Abstract
The “other-race” effect describes the phenomenon in which faces are difficult to distinguish from one another if they belong to an ethnic or racial group to which the observer has had little exposure. Adult observers typically display multiple forms of recognition error for other-race faces, and infants exhibit behavioral evidence of a developing other-race effect at about 9 months of age. The neural correlates of the adult other-race effect have been identified using ERPs and fMRI, but the effects of racial category on infants’ neural response to face stimuli have to date not been described. We examine two distinct components of the infant ERP response to human faces and demonstrate through the use of computer-generated “hybrid” faces that the observed other-race effect is not the result of low-level sensitivity to 3D shape and color differences between the stimuli. Rather, differential processing depends critically on the joint encoding of race-specific features.
Introduction
Adults and older infants alike find it difficult to recognize faces that belong to a category to which they have little exposure. By gaining expertise with the faces that dominate their environment, observers become specialists with commonly-seen face categories to the detriment of their ability to distinguish between more rarely seen face types. One of the most consistent manifestations of experience-dependent recognition is the “other-race effect” (Malpass & Kravtiz, 1969; Malpass, 1981; Meissner & Brigham, 2001; Sporer, 2001), in which other-race faces tend to look more alike to observers than faces of their own race and are individuated with less accuracy. The reduction in face-recognition abilities for other-race faces (and other categories of out-group faces) appears to be acquired during the first year of life, following a process that has been called perceptual narrowing (Nelson, 2001). Similar to the developmental trajectory followed in speech perception (Kuhl et al., 2006), infants initially possess broad capabilities to recognize faces that become increasingly specialized by the age of 9–12 months. At earlier ages, infants tend to have approximately equal face processing abilities across a wide range of categories. Late in the first year of life, differential processing according to face categories begins to emerge.
Our goal in the current study was to characterize the neural basis of the other-race effect (or ORE) in 9-month-old infants. We used event-related potentials (ERPs) as a sensitive tool for assessing differences in face processing by racial category, and also as a means of examining distinct stages of face processing (represented by distinct ERP components) that may be very difficult to separate using behavioral paradigms. We also concentrated on older infants to maximize the likelihood that we would be able to see differences in the neural response to own- and other-race faces. Behaviorally, 3-month old infants exhibit race-dependent face preferences (Bar-Haim et al., 2006; Kelly et al., 2005), but the fact that infants show a preference for one race over another does not necessarily imply that individuation is impaired as in the adult ORE. While there is some evidence that infants at this age do show race-dependent discrimination performance (Sangrigoli & de Schonen, 2004; Hayden et al., 2007) other data suggests that it is not until approximately 9 months that infants begin to reliably display behavior consistent with a reduced ability to discriminate between other-race faces (Kelly et al., 2007, Hayden et al., 2009; Anzures et al., 2010). We thus did not attempt to describe the trajectory of perceptual narrowing for race in this study, but rather focused on an age group in which previous behavioral results indicate that differential face processing based on race should be evident.
Beyond describing the existence of a neural other-race effect in infancy, we also wished to describe what exactly is being learned about facial appearance and how that learning is manifest in the infant brain. Specifically, what visual cues do infants use to assign faces to different groups and how do those visual features modulate neural responses to own-group and out-group faces? Faces are highly multidimensional stimuli and multiple cues are known to be important for effective recognition, including the internal features of the face (Ellis, Shepherd, & Davies, 1979), the external outline of the head (Sinha & Poggio, 1996), the configural geometry of facial features (Young, Hellawell, & Hay, 1987), and the surface properties of the skin (Russell et al., 2006; Russell & Sinha, 2007). Our goal was to ask a fundamental question regarding the “tuning” of neural face representations in infants along multiple diagnostic dimensions of facial appearance: To what extent do distinct cues contribute independently to differential processing of other-race faces? We emphasize that this question asks how ecologically-relevant physical stimulus differences affect subsequent processing. This is in contrast to many previous studies in which image-level differences between faces belonging to “in-group” and “out-group” categories are equalized to isolate higher-level influences (Shutts & Kinzler, 2007).
We chose own- and other-race face processing as our test domain since differences in face processing based on racial category are well established in the adult and infant literature. We also chose to examine just two broad aspects of facial appearance, face shape and face pigmentation, that together encompass mutually exclusive dimensions of face variability. Face pigmentation and face shape are known to make independent contributions to adult face recognition (Russell, 2003; Russell et al., 2006) and gender classification (Nestor & Tarr, 2008), making these aspects of appearance a useful target for studying the visual basis of differential face processing in infancy. Other-race face perception and the dissociation of shape and pigmentation is a particularly good match, since perceived category membership (deciding that a face is White or Black, for example) is clearly a function of both shape and pigmentation data. Previous work has examined the extent to which the processing of ambiguous faces (that are not clearly White or Black) can be affected by various manipulations of the stimulus (Levin, 2000), suggesting that particular shape features (such as hair style) can dramatically influence processing. Of particular relevance is a recent study by Balas & Nelson (2010) who reported independent contributions of face shape and color at the adult N170 and N250 ERP components.
