Abstract
Background:
Acellular dermal matrices (ADMs) in plastic surgery have become increasingly popular particularly for breast reconstruction. Despite their advantages, questions exist regarding their association with a possible increased incidence of complications. We describe a collective experience of plastic surgeons’ use of ADMs in reconstructive breast surgery using an internet-based survey.
Methods:
Members of the American Society of Plastic Surgeons were recruited through voluntary, anonymous participation in an online survey. The web-based survey garnered information about participant demographics and their experience with ADM use in breast reconstruction procedures. After responses were collected, all data were anonymously processed.
Results:
Data were ascertained through 365 physician responses of which 99% (n = 361) completed the survey. The majority of participants were men (84.5%) between 51 and 60 years (37.4%); 84.2% used ADM in breast reconstruction, including radiated patients (79.7%). ADM use was not favored for nipple reconstruction (81.5%); 94.6% of participants used drains, and 87.8% administered antibiotics postoperatively. The most common complications were seroma (70.9%) and infection (16%), although 57.4% claimed anecdotally that overall complication rate was unchanged after incorporating ADM into their practice. High cost was a deterrent for ADM use (37.5%).
Conclusions:
Plastic surgeons currently use ADM in breast reconstruction for both immediate and staged procedures. Of those responding, a majority of plastic surgeons will incorporate drains and use postoperative antibiotics for more than 48 hours.
Acellular dermal matrices (ADMs) in plastic surgery have become increasingly popular.1 The use of ADMs together with improved techniques has helped to solve surgical problems lacking simple surgical solutions.2 Not only do these biologic meshes provide increased structural strength but also they promote rapid vascular ingrowth potentially serving as a scaffold for formation of new tissue.3 Since their availability in the 1990s, the list of indications for their use continues to grow.1 Among other indications, ADMs have been incorporated into abdominal wall reconstruction, extremity surgery, eyelid reconstruction, and nasal reconstruction.4–7
Increased interest in ADM use for reconstructive breast surgery has mirrored the introduction of new products.1 Initially, ADM was reported for use in secondary breast deformities, such as contracture and rippling, but has since evolved to address other shortcomings of implant-based reconstruction.8 ADMs have been described as the most significant innovation impacting prosthetic breast reconstruction in recent years.9 This statement can be attributed to its numerous potential benefits, including improved aesthetic outcome, reduction in postoperative pain, and decreased operative time.10 Furthermore, it has been reported to provide better control of the mastectomy space, optimize implant positioning, allow for increased intraoperative expansion, and prevent superior migration of the implant.9 Prior studies have evaluated the outcomes of ADM use in breast reconstruction.9,11 Despite their advantages, there is literature implicating their association with an increased incidence of postoperative complications, particularly infection and seroma formation.12,13
Although it is not common, ADMs can also be employed in delayed breast reconstruction provided there is an adequate degree of skin laxity. Moreover, ADMs have shown to be successful in patients with a variety of breast volumes.9 ADMs have proven less effective in patients with delayed reconstruction, in exposure to radiation, in those with a history of smoking, when vascularity to skin flaps has been compromised immediately following mastectomy, and in the morbidly obese.9,14,15
Although ADMs provide an added tool for plastic surgeons, their use has a learning curve requiring effort to attain desired outcomes.9 Another drawback is their high cost.1,16 In this instance, and in today’s economic environment, cost-benefit and cost analysis are essential.17 In light of the pros and cons of ADM use in breast reconstruction, the aim of this study was to gain a better understanding of the collective experience of plastic surgeons with ADM in postmastectomy breast reconstruction in terms of the popularity of its use, the most commonly incorporated meshes, patient satisfaction, surgical outcomes, complications, and application in breast revision procedures. In addition, we sought to investigate the impact of ADM in the setting of drains, antibiotics, nipple reconstruction, and in previously radiated patients.
METHODS
Recruitment
A survey was created using http://www.surveymonkey.com. An invitation containing a generic link to the survey (that ensured anonymity and prevented tracking) was distributed by e-mail to 365 plastic surgeons who are members of the American Society of Plastic Surgeons. Participation was voluntary. After responses were collected, all data were anonymously processed. Only a small cohort of plastic surgeons who are members of the American Society of Plastic Surgeons was included owing to the difficulty in obtaining member e-mails (lack of an e-mail repository) and based on omission of e-mails that were either no longer in use by the plastic surgeon (referred to an administrative e-mail) or inactive, in which case the e-mail was not delivered (a delivery status notification failure was received). There were no incentives given for participation in this study, and those who did participate were completely random with no preselection based on practice patterns or ADM use.
