Abstract
Background
We aim to identify optimal strategies for deploying pre-exposure prophylaxis among men who have sex with men in the US and Peru to maximize population-level effectiveness in an efficient manner. We use epidemic models to simulate the impact of targeting strategies. Most studies have focused on targeting either the general population or high-risk MSM. Alternative strategies, including serodiscordant couples, may better balance effectiveness and efficiency.
Methods
We use dynamic, stochastic sexual network models based in exponential-family random graph modeling, parameterized from behavioral surveys of MSM in the US and Peru. These models represent main partnerships and casual contacts separately, permitting modeling of interventions targeting men whose risk derives from combinations of relational types. We also model varying rates of uptake and adherence to PrEP. We assess sensitivity of results to risk compensation via increases in condomless casual contacts and condomless sex in main partnerships.
Results
Targeting all men who are not exclusively insertive has the largest impact on HIV incidence, but targeting only those with high levels of casual activity yields comparable results using fewer person-years on PrEP. The effect is robust to risk compensation in the US, but less so in Peru. Targeting serodiscordant main partnerships does not significantly impact incidence, but requires fewer person-years on PrEP per infection averted than other strategies.
Conclusions
PrEP could be effective in reducing new infections at the population level in both settings. Serodiscordant partnerships are an attractive component of a targeting program, but targeting should include other high-risk men.
Introduction
Three decades into the epidemic, HIV in the developed world and much of Latin America remains concentrated among men who have sex with men (MSM) [1-3]. Evidence suggests that incidence is rising among young MSM in the US [4]. While great strides have been made in HIV treatment in that time [5-8], research on biomedical interventions for prevention has hit frequent roadblocks.
One promising intervention for MSM is pre-exposure prophylaxis (PrEP). PrEP has been evaluated for MSM in a randomized controlled trial in six countries, including the US and Peru. The trial showed significant reductions in HIV acquisition (∼44%) for men in the treatment arm, with subgroup analyses suggesting that men with detectable drug levels achieve extremely high rates of protection (∼92%) [9]. PrEP has been found significantly protective in other populations as well [10-13]. Continuing follow-up from PrEP trials shows extremely high protection among those with high adherence [14]. Based on these results, implementation studies and projects are planned or underway [15, 16].
While these results show great promise for PrEP as an intervention to prevent HIV acquisition, it remains to be seen how demonstrated individual-level efficacy will translate to population-level effectiveness. Evaluating the potential population-level impact of PrEP and how targeting, uptake, adherence, and risk compensation can affect this will help guide implementation efforts. A number of PrEP implementation models have appeared recently, examining possible population-level reductions in incidence depending on PrEP efficacy, or for different targeting strategies [17-28]. Most employ compartmental models of HIV transmission and disease progression, the exception being the microsimulation of Hallett et al. [19]. Compartmental models are practically limited in their complexity, as the number of compartments required increases exponentially with the number of variables considered. Microsimulation is a catch-all term for models that track individuals explicitly, as opposed to compartmental models, which are concerned only with rates of movement between states in the population. Ours is a particular type of microsimulation that can account for complex relational structures.
Some of these studies consider MSM in the US [24-28] and one models transmission among MSM and transgender women in Peru [29]. These studies model PrEP targeted to either the general population or high-risk men (with risk almost exclusively defined by the number of partners), with a constant rate of protection. Results are mixed; all find a moderate-to-substantial reduction in incidence, but but the number of men on treatment needed to achieve that reduction varies. In general, when targeting high-risk men only, efficiency is improved, indicating the central importance of identifying optimal targeting strategies.
Risk compensation is a major concern in HIV prevention [30], and various studies have suggested that some compensation occurs with different interventions (see Eaton and Kalichman [31] for a review). Since PrEP is a recent development, risk compensation assessments specific to it are not well established. Behavioral data from PrEP clinical trials participants show little evidence of risk compensation [32-34], in some cases even after unblinding [35]. This may not reflect behavior changes in practice, however. Surveys of MSM in the US assessing likelihood of PrEP use and associated decision-making suggest that a substantial proportion of men would decrease condom use to some extent when taking PrEP, especially if it is known to be highly effective [36, 37].
This paper models the likely population-level impact of large-scale PrEP rollout among urban MSM populations in the US and Peru under several different targeting strategies.
Both countries have HIV epidemics concentrated among MSM. It differs from previous work in using a dynamic, stochastic network model of contacts, described in Goodreau et al. [38], allowing for greater flexibility in the handling of individual attributes, as well as a more explicit accounting for down-stream effects of prevention. We allow for differential protection by adherence level, rather than a single rate of protection for all men on PrEP. This allows us to compare changes in incidence from increasing population coverage by increasing uptake or by increasing adherence of those on PrEP, and thus to assess the relative benefit of expending resources on one or the other.