Similar to the effects reported in Balas & Nelson’s (2010) study with adult observers, we wished to describe the independent contributions of both shape and pigmentation to own- and other-race face processing in infancy. Typically, faces belonging to different races will differ substantially in both shape and pigmentation, making it difficult to assess the individual contribution of each attribute using natural stimuli. To enact a dissociation between shape and pigmentation, we constructed synthetic 3D models of Caucasian and African faces. We manipulated these models such that the skin tone from a particular model was transferred to another, resulting in hybrid stimuli that possessed both the shape of a Caucasian individual and the darker pigmentation of an African individual. By showing infants both the congruent White and Black faces and the incongruent hybrid faces, we are able to ask how distinct neural responses to own- and other-race faces are affected by independent changes in face shape and surface pigmentation. Our first question is basic: Do 9-month olds exhibit a neural ORE? Our second question: Can differential processing of White and Black faces be induced solely by a difference in skin color, or solely by a difference in shape? Alternatively, 9-month old infants may have learned an ecologically valid conjunction of these features rather than independent sensitivity to any difference in appearance that is consistent with an other-race face.
To examine the neural basis and tuning properties of the infant other-race effect, infants viewed our computer-generated faces while event-related potentials (ERPs) were recorded. 9-month-olds viewed synthetic faces constructed to have either White or Black shape as well as either White or Black pigmentation, resulting in four distinct types of stimuli (Figure 1a). We recruited Caucasian infants whose parents reported no significant exposure to non-white faces and assigned each baby to one of three groups: (1) The “Classic ORE” group was shown pictures of faces with White shape and pigmentation, and faces with Black shape and pigmentation. (2) The “Color-matched” group was shown pictures of faces with White shape and pigmentation, and faces with Black shape and White pigmentation. (3) The “Shape-matched” group viewed faces with White shape and pigmentation, as well as faces with White shape and Black pigmentation. In each group, infants viewed a randomly-ordered sequence of faces from the two categories assigned to them (Figure 1b). This between-subjects design offered substantial power to identify an ORE using comparisons of the congruent-cue White face with each potential other-race category.
Figure 1. Synthetic other-race stimuli and experimental design.
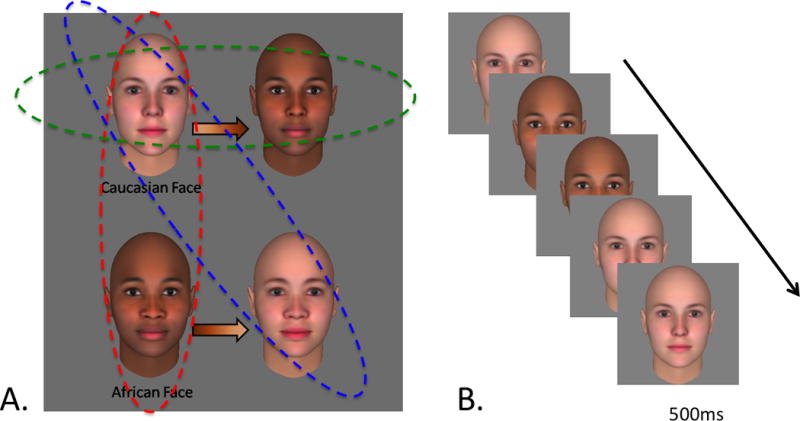
We dissociated 3-D face shape from 2-D surface properties through the use of a commercial computer graphics model of facial appearance (FaceGen, Singular Inversions). White faces were given Black pigmentation (and vice versa) to create two sets of incongruent face stimuli, yielding three potential “other-race” face types. Infants were split into three experimental groups (dashed ovals), with each group seeing the veridical White faces and one of the other-race categories. (A) Infants viewed the faces from the two groups assigned to them while ERPs were recorded. Faces were presented in an upright orientation and in a randomized order. (B)
We examined two components of the ERP response, the N290 and the P400, which are putative markers of infant face processing (de Haan, Johnson, & Halit, 2002). Both of these components appear to reflect important aspects of face-specific processing during infancy and are thus important indices of how race-specific face shape and pigmentation affect the neural response to face stimuli. In each case, we wished to know whether the amplitude of the N290 or the P400 could be modulated by race-specific appearance at all, and subsequently whether that modulation could be shown to be largely due either to 3-D shape properties or 2-D surface properties. Further, it is not obvious whether differential processing of the veridical White and Black faces should lead the incongruent stimuli to be processed more like an own-race face or an other-race face. Put another way, have 9-month old infants excluded a face from typical face processing based on one other-race feature, or do they include that face based on one own-race feature?