Survey
The web-based survey gathered information about participant demographics, including gender, age, practice type, years in practice, and geographic setting. Participants were then asked if they utilized ADM in breast reconstruction. Plastic surgeons who did not use ADMs were asked about the use of biologic meshes in other procedures and questioned about their decision not to use ADM in breast procedures. For participants who used ADM in breast reconstruction, inquiries were made about the type of mesh, the reasons for their particular choice, application in either immediate or delayed setting, patient satisfaction with surgical outcomes, and the utilization of ADM in breast revision procedures. Furthermore, to better understand the scope of ADM application, inquiries were made regarding the use of ADM for nipple reconstruction, drain usage, administration of antibiotics postoperatively, and in previously radiated patients. Finally, participants provided insight into their experience with infection rates and complications following ADM use and their opinion of the literature concerning the current evidence pertaining to ADM use in plastic surgery.
Statistical Analysis
Statistical analyses were performed using SPSS software version 21.0 (SPSS Inc., Chicago, Ill.). Associations between the use of ADM (dependent variable) and different independent variables were determined using chi-square and Fisher’s exact tests. Two-sided P value of <0.05 was deemed statistically significant. Information about plastic surgeons was deidentified before analysis.
RESULTS
Patient Demographics
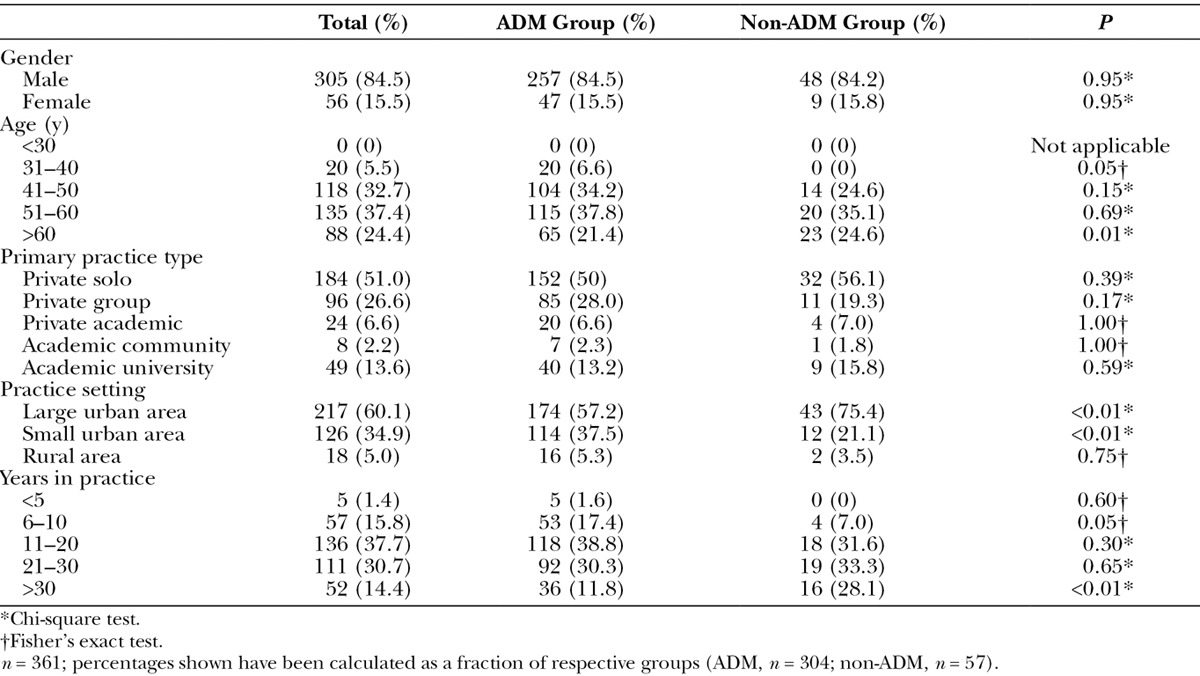
The data for this study were ascertained through 365 physician responses of which 99% (n = 361) of the respondents completed the survey. Responses of plastic surgeons who did not complete the survey were excluded (n = 4) (Fig. 1). The majority of participants were men (84.5%) who worked as solo practitioners in private practice (51%) in a large urban area (60.1%). Most plastic surgeons were between the ages of 51 and 60 (37.4%) and had been in practice for 11–20 years (37.7%). Participant demographics are summarized in Table 1.
Fig. 1.
