Abstract
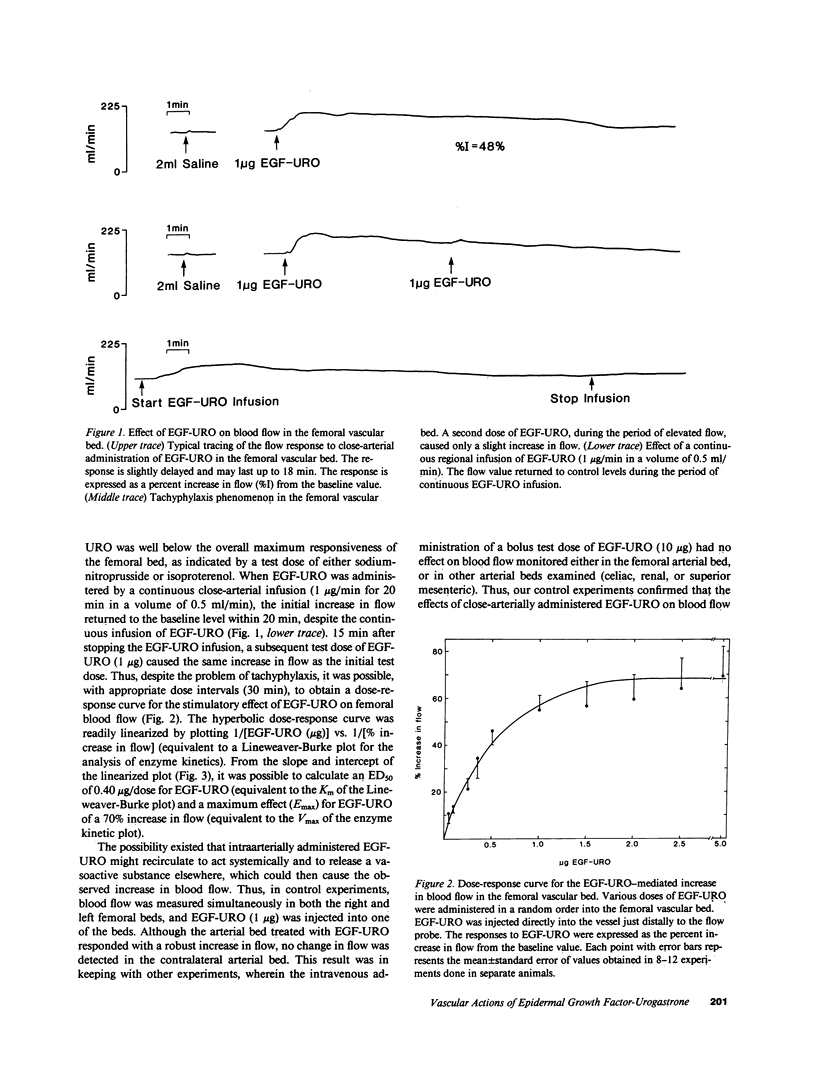
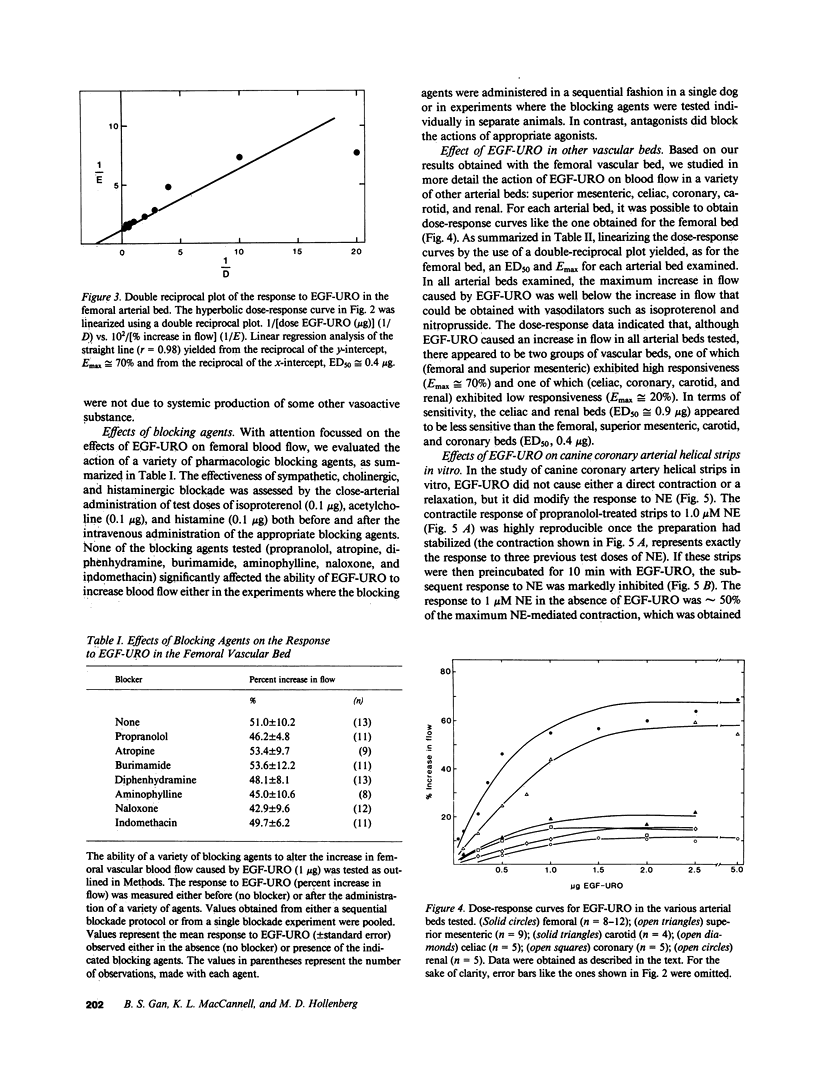
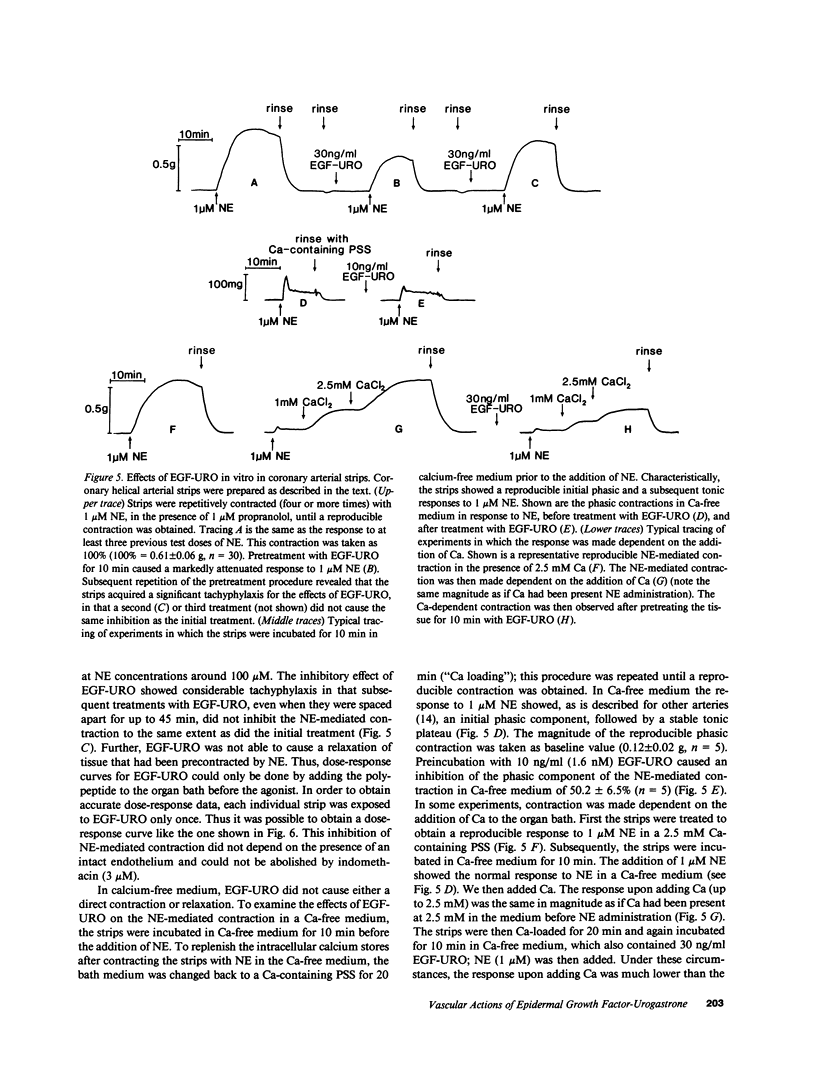
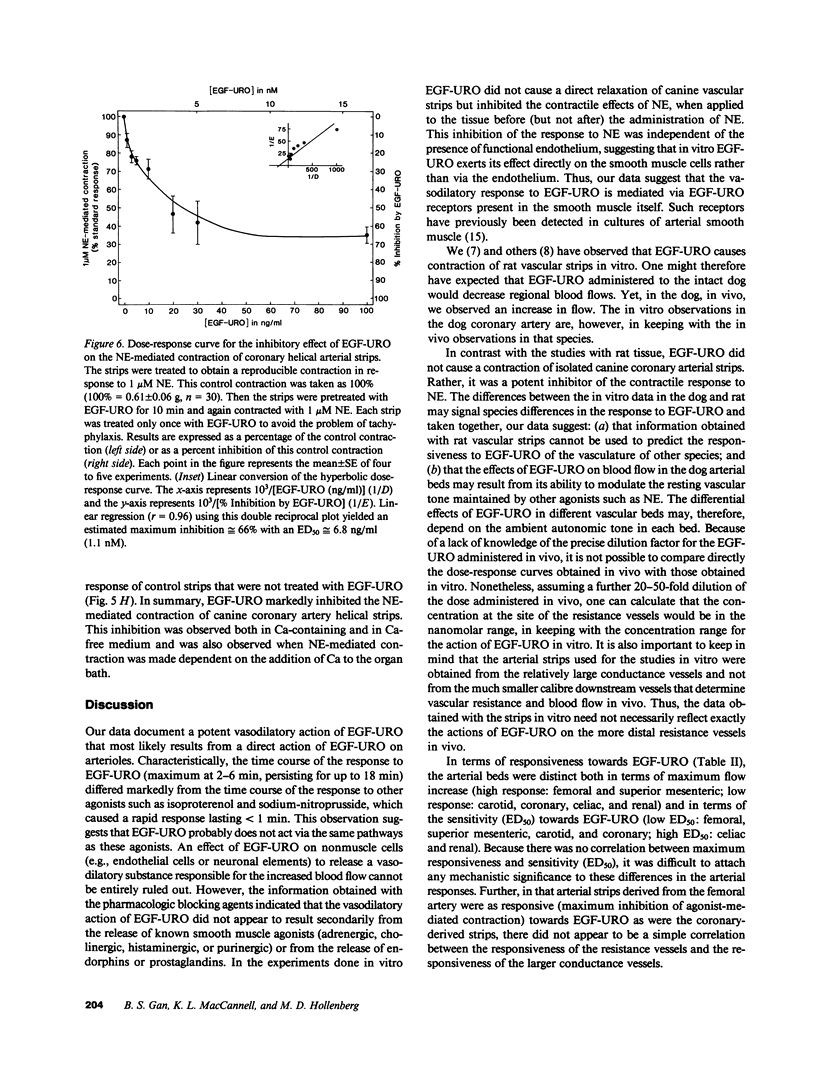
Epidermal growth factor-urogastrone (EGF-URO) administered intraarterially was a potent dilator in dog femoral (FEM), superior (cephalic) mesenteric (SMA), celiac (CAC), coronary (COR), carotid (CAR), and renal (REN) vascular beds. The effects of EGF-URO, which exhibited tachyphylaxis, could not be attributed either to recirculating EGF-URO or to the secondary release of other agonists or products of the cyclooxygenase pathway. Two vascular beds (FEM, SMA) showed a high maximum responsiveness to EGF-URO (maximum effect [Emax] approximately equal to 70% increase in flow) whereas another group (CAC, COR, CAR, and REN) exhibited lower responsiveness (Emax approximately equal to 20%). The ED50 for this effect of EGF-URO was in the range of 0.4 micrograms (FEM, SMA, CAR, and COR) to 0.9 micrograms (CAC and REN). In isolated dog COR helical strips, EGF-URO did not exhibit either a direct relaxing or a contractile effect. However, preincubation of strips with EGF-URO caused up to a 66% inhibition of contraction in response to norepinephrine (1 microM), with an ED50 for EGF-URO of 1 nM. This action of EGF-URO also showed marked tachyphylaxis. Our data point to a potential role for EGF-URO (and possibly for the structurally related alpha-transforming growth factor) in the regulation of blood flow in vivo.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk B. C., Alexander R. W., Brock T. A., Gimbrone M. A., Jr, Webb R. C. Vasoconstriction: a new activity for platelet-derived growth factor. Science. 1986 Apr 4;232(4746):87–90. doi: 10.1126/science.3485309. [DOI] [PubMed] [Google Scholar]
