Abstract
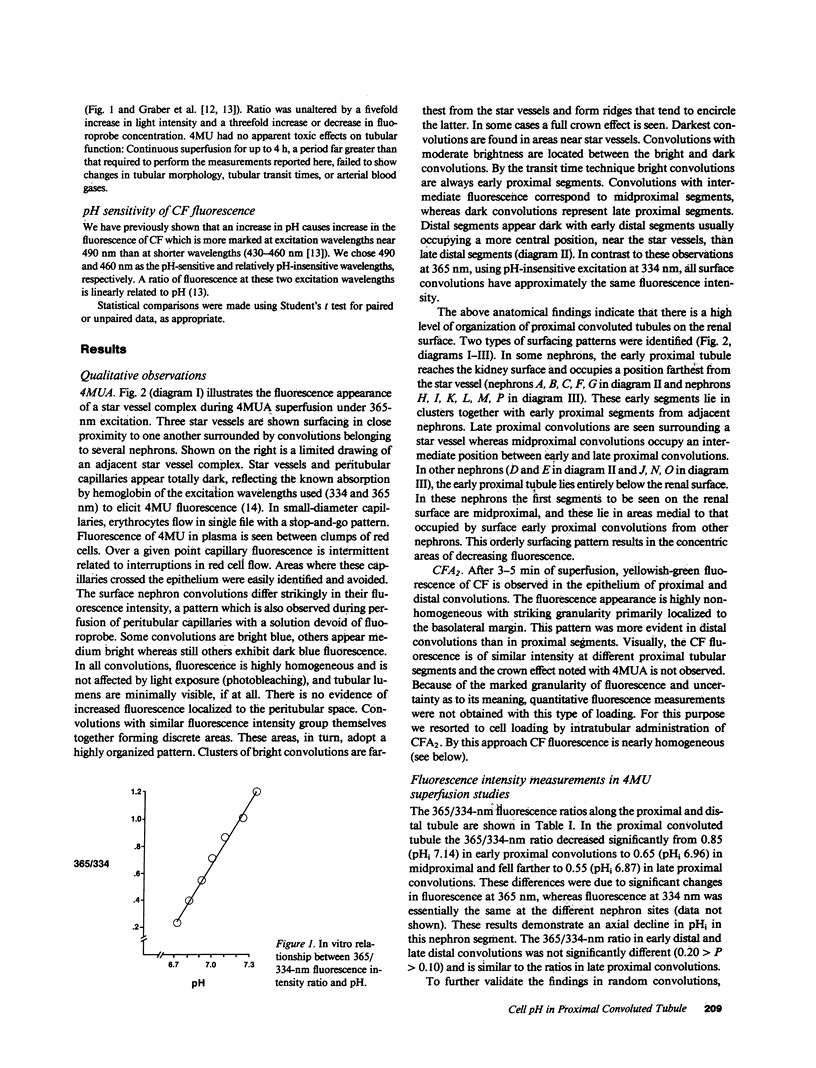
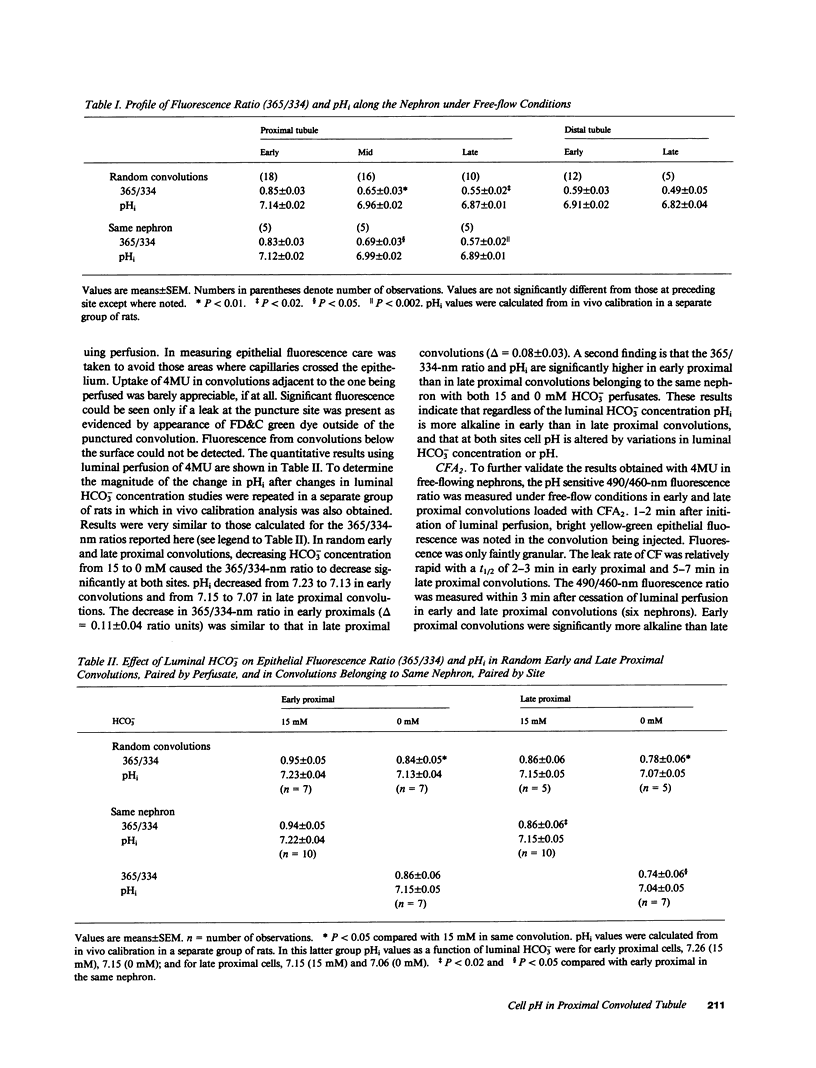
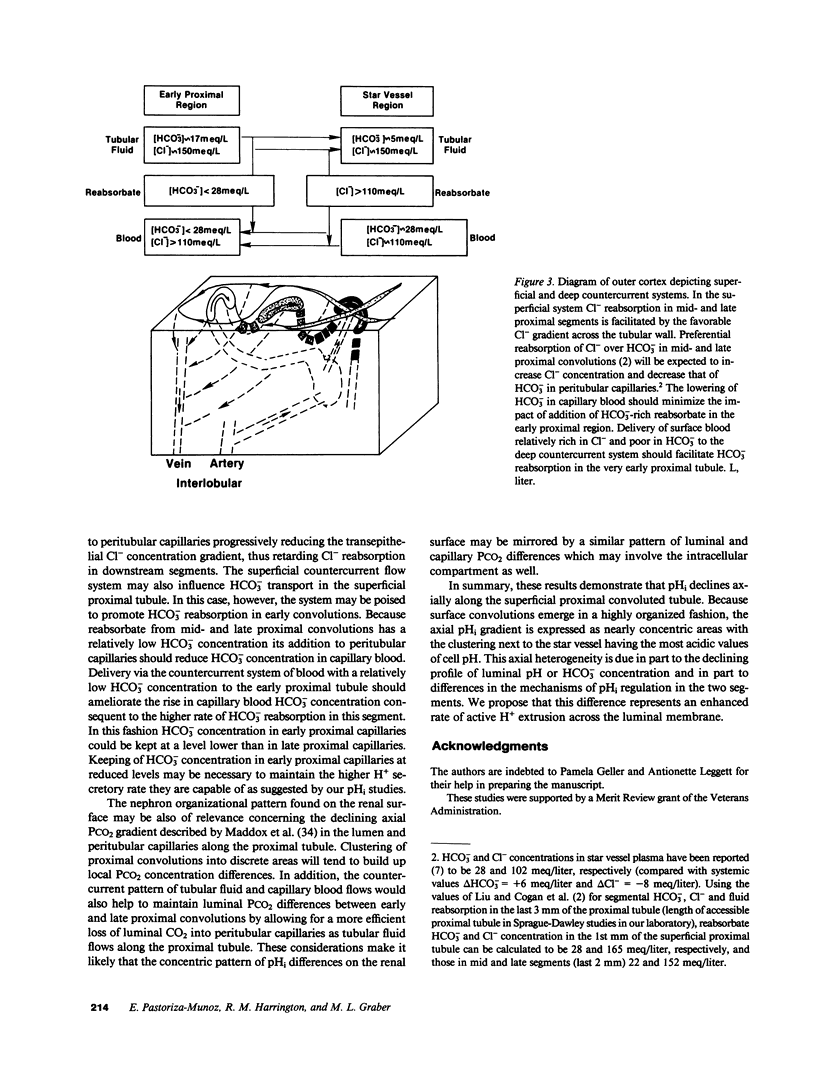
In the proximal convoluted tubule (PT), the HCO3- reabsorptive rate is higher in early (EPS) compared with late proximal segments (LPS). To examine the mechanism of this HCO3- reabsorption profile, intracellular pH (pHi) was measured along the superficial PT of the rat under free-flow and stationary microperfusion using the pH-sensitive fluorescence of 4-methylumbelliferone (4MU). With 4MU superfusion, pHi was found to decline along the PT. Observation with 365-nm excitation revealed that EPS were brightly fluorescent and always emerged away from their star vessel. Midproximal segments were darker and closer to the star vessel which was surrounded by the darkest LPS. Decreasing luminal HCO3- from 15 to 0 mM lowered pHi in both EPS and LPS, but pHi remained more alkaline in EPS with both perfusates. Thus the axial decline in pHi along the PT is due to both luminal factors and intrinsic differences in luminal H+ extrusion in PT cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern R. J., Chambers M. Cell pH in the rat proximal convoluted tubule. Regulation by luminal and peritubular pH and sodium concentration. J Clin Invest. 1986 Aug;78(2):502–510. doi: 10.1172/JCI112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J., Cogan M. G., Rector F. C., Jr Effect of luminal bicarbonate concentration on proximal acidification in the rat. Am J Physiol. 1982 Jul;243(1):F53–F59. doi: 10.1152/ajprenal.1982.243.1.F53. [DOI] [PubMed] [Google Scholar]
- Alpern R. J., Cogan M. G., Rector F. C., Jr Flow dependence of proximal tubular bicarbonate absorption. Am J Physiol. 1983 Oct;245(4):F478–F484. doi: 10.1152/ajprenal.1983.245.4.F478. [DOI] [PubMed] [Google Scholar]