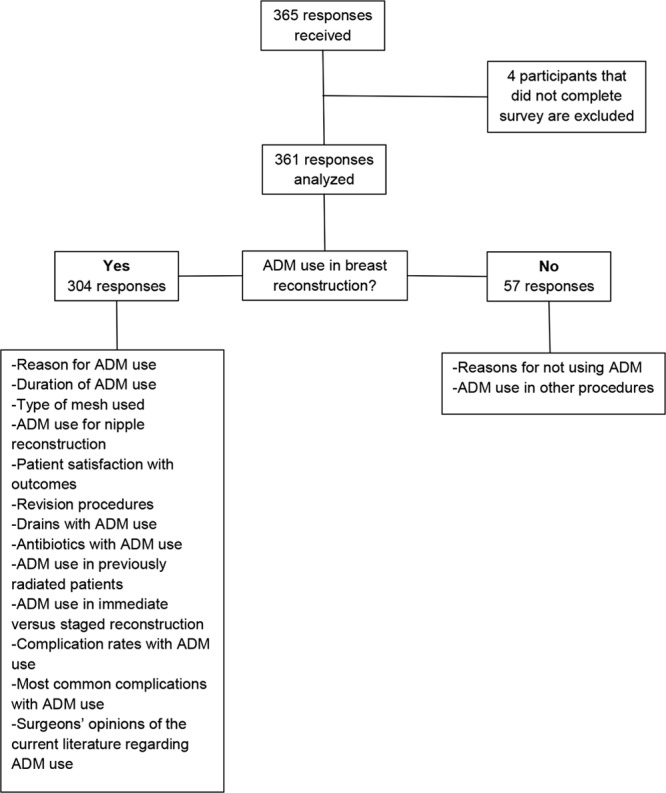
Survey participant selection process.
Table 1.
Demographic Characteristics of Respondents (n = 361)
Participants Who Did Not Use ADM in Breast Reconstruction
Of the 361 participants who completed the survey, 57 (15.8%) stated that they did not use ADM in breast reconstruction. When these 57 participants were queried as to whether they used ADM for any other procedures (such as abdominal wall reconstruction, head and neck reconstruction, burn surgery, lower limb coverage, and hand surgery), 42 (73.7%) claimed that they did not incorporate ADM into any aspect of their practice. The most reported reasons for this were due to cost (n = 15), surgeon preference (n = 10), and increased complications with previous experience (n = 11). Only 15 of the 57 participants (26.3%) stated their use of ADM for other procedures, the most common of which were abdominal wall reconstruction (n = 5) followed by extremity surgery, lower limb coverage (n = 4), and hand surgery (n = 3). When asked about the decision not to incorporate ADM in breast procedures, most of the respondents attributed it to the absence of breast reconstruction in practice (n = 7), no clear indication of benefit (n = 3), and cost (n = 2).
Participants Who Use ADM in Breast Reconstruction
Three hundred four participants (84.2%) stated routinely using ADM in breast reconstruction. The majority of respondents in this group had been using ADM in breast procedures for the last 6–10 years (69.5%). The most popular mesh for use in practice was AlloDerm (LifeCell Corporation, Branchburg, N.J.; 71.6%) (Fig. 2). The main reason for its popularity was reported to be adequate long-term experience and AlloDerm being well described in the literature (68.1%). The data suggest that this group of participants chose to incorporate ADM in breast reconstruction to allow for better control of the implant pocket (81.4%), improved aesthetic outcomes (70.1%), a quicker expansion (43.9%), and being able to reduce the incidence of capsular contracture and breast deformities (40.2% and 25.6%, respectively).
Fig. 2.
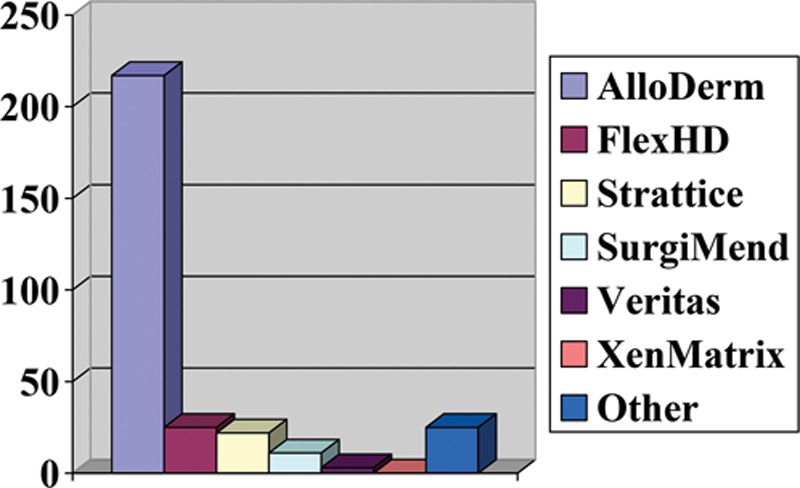
Most common meshes used for breast reconstruction.
The majority of responding plastic surgeons (81.5%) did not use ADM for nipple reconstruction. When asked about patient satisfaction, 86.4% of the surveyed plastic surgeon population reported that patients were satisfied with aesthetic outcomes; furthermore, 77.9% stated that patients rarely come back for further revisions. In the circumstance when a patient is unsatisfied and ADM has been used for reconstruction, the most commonly performed revision procedures include symmetry procedures (53.9%), fat grafting (19.5%), and capsulotomy/capsulectomy (17.9%).