We also examine a variety of targeting strategies beyond those already considered in the literature. In particular, we represent main partnerships and casual contacts separately, allowing us to model PrEP interventions that target men whose HIV risk derives from different combinations of these relational types. Finally, we consider levels of risk compensation needed in each relationship type to turn the intervention from beneficial to harmful.
Methods
We model the percent of new infections averted due to PrEP roll-out and person-years on PrEP per infection averted using the dynamic, stochastic network models first described in Goodreau et al. [38]. These models perform day-by-day simulations of vital, relational and disease dynamics on populations of initial size 10,000 MSM over 10 years. In general, to assess stochastic variation, we repeat the simulation for each set of inputs ten times.
The MSM in each run of the simulation are characterized by a wide variety of attributes, but two are of particular relevance here: sexual role preference and propensity for condomless (denoted “nc”) anal intercourse in casual relationships (CAI). Sexual role preference can be exclusively insertive, exclusively receptive, or versatile (with an insertivity preference between 0-100%); the proportion of men with each preference varies by country based on country-specific data. Data indicate that the distribution of propensity for ncCAI is highly skewed in both countries. We characterize this by dividing men into quintiles of casual activity, with rates for each quintile estimated from country-specific data.
The network model consists of two components: a main partnership network and a network of casual contacts. Once a relationship is formed in the main partnership network, it has a constant daily probability of dissolution; the resulting geometric distribution for relationship duration allows for both short- and long-term relationships. Within these relationships there is a certain daily probability of CAI. In contrast, the casual contact network is formed independently each day, with no carryover across time (though pairings can repeat with some small probability). The probability of a contact in this network is a function of the propensity of each man to engage in ncCAI, as well as other characteristics.
Treatment status of HIV positive men depends on diagnosis status (only those who are diagnosed positive can start treatment), CD4 count (treatment can only be initiated after reaching a country-specific threshold), and trajectory; however, we did not model additional dependence on partner PrEP use within serodiscordant long-term relationships. We consider three lifetime trajectories: never on treatment, treated but partially suppressed, and treated and fully suppressed. Infectiousness depends on time since infection and treatment status.
We investigate a variety of PrEP roll-out scenarios, each defined by a combination of targeting, uptake, and adherence. Table 1 lists our targeting strategies. The RECEP strategy targets men based on their sexual role; eligibility extends to all negative men who are versatile or exclusively receptive. This broad strategy should optimize population-level PrEP effectiveness, at the expense of efficiency. Other behavior-based strategies condition only on the number of condomless casual AI contacts, regardless of role (so exclusively insertive men are now potentially included). These include targeting men in the top two quintiles of the distribution of numbers of condomless CAI contacts (CAI40), or those in the uppermost fifth quintile only (CAI20). We also consider targeting serodiscordant main partnerships, both alone (MAIN) and in combination with the CAI20 strategy (MAIN+CAI20).
Table 1.
Definitions of targeting strategies.
| Name | Eligibility criteria for intervention | |
|---|---|---|
|
| ||
| Relevant behavioral dimension | Description | |
|
| ||
| RECEP | Sexual role | All men with versatile or exclusively receptive role, i.e. anyone who engages in receptive anal intercourse |
| CAI40 | Casual condomless AI | All men in the top two quintiles (ie 40%) of the distribution of amount of condomless AI with casual partners |
| CAI20 | Casual condomless AI | All men in the top quintile (ie 20%) of the distribution of amount of condomless AI with casual partners |
| MAIN | Main partnership | All negative men in a diagnosed serodiscordant main partnership |
| MAIN+CAI20 | Main partnership or casual condomless AI | All men fulfilling the description of either CAI20 or MAIN; ie highest 20% of casual condomless AI or with serodiscodant main partner |
In addition, we consider different levels of uptake and adherence to PrEP. Defining uptake as accepting at least one prescription, we consider uptake rates of 20%, 40%, and 60%. Men who uptake PrEP are then assigned to one of three adherence categories: negligible, low and high, corresponding to roughly zero, two and four doses/week [39]. We assume that negligible adherence provides no protection, low adherence reduces per-contact risk by 75%, and high adherence by 90%, similar rates to those found by Anderson et al. [39, 40].
