use azithromycin as a vaginal suppository to clear vaginal Lactobacillus as it is acid stable (which is important in a healthy vaginal setting) and is better tolerated by animals and humans [17, 24]. By administering a vaginal suppository of azithromycin for 5 consecutive days, the exogenous human vaginal lactobacilli previously administered to the animals were readily cleared from the vaginal flora of Chinese rhesus macaques (Fig. 4B). In addition, no lactobacilli were recovered up to at least 36 days after the final azithromycin administration (Fig. 4B). Furthermore, endogenous vaginal L. johnsonii could be restored in these macaques. Respective isolates of L. johnsonii from individual macaques were reintroduced intravaginally into each of the two macaques. Approximately 107 CFU per vaginal swab of L. johnsonii were recovered 12 days after the final bacterial inoculation (Fig. 4B), demonstrating that endogenous L. johnsonii was restored in the Chinese rhesus macaques. On the contrary, no human vaginal Lactobacillus isolates were recovered from the ‘reconstructed’ microflora after azithromycin treatment, showing effective clearance of the pre-existing lactobacilli by the antibiotic.
Discussion
Macaques are commonly used for evaluation of the safety and efficacy of topical vaginal microbicides for preventing sexually transmitted diseases in humans [26, 45, 47–49]. The similarities between the reproductive anatomy and physiology of human and rhesus macaques [36] prompted us to establish a nonhuman primate model suitable to evaluate the colonization of our Lactobacillus-based topical microbicide candidates. This study describes the use of the Chinese rhesus macaque as a potential model for persistent vaginal colonization of human Lactobacillus, and for pre-clinical evaluation of Lactobacillus-based topical microbicides. Our data demonstrated that Chinese rhesus macaques naturally harbor endogenous L. johnsonii among their vaginal flora.
Although several small animal models (such as rabbits, mice and rats) could possibly support transient vaginal colonization of Lactobacillus [7, 31, 34], they are not suitable or relevant for our purpose. All of these small animals require treatment with estrogen, and the profiles of their indigenous microflora show no resemblance to the microflora of humans [22, 34]. Rabbits in particular are not suitable, as it has been reported that laboratory rabbits are usually in precoital status. They do not undergo estrous cycle stages and have little mucous secretion before mating. Vaginal environments with poor mucus secretion are inadequate for bacterial proliferation. Thus, the vaginal environment in precoital rabbits was suggested to be comparable to that during diestrus or anestrus in other animal species [34]. We found that diestrus does not support Lactobacillus colonization in a mouse model [28]. It also appears that the predominance of lactobacilli and low vaginal pH are distinct characteristics of the human vaginal microflora [5, 20]. However, small animals have shown minimal numbers of vaginal lactobacilli and neutral vaginal pH [22, 31, 34]. In addition, anaerobic bacteria were the predominant flora in vagina of humans, and the total number of bacteria was higher than that in small laboratory animal models [18, 34]. The lack of similarity prompted us to look for a primate model that better mimics the human vaginal environment for the testing of live topical vaginal microbicides.
The normal vaginal flora we identified from the Chinese rhesus macaques in this study was generally similar to the findings of Doyle et al. [12] and Scorpio et al. [39] (Table 1). However, there are several major differences in the findings of these studies. First, we isolated and identified vaginal Lactobacillus in 12 of 13 of the animals we studied, and some of these macaques harbored more than one species of Lactobacillus over the course of the study. Lactobacillus johnsonii was the predominant endogenous vaginal Lactobacillus species we recovered, and it was isolated from animals housed at both ABL and Southern Research Institute. On the contrary, Doyle et al. did not find any lactobacilli in 37 rhesus macaques, and instead, reported Gardnerella-like or Gardnerella-probable organisms in about one-third of the animals, and Mobiluncus curtisii in half of the animals they studied. Although sampling time and bacterial identification methods were different in these two studies, it is difficult to determine whether these reasons alone contributed to the discrepancies. It was also unclear whether naïve animals were used in the studies of Doyle et al. For instance, endogenous vaginal Lactobacillus is sensitive to certain antibiotic treatments, as evidenced in our studies. Although Scorpio et al. reported Lactobacillus spp. as one of the most commonly cultured bacteria from the 11 Chinese rhesus macaques in their study, it is unclear what species these lactobacilli are and how many animals harbor them [39].