Methods
Participants
Participants were 9-month-old Caucasian infants that were born full-term with no known pre- or perinatal complications, and tested within two weeks of their 9-month birthday. Infants were assigned to one of three groups: (1) “Classic” Other-Race faces (N=13, 8 female), (2) Color-matched faces (N=13,9 female), and (3) Shape-matched faces (N=12,7 female). A total of 27 additional infants were tested across all three of these groups, but were excluded from the final sample due to fussiness or eye and body movements that caused extreme artifacts in the EEG signal.
Stimuli
We created 4 sets of face images depicting: (1) Individuals with White face shape and pigmentation, (2) Individuals with White face shape and Black pigmentation, (3) Individuals with Black face shape and White pigmentation, or (4) Individuals with Black shape and Black pigmentation. These images were created using 3-D graphics software (FaceGen, Singular Inversions Inc.) that allowed for independent manipulation of face shape and reflectance data. The program’s estimates of facial appearance across racial groups are based on physical measurements of 3D faces obtained from individuals belonging to the multiple groups supported by the application’s interface. Generating random faces involves drawing samples from the distributions of faces learned from these data. Our use of artificial faces generated in this manner raises the possible concern that the results obtained from computer-generated stimuli may not generalize to natural stimuli, but there are two primary reasons why we feel confident using these stimuli in this context. First, as already noted, the model used to generate the faces in our experiment is based on anthropometric data describing real variation in shape and pigmentation (Blanz & Vetter, 1999). Second, faces generated in the same manner are increasingly being used as a tool for studying face perception (Russell et al., 2006; Balas & Nelson, 2010) as are other parametrized models of facial appearance that are far more simplistic than the model we have used (Wilson, Loffler, & Wilkinson, 2002). Complex stimuli like faces inevitably force researchers to consider whether uncontrolled natural stimuli are more appropriate than controlled, artificial stimuli. In this case, we chose to maximize our control over the shape and pigmentation parameters we were primarily interested in by using synthetic faces. Alternatively, one could use various morphing methods to achieve a similar design with photographic faces, which would raise new concerns about artifice, but allay some concerns regarding the generalizability of the stimuli.
When manipulating shape/pigmentation relationships, we maintained the variability of pigmentation across all conditions by always creating “hybrid” stimuli using a particular individual’s shape information and a different individual’s pigmentation information (Figure 1). Infants in the “Classic” Other-Race group were only shown faces with congruent shape and skin color. Infants in the “Color-Matched” group were shown faces with White shape and skin color, as well as faces with Black shape, but White skin color. Finally, infants in the “Shape-Matched” group were also shown faces with White shape and skin color, as well as faces with White shape and Black skin color.
Procedure
Testing took place in a darkened, quiet room after application of the sensor net. Infants were tested while sitting on their parent’s lap. Stimuli were presented using E-Prime software v2.0 (Psychology Software Tools Inc., Pittsburgh, PA). The faces were presented on the center of the screen on a black background. The computer monitor was 48 cm wide and 31 cm high. When viewed from a distance of ~50 cm, the faces on average subtended approximately 12×12 degrees of visual angle. A digital video camera mounted above the monitor and centered on the infant’s face allowed for observation of the infant at all times during the testing session. On-line judgments were made by the experimenter to present the pictures only when the infant was attending to the monitor. Trials were immediately rejected by the experimenter if the infant looked away during stimulus presentation.
Stimuli were presented for 500 ms followed by an experimenter-controlled inter-stimulus interval of at least 1000 ms during which time the screen was black. After the presentation of each stimulus, the experimenter waited until the infant was attending to the display before presenting the next image. This means that the inter-trial interval was a minimum of 1500ms, but was frequently longer to accommodate the looking behavior of the subject. The two types of faces that were included in each testing session were randomly presented with equal probability. Stimulus presentation continued until the infant became bored or too fussy to attend, with a maximum of 100 trials.
ERP Recording and Analysis
ERPs were recorded using a 64-channel Geodesic Sensor Net v2.0, (Electrical Geodesics Inc., Eugene, OR). EEG was recorded continuously and referenced to a single vertex electrode (Cz). Signals were amplified using an EGI NetAmps 200 amplifier with a band-pass filter of 0.1–100 Hz and a sampling rate of 200 Hz. Impedances were checked on-line prior to beginning the session and were considered acceptable if lower than 50 KOhm.
Continuous EEG data were processed offline using NetStation v4.3.1 (Eugene, OR). A 30-Hz lowpass filter was applied and trials were constructed that consisted of a 100 ms baseline period and 1500 ms period following stimulus onset. Data were baseline corrected to the average voltage during the 100 ms prior to stimulus onset. Segmented data were hand-edited for EOG and motion artifact. Specifically, trials in which eye-blinks or eye-movements were clearly visible in the ERP data were excluded as were trials during which substantial drift was visually evident across the entire sensor array. Data from individual sensors was also rejected if there was artifact resulting from poor contact or movement, again by visual inspection. Trials were excluded if more than nine sensors had been rejected, or if an eye-blink or other significant artifact had occurred. Of the remaining trials, individual channels containing artifact were replaced using spherical spline interpolation. Individual subject averages were constructed separately for the two experimental conditions and data were re-referenced to the average reference. The average number of trials contributing to the average ERP in each condition following post-processing was as follows: “Classic ORE group” – White faces; M=31.7, SD=10.2; Black faces; M=33.6, SD=10.6. “Color-Matched group” – White faces; M=34.7, SD=11.8, Black faces; M=37.5, SD=12.0. “Shape-Matched group” – White faces; M=32.0, SD=10.6, Black faces; M=30.1, SD=11.5. We observed no significant differences in the number of trials contributing to the final analysis across subject groups or experimental conditions.