- Berk B. C., Brock T. A., Webb R. C., Taubman M. B., Atkinson W. J., Gimbrone M. A., Jr, Alexander R. W. Epidermal growth factor, a vascular smooth muscle mitogen, induces rat aortic contraction. J Clin Invest. 1985 Mar;75(3):1083–1086. doi: 10.1172/JCI111772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava G., Rifas L., Makman M. H. Presence of epidermal growth factor receptors and influence of epidermal growth factor on proliferation and aging in cultured smooth muscle cells. J Cell Physiol. 1979 Aug;100(2):365–374. doi: 10.1002/jcp.1041000217. [DOI] [PubMed] [Google Scholar]
- Byyny R. L., Orth D. N., Cohen S., Doyne E. S. Epidermal growth factor: effects of androgens and adrenergic agents. Endocrinology. 1974 Sep;95(3):776–782. doi: 10.1210/endo-95-3-776. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Zendegui J. G. Epidermal growth factor, its receptor, and related proteins. Exp Cell Res. 1986 May;164(1):1–10. doi: 10.1016/0014-4827(86)90449-0. [DOI] [PubMed] [Google Scholar]
- Chiba T., Hirata Y., Taminato T., Kadowaki S., Matsukura S., Fujita T. Epidermal growth factor stimulates prostaglandin E release from isolated perfused rat stomach. Biochem Biophys Res Commun. 1982 Mar 15;105(1):370–374. doi: 10.1016/s0006-291x(82)80054-5. [DOI] [PubMed] [Google Scholar]