Few data are available on likely adherence to PrEP. Studies of antiretroviral treatment adherence in HIV-infected patients suggest that roughly one-quarter to one-third of patients are highly adherent [41-43]. One study showed 50% of patients as completely non-adherent and 25% with low adherence. Other work finds generally low adherence and persistence on medications for chronic conditions, with percentages of patients continuing to fill prescriptions 6 months into treatment ranging from 11-73% for various conditions and medication classes [44, 45]. Together, these results led us to choose a baseline adherence profile of 50-25-25% in the negligible, low, and high categories.
To evaluate the relative benefits of increasing uptake versus adherence, we require a measure that combines both dimensions into a single scale related to expected effectiveness. We use the expected percent reduction in per-contact risk (PRPCR). For example, with 20% uptake, 25% low adherence, and 25% high adherence, the PRPCR is 0.2*(0.75*0.25 + 0.9*0.25)*100 = 8.25%. Note that the percent of cases averted directly may be lower than the PRPCR, due to repeated exposures. However, this measure does not account for indirect effects - infections averted in men not taking PrEP because a partner who would have infected them was protected by PrEP. Thus, the total population effect of a scenario may differ substantially from its PRPCR.
Table 2 lists the PRPCRs for each combination of uptake and adherence we consider, with PRPCR values ranging from 6% to 36%. By design, several of the scenarios have similar or equal PRPCRs; some of these place greater emphasis on uptake (more low-adherent individuals), while others place more emphasis on adherence (more high-adherent individuals).
Table 2.
Adherence scenarios considered, including population percent reduction in risk by uptake level. Values given in first column are percent of uptakers who are non-, partially, and fully adherent. Population percent reduction in risk is defined as the overall per-act reduction in the probability of HIV transmission within the target population.
| Adherence (0-low-high) |
% reduction in per-contact risk (PRPCR) | ||
|---|---|---|---|
| 20% uptake | 40% uptake | 60% uptake | |
| 65-10-25 | 6% | 12% | 18% |
| 50-25-25 | 8% | 17% | 25% |
| 45-30-25 | 9% | 18% | 27% |
| 47-18-35 | 9% | 18% | 27% |
| 25-50-25 | 12% | 24% | 36% |
| 27.5-37.5-35 | 12% | 24% | 36% |
In initial exploratory analyses (results not shown), we found that scenarios with similar PRPCRs yielded qualitatively similar reductions in percent of new infections in all cases, indicating no strong evidence in favor of focusing on either adherence or uptake. Thus, we present average reductions in new infections for each targeting strategy as a function only of PRPCR (for unpooled results see Figures, Supplemental Digital Content 1 & 2). Similarly, linear regression showed that efficiency, as measured by person-years on PrEP per infection averted, was not statistically significantly associated with PRPCR, adherence, or uptake. Thus we also pool these results for each targeting strategy.
Finally, we modeled risk compensation as increases of 50 to 300% in condomless anal intercourse, both within main partnerships and with casual contacts. These increases could result from reductions in condom use, increases in the number of sex acts, or both. In summarizing results, we present the estimated change in incidence under each scenario. To account for stochastic error, we use the t-statistic to evaluate the strength of evidence against the null hypothesis of no net effect of PrEP on incidence, after accounting for risk compensation.
Results
Figure 1 (left panel) gives the average over 10 simulations in the US model of the percent reduction in incident infections compared to no intervention across targeting strategies, plotted against PRPCR. As expected, effectiveness increases more or less monotonically in PRPCR under all targeting strategies. Percent of infections averted ranges from <1% with low uptake and adherence in the MAIN targeting strategy, to ∼50% with high uptake and adherence under the RECEP strategy. The right panel shows person-years on PrEP per infection averted for each targeting strategy. The reduction in infections for the RECEP strategy (targeting 91% of the population in the US or 77% in Peru) is only modestly higher than for CAI40 (where only 40% of the population is targeted). These diminishing returns are reflected in the substantially greater efficiency of CAI40, measured by person-years on PrEP per infection averted. The MAIN strategy, on the other hand, is relatively ineffective in averting infections, with reductions ranging from 2-13%, but relatively efficient.
Figure 1.
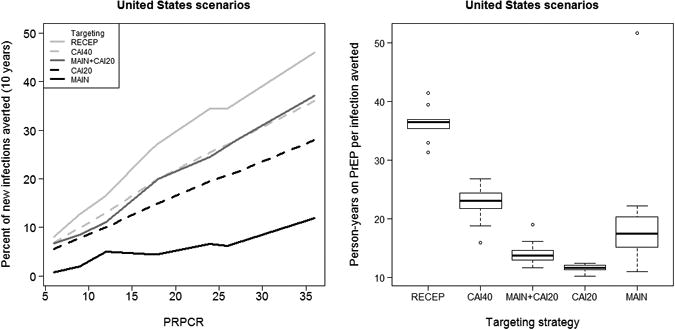
Percent of new infections averted plotted against the population percent reduction in per-contact risk (PRPCR) in the targeted population (L) and person-years on PrEP per infection averted by targeting strategy (R) for scenarios in US.