Inspection of the grand-averaged waveforms revealed a well-defined P400 component that was subsequently analyzed within a time window 340–600 ms. The N290 component was identified and measured within a time window of 120–240ms. Electrode groupings over occipital sensors and time windows were selected based on visual inspection of the grand-averaged and individual waveforms. We grouped the selected sensors into left, middle, and right regions, comprising sensors 32,33, & 36 on the left, sensors 37, 38, 39, & 40, and sensors 41, 44, & 45 on the right (Figure 2). Within these groups of sensors the ERP signal was averaged across the individual sensors to yield a single average waveform per subject for the left, middle, and right groups.
Figure 2. Sensor selection for other-race analysis.
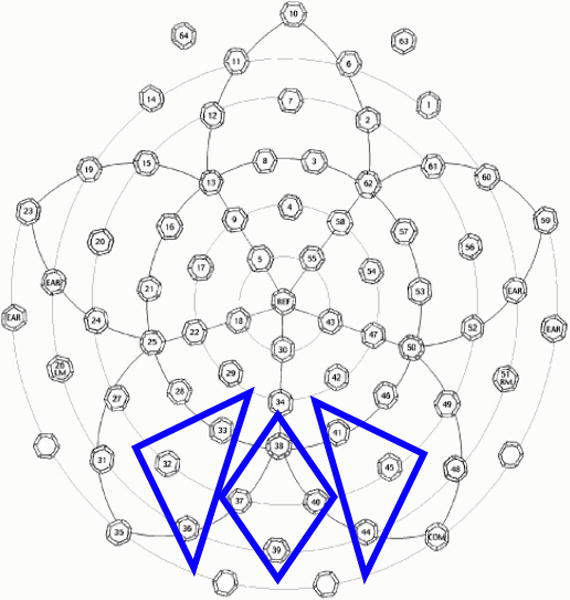
The N290 and the P400 were measured over the left, middle, and right groups of electrodes outlined in blue (the top of the array is the front of the head, the bottom is the back). The signal at each electrode was averaged across trials and across sensors within a group to yield an average waveform for each subject.
We described each component in terms of the average amplitude within the pre-defined time window for analysis. For each group, we conducted a two-way ANOVA using face category (own- or other-race) and region (left, middle, right) as within-subject factors. We present the data in this fashion both because an omnibus ANOVA across all experimental groups proved problematic numerically due to the nature of our design (necessary terms in the ANOVA were not full rank), and also to highlight the fundamental questions we wished to address. The results obtained from our “Classic ORE” group indicate the extent to which we observe a neural ORE at the selected components using representative White and Black faces, while the remaining subject groups allow us to ask whether similar results obtain when shape and pigmentation are separately controlled for. We acknowledge that this limits the strength of our conclusions somewhat, but our data nonetheless allows us to separately consider whether: (1) An ORE is observed at either the N290 or the P400 using congruent faces, (2) An ORE is observed in either control group when only one race-specific feature is present in the “other-race” condition.
Results
In the following, we report average amplitudes because peak amplitude and latency computations that rely upon peak response are known to be highly sensitive to noise (Luck, 2005). We note briefly that we observed no significant effects of peak latency in any of our subject groups or components, potentially due in part to high variability in localizing the peak.
N290
The “Classic ORE” group exhibited a main effect of race (F(1,24)=5.96, p = 0.030), but no main effect of region (F(2,24)=0.29, p=0.75), nor an interaction between race and region (F(2,24)=0.02, p=0.98). In Figure 3, we present the left, middle, and right grand average waveforms for both the White and Black faces presented to the Classic ORE group. Notably, the N290 appears to be largely absent in the average waveform derived from this group’s responses to Black faces. By contrast, the N290 to White faces is very robust over the middle and right regions. To the best of our knowledge, this represents the first report of an other-race effect in infant ERPs.
Figure 3. The Other-Race effect for congruent White and Black faces.