- Chinkers M., McKanna J. A., Cohen S. Rapid rounding of human epidermoid carcinoma cells A-431 induced by epidermal growth factor. J Cell Biol. 1981 Feb;88(2):422–429. doi: 10.1083/jcb.88.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto K., Cossu M. F., Maeno T., Edwards C., Oka T. Involvement of the Ca2+-dependent K+ channel activity in the hyperpolarizing response induced by epidermal growth factor in mammary epithelial cells. FEBS Lett. 1986 Jul 28;203(2):181–184. doi: 10.1016/0014-5793(86)80738-4. [DOI] [PubMed] [Google Scholar]
- Goustin A. S., Leof E. B., Shipley G. D., Moses H. L. Growth factors and cancer. Cancer Res. 1986 Mar;46(3):1015–1029. [PubMed] [Google Scholar]
- Gregory H., Walsh S., Hopkins C. R. The identification of urogastrone in serum, saliva, and gastric juice. Gastroenterology. 1979 Aug;77(2):313–318. [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D. Epidermal growth factor-urogastrone, a polypeptide acquiring hormonal status. Vitam Horm. 1979;37:69–110. doi: 10.1016/s0083-6729(08)61068-7. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Gregory H. Epidermal growth factor-urogastrone: biological activity and receptor binding of derivatives. Mol Pharmacol. 1980 May;17(3):314–320. [PubMed] [Google Scholar]
- Lopez-Rivas A., Rozengurt E. Serum rapidly mobilizes calcium from an intracellular pool in quiescent fibroblastic cells. Biochem Biophys Res Commun. 1983 Jul 18;114(1):240–247. doi: 10.1016/0006-291x(83)91619-4. [DOI] [PubMed] [Google Scholar]