Figure 1 also shows that the CAI20 strategy is less effective than CAI40, as measured by the percent of infections averted, but with substantially greater efficiency. Indeed, the efficiency for CAI20 is even greater than that for MAIN. Also targeting men in serodiscordant main partnerships (MAIN+CAI20) increases effectiveness to levels comparable to CAI40, while only decreasing efficiency to midway between CAI20 and CAI40.
Combining the MAIN and CAI20 interventions (MAIN+CAI20) results in an overall reduction in incidence that is slightly smaller than the sum of the effects of the two interventions alone. The slight penalty in net effectiveness is likely due to the fact that some men in serodiscordant main partnerships also have many casual CAI contacts, and thus would be targeted by both strategies.
Notably, for the RECEP and CAI40 scenarios, the percent of new infections averted is greater than the PRPCR. For example, in the CAI40 strategy, with 20% uptake and the baseline (50-25-25) adherence profile, PRPCR is only 8.3% among the 40% of men in the targeted population, but incidence is reduced by 12.4%. This occurs despite the attenuation due to repeated exposures, and is attributable to indirect long term effects.
Figure 2 shows analogous results for Peru; they are broadly similar to results in Figure 1 for the US. The CAI20 strategy appears to be somewhat less effective in reducing new infections at the population level than the same strategy in the US. We again see MAIN+CAI20 as a targeting strategy giving similar reductions in incidence to CAI40, but at a level of efficiency more similar to CAI20. Overall, the reductions in incidence for any given scenario are slightly smaller in Peru, and the person-years on PrEP per infection averted are slightly higher, but substantively the conclusions remain the same.
Figure 2.
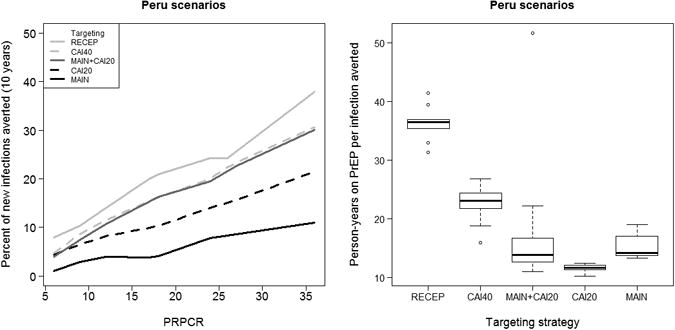
Percent of new infections averted plotted against the population percent reduction in per-contact risk (PRPCR) in the targeted population (L) and person-years on PrEP per infection averted by targeting strategy (R) for scenarios in Peru.
In assessing risk compensation, we focused on the most efficient scenario (MAIN+CAI20), with 40% uptake and an adherence profile of 25-50-25. Figure 3 is a contour plot showing the percent change in the number of new infections over 10 years for each risk compensation scenario for the US. The contour at 0 indicates scenarios where PrEP has no net effect on incidence after accounting for risk compensation, while those with negative values indicate a decrease in incidence on average relative to no PrEP. Darker shading indicates stronger evidence for a net effect, while results in the white region are consistent with no PrEP effect. The figure shows evidence for a beneficial effect of PrEP despite increases of up to 150% in casual CAI for almost any level of increase in CAI in main partnerships, which has a much weaker effect. Significant harm is observed only with 3-fold increases in both. These results suggest that the benefits of PrEP would be quite robust to risk compensation in the US.
Figure 3.