- Alpern R. J. Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985 Nov;86(5):613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. E., Michenfelder J. D., Sundt T. M., Jr Brain intracellular pH, blood flow, and blood--brain barrier differences with barbiturate and halothane anesthesia in the cat. Anesthesiology. 1980 Mar;52(3):201–206. doi: 10.1097/00000542-198003000-00002. [DOI] [PubMed] [Google Scholar]
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Bank N., Aynedjian H. S., Mutz B. F. Evidence for a DCCD-sensitive component of proximal bicarbonate reabsorption. Am J Physiol. 1985 Nov;249(5 Pt 2):F636–F644. doi: 10.1152/ajprenal.1985.249.5.F636. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Sackin H. Measurement of intracellular ionic composition and activities in renal tubules. Annu Rev Physiol. 1983;45:483–496. doi: 10.1146/annurev.ph.45.030183.002411. [DOI] [PubMed] [Google Scholar]
- Cogan M. G., Alpern R. J. Regulation of proximal bicarbonate reabsorption. Am J Physiol. 1984 Sep;247(3 Pt 2):F387–F395. doi: 10.1152/ajprenal.1984.247.3.F387. [DOI] [PubMed] [Google Scholar]
- DuBose T. D., Jr, Pucacco L. R., Lucci M. S., Carter N. W. Micropuncture determination of pH, PCO2, and total CO2 concentration in accessible structures of the rat renal cortex. J Clin Invest. 1979 Aug;64(2):476–482. doi: 10.1172/JCI109485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz K. H., Mangos J. A., Braun G., Pagel H. D. Pressure in the glomerular capillaries of the rat kidney and its relation to arterial blood pressure. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;288(4):369–374. doi: 10.1007/BF00362581. [DOI] [PubMed] [Google Scholar]
- Graber M. L., DiLillo D. C., Friedman B. L., Pastoriza-Munoz E. Characteristics of fluoroprobes for measuring intracellular pH. Anal Biochem. 1986 Jul;156(1):202–212. doi: 10.1016/0003-2697(86)90174-0. [DOI] [PubMed] [Google Scholar]