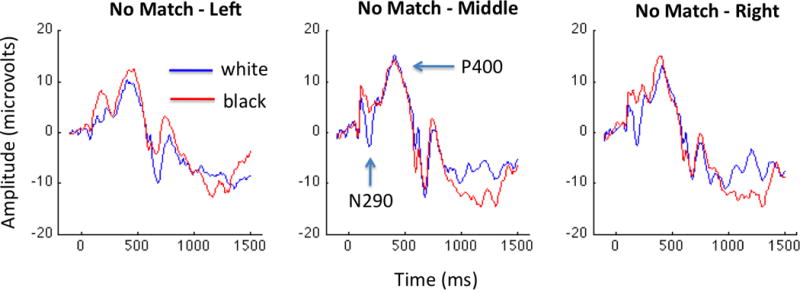
The average ERP to White and Black faces with congruent shape and pigmentation is presented for the left, middle, and right electrode groups. We find a main effect of race such that White faces elicit a reliable N290 and Black faces do not. The P400 does not exhibit a race-dependent difference in response.
The “Color-Matched” and “Shape-Matched” groups presented a different pattern of results than the “Classic ORE” group. Neither group exhibited a main effect of race – “Color-Matched” group: F(1,24)=3.35, p=0.10, “Shape-Matched” group: F(1,20)=0.06, p=0.82). However, we did obtain a main effect of region in both groups (“Color-Matched” group: F(2,24)=7.13, p=0.004, “Shape-Matched” group: F(2,20)=4.14, p=0.03) and an interaction between region and race in the “Shape-Matched” group (F(2,20)=13.9, p<0.001). We analyzed this interaction in more detail using Bonferroni-corrected post-hoc comparisons and found that the interaction reflected a significant region difference between the responses measured over the left and middle regions in response to other-race faces (p=0.004) that was not evident for own-race faces. In particular, we note that the apparent difference between and own- and other-race N290 responses over the right hemisphere region in this group does not reach significance (Figure 4). Beyond our report of an ORE at the N290 in the Classic ORE group, these results imply that other-race shape and pigmentation alone are in general not sufficient to drive a robust ORE at this component.
Figure 4. Shape- and color-matched ERP results.
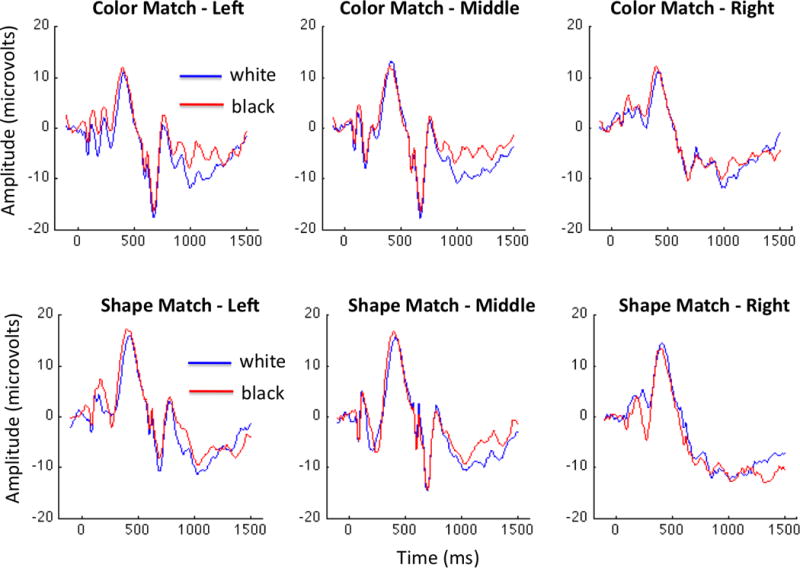
Average ERPs over the left, middle, and right electrode groups in the color-matched (top row) and shape-matched (bottom row) groups are presented here. We found no main effect of race in either group, but significant effects of region in both cases (favoring middle electrodes over left). The shape-matched group also yielded an interaction between race and region, which was due to region effects that were only evident for Black faces. Post-hoc tests revealed no significant effects of race within any region (including the apparent difference in the right sensor group).
P400
The P400 was not significantly modulated by any condition differences. Though a robust peak was observed in all infants, we observed no significant main effects (F<1) in either the “Classic ORE” group or the “Color-matched” and “Shape-matched” groups. Similarly, we observed no significant interaction between race and region in any of the three groups. We take this to mean that the P400 does not reflect race-dependent differential face processing.
Slow-wave activity
Though our primary focus in the current study was a comparison of the N290 and the P400, we also observed what appeared to be a difference in slow-wave activity across our stimulus conditions. We thus also compared slow-wave activity between our experimental groups to determine if this was indeed an additional difference between the processing of our various shape and color conditions. Ultimately, we found no significant differences across conditions in late time windows that incorporated the apparent differences that can be seen in the grand averages over our sensors of interest.