Drains were used by 95% of respondents in conjunction with ADM in breast reconstruction; 81.5% reported using drains in all breast procedures involving ADM; typically, either 1 (45%) or 2 drains (49.6%) were used, and 57.5% of respondents stated to have left drains in for a longer period of time when they used ADM. Most participants (87.8%) routinely used antibiotics in the postoperative period; however, the number of days of antibiotic use varied from less than 5 days (31.5%), 6–10 days (45%), 11–14 days (15.8%), to more than 14 days (7.7%). We found that 79.7% of respondents had used ADM in previously radiated patients. Of these, 37.4% experienced no change in complication rates, an almost equal number of respondents suspected an increase or decrease in complication rate (24.3% versus 23%), and 15.3% were unsure. Those who reported an increase in complications implicated seroma (n = 45) and surgical site infection (n = 28), necessitating return to the operating room 11–30% of the time. The majority of participants (72.8%) used ADM in both immediate implant and staged reconstruction (tissue expander/implant). Regardless of the type of procedure, the most commonly reported complications were seroma (70.9%) followed by surgical site infection (16%) and wound dehiscence (9.4%) (Fig. 3). When asked whether ADM use in breast reconstruction contributed to an increased rate of infection, participants responded that it remained unchanged (57.4%); 26.4% of respondents reported an increase and 5.4% a decrease in the rate of infection. Finally, when questioned about their opinion of the literature with regard to current evidence for the use of ADM in plastic surgery, 38.7% suggested that the use of ADM is safe and effective in preventing complications while 28.4% reported that the evidence against the use of ADM is weak (Fig. 4).
Fig. 3.
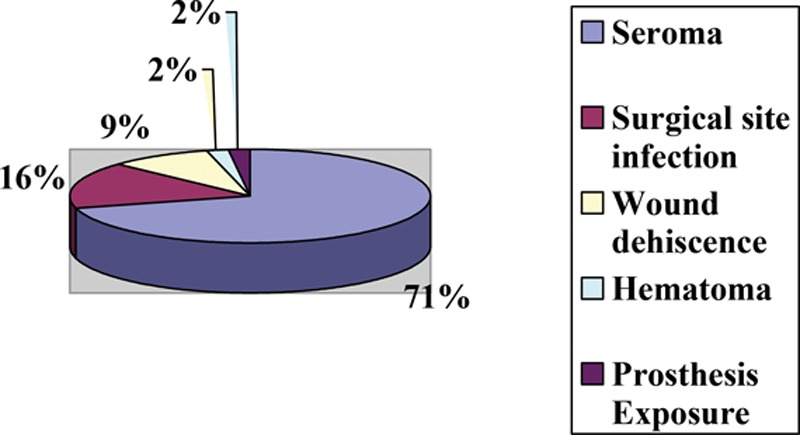
Most common complications with ADM use in breast reconstruction.
Fig. 4.
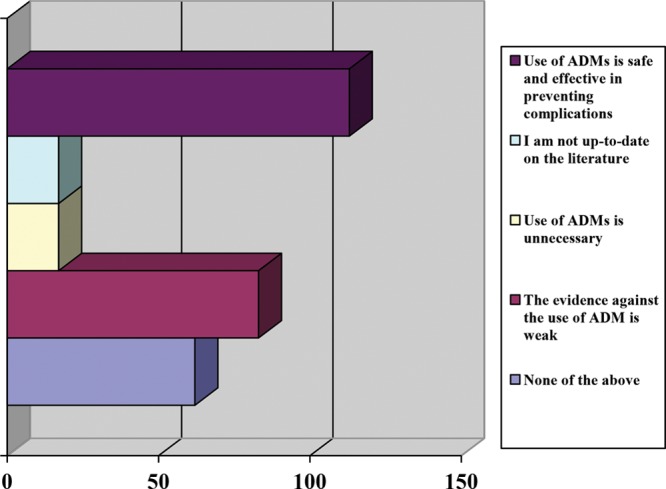
Summary of plastic surgeon opinion of evidence in the current literature for use of ADMs.
DISCUSSION
The emergence of ADMs has altered the practice patterns of many surgeons in various surgical disciplines.2 As this technology continues to advance, an abundance of new applications will develop. Currently, ADMs are used in a large spectrum of surgical procedures, including abdominal wall surgery, chest wall reconstruction, head and neck reconstruction, burns, and injuries of the extremities.5,6,18,19 ADMs have gained in popularity for use in breast reconstruction procedures, and they are now common practice in implant-based surgery.20 The rationale behind their application is to improve positioning of the prosthesis, to provide coverage of compromised muscle and fascia (pectoralis or serratus), to reduce the incidence of capsular contracture, to create a more defined inframammary fold, and notably to enhance the aesthetic outcome resulting from improved lower pole expansion.1,21,22 Despite their widespread use, there is an ongoing debate about incorporating ADM in breast reconstructive surgery, which primarily is attributed to a possible increased risk of postoperative complications. In this study, the practice pattern of plastic surgeons with ADM use in breast reconstruction was investigated.
