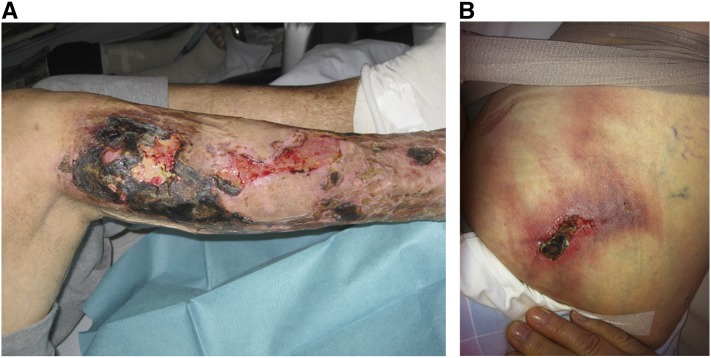
Calciphylaxis or calcific uremic arteriolopathy is a rare but disastrous and life-threatening disease mostly affecting patients on dialysis. It is characterized by very painful, ulcerating, and, finally, necrotic skin lesions (Figure 1), which in histology, show calcifications in the media of small cutaneous arterioles, nerve sheaths, and sometimes, adipose and soft tissues (1). One-year mortality may be approximately 50%, and deaths are mostly related to superinfection and consecutive episodes of sepsis or cardiovascular events. Epidemiologic information was so far provided by case reports, case studies, or registry data, always leaving questions with regard to representativeness and real-life incidence.
Figure 1.
Clinical manifestations of calciphylaxis. (A) Peripheral calciphylaxis in an intermediate stage, with an acute necrotic lesion below the right knee and some partially healing lesions farther below on the lower right leg. (B) Central abdominal calciphylaxis associated with obesity—this manifestation shows the typical indurated and very painful livedo reticularis manifestations.
Considering this background, the report by Floege et al. (2) on the randomized, controlled Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial can be regarded as a significant contribution to the epidemiologic understanding of calciphylaxis, because this trial investigated a large cohort of patients at high risk for developing this complication (3). In fact, just one or two decades ago, secondary hyperparathyroidism (sHPT) had been regarded as the most abundant key trigger of calciphylaxis, and therefore, a study targeting a dialysis population with moderate to severe sHPT over about 5 years should have been optimal to learn about incidence and clinical fate of this disease.
In the EVOLVE Trial, 24 of 3861 participants developed calciphylaxis during follow-up: six participants in the cinacalcet arm and 18 participants in the placebo arm. As Floege et al. (2) correctly pointed out, because of the severity of the disease, it seems unlikely that patients were overlooked; calciphylaxis should always qualify as a serious adverse event with a very typical clinical presentation. Although the data reflect a 69%–75% therapeutic calciphylaxis risk reduction by cinacalcet, the absolute numbers obviously remain low and actually, were lower than anticipated from former epidemiologic estimates. Could this potentially mean that sHPT may be a lesser calciphylaxis risk factor than previously expected? This may, indeed, be the case—in the German calciphylaxis registry (www.calciphylaxie.de), a total of 253 patients had currently been included, of which about 46% had immunoreactive parathyroid hormone (iPTH) levels of <150 pg/ml and close to 75% had iPTH levels of <300 pg/ml at the time of the calciphylaxis manifestation (V. Brandenburg, personal communication). Thus, approximately three quarters of registry-related cases would, by definition, not have been enrolled in the EVOLVE Trial. This observation could mean that calciphylaxis (or possibly, vascular calcification in general) is rather a complication associated more with adynamic than high bone turnover. However, this relatively low incidence could also have related to the rather typical situation that study participants frequently are healthier than a common average comparator population.
Other risk factors showing up in the EVOLVE Trial corresponded well with previously published literature, especially women, diabetes mellitus, and use of vitamin K antagonists, such as warfarin (1). The latter observation is of high pathobiologic relevance, because vitamin K deficiency inactivates the calcification inhibitory protein matrix Gla protein in the arterial vessel wall (4), thus adding up to a complex calcification risk scenario together with hyperphosphatemia, calcium loads, inflammation, etc. In this report by Floege et al. (2), 46% of patients with calciphylaxis were on warfarin therapy, closely comparable with >50% of patients in the German calciphylaxis registry (V. Brandenburg, personal communication) and a 10.1-fold elevated warfarin-associated risk reported from a Japanese case control study (2,5). The use of vitamin K antagonists in dialysis populations varies, and reliable proportions are not known but may overall be in a range of 8%–15% (6). Furthermore, vitamin K deficiencies were recently reported in majorities of CKD and dialysis populations, even in patients without anticoagulation therapy (7,8). Therapeutic or nutritional vitamin K deficiencies, however, may well qualify as modifiable risk factors, and, if this is the case, more in-depth research will be supportive in this direction. Finally, new and direct anticoagulants act independently of vitamin K and may, thus, qualify as alternatives, especially given the relatively high prevalence of atrial fibrillation in patients on dialysis (6). Despite some serious concerns against their administration in patients with advanced renal insufficiency, the Food and Drug Administration recently approved a dosage note on the potential use of apixaban (even in patients on hemodialysis) in the prescribing information (9).