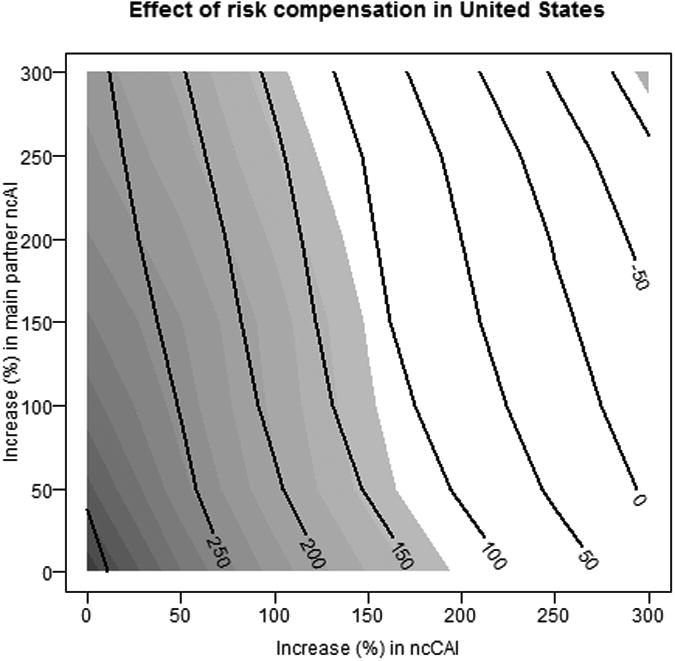
Effects of risk compensation on cases averted in the United States. Contour lines represent the expected percent change in the number of new infections, while the shading indicate the strength of evidence for the effect.
By contrast, the population level impact of PrEP appears to be much more sensitive to risk compensation in Peru. Figure 4 shows that PrEP would be beneficial only if risk compensation were fairly weak, and that significant harm would be expected at much lower levels of risk compensation than in the United States.
Figure 4.
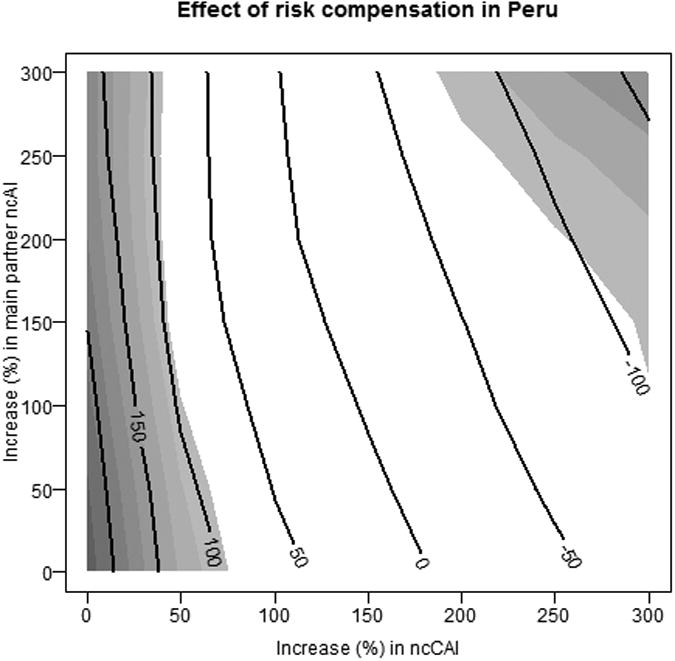
Effects of risk compensation on cases averted in Peru. Contour lines represent the expected percent change in the number of new infections, while the shading indicate the strength of evidence for the effect.
Discussion
The results of our dynamic stochastic modeling show that PrEP has the potential to be a highly effective tool in the fight against HIV among MSM in the Americas, in keeping with results of previous modeling work [17-28]. Consistent with analyses of the iPrEX trial [46], we find that targeting all men who may engage in condomless receptive anal intercourse results in the greatest population-level effect, but more focused approaches yield greater efficiency. Targeting PrEP to men with the highest level of condomless casual sex (approximately 1.5 to 7 contacts per month) could lead to as much as a 30% reduction in new infections over ten years. In general, targeting men with high rates of casual ncAI is much more efficient than targeting all versatile or receptive men, without much loss of effectiveness population-wide.
This work goes beyond earlier modeling efforts in the breadth of targeting strategies considered. We explicitly consider the role of main partnerships in mediating the effectiveness of PrEP at a population level, a contribution thus far neglected in the literature. Targeting serodiscordant couples alone is insufficient to substantially affect the course of the HIV epidemic. It is, however, an important tool for serodiscordant couples desiring to protect the negative partner, and could be a useful component of a composite targeting strategy, as it increases the overall efficiency of a wider roll-out because of the low person-years on PrEP required per infection averted.
Using a model that includes sustained partnerships also allows us to measure downstream effects of PrEP – new infections averted by preventing the infection of individuals farther down the transmission chain from the person taking PrEP. One of the most remarkable results found here is that targeting versatile and exclusively receptive men or men with the top 40% of casual ncAI rates yields a greater percentage reduction in incidence in the full population than the percent reduction in per-contact risk in the target population (a conservative estimate of the direct effect of taking PrEP), indicating substantial downstream effects. This points to PrEP as a valuable public health intervention – its use protects not only those taking medication, but also the population as a whole.