An amount of literature has been published about the use of ADM in breast reconstruction. However, data about the demographics and its application in breast reconstructive surgery in the general plastic surgery population are less clear. In our study, the majority of responding plastic surgeons performing breast reconstruction have incorporated ADM into their practice (84.2%) regardless of practice type. This support for ADM use was attributed by most surgeons to its published safety and efficacy in the surgical literature. Interestingly, 100% of respondents aged 31–40 years, or less than 5 years in practice, use ADM in breast reconstruction, which may suggest a practice pattern that newer graduates are learning and/or choose to use ADM in breast reconstruction at greater rates than surgeons who did not learn to use it during their training. This assertion is somewhat confirmed by the finding that 57.4% of respondents suspected that the rate of infection did not change with ADM. However, a careful review of recent evidence indicates that opinion is divided on whether or not the use of ADM is associated with a higher incidence of postoperative complications.13,20,23–27 A chart review of 41 patients (65 breasts) by Bindingnavele et al26 found extremely low complication rates with biologic mesh use in postmastectomy breast reconstruction: seroma in 3 patients, wound infection in 2 patients, and hematoma and expander removal in 1 patient each. This finding is corroborated in a study by Preminger et al28 who reported that AlloDerm did not increase the risk of postoperative complications. In contrast, Chun et al12 and Liu et al13 observed statistically significant increases in infection rate and seroma rate with AlloDerm use, respectively. Only 26.4% of our survey participants reported an increase in postoperative complications, the most common of which were seroma (70.9%), surgical site infection (16%), and wound dehiscence (9.4%). A systematic review by Ho et al10 showed higher likelihood of seroma and infection in prosthetic-based breast reconstructions using traditional musculofascial flaps, whereas Adetayo et al29 identified the most common complications as wound infection (16%), seroma formation (8%), and breast implant failure (6%).
The ability of ADM to tolerate exposure to radiation is still being debated.21,30 In our study, 79.7% of participants claimed to have used ADM in previously radiated patients with most (37.4%) reporting no change in complication rate and equal numbers suspecting an increase (24.3%) or decrease (23%). Nahabedian24 reported that ADM is able to tolerate radiation exposure, demonstrating that the risk of infection did not vary with or without AlloDerm. Komorowska-Timek et al25 noted that AlloDerm reduced the rate of radiation-related inflammation. Colwell et al31 found that radiation therapy following stage 1 of tissue expander/implant-based reconstruction had a significantly lower complication rate than radiation therapy in the setting of breast conserving therapy. On the other hand, Spear et al23 and Salzberg et al32 observed 11-fold and 4-fold higher complication rates, respectively, in irradiated versus nonirradiated breasts. Despite these high complication rates, Ayeni et al,21 in their review reported that compared with plain tissue expander reconstructions, ADM-assisted tissue expander reconstruction seemed to have better resistance to radiation or at least have similar complication rates.
No consensus exists on antibiotics use following breast reconstructive surgery. Despite indications that there is no benefit in patients who receive treatment for more than 24 hours,33 a majority of plastic surgeons (87.8%) routinely use antibiotics for 6–10 days (45%) in the postoperative period. Avashia et al34 demonstrated a significant decrease in the rate of infection when postoperative antibiotics were taken for at least 48 hours following implant-based breast reconstruction with ADM. In a series of 321 implant-based reconstructions of which AlloDerm was used in 75, Nguyen et al35 reported no variations in the readmission rates for intravenous antibiotics. However, development of infected fluid collections resulting in explantation was found to be significantly higher in the AlloDerm group compared with the control group. The use of drains was also prevalent in our surveyed population (94.6%) with just over half (57.5%) leaving drains in for a longer period of time when they used ADM. This finding is consistent with findings by Collis et al36 who reported drains to have remained in situ for a significantly longer duration when using ADM.
Few studies have implemented ADM to aid in reconstruction of the nipple-areola complex with the goal of improving nipple projection.37–39 The survey found that only 18.5% of respondents reported to have used ADM for nipple reconstruction. Although experience with ADM in nipple reconstruction is limited, results thus far may be promising.39 Garramone and Lam37 demonstrated that AlloDerm use in a modified dermal flap pattern for 30 nipple reconstructions was a safe, reproducible, and easily performed approach for enhancing nipple projection. In contrast, a review of ADM use in nipple reconstruction by Israeli20 suggests limited success due to loss of nipple projection over time.