Discussion
Nine-month old Caucasian infants exhibit a neural other-race effect. As we stated above, our data represent the first report of race-dependent face processing in the infant brain using ERPs. Moreover, our use of synthetic faces that provided a clean dissociation of 3-D shape from 2-D surface properties revealed that robust differential processing for White and Black faces does not appear to result solely from differences in shape or differences in skin pigmentation. Rather, the conjunction of other-race shape and surface properties is required to obtain a robust difference in the ERP response. This suggests that the effects we observe are unlikely to be solely driven by low-level stimulus differences percolating forward to the components of interest. The differences in local contrast, etc. brought on by darkening the skin or changing the shape of the head are present in the shape-matched and color-matched groups as well, making them highly useful controls in this regard. The ORE we observe in our data thus likely reflects face processing rather than more basic visual processes, indicating that infants possess a representation of facial appearance that jointly encodes shape and color.
That we observe the ORE at the N290, but not the P400, is consistent with the results of several previous studies. The P400 is known to be insensitive to the difference between human and monkey faces (de Haan, Pascalis & Johnson, 2002), intact vs. distorted faces (Gliga & Dehaene-Lamberz, 2005), and also does not appear to robustly differentiate between faces and noise patterns with a matched power-spectrum (Halit et al., 2004). While familiarity effects for the P400 have been reported (Scott, Shannon, Nelson, 2006; Scott & Nelson, 2006; Balas et al., 2010) overall this component appears to be largely insensitive to profound category differences between stimuli. By contrast, the N290 has been shown to be sensitive to face category in several experimental settings. Its amplitude is known to be larger for faces than matched noise patterns (Halit et al., 2004), and it is larger in response to human faces than monkey faces (Halit, de Haan, & Johnson, 2003). It has also been observed to be larger in response to familiar stimuli than unfamiliar stimuli (Scott & Nelson, 2006). All of these results are consistent with the data we report here, with our main contribution being the report of a similar pattern of responses for race processing and the implication of selectivity for the joint properties of race-specific facial appearance.
The current results describe a robust neural ORE in infant face processing and raise several intriguing questions. First, it is of interest that the response of the N290 across the four face categories we defined in our task is more inclusive than exclusive. That is, 3 of 4 face categories elicit a large N290, with only the “real” Black faces eliciting a reliably different response. While we would expect that infants at 9 months of age have begun to lose face recognition abilities for rarely seen classes, our data suggest that it may only be maximally different stimuli that elicit differential neural processing. One can easily imagine that this could proceed in exactly the opposite manner, e.g. all categories but “real” White faces could be excluded. Indeed, recent behavioral data suggests adult observes behave in this manner (Balas & Nelson, 2010).
There are several important potential extensions of the work we have described here. Perhaps most important is the fact that the current study lacks an African-American control group, though the inclusion of the color-matched and shape-matched faces ameliorate the usual concerns regarding the possibility that one subset of faces is objectively harder than the other to recognize. Nonetheless, it would undoubtedly be useful to characterize how the N290 response changes as a function of participant race and increased experience with Black faces. In a similar vein, comparing the response properties of these components across a wider range of face categories would also more clearly characterize the boundaries of in-group and out-group faces in face space (Valentine, 1991). Are all “other” faces treated the same way, or does an inter-species difference manifest differently than an inter-race or inter-gender difference? An interesting extension of our work would be the inclusion of inverted faces as an additional control for low-level differences, as well as a potential index of face-specific processing. Balas & Nelson (2010) observed an orientation-specific ORE at the adult N250, and Balas et al. (2010) reported a familiarity-dependent orientation effect at the P400. Both of these results suggest that portions of the infant ERP response may select for stimulus orientation as a function of race category, and the manipulation of stimulus orientation may lead to a means of examining what (if any) category selectivity the P400 may have.
It would also be useful to examine the developmental trajectory of the effects reported here. Typical “perceptual narrowing” designs compare infants at 9–10 months of age to younger infants at 6 months of age (Pascalis, de Haan, & Nelson 2002; Pascalis et al., 2005). Though describing the timecourse of perceptual narrowing for race was not the main goal of the current study, it is clearly of interest to characterize this trajectory in detail with regard to results we have reported. Specifically, given the ambiguity noted in the introduction as to when the behavioral ORE (as defined by individuation abilities) emerges during the first year of life, studying 3- and 6-month old infants using our paradigm would be an important contribution. Similarly, it would be very interesting to examine the relationship between the neural ORE reported here and a behavioral ORE using infants’ behavioral performance in standard visual paired-comparison tasks.
