Abstract
Background and objectives
Data on mineral metabolism in pediatric renal transplant recipients largely arise from small single-center studies. In adult patients, abnormal mineral levels are related to a higher risk of graft failure. This study used data from the European Society for Paediatric Nephrology/European Renal Association–European Dialysis and Transplant Association Registry to study the prevalence and potential determinants of mineral abnormalities, as well as the predictive value of a disturbed mineral level on graft survival in a large cohort of European pediatric renal transplant recipients.
Design, setting, participants, & measurements
This study included 1237 children (0–17 years) from 10 European countries, who had serum calcium, phosphorus, and parathyroid hormone measurements from 2000 onward. Abnormalities of mineral metabolism were defined according to European guidelines on prevention and treatment of renal osteodystrophy in children on chronic renal failure.
Results
Abnormal serum phosphorus levels were observed in 25% (14% hypophosphatemia and 11% hyperphosphatemia), altered serum calcium in 30% (19% hypocalcemia, 11% hypercalcemia), and hyperparathyroidism in 41% of the patients. A longer time since transplantation was associated with a lower risk of having mineral levels above target range. Serum phosphorus levels were inversely associated with eGFR, and levels above the recommended targets were associated with a higher risk of graft failure independently of eGFR.
Conclusions
Abnormalities in mineral metabolism are common after pediatric renal transplantation in Europe and are associated with graft dysfunction.
Keywords: pediatric kidney transplantation, calcium, hyperphosphatemia, transplant outcomes, epidemiology and outcomes
Introduction
Abnormalities in calcium-phosphorus metabolism and consequent bone mineral disorders are a frequent and severe complication in children with ESRD (1). Maintaining normal mineral levels is important to not only ensure adequate linear growth (2) but to also avoid cardiovascular complications (3).
Although disturbances in mineral metabolism are highly prevalent in pediatric dialysis patients (4), mineral abnormalities generally improve after transplantation. However, altered mineral metabolism appears to persist in some patients (5) with a potential effect on osteoporosis (6) and graft function. A high phosphorus load might promote deposition of calcium-phosphate crystals in the renal tubules, leading to microvascular and interstitial calcifications, which may contribute to the risk of graft failure (7). Indeed, in children (8) and adult patients with early (9) or advanced CKD (10), serum phosphorus was independently associated with a decline in renal function. Moreover, donor (11), pretransplant (12,13), and post-transplant (14–16) mineral levels have been associated with graft failure in adult renal graft recipients. Information on mineral metabolism in pediatric graft recipients mainly originates from small single-center studies with relatively short follow-up time (5,17,18). Therefore, we aimed to study the prevalence and potential determinants of a disturbed mineral metabolism in a large cohort of European pediatric renal transplant patients. Moreover, we studied the effect of mineral levels on graft function.
Materials and Methods
Study Participants
Data on mineral levels were collected within the European Society for Paediatric Nephrology/European Renal Association–European Dialysis and Transplant Association (ESPN/ERA-EDTA) Registry. On an annual basis, the registry collects individual patient data on date of birth, sex, treatment modality at start of RRT, and subsequent modality changes of all European children requiring RRT. Furthermore, a variable set of anthropometric, clinical, and medication-related parameters is collected. For these analyses, all measurements on serum calcium, phosphorus, and parathyroid hormone (PTH) collected from 2000 onward from patients aged <18 years with a functioning graft were included. This included data from the following countries and periods: Belarus (2009–2010), Belgium (2010–2011), Denmark (2006–2011), Finland (2000–2011), Hungary (2010), Norway (2009–2011), Portugal (2008–2011), Slovenia (2007–2011), Turkey (2011–2012), and the United Kingdom (2002–2012).