One limitation of our work is that we do not explicitly consider the treatment status of the positive partner in our main partnership interventions. As in the baseline model [38], most men initiate treatment at some point after diagnosis, with variation in adherence, and thus in viral load and transmissibility. Thus, some men on PrEP will have partners who are on treatment, either with or without full suppression. Future work will consider the relative merits of treatment as prevention and PrEP in main partnerships and optimal combinations of treatment protocols.
Our results provide evidence of the beneficial effects of both adherence support and expanding PrEP access to improve population-level outcomes, with similar benefits from either approach, assuming equal reductions in average per-contact risk. This equality may not be realistic if the two approaches differ in their potential effects or cost-effectiveness. Moreover, our model ignores drug resistance. If low adherence contributes to increased prevalence of drug resistance, then it would be beneficial to increase adherence among those taking PrEP rather than getting more men to take PrEP at lower levels of adherence, given the relative equivalence in terms of effectiveness.
The impact of PrEP appears to be robust to risk compensation in the United States, but less so in Peru. There are several reasons why we might see this pattern. Our source data suggest greater heterogeneity in casual CAI risks among US MSM, with more men with very low and with very high rates of ncAI than in Peru. As a result, the mean number of condomless casual contacts per year for men in the highest quintiles of activity is about 10% higher in the US than in Peru (52 ncCAI contact per year in the US, compared to 47 in Peru). This could contribute to the increased sensitivity of results for the Peru model to risk compensation: additional partners will add proportionately more risk at lower base partner counts. Also, although the proportion of men in a main partnership at any moment in time is qualitatively similar in the US and Peru (40.0% and 42.5%, respectively), Peruvian men report considerably shorter main partnership durations and less frequent ncAI within those partnerships. This leads to a greater influence of increased main partner ncAI in Peru relative to the US. For a more detailed exposition of similarities and differences between the two populations, please see Goodreau et al. [38] and Beyrer et al. [2].
We have not conducted a cost-effectiveness analysis, since we do not calculate costs of treatment. Thus, we cannot say whether the targeting strategies analyzed here are cost-effective (or cost-saving). We can, however, say which strategies should lead to the lowest required person-years on PrEP per infection averted. By this metric, jointly targeting the men with the highest rates of casual CAI (6-7 contacts per month) and those in serodiscordant main partnerships (particularly if the partner is not virally suppressed) will be the most efficient approach. This public health approach does not address the provider-patient decision-making that will address the health of the individual, rather than the community.
Our results for risk compensation suggest that strategies to reduce risk compensation will be critical to the success of PrEP in settings like Peru, whereas PrEP is likely to be beneficial in the US even under significant risk compensation. Recall, however, that the lowest level of risk compensation considered is a 50% increase in overall risk, and even in Peru, there is still a significant reduction in incidence at this level. Surveys of US MSM suggest that a significant proportion of men (30 to 80%) would not change condom use behaviors while using PrEP [36, 37]. A meta-analysis of the relationship of beliefs about the effectiveness of HAART and HIV transmission found only a 29% increase in the median prevalence of condomless sex, from 38% to 49%, for people who believed that HAART reduced or eliminated the transmission of HIV [47]. While none of these results directly inform the likely amount of risk compensation with actual PrEP rollout, they are suggestive that the reality would be within the range where PrEP is effective both in Peru and the US.
Supplementary Material
Acknowledgments
This work is supported by NIH grants R01-AI083060, R01-AI51164 and R01-HD068395 from the National Institute of Allergy and Infectious Disease (NIAID), the National Institute of Mental Health (NIMH), and the National Institute of Child Health and Human Development (NICHD), National Institutes of Health (NIH).
Contributor Information
Nicole Bohme Carnegie, Harvard School of Public Health.
Steven M. Goodreau, University of Washington.
Albert Liu, San Francisco Department of Public Health.
Eric Vittinghoff, University of California San Francisco.
Jorge Sanchez, Asociación Civil Impacta Salud y Educación.
Javier R. Lama, Asociación Civil Impacta Salud y Educación.
Susan Buchbinder, San Francisco Department of Public Health.