The observed calciphylaxis risk association with dyslipidemia in the EVOLVE Trial may also be of interest because of the recent report by Nigwekar et al. (10) that showed a decreased risk with statin use in patients on dialysis. The arteriolar lesions in calciphylaxis certainly do not resemble atherosclerosis or plaque manifestations; however, statins may affect and suppress inflammation or modify oxidative stress, and many patients with calciphylaxis also suffer from large-artery disease.
Unfortunately, data are missing on the intermediate and long-term outcomes of 24 patients with calciphylaxis in the EVOLVE Trial and additional medical and therapeutic consequences (e.g., warfarin withdrawal, vitamin K supplementation, changes in phosphate binder treatment and dialysis intensity, and sodium thiosulfate treatment) after the diagnosis had been established. Such information would have been very welcome concerning the epidemiologic completeness of the report (2).
Probably, the most interesting and challenging question, however, is related to the observation that cinacalcet seemed to be superior to standard treatment with regard to preventing calciphylaxis episodes. Given the wide iPTH ranges (71–4957 pg/ml) under which calciphylaxis occurred in the affected individuals, cinacalcet’s PTH-lowering propensity does not seem to be the most likely explanation considering this effect. Is it, therefore, possible that cinacalcet possesses pleiotropic vasculoprotective properties by direct actions on the vascular wall or indirectly by interfering with other regulatory systems involved in vascular integrity or calcification control (e.g., fibroblast growth factor-23)? Answers will have to be approached by intelligent experimental research, which could then be translationally transferred into the clinic. If it were the case that calcimimetics have vasculoprotective actions beyond PTH lowering, a new field of indication may arise in patients on dialysis.
Will such clinical studies also be feasible in patients with calciphylaxis? Some clinical research groups currently attempt to implement prospective registry–like approaches on broader platforms. Supported by the European Renal Association-European Dialysis and Transplantation Association, the CKD–Mineral and Bone Disorder Working Group started to extend the German calciphylaxis registry into a pan-European approach, and the respective online website for data capture can already be accessed (www.calciphylaxis.net). Until the end of 2014, an annual rate of approximately 35 patients with calciphylaxis was recorded in Germany alone, but with this European extension including Belgium, France, Italy, The Netherlands, Portugal, and Spain, this number may rise to >100 per year. With such a volume, clinical researchers may find feasible ways to conduct randomized clinical studies investigating promising therapeutics, such as calcimimetics, but also, other candidate compounds, such as thiosulfate, phytate, etc., even in such a rare disease scenario.
Disclosures
M.K. and P.H.B. received honoraria with regard to lecturing and advisory tasks from Abbvie and Amgen.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related article, “The Effect of Cinacalcet on Calcific Uremic Arteriolopathy Events in Patients Receiving Hemodialysis: The EVOLVE Trial,” on pages 800–807.
References
- 1.Brandenburg VM, Sinha S, Specht P, Ketteler M: Calcific uraemic arteriolopathy: A rare disease with a potentially high impact on chronic kidney disease-mineral and bone disorder. Pediatr Nephrol 29: 2289–2298, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Floege J, Kubo Y, Floege A, Chertow GM, Parfrey PS: The effect of cinacalcet on calcific uremic arteriolopathy events in patients receiving hemodialysis: The EVOLVE Trial. Clin J Am Soc Nephrol 10: 800–807, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS; EVOLVE Trial Investigators: Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 367: 2482–2494, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Krueger T, Westenfeld R, Ketteler M, Schurgers LJ, Floege J: Vitamin K deficiency in CKD patients: A modifiable risk factor for vascular calcification? Kidney Int 76: 18–22, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Hayashi M, Takamatsu I, Kanno Y, Yoshida T, Abe T, Sato Y; Japanese Calciphylaxis Study Group: A case-control study of calciphylaxis in Japanese end-stage renal disease patients. Nephrol Dial Transplant 27: 1580–1584, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Reinecke H, Brand E, Mesters R, Schäbitz WR, Fisher M, Pavenstädt H, Breithardt G: Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 20: 705–711, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Holden RM, Morton AR, Garland JS, Pavlov A, Day AG, Booth SL: Vitamins K and D status in stages 3-5 chronic kidney disease. Clin J Am Soc Nephrol 5: 590–597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlieper G, Westenfeld R, Krüger T, Cranenburg EC, Magdeleyns EJ, Brandenburg VM, Djuric Z, Damjanovic T, Ketteler M, Vermeer C, Dimkovic N, Floege J, Schurgers LJ: Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. J Am Soc Nephrol 22: 387–395, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202155s002lbl.pdf. Accessed
- 10.Nigwekar SU, Bhan I, Turchin A, Skentzos SC, Hajhosseiny R, Steele D, Nazarian RM, Wenger J, Parikh S, Karumanchi A, Thadhani R: Statin use and calcific uremic arteriolopathy: A matched case-control study. Am J Nephrol 37: 325–332, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]