- MacCannell K. L., Hamilton P. L., Lederis K., Newton C. A., Rivier J. Corticotropin releasing factor-like peptides produce selective dilatation of the dog mesenteric circulation. Gastroenterology. 1984 Jul;87(1):94–102. [PubMed] [Google Scholar]
- MacCannell K., Lederis K. Dilatation of the mesenteric vascular bed of the dog produced by a peptide, urotensin I. J Pharmacol Exp Ther. 1977 Oct;203(1):38–46. [PubMed] [Google Scholar]
- Macara I. G. Activation of 45Ca2+ influx and 22Na+/H+ exchange by epidermal growth factor and vanadate in A431 cells is independent of phosphatidylinositol turnover and is inhibited by phorbol ester and diacylglycerol. J Biol Chem. 1986 Jul 15;261(20):9321–9327. [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Growth factors immediately raise cytoplasmic free Ca2+ in human fibroblasts. J Biol Chem. 1984 Jul 10;259(13):8066–8069. [PubMed] [Google Scholar]
- Morris J. D., Metcalfe J. C., Smith G. A., Hesketh T. R., Taylor M. V. Some mitogens cause rapid increases in free calcium in fibroblasts. FEBS Lett. 1984 Apr 24;169(2):189–193. doi: 10.1016/0014-5793(84)80316-6. [DOI] [PubMed] [Google Scholar]
- Muramatsu I., Hollenberg M. D., Lederis K. Modulation by epidermal growth factor--urogastrone of contraction in isolated canine helical mesenteric arterial strips. Can J Physiol Pharmacol. 1986 Dec;64(12):1561–1565. doi: 10.1139/y86-262. [DOI] [PubMed] [Google Scholar]
- Muramatsu I., Hollenberg M. D., Lederis K. Vascular actions of epidermal growth factor-urogastrone: possible relationship to prostaglandin production. Can J Physiol Pharmacol. 1985 Aug;63(8):994–999. doi: 10.1139/y85-164. [DOI] [PubMed] [Google Scholar]