Our data also invites the comparison between neural correlates of adult and infant other-race processing. To what extent can we relate our data from the N290 to previous reports regarding other-race processing at analogous components in adults (e.g. the N170)? The ERP literature regarding the neural basis of the other-race effect is complex; both the tasks employed by different researchers and the particular races used vary substantially. Regarding the N170, previous results are mixed with regard to the sensitivity of this component for race-specific appearance. Caldara et al. (2003) for example, report no difference between White and East Asian faces at the N170, while Stahl, Wiese, & Schweinberger (2008) do report effects with the same races. As to the comparison of White and Black faces, both Ito & Urland (2005) and Balas & Nelson (2010) report an effect of White vs. Black skin pigmentation at the N170, though their effects do not agree with regard to the direction of the effect. Clearly a great deal of work remains to specify exactly how sensitive the N170 is to race-specific visual information. Nonetheless, we must consider how the infant N290, which appears to jointly select for race-specific shape and color, matures into the adult N170, which may be sensitive either to only one “race feature” or none at all. Continued study of the adult N170 with regard to race is clearly still necessary, and a characterization of the extended developmental trajectory of the N290 race effect would also be very useful here. In particular, it has been proposed that the adult N170 may result from the integration of both the N290 and the P400 over development (Halit, de Haan, & Johnson, 2003). This proposal is interesting to consider in light of our current data, since the N290 exhibits an ORE that is somewhat “stricter” than that reported by Balas & Nelson (2010) in adults, and the P400 appears to encode no race-specific information. Though it is not obvious exactly how the integration of these components might be realized, a potentially compelling account is that the joint selectivity of the N290 combined with the insensitivity of the P400 may combine to provide adults with an N170 that is only sensitive to one feature dimension.
Presently, we have provided evidence of a robust neural other-race effect in 9-month old infants that is jointly tuned to 3-D shape and 2-D surface properties. Our results suggest that these infants do exhibit differential processing of own- and other-race faces, so long as other-race faces are defined by both race-specific shape and skin pigmentation. Our study provides a first account of the neural basis of the other-race effect in infancy and suggests multiple avenues for future work.
References
- Anzures G, Quinn PC, Pascalis O, Slater AM, Lee K. Categorization, categorical perception, and asymmetry in infants’ representation of face race. Developmental Science. doi: 10.1111/j.1467-7687.2009.00900.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balas BJ, Nelson CA. The role of face shape and pigmentation in other-race face perception: An electrophysiological study. Neuropsychologia. 2010;48:498–506. doi: 10.1016/j.neuropsychologia.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balas BJ, Nelson CA, Westerlund A, Vogel-Farley V, Riggins T, Kuefner D. Personal familiarity influences the processing of upright and inverted faces in infants. Frontiers in Human Neuroscience. 2010 doi: 10.3389/neuro.09.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Ham Y, Ziv T, Lamy D, Hodes RM. Nature and nurture in own-race face processing. Psychological Science. 2006;17:159–163. doi: 10.1111/j.1467-9280.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- Blanz V, Vetter T. Proc of the SIGGRAPH ’99. Los Angeles, USA: 1999. A Morphable Model for the Synthesis of 3D Faces; pp. 187–194. [Google Scholar]
- Caldara R, Thut G, Servoir P, Michel CM, Bovet PM, Renault B. Face versus non face object perception and the “other-race” effect: a spatio-temporal event-related potential study. Clinical Neuropsychology. 2003:515–528. doi: 10.1016/s1388-2457(02)00407-8. [DOI] [PubMed] [Google Scholar]
- De Haan M, Johnson MH, Halit H. Development of face sensitive event-related potentials during infancy: A review. Journal of Psychophysiology. 2002;51:45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- De Haan M, Pascalis O, Johnson MH. Specialization of neural mechanisms of face recognition in human infants. Journal of Cognitive Neuroscience. 2002;12:199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- Ellis HD, Shepherd JW, Davies GM. Identification of familiar and unfamiliar faces from internal and external features: some implications for theories of face recognition. Perception. 1979;8:431–439. doi: 10.1068/p080431. [DOI] [PubMed] [Google Scholar]
- Gliga T, Dehaene-Lamberz G. Structural encoding of body and face in human infants and adults. Journal of Cognitive Neuroscience. 2005;17:1328–1340. doi: 10.1162/0898929055002481. [DOI] [PubMed] [Google Scholar]
- Halit H, Csibra G, Volein A, Johnson MH. Face-sensitive cortical processing in early infancy. Journal of Child Psychology and Psychiatry. 2004;45:1228–1234. doi: 10.1111/j.1469-7610.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- Halit H, de Haan M, Johnson MH. Cortical specialization for face processing: face-sensitive event-related potential components in 3- and 12- month old infants. Neuroimage. 2003;19:1180–1193. doi: 10.1016/s1053-8119(03)00076-4. [DOI] [PubMed] [Google Scholar]