The most frequently used ADM is AlloDerm (71.6%), which is not surprising given it is the most commonly reported mesh for use in breast reconstruction in the literature.1 Moreover, 72.8% of surgeons used ADM in both immediate and staged breast reconstruction procedures owing to its many reported benefits.1,21,40 One major deterrent against ADM use in breast procedures is cost, which can range from $3536 to $4856 per breast16; it was implicated as the main reason for not using ADM in practice at all by 37.5% of participants. Regardless, Salzberg40 found AlloDerm use in immediate reconstruction to be less costly than transverse rectus abdominis myocutaneous flap surgery and expander/implant reconstruction after mastectomy. Although ADM can be expensive, various reports have demonstrated that in the long-term, it is cost effective in breast reconstruction.1,17,40
There are limitations to our study. Despite the number of plastic surgeons who completed the survey, only 57 did not use ADM for breast reconstruction. This finding was perhaps due to the voluntary sampling of participants which was utilized and may have resulted in bias toward ADM use. A small sample size may reduce the chances of detecting a true effect by overestimating it. Also, it may decrease the likelihood that a statistically significant result reflects a true effect. Therefore, this may be an incomplete assessment of the actual prevalence of plastic surgeons who do not use ADM in practice. Patient satisfaction with the aesthetic result was solely based on surgeon opinion, which may have also contributed toward bias with ADM use. Another limitation may be interpretation of the term “breast reconstruction” used in our survey, which some surgeons may have found to mean reconstruction following mastectomy only rather than also its use in aesthetic cases. Furthermore, the term “revision” may be have been interpreted differently by the study participants with some considering it to be further surgical intervention in the operating room and others deeming it a simple outpatient “touch-up.” Nevertheless, a majority of our respondents claimed that patients rarely came back for a revision maintaining consistency of our result. Finally, recall bias of the surveyed plastic surgeons may be a factor; however, this issue could potentially be overcome by our sample size and subgroup analysis excluding patients who did not use ADM for breast reconstruction.
CONCLUSIONS
Plastic surgeons use ADM in breast reconstruction for both immediate and staged procedures. Younger generations of plastic surgeons seem more willing to include ADMs in their practice. A majority of responding plastic surgeons incorporate drains and use postoperative antibiotics for more than 48 hours. ADM use for nipple reconstruction is not yet widely accepted. The occurrence of seroma, surgical site infection, and wound dehiscence are the most commonly implicated complications when ADM is incorporated necessitating return to the operating room. Despite this, a good number of respondents believe that overall infection rate remains unchanged. In addition, the majority of participants who reported use of ADM in previously radiated patients found that it did not contribute to a difference in complication rate. Most responding plastic surgeons believe that based on existing evidence, ADM use is safe and effective in preventing complications and that the data against its use are weak. The main deterrent against ADM use is its cost. In future studies, a larger participant population is needed to eliminate potential bias regarding ADM use.
Footnotes
Drs. Ibrahim and Koolen are co-first authors.
Disclaimer: The web-based survey site http://www.surveymonkey.com was used to obtain the necessary information. Data extrapolation, statistical analysis, and conclusions reached are the result of the work done by authors of this study.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Ibrahim AM, Ayeni OA, Hughes KB, et al. Acellular dermal matrices in breast surgery: a comprehensive review. Ann Plast Surg. 2013;70:732–738. doi: 10.1097/SAP.0b013e31824b3d30. [DOI] [PubMed] [Google Scholar]
- 2.Janis JE, Nahabedian MY. Acellular dermal matrices in surgery. Plast Reconstr Surg. 2012;130(5 Suppl 2):7S–8S. doi: 10.1097/PRS.0b013e31825f2d20. [DOI] [PubMed] [Google Scholar]