References
- 1.Beyrer C, Baral SD, Walker D, et al. The expanding epidemics of HIV type 1 among men who have sex with men in low-and middle-income countries: diversity and consistency. Epidemiologic Reviews. 2010;32(1):137–151. doi: 10.1093/epirev/mxq011. [DOI] [PubMed] [Google Scholar]
- 2.Beyrer C, Baral SD, van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. The Lancet. 2012;380(9839):367–377. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips AN, Cambiano V, Nakagawa F, et al. Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: analysis of an extensively documented epidemic. PLoS ONE. 2013;8(2):e55312. doi: 10.1371/journal.pone.0055312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006-2009. PLoS ONE. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the United States. Journal of Infectious Diseases. 2006;194(1):11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 6.PLoS Medicine Editors et al. HIV treatment proceeds as prevention research confounds. PLoS Medicine. 2007;4(12):e347. doi: 10.1371/journal.pmed.0040347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Research. 2010;85:1–18. doi: 10.1016/j.antiviral.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UNAIDS. UNAIDS report on the global AIDS epidemic. [Accessed: 2014-01-30];2013 http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS.
- 9.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. New England Journal of Medicine. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. New England Journal of Medicine. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 2013;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 13.Thigpen MC, Kebaabetswe PM, Smith DK, et al. Daily oral antiretroviral use for the prevention of HIV infection in heterosexually active young adults in Botswana: results from the TDF2 study. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention. 2011:17–20. [Google Scholar]
- 14.Grant RM, Anderson PL, McMahan V, et al. Results of the iPrEx open-label extension (iPrEx OLE) in men and transgener women who have sex with men: PrEP uptake, sexual practices, and HIV incidence. XX International AIDS Conference; Melbourne, Australia. 2014. [Google Scholar]
- 15.Baeten JM, Haberer JE, Liu AY, et al. Preexposure prophylaxis for HIV prevention: where have we been and where are we going? Journal of Acquired Immune Deficiency Syndromes. 2013;63:S122–S129. doi: 10.1097/QAI.0b013e3182986f69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beyrer C. Strategies to manage the HIV epidemic in gay, bisexual, and other men who have sex with men. Current opinion in infectious diseases. 2014;27(1):1–8. doi: 10.1097/QCO.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 17.Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral chemoprophylaxis on HIV-1 transmission in resource-limited settings. PLoS ONE. 2007;2(9):e875. doi: 10.1371/journal.pone.0000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbas UL, Hood G, Wetzel AW, et al. Factors influencing the emergence and spread of HIV drug resistance arising from rollout of antiretroviral pre-exposure prophylaxis (PrEP) PLoS ONE. 2011;6(4):e18165. doi: 10.1371/journal.pone.0018165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallett TB, Baeten JM, Heffron R, et al. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study. PLoS Medicine. 2011;8(11):e1001123. doi: 10.1371/journal.pmed.1001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pretorius C, Stover J, Bollinger L, et al. Evaluating the cost-effectiveness of pre-exposure prophylaxis (PrEP) and its impact on HIV-1 transmission in South Africa. PLoS ONE. 2010;5(11):e13646. doi: 10.1371/journal.pone.0013646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Supervie V, Barrett M, Kahn JS, et al. Modeling dynamic interactions between pre-exposure prophylaxis interventions and treatment programs: predicting HIV transmission and resistance. Scientific Reports. 2011;1:185. doi: 10.1038/srep00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Vijver DAM, Derdelinckx I, Boucher CAB. Circulating HIV type 1 drug resistance will have limited impact on the effectiveness of preexposure prophylaxis among young women in Zimbabwe. Journal of Infectious Diseases. 2009;199:1310–1317. doi: 10.1086/597804. [DOI] [PubMed] [Google Scholar]
- 23.Vissers DCJ, Voeten HACM, Nagelkerke NJD, et al. The impact of pre-exposure prophylaxis (PrEP) on HIV epidemics in Africa and India: a simulation study. PLoS ONE. 2008;3(5):e2077. doi: 10.1371/journal.pone.0002077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai K, Sansom SL, Ackers ML, et al. Modeling the impact of HIV chemoprophylaxis strategies among men who have sex with men in the United States: HIV infections prevented and cost-effectiveness. AIDS. 2008;22(1):1–11. doi: 10.1097/QAD.0b013e32830e00f5. [DOI] [PubMed] [Google Scholar]
- 25.Koppenhaver RT, Sorensen SW, Farnham PG, et al. The cost-effectiveness of pre-exposure prophylaxis in men who have sex with men in the United States: an epidemic model. Journal of Acquired Immune Deficiency Syndromes. 2011;58(2):e51–e52. doi: 10.1097/QAI.0b013e31822b74fe. [DOI] [PubMed] [Google Scholar]