In healthy women of childbearing age, the vaginal flora is dominated by Lactobacillus (107–109 CFU per gram of fluid) [41]. The species of Lactobacillus most commonly isolated from the reproductive tracts of healthy women worldwide belong to L. acidophilus complex that has been subdivided into six distinct species: L. acidophilus, L. crispatus, L. gasseri, L. gallinarum, L. amylovorous, and L. johnsonii [15, 23, 25, 50]. With the advancement of molecular biology techniques, it was revealed that L. gasseri, L. jensenii, L. crispatus, and Lactobacillus iners are the most commonly isolated endogenous Lactobacillus species from healthy women at child-bearing age [1, 16, 46, 50]. It is interesting that three of the four endogenous Lactobacillus species, L. johnsonii, L. amylovorous, and L. acidophilus that were recovered from Chinese rhesus macaques belong to the L. acidophilus complex, with L. johnsonii as the most frequently isolated and the most prevalent vaginal Lactobacillus species in these macaques. Lactobacillus johnsonii is more closely related to L. gasseri phylogenetically than to L. jensenii or L. crispatus (Fig. 2 and [37]). The finding of L. acidophilus complex in the vagina of Chinese rhesus macaques may indicate that the microenvironmental niche for the growth and persistence of vaginal lactobacilli is similar in human and macaque. Indeed, the carbohydrate utilization profiles of the L. johnsonii isolates from macaques (Table 2) and the human vaginal Lactobacillus isolates we collected are very similar (unpublished data). This may explain why the human vaginal L. jensenii isolates could colonize in the vagina of macaques. In a different study, we also isolated vaginal L. johnsonii from seven out of nine rhesus macaques of Indian-origin and successfully colonized human vaginal Lactobacillus in these animals (unpublished data), which further supports our hypothesis that the similarity between rhesus macaque and human vaginal microflora makes these animals a relevant model for assessing the safety, efficacy and distribution of Lactobacillus-based vaginal microbicides.
Antonio et al. suggested that Lactobacillus from the rectum could be a potential source to seed the vagina in women [3]. However, we found that although the same species of Lactobacillus could be isolated from both the vagina and rectum of the macaques, they are not identical, suggesting that the rectal microflora may not be the reservoir for the endogenous vaginal Lactobacillus. While we could not rule out that our analysis may miss a minor Lactobacillus present at a very low quantity in the vaginal swabs, the origin of endogenous vaginal Lactobacillus remains to be elucidated.
In this study, we demonstrated the presence of abundant endogenous vaginal Lactobacillus in rhesus macaques that have been widely used to test various candidate microbicides. In this animal model, endogenous vaginal Lactobacillus microflora could serve as a complimentary marker for preclinical toxicity evaluation of potential vaginal microbicide candidates. In addition, this is a potentially useful model for vaginal Lactobacillus colonization to support the pre-clinical evaluation of Lactobacillus-based topical microbicides. Indeed, we have successfully colonized a genetically modified L. jensenii strain that produces a heterologous protein for at least 87 days in this rhesus macaque model (unpublished data). We also determined that it is unnecessary to clear the endogenous flora for the colonization of exogenous human Lactobacillus in Chinese rhesus macaques (unpublished data). The decrease in the quantity of vaginal L. johnsonii recovered during or immediately after menses in macaques was similar to what has been reported with humans [13]. Therefore, immediately after menses should be the best time to intravaginally administer ecologically appropriate Lactobacillus.
Acknowledgments
This work was supported in part by NIH Integrated Preclinical/Clinical Program for Topical Microbicides U19AI60615 and NIH Partnerships for Topical Microbicides U01AI066708. The authors thank Lorna K. Rabe at Magee-Womens Research Institute for technical advice in quantitative microbiology, Dr. Dusty Rhodes and Dawn Golightly at Southern Research Institute for their guidance and assistance in macaque studies, and Dr. Xiaowen Liu for critical reading of this manuscript.