- Graber M. L., Dixon T. E., Coachman D., Herring K., Ruenes A., Gardner T., Pastoriza-Munoz E. Fluorescence identifies an alkaline cell in turtle urinary bladder. Am J Physiol. 1986 Jan;250(1 Pt 2):F159–F168. doi: 10.1152/ajprenal.1986.250.1.F159. [DOI] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Properties of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1980 Jun;238(6):F461–F469. doi: 10.1152/ajprenal.1980.238.6.F461. [DOI] [PubMed] [Google Scholar]
- Liu F. Y., Cogan M. G. Axial heterogeneity in the rat proximal convoluted tubule. I. Bicarbonate, chloride, and water transport. Am J Physiol. 1984 Nov;247(5 Pt 2):F816–F821. doi: 10.1152/ajprenal.1984.247.5.F816. [DOI] [PubMed] [Google Scholar]
- Maddox D. A., Gennari F. J. Load dependence of HCO3 and H2O reabsorption in the early proximal tubule of the Munich-Wistar rat. Am J Physiol. 1985 Jan;248(1 Pt 2):F113–F121. doi: 10.1152/ajprenal.1985.248.1.F113. [DOI] [PubMed] [Google Scholar]
- Malnic G., de Mello-Aires M. Kinetic study of bicarbonate reabsorption in proximal tubule of the rat. Am J Physiol. 1971 Jun;220(6):1759–1767. doi: 10.1152/ajplegacy.1971.220.6.1759. [DOI] [PubMed] [Google Scholar]
- Pastoriza-Muñoz E., Colindres R. E., Lassiter W. E., Lechene C. Effect of parathyroid hormone on phosphate reabsorption in rat distal convolution. Am J Physiol. 1978 Oct;235(4):F321–F330. doi: 10.1152/ajprenal.1978.235.4.F321. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms D., Lieberman M. Microfluorometric monitoring of pHi in cultured heart cells: Na+-H+ exchange. Am J Physiol. 1983 May;244(5):C422–C428. doi: 10.1152/ajpcell.1983.244.5.C422. [DOI] [PubMed] [Google Scholar]
- Renault G., Raynal E., Sinet M., Muffat-Joly M., Berthier J. P., Cornillault J., Godard B., Pocidalo J. J. In situ double-beam NADH laser fluorimetry: choice of a reference wavelength. Am J Physiol. 1984 Apr;246(4 Pt 2):H491–H499. doi: 10.1152/ajpheart.1984.246.4.H491. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J. Na+-dependent H+ efflux from proximal tubule: evidence for reversible Na+-H+ exchange. Am J Physiol. 1981 Oct;241(4):F380–F385. doi: 10.1152/ajprenal.1981.241.4.F380. [DOI] [PubMed] [Google Scholar]