- Hayden A, Bhatt RS, Joseph J, Tanaka JW. The Other-Race Effect in Infancy: Evidence Using a Morphing Technique. Infancy. 2007;12:95–104. doi: 10.1111/j.1532-7078.2007.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Hayden A, Bhatt RS, Zieber N, Kangas A. Race-based perceptual asymmetries underlying face processing in infancy. Psychonomic Bulletin & Review. 2009;16:270–275. doi: 10.3758/PBR.16.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito TA, Urland GR. The influence of processing objectives on the perception of faces: An ERP study of race and gender perception. Cognitive, Affective, & Behavioral Neuroscience. 2005;5:21–36. doi: 10.3758/cabn.5.1.21. [DOI] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Ge L, Pascalis O. The other-race effect develops during infancy: Evidence of perceptual narrowing. Psychological Science. 2007;18:1084–1089. doi: 10.1111/j.1467-9280.2007.02029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DJ, Quinn PC, Slater AM, Lee K, Gibson A, Smith M, Ge L, Pascalis O. Three-month-olds, but not newborns, prefer own-race faces. Developmental Science. 2005;8:F31–F36. doi: 10.1111/j.1467-7687.2005.0434a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Stevens E, Hayashi A, Deguchi T, Kiritani S, Iverson P. Infants show a facilitation effect for nature language phonetic perception between 6 and 12 months. 2006 doi: 10.1111/j.1467-7687.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ. An Introduction to the Event-Related Potential Technique. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Malpass RS. Training in face recognition. In: Davies GM, Ellis HD, Shepherd JW, editors. Perceiving and remembering faces. London: Academic Press; 1981. pp. 271–285. [Google Scholar]
- Malpass RS, Kravitz J. Recognition of faces of own and other race. Journal of Personality and Social Psychology. 1969;13:330–334. doi: 10.1037/h0028434. [DOI] [PubMed] [Google Scholar]
- Meissner CA, Brigham JC. Thirty years of investigating the own-race bias memory for faces: a meta-analytic review. Psychology, Public Policy, & Law. 2001;7:3–35. [Google Scholar]
- Nelson CA. The development and neural bases of face recognition. Infant and Child Development. 2001;10:3–18. [Google Scholar]
- Nestor A, Tarr MJ. Gender recognition of human faces using color. Psychological Science. 2008;19:1242–1246. doi: 10.1111/j.1467-9280.2008.02232.x. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA. Is face processing species-specific during the first year of life. Science. 2002;296:1321–1323. doi: 10.1126/science.1070223. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Scott LS, Kelly DJ, Shannon RW, Nicholson E, Coleman M, Nelson CA. Plasticity of face processing in infancy. PNAS. 2005;102:5297–5300. doi: 10.1073/pnas.0406627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R, Sinha P. Real world face recognition: The importance of surface reflectance properties. Perception. 2007;36:1368–1374. doi: 10.1068/p5779. [DOI] [PubMed] [Google Scholar]
- Russell R. Sex, beauty, and the relative luminance of facial features. Perception. 2003;(32):1093–1107. doi: 10.1068/p5101. [DOI] [PubMed] [Google Scholar]
- Russell R, Sinha P, Biederman I, Nederhouser M. Is pigmentation important for face recognition? Evidence from contrast negation. Perception. 2006;(35):749–759. doi: 10.1068/p5490. [DOI] [PubMed] [Google Scholar]
- Sangrigoli S, De Schonen S. Recognition of own- and other-race faces by three-month-old infants. Journal of Child Psychology & Psychiatry. 2004;45:1219–1227. doi: 10.1111/j.1469-7610.2004.00319.x. [DOI] [PubMed] [Google Scholar]
- Scott LS, Nelson CA. Featural and Configural Face Processing in Adults and Infants: A Behavioral and Electrophysiological Investigation. Perception. 2006;35(8):1107–1128. doi: 10.1068/p5493. [DOI] [PubMed] [Google Scholar]
- Scott LS, Shannon RW, Nelson CA. Neural correlates of human and monkey face processing in 9-month-old infants. Infancy. 2006;10(2):171–186. doi: 10.1207/s15327078in1002_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutts K, Kinzler K. An ambiguous-race illusion in children’s face memory. Psychological Science. 2007;18:763–767. doi: 10.1111/j.1467-9280.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- Sinha P, Poggio T. I think I know that face…. Nature. 1996;384:404. doi: 10.1038/384404a0. [DOI] [PubMed] [Google Scholar]
- Sporer SL. Recognizing faces of other ethnic groups: An integration of theories. Psychology, Public Policy & Law. 2001;7:36–97. [Google Scholar]
- Stahl J, Wiese H, Schweinberger SR. Expertise and own-race bias in face processing: an event-related potential study. Neuroreport. 2008;19:583–587. doi: 10.1097/WNR.0b013e3282f97b4d. [DOI] [PubMed] [Google Scholar]
- Sugita Y. Face perception in monkeys reared with no exposure to faces. PNAS. 2008;105:394–398. doi: 10.1073/pnas.0706079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine TA. Unified account of the effects of distinctiveness, inversion, and race in face recognition. The Quarterly Journal of Experimental Psychology. 1991;43:161–204. doi: 10.1080/14640749108400966. [DOI] [PubMed] [Google Scholar]
- Wilson HR, Loffler G, Wilkinson F. Synthetic faces, face cubes, and the geometry of face space. Vision Research. 2002;42:2909–2923. doi: 10.1016/s0042-6989(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Young AW, Hellawell D, Hay DC. Configurational information in face perception. Perception. 1987;16:747–759. doi: 10.1068/p160747. [DOI] [PubMed] [Google Scholar]