References
- 1.Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999;180:1950–6. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- 2.Antonio MA, Hillier SL. DNA fingerprinting of Lactobacillus crispatus strain CTV-05 by repetitive element sequence-based PCR analysis in a pilot study of vaginal colonization. J Clin Microbiol. 2003;41:1881–7. doi: 10.1128/JCM.41.5.1881-1887.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonio MA, Rabe LK, Hillier SL. Colonization of the rectum by Lactobacillus species and decreased risk of bacterial vaginosis. J Infect Dis. 2005;192:394–8. doi: 10.1086/430926. [DOI] [PubMed] [Google Scholar]
- 4.Baron EJ, Vaisanen ML, McTeague M, Strong CA, Norman D, Finegold SM. Comparison of the Accu-CulShure system and a swab placed in a B-D Port-a-Cul tube for specimen collection and transport. Clin Infect Dis. 1993;16:S325–7. doi: 10.1093/clinids/16.supplement_4.s325. [DOI] [PubMed] [Google Scholar]
- 5.Boskey ER, Cone RA, Whaley KJ, Moench TR. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001;16:1809–13. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 6.Boyd MA, Antonio MA, Hillier SL. Comparison of API 50 CH strips to whole-chromosomal DNA probes for identification of Lactobacillus species. J Clin Microbiol. 2005;43:5309–11. doi: 10.1128/JCM.43.10.5309-5311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catalone BJ, Kish-Catalone TM, Budgeon LR, Neely EB, Ferguson M, Krebs FC, Howett MK, Labib M, Rando R, Wigdahl B. Mouse model of cervicovaginal toxicity and inflammation for preclinical evaluation of topical vaginal microbicides. Antimicrob Agents Chemother. 2004;48:1837–47. doi: 10.1128/AAC.48.5.1837-1847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang TL, Chang CH, Simpson DA, Xu Q, Martin PK, Lagenaur LA, Schoolnik GK, Ho DD, Hillier SL, Holodniy M, Lewicki JA, Lee PP. Inhibition of HIV infectivity by a natural human isolate of Lactobacillus jensenii engineered to express functional two-domain CD4. Proc Natl Acad Sci USA. 2003;100:11672–7. doi: 10.1073/pnas.1934747100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherpes TL, Melan MA, Kant JA, Cosentino LA, Meyn LA, Hillier SL. Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B streptococcus colonization. Clin Infect Dis. 2005;40:1422–8. doi: 10.1086/429622. [DOI] [PubMed] [Google Scholar]
- 10.Cohn MA, Frankel SS, Rugpao S, Young MA, Willett G, Tovanabutra S, Khamboonruang C, VanCott T, Bhoopat L, Barrick S, Fox C, Quinn TC, Vahey M, Nelson KE, Weissman D. Chronic inflammation with increased human immunodeficiency virus (HIV) RNA expression in the vaginal epithelium of HIV-infected Thai women. J Infect Dis. 2001;184:410–7. doi: 10.1086/322780. [DOI] [PubMed] [Google Scholar]
- 11.Coolen MJ, Post E, Davis CC, Forney LJ. Characterization of microbial communities found in the human vagina by analysis of terminal restriction fragment length polymorphisms of 16S rRNA genes. Appl Environ Microbiol. 2005;71:8729–37. doi: 10.1128/AEM.71.12.8729-8737.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle L, Young CL, Jang SS, Hillier SL. Normal vaginal aerobic and anaerobic bacterial flora of the rhesus macaque (Macaca mulatta) J Med Primatol. 1991;20:409–13. [PubMed] [Google Scholar]
- 13.Eschenbach DA, Thwin SS, Patton DL, Hooton TM, Stapleton AE, Agnew K, Winter C, Meier A, Stamm WE. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin Infect Dis. 2000;30:901–7. doi: 10.1086/313818. [DOI] [PubMed] [Google Scholar]
- 14.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353:1899–911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 15.Fujisawa T, Benno Y, Yaeshima T, Mitsuoka T. Taxonomic study of the Lactobacillus acidophilus group, with recognition of Lactobacillus gallinarum sp. nov. and Lactobacillus johnsonii sp. nov. and synonymy of Lactobacillus acidophilus group A3 (Johnson et al. 1980) with the type strain of Lactobacillus amylovorus (Nakamura 1981) Int J Syst Bacteriol. 1992;42:487–91. doi: 10.1099/00207713-42-3-487. [DOI] [PubMed] [Google Scholar]