- 26.Paltiel AD, Freedberg KA, Scott CA, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clinical Infectious Diseases. 2009;48:806–815. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Supervie V, Garcia-Lerma JG, Heneine W, et al. HIV, transmitted drug resistance and the paradox of preexposure prophylaxis. Proceedings of the National Academy of Sciences. 2010;107(27):12381–12386. doi: 10.1073/pnas.1006061107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juusola JL, Brandeau ML, Owens DK, Bendavid Eran. The cost-effectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Annals of Internal Medicine. 2012;156:541–550. doi: 10.1059/0003-4819-156-8-201204170-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez GB, Borquez A, Caceres CF, et al. The potential impact of pre-exposure prophylaxis for HIV prevention among men who have sex with men and transwomen in Lima, Peru: a mathematical modelling study. PLoS Medicine. 2012;9(10):e1001323. doi: 10.1371/journal.pmed.1001323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cassell MM, Halperin DT, Shelton JD, et al. Risk compensation: the Achilles' heel of innovations in HIV prevention? British Medical Journal. 2006;332:605–607. doi: 10.1136/bmj.332.7541.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eaton LA, Kalichman SC. Risk compensation in HIV prevention: Implications for vaccines, microbicides, and other biomedical HIV prevention technologies. Current HIV/AIDS Reports. 2007;4:165–172. doi: 10.1007/s11904-007-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guest G, Shattuck D, Johnson L, et al. Changes in sexual risk behavior among participants in a PrEP HIV prevention trial. Sexually Transmitted Diseases. 2008;35(12):1002–1008. [PubMed] [Google Scholar]
- 33.Liu AY, Vittinghoff E, Chillag K, et al. Sexual risk behavior among HIV-uninfected men who have sex with men (MSM) participating in a tenofovir pre-exposure prophylaxis (PrEP) randomized trial in the United States. Journal of Acquired Immune Deficiency Syndromes. 2013;64(1):87–94. doi: 10.1097/QAI.0b013e31828f097a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcus JL, Glidden DV, Mayer KH, et al. No evidence of sexual risk compensation in the iPrEx trial of daily oral HIV preexposure prophylaxis. PLoS ONE. 2013;8(12):e81997. doi: 10.1371/journal.pone.0081997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mugwanya KK, Donnell D, Celum C, et al. Sexual behaviour of heterosexual men and women receiving antiretroviral pre-exposure prophylaxis for HIV prevention: a longitudinal analysis. The Lancet Infectious Diseases. 2013;13(12):1021–1028. doi: 10.1016/S1473-3099(13)70226-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Golub SA, Kowalczyk W, Weinberger CL, et al. Preexposure prophylaxis and predicted condom use among high-risk men who have sex with men. Journal of Acquired Immune Deficiency Syndromes. 2010;54(5):548–555. doi: 10.1097/QAI.0b013e3181e19a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koblin BA, Mansergh G, Frye V, et al. Condom-use decision making in the context of hypothetical pre-exposure prophylaxis efficacy among substance-using men who have sex with men: Project MIX. Journal of Acquired Immune Deficiency Syndromes. 2011;58(3):319–327. doi: 10.1097/QAI.0b013e31822b76d2. [DOI] [PubMed] [Google Scholar]
- 38.Goodreau SM, Carnegie NB, Vittinghoff E, et al. What drives the US and Peruvian HIV epidemics in men who have sex with men (MSM)? PLoS ONE. 2012;7(11):e50522. doi: 10.1371/journal.pone.0050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and preexposure prophylaxis efficacy in men who have sex with men. Science Translational Medicine. 2012;4(151):151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson PL, Liu A, Buchbinder S, et al. Intracellular tenofovir-diphosphate (TFV-DP) concentrations associated with PrEP efficacy in men who have sex with men (MSM) from iPrEx. 19th Conference on Retroviruses and Opportunistic Infections. 2012:5–8. [Google Scholar]
- 41.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Annals of Internal Medicine. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 42.Hinkin CH, Hardy DJ, Mason KI, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004;18(Suppl 1):S19–S25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 44.Yeaw J, Benner JS, Walt JG, et al. Comparing adherence and persistence across 6 chronic medication classes. Journal of Managed Care Pharmacy. 2009;15(9):724–736. doi: 10.18553/jmcp.2009.15.9.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantrell CR, Priest JL, Cook CL, et al. Adherence to treatment guidelines and therapeutic regimens: a US claims-based benchmark of a commercial population. Population Health Management. 2011;14(1):33–41. doi: 10.1089/pop.2010.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buchbinder SP, Glidden DV, Liu AY, et al. HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial. Lancet Infectious Diseases. 2014;14(6):468–475. doi: 10.1016/S1473-3099(14)70025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior. The Journal of the American Medical Association. 2004;92(2):224–236. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