- 3.JoAnna Nguyen T, Carey JN, Wong AK. Use of human acellular dermal matrix in implant-based breast reconstruction: evaluating the evidence. J Plast Reconstr Aesthet Surg. 2011;64:1553–1561. doi: 10.1016/j.bjps.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Chang HS, Lee D, Taban M, et al. “En-glove” lysis of lower eyelid retractors with AlloDerm and dermis-fat grafts in lower eyelid retraction surgery. Ophthal Plast Reconstr Surg. 2011;27:137–141. doi: 10.1097/IOP.0b013e3181c53d38. [DOI] [PubMed] [Google Scholar]
- 5.Iorio ML, Shuck J, Attinger CE. Wound healing in the upper and lower extremities: a systematic review on the use of acellular dermal matrices. Plast Reconstr Surg. 2012;130(5 Suppl 2):232S–241S. doi: 10.1097/PRS.0b013e3182615703. [DOI] [PubMed] [Google Scholar]
- 6.Janis JE, O’Neill AC, Ahmad J, et al. Acellular dermal matrices in abdominal wall reconstruction: a systematic review of the current evidence. Plast Reconstr Surg. 2012;130:183S–193S. doi: 10.1097/PRS.0b013e3182605cfc. [DOI] [PubMed] [Google Scholar]
- 7.Sajjadian A, Naghshineh N, Rubinstein R. Current status of grafts and implants in rhinoplasty: part II. Homologous grafts and allogenic implants. Plast Reconstr Surg. 2010;125:99e–109e. doi: 10.1097/PRS.0b013e3181cb662f. [DOI] [PubMed] [Google Scholar]
- 8.Haddock N, Levine J. Breast reconstruction with implants, tissue expanders and AlloDerm: predicting volume and maximizing the skin envelope in skin sparing mastectomies. Breast J. 2010;16:14–19. doi: 10.1111/j.1524-4741.2009.00866.x. [DOI] [PubMed] [Google Scholar]
- 9.Nahabedian MY. Acellular dermal matrices in primary breast reconstruction: principles, concepts, and indications. Plast Reconstr Surg. 2012;130:44S–53S. doi: 10.1097/PRS.0b013e31825f2215. [DOI] [PubMed] [Google Scholar]
- 10.Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg. 2012;68:346–356. doi: 10.1097/SAP.0b013e31823f3cd9. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy CM, Lee CN, Halvorson EG, et al. The use of acellular dermal matrices in two-stage expander/implant reconstruction: a multicenter, blinded, randomized controlled trial. Plast Reconstr Surg. 2012;130(5 Suppl 2):57S–66S. doi: 10.1097/PRS.0b013e31825f05b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun YS, Verma K, Rosen H, et al. Implant-based breast reconstruction using acellular dermal matrix and the risk of postoperative complications. Plast Reconstr Surg. 2010;125:429–436. doi: 10.1097/PRS.0b013e3181c82d90. [DOI] [PubMed] [Google Scholar]
- 13.Liu AS, Kao HK, Reish RG, et al. Postoperative complications in prosthesis-based breast reconstruction using acellular dermal matrix. Plast Reconstr Surg. 2011;127:1755–1762. doi: 10.1097/PRS.0b013e31820cf233. [DOI] [PubMed] [Google Scholar]
- 14.Hvilsom GB, Hölmich LR, Steding-Jessen M, et al. Delayed breast implant reconstruction: is radiation therapy associated with capsular contracture or reoperations? Ann Plast Surg. 2012;68:246–252. doi: 10.1097/SAP.0b013e318214e69c. [DOI] [PubMed] [Google Scholar]
- 15.Padubidri AN, Yetman R, Browne E, et al. Complications of postmastectomy breast reconstruction in smokers, ex-smokers, and nonsmokers. Plast Reconstr Surg. 2001;107:350–351. doi: 10.1097/00006534-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Hartzell TL, Taghinia AH, Chang J, et al. The use of human acellular dermal matrix for the correction of secondary deformities after breast augmentation: results and costs. Plast Reconstr Surg. 2010;126:1711–1720. doi: 10.1097/PRS.0b013e3181ef900c. [DOI] [PubMed] [Google Scholar]
- 17.Jansen LA, Macadam SA. The use of AlloDerm in postmastectomy alloplastic breast reconstruction: part II. A cost analysis. Plast Reconstr Surg. 2011;127:2245–2254. doi: 10.1097/PRS.0b013e3182131c6b. [DOI] [PubMed] [Google Scholar]
- 18.Askari M, Cohen MJ, Grossman PH, et al. The use of acellular dermal matrix in release of burn contracture scars in the hand. Plast Reconstr Surg. 2011;127:1593–1599. doi: 10.1097/PRS.0b013e31820a6511. [DOI] [PubMed] [Google Scholar]
- 19.Sodha NR, Azoury SC, Sciortino C, et al. The use of acellular dermal matrices in chest wall reconstruction. Plast Reconstr Surg. 2012;130(5 Suppl 2):175S–182S. doi: 10.1097/PRS.0b013e31825f26b7. [DOI] [PubMed] [Google Scholar]
- 20.Israeli R. Complications of acellular dermal matrices in breast surgery. Plast Reconstr Surg. 2012;130(5 Suppl 2):159S–172S. doi: 10.1097/PRS.0b013e3182634e62. [DOI] [PubMed] [Google Scholar]