- 16.Giorgi A, Torriani S, Dellaglio F, Bo G, Stola E, Bernuzzi L. Identification of vaginal lactobacilli from asymptomatic women. Microbiologica. 1987;10:377–84. [PubMed] [Google Scholar]
- 17.Girard AE, Girard D, English AR, Gootz TD, Cimochowski CR, Faiella JA, Haskell SL, Retsema JA. Pharmacokinetic and in vivo studies with azithromycin (CP-62,993), a new macrolide with an extended half-life and excellent tissue distribution. Antimicrob Agents Chemother. 1987;31:1948–54. doi: 10.1128/aac.31.12.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammann R, Kronibus A, Lang N, Werner H. Quantitative studies on the vaginal flora of asymptomatic women and patients with vaginitis and vaginosis. Zentralbl Bakteriol Mikrobiol Hyg [A] 1987;265:451–61. doi: 10.1016/s0176-6724(87)80264-x. [DOI] [PubMed] [Google Scholar]
- 19.Harouse JM, Gettie A, Eshetu T, Tan RC, Bohm R, Blanchard J, Baskin G, Cheng-Mayer C. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV (SF162P3) J Virol. 2001;75:1990–5. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hillier SL, Krohn MA, Rabe LK, Klebanoff SJ, Eschenbach DA. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clin Infect Dis. 1993;16:S273–81. doi: 10.1093/clinids/16.supplement_4.s273. [DOI] [PubMed] [Google Scholar]
- 21.Hyman RW, Fukushima M, Diamond L, Kumm J, Giudice LC, Davis RW. Microbes on the human vaginal epithelium. Proc Natl Acad Sci USA. 2005;102:7952–7. doi: 10.1073/pnas.0503236102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacques M, Olson ME, Crichlow AM, Osborne AD, Costerton JW. The normal microflora of the female rabbit’s genital tract. Can J Vet Res. 1986;50:272–4. [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson JL, Phelps CF, Cummins CS, London J, Gasser F. Taxonomy of the Lactobacillus acidophilus group. Int J Syst Bacteriol. 1980;30:53–68. [Google Scholar]
- 24.Langley JM, Halperin SA, Boucher FD, Smith B. Azithromycin is as effective as and better tolerated than erythromycin estolate for the treatment of pertussis. Pediatrics. 2004;114:e96–101. doi: 10.1542/peds.114.1.e96. [DOI] [PubMed] [Google Scholar]
- 25.Lauer E, Helming C, Kandler O. Heterogeneity of the species Lactobacillus acidophilus (Moro) Hansen and Moquot as revealed by biochemical characteristics and DNA–DNA hybridization. Zentralbl Bakteriol Mikrobiol Hyg [A] 1980;1:150–68. [Google Scholar]
- 26.Lederman MM, Veazey RS, Offord R, Mosier DE, Dufour J, Mefford M, Piatak M, Jr, Lifson JD, Salkowitz JR, Rodriguez B, Blauvelt A, Hartley O. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–7. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Lagenaur LA, Lee PP, Xu Q. Engineering human vaginal Lactobacillus to surface expression of two-domain CD4. Appl Environ Microbiol. 2008;74:4626–35. doi: 10.1128/AEM.00104-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X, Lagenaur LA, Simpson DA, Essenmacher KP, Frazier-Parker CL, Liu Y, Tsai D, Rao SS, Hamer DH, Parks TP, Lee PP, Xu Q. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob Agents Chemother. 2006;50:3250–9. doi: 10.1128/AAC.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y, Brosio P, Lafaile M, Li J, Collman RG, Sodroski J, Miller CJ. Vaginal transmission of chimeric simian/human immunodeficiency viruses in rhesus macaques. J Virol. 1996;70:3045–50. doi: 10.1128/jvi.70.5.3045-3050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–8. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 31.Meysick KC, Garber GE. Interactions between Trichomonas vaginalis and vaginal flora in a mouse model. J Parasitol. 1992;78:157–60. [PubMed] [Google Scholar]
- 32.Munson MA, Nedwell DB, Embley TM. Phylogenetic diversity of Archaea in sediment samples from a coastal salt marsh. Appl Environ Microbiol. 1997;63:4729–33. doi: 10.1128/aem.63.12.4729-4733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. 5. Wayne, PA, USA: NCCLS; 2000. Approved Standard M7-A5. [Google Scholar]