- Oka Y., Orth D. N. Human plasma epidermal growth factor/beta-urogastrone is associated with blood platelets. J Clin Invest. 1983 Jul;72(1):249–259. doi: 10.1172/JCI110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen N. E., Villereal M. L. Efflux of 45Ca2+ from human fibroblasts in response to serum or growth factors. J Cell Physiol. 1983 Oct;117(1):23–29. doi: 10.1002/jcp.1041170105. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Sawyer S. T., Cohen S. Enhancement of calcium uptake and phosphatidylinositol turnover by epidermal growth factor in A-431 cells. Biochemistry. 1981 Oct 13;20(21):6280–6286. doi: 10.1021/bi00524a057. [DOI] [PubMed] [Google Scholar]
- Shupnik M. A., Tashjian A. H., Jr Epidermal growth factor and phorbol ester actions on human osteosarcoma cells. Characterization of response and nonresponsive cell lines. J Biol Chem. 1982 Oct 25;257(20):12161–12164. [PubMed] [Google Scholar]
- Stoscheck C. M., King L. E., Jr Role of epidermal growth factor in carcinogenesis. Cancer Res. 1986 Mar;46(3):1030–1037. [PubMed] [Google Scholar]
- Vanhoutte P. M., Rubanyi G. M., Miller V. M., Houston D. S. Modulation of vascular smooth muscle contraction by the endothelium. Annu Rev Physiol. 1986;48:307–320. doi: 10.1146/annurev.ph.48.030186.001515. [DOI] [PubMed] [Google Scholar]
- Voelkel E. F., Tashjian A. H., Jr, Levine L. Cyclooxygenase products of arachidonic acid metabolism by mouse bone in organ culture. Biochim Biophys Acta. 1980 Dec 5;620(3):418–428. doi: 10.1016/0005-2760(80)90133-2. [DOI] [PubMed] [Google Scholar]