- Shiuan D., Weinstein S. W. Evidence for electroneutral chloride transport in rabbit renal cortical brush border membrane vesicles. Am J Physiol. 1984 Nov;247(5 Pt 2):F837–F847. doi: 10.1152/ajprenal.1984.247.5.F837. [DOI] [PubMed] [Google Scholar]
- Steinhausen M., Eisenbach G. M., Galaske R. A counter-current system of the surface of the renal cortex of rats. Pflugers Arch. 1970;318(3):244–258. doi: 10.1007/BF00593664. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Baumann K. Renal proximal tubular buffer-(glycodiazine) transport. Inhomogeneity of local transport rate, dependence on sodium, effect of inhibitors and chronic adaptation. Pflugers Arch. 1975 Jun 26;357(3-4):149–163. doi: 10.1007/BF00585971. [DOI] [PubMed] [Google Scholar]
- Warnock D. G., Yee V. J. Chloride uptake by brush border membrane vesicles isolated from rabbit renal cortex. Coupling to proton gradients and K+ diffusion potentials. J Clin Invest. 1981 Jan;67(1):103–115. doi: 10.1172/JCI110002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S. W., Szyjewicz J. Early postglomerular plasma concentrations of chloride, sodium, and inulin in the rat kidney. Am J Physiol. 1976 Sep;231(3):822–831. doi: 10.1152/ajplegacy.1976.231.3.822. [DOI] [PubMed] [Google Scholar]
- Weinstein S. W., Szyjewicz J. Superficial nephron tubular-vascular relationships in the rat kidney. Am J Physiol. 1978 Mar;234(3):F207–F214. doi: 10.1152/ajprenal.1978.234.3.F207. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Frömter E. Cell pH of rat renal proximal tubule in vivo and the conductive nature of peritubular HCO3- (OH-) exit. Pflugers Arch. 1984 Nov;402(3):300–305. doi: 10.1007/BF00585513. [DOI] [PubMed] [Google Scholar]