- 34.Noguchi K, Tsukumi K, Urano T. Qualitative and quantitative differences in normal vaginal flora of conventionally reared mice, rats, hamsters, rabbits, and dogs. Comp Med. 2003;53:404–12. [PubMed] [Google Scholar]
- 35.Patton DL, Sweeney YC, Rabe LK, Hillier SL. The vaginal microflora of pig-tailed macaques and the effects of chlorhexidine and benzalkonium on this ecosystem. Sex Transm Dis. 1996;23:489–93. doi: 10.1097/00007435-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Poonia B, Walter L, Dufour J, Harrison R, Marx PA, Veazey RS. Cyclic changes in the vaginal epithelium of normal rhesus macaques. J Endocrinol. 2006;190:829–35. doi: 10.1677/joe.1.06873. [DOI] [PubMed] [Google Scholar]
- 37.Pot B, Hertel C, Ludwig W, Descheemaeker P, Kersters K, Schleifer KH. Identification and classification of Lactobacillus acidophilus, L. gasseri and L. johnsonii strains by SDS-PAGE and rRNA-targeted oligonucleotide probe hybridization. J Gen Microbiol. 1993;139:513–7. doi: 10.1099/00221287-139-3-513. [DOI] [PubMed] [Google Scholar]
- 38.Rush CM, Hafner LM, Timms P. Genetic modification of a vaginal strain of Lactobacillus fermentum and its maintenance within the reproductive tract after intravaginal administration. J Med Microbiol. 1994;41:272–8. doi: 10.1099/00222615-41-4-272. [DOI] [PubMed] [Google Scholar]
- 39.Scorpio DG, Ruben DS, Liao Z, Hildreth JE, Fletcher CA. Cervicovaginal evaluation in macaques used as a model for topical microbicide safety studies. J Med Primatol. 2008;37(Suppl 1):65–73. doi: 10.1111/j.1600-0684.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 40.Sha BE, Zariffard MR, Wang QJ, Chen HY, Bremer J, Cohen MH, Spear GT. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis. 2005;191:25–32. doi: 10.1086/426394. [DOI] [PubMed] [Google Scholar]
- 41.Sobel JD, Chaim W. Vaginal microbiology of women with acute recurrent vulvovaginal candidiasis. J Clin Microbiol. 1996;34:2497–9. doi: 10.1128/jcm.34.10.2497-2499.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song YL, Kato N, Matsumiya Y, Liu CX, Kato H, Watanabe K. Identification of and hydrogen peroxide production by fecal and vaginal lactobacilli isolated from Japanese women and newborn infants. J Clin Microbiol. 1999;37:3062–4. doi: 10.1128/jcm.37.9.3062-3064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, Liomba GN, Broadhead RL, Chiphangwi JD, Miotti PG. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS. 1998;12:1699–706. doi: 10.1097/00002030-199813000-00019. [DOI] [PubMed] [Google Scholar]
- 44.Tien D, Schnaare RL, Kang F, Cohl G, McCormick TJ, Moench TR, Doncel G, Watson K, Buckheit RW, Lewis MG, Schwartz J, Douville K, Romano JW. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21:845–53. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- 45.Tsai CC, Emau P, Jiang Y, Agy MB, Shattock RJ, Schmidt A, Morton WR, Gustafson KR, Boyd MR. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res Hum Retroviruses. 2004;20:11–8. doi: 10.1089/088922204322749459. [DOI] [PubMed] [Google Scholar]
- 46.Vasquez A, Jakobsson T, Ahrne S, Forsum U, Molin G. Vaginal Lactobacillus flora of healthy Swedish women. J Clin Microbiol. 2002;40:2746–9. doi: 10.1128/JCM.40.8.2746-2749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veazey RS, Klasse PJ, Ketas TJ, Reeves JD, Piatak M, Jr, Kunstman K, Kuhmann SE, Marx PA, Lifson JD, Dufour J, Mefford M, Pandrea I, Wolinsky SM, Doms RW, DeMartino JA, Siciliano SJ, Lyons K, Springer MS, Moore JP. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J Exp Med. 2003;198:1551–62. doi: 10.1084/jem.20031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veazey RS, Klasse PJ, Schader SM, Hu Q, Ketas TJ, Lu M, Marx PA, Dufour J, Colonno RJ, Shattock RJ, Springer MS, Moore JP. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 49.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–6. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 50.Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004;150:2565–73. doi: 10.1099/mic.0.26905-0. [DOI] [PubMed] [Google Scholar]