- 21.Ayeni OA, Ibrahim AM, Lin SJ, et al. Acellular dermal matrices in breast surgery: tips and pearls. Clin Plast Surg. 2012;39:177–186. doi: 10.1016/j.cps.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Kim JY, Davila AA, Persing S, et al. A meta-analysis of human acellular dermis and submuscular tissue expander breast reconstruction. Plast Reconstr Surg. 2012;129:28–41. doi: 10.1097/PRS.0b013e3182361fd6. [DOI] [PubMed] [Google Scholar]
- 23.Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32:418–425. doi: 10.1007/s00266-008-9128-8. [DOI] [PubMed] [Google Scholar]
- 24.Nahabedian MY. AlloDerm performance in the setting of prosthetic breast surgery, infection, and irradiation. Plast Reconstr Surg. 2009;124:1743–1753. doi: 10.1097/PRS.0b013e3181bf8087. [DOI] [PubMed] [Google Scholar]
- 25.Komorowska-Timek E, Oberg KC, Timek TA, et al. The effect of AlloDerm envelopes on periprosthetic capsule formation with and without radiation. Plast Reconstr Surg. 2009;123:807–816. doi: 10.1097/PRS.0b013e318199eef3. [DOI] [PubMed] [Google Scholar]
- 26.Bindingnavele V, Gaon M, Ota KS, et al. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg. 2007;60:1214–1218. doi: 10.1016/j.bjps.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim AM, Shuster M, Koolen PG, et al. Analysis of the National Surgical Quality Improvement Program database in 19,100 patients undergoing implant-based breast reconstruction: complication rates with acellular dermal matrix. Plast Reconstr Surg. 2013;132:1057–1066. doi: 10.1097/PRS.0b013e3182a3beec. [DOI] [PubMed] [Google Scholar]
- 28.Preminger BA, McCarthy CM, Hu QY, et al. The influence of AlloDerm on expander dynamics and complications in the setting of immediate tissue expander/implant reconstruction: a matched-cohort study. Ann Plast Surg. 2008;60:510–513. doi: 10.1097/SAP.0b013e31816f2836. [DOI] [PubMed] [Google Scholar]
- 29.Adetayo OA, Salcedo SE, Gupta SC. The use of acellular dermal matrix in breast and abdominal wall surgery: a meta-analysis of outcomes and risk factors predictive of complications. Plast Reconstr Surg. 2011;127:9. doi: 10.1097/SAP.0b013e31822afae5. [DOI] [PubMed] [Google Scholar]
- 30.Clemens MW, Kronowitz SJ. Acellular dermal matrix in irradiated tissue expander/implant-based breast reconstruction: evidence-based review. Plast Reconstr Surg. 2012;130(5 Suppl 2):27S–34S. doi: 10.1097/PRS.0b013e318265f690. [DOI] [PubMed] [Google Scholar]
- 31.Colwell AS, Damjanovic B, Zahedi B, et al. Retrospective review of 331 consecutive immediate single-stage implant reconstructions with acellular dermal matrix: indications, complications, trends, and costs. Plast Reconstr Surg. 2011;128:1170–1178. doi: 10.1097/PRS.0b013e318230c2f6. [DOI] [PubMed] [Google Scholar]
- 32.Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plast Reconstr Surg. 2011;127:514–524. doi: 10.1097/PRS.0b013e318200a961. [DOI] [PubMed] [Google Scholar]
- 33.Phillips BT, Bishawi M, Dagum AB, et al. A systematic review of antibiotic use and infection in breast reconstruction: what is the evidence? Plast Reconstr Surg. 2013;131:1–13. doi: 10.1097/PRS.0b013e3182729c39. [DOI] [PubMed] [Google Scholar]
- 34.Avashia YJ, Mohan R, Berhane C, et al. Postoperative antibiotic prophylaxis for implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;131:453–461. doi: 10.1097/PRS.0b013e31827c6d90. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen MD, Chen C, Colakoğlu S, et al. Infectious complications leading to explantation in implant-based breast reconstruction with AlloDerm. Eplasty. 2010;10:e48. [PMC free article] [PubMed] [Google Scholar]
- 36.Collis GN, TerKonda SP, Waldorf JC, et al. Acellular dermal matrix slings in tissue expander breast reconstruction: are there substantial benefits? Ann Plast Surg. 2012;68:425–428. doi: 10.1097/SAP.0b013e318225833f. [DOI] [PubMed] [Google Scholar]
- 37.Garramone CE, Lam B. Use of AlloDerm in primary nipple reconstruction to improve long-term nipple projection. Plast Reconstr Surg. 2007;119:1663–1668. doi: 10.1097/01.prs.0000258831.38615.80. [DOI] [PubMed] [Google Scholar]
- 38.Nahabedian MY. Secondary nipple reconstruction using local flaps and AlloDerm. Plast Reconstr Surg. 2005;115:2056–2061. doi: 10.1097/01.prs.0000164490.99581.f9. [DOI] [PubMed] [Google Scholar]
- 39.Chen WF, Barounis D, Kalimuthu R. A novel cost-saving approach to the use of acellular dermal matrix (AlloDerm) in postmastectomy breast and nipple reconstructions. Plast Reconstr Surg. 2010;125:479–481. doi: 10.1097/PRS.0b013e3181c82da6. [DOI] [PubMed] [Google Scholar]
- 40.Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg. 2006;57:1–5. doi: 10.1097/01.sap.0000214873.13102.9f. [DOI] [PubMed] [Google Scholar]