Definition of Variables
All biochemical variables (e.g., serum creatinine and mineral levels) are determined according to local practice. Because we included measurements from 2000 onward, we assumed serum creatinine levels were determined with the enzymatic method. The eGFR was calculated with the new bedside Schwartz equation (19). Abnormalities in calcium, phosphorus, and PTH were assessed using European Pediatric Dialysis Working Group (EPDWG) guidelines on prevention and treatment of renal osteodystrophy in children on chronic renal failure (20) (Table 1). Although these guidelines are largely opinion based, they take into account the age dependency of mineral metabolism during childhood. Calcium levels were assumed to be uncorrected for serum albumin and were therefore corrected as follows: calcium (milligrams per decaliter)+0.8 (4 − serum albumin [grams per decaliter]). Calcium and phosphorus levels are age dependent. Therefore, to make meaningful comparisons across the pediatric age range, we calculated SD scores using pediatric reference values (21). Missing values for albumin (18.6%), height (13.2%), and serum creatinine (2.2%) were imputed using multiple imputation creating five imputed data sets, thereby taking into account the uncertainty in the measurements (22,23). To test the validity of the Schwartz eGFR using imputed heights, we performed sensitivity analyses calculating eGFR according to the height-independent equation of Pottel et al. (24), which showed similar associations between mineral metabolism and eGFR according to both equations.
Table 1.
Target levels for PTH, calcium, and phosphate according to European guidelines on prevention and treatment of renal osteodystrophy in children with chronic renal failure
| Variable | Target Level |
|---|---|
| PTH (pg/ml) | |
| GFR (ml/min per 1.73 m2) | |
| >59 | 10–65 |
| 29< GFR≤59 | 10–65 |
| 15≤ GFR≤29 | 130–195 |
| <15 | 130–195 |
| Calcium (mg/dl) | |
| Age (yr) | |
| 0–2 | 8.8–11.3 |
| 3–5 | 9.4–10.8 |
| 6–12 | 9.4–10.3 |
| 13–18 | 8.8–10.2 |
| Phosphate (mg/dl) | |
| Age (yr) | |
| 0–2 | 4.8–7.4 |
| 3–5 | 4.5–6.5 |
| 6–12 | 3.6–5.8 |
| 13–18 | 2.3–4.5 |
PTH, parathyroid hormone.
Statistical Analyses
To satisfy normality assumptions, PTH values were log-transformed. Because mineral levels were repeatedly measured within the same patient, we used multinomial generalized estimating equation models (25) with clusters on country and patient level and an autoregressive working correlation structure to estimate the prevalence of, and factors associated with, mineral levels outside target range. Risk of graft failure was calculated as hazard ratios (HRs) using time-dependent Cox proportional hazards regression models adjusting for late entry into the risk set. To investigate the confounding effect of low eGFR on the association between mineral levels and risk of graft failure, we performed two sensitivity analyses: one excluding eGFR measurements below 20 ml/min per 1.73 m2 and one using marginal structural models. Marginal structural models adjust for bias caused by time-varying confounders (i.e., eGFR) that may affect changes in the parameter of interest (mineral level) (26). We adjusted all analyses for potential confounders according to criteria for confounding (27). Variables included in the adjusted analyses were as follows: age, sex, year of transplantation, time since transplantation, and eGFR. P values <0.05 were considered statistically significant. All statistical analyses were performed in SAS software (version 9.3; SAS Institute Inc, Cary, NC).
Results
Description
Data on mineral levels were available for 1237 renal transplant patients from 10 different countries, contributing to a total of 3358 measurements (median 2; range, 1–12 measurements per patient). Median time since transplantation was 3.0 years (interquartile range, 1.1–6.2 years). Most patients were male (59.9%), started RRT on peritoneal dialysis (52.7%), and received a kidney from a deceased donor (55.6%). Congenital anomalies of the kidney and the urinary tract were the most common cause of renal failure (44.4%). At time of measurement, most patients were between 13 and 17 years of age (58.9%) and had a median eGFR of 62 ml/min per 1.73 m2 (interquartile range, 49–78). Overall, the mean SD score for calcium was −0.07±1.16, whereas the mean phosphorus SD score was −0.50±0.98 (Table 2).
Table 2.
Patient characteristics
| Characteristic | Patients (n=1237), n (%) | Measurements (n=3358) |
|---|---|---|
| Age at start of RRT (yr) | ||
| 0–2 | 402 (32.5) | 1416 |
| 3–5 | 185 (15.0) | 464 |
| 6–12 | 407 (32.9) | 1021 |
| 13–17 | 243 (19.6) | 457 |
| Age at measurement (yr) | ||
| 0–2 | 35 (2.9) | 81 |
| 3–5 | 112 (9.1) | 335 |
| 6–12 | 361 (29.1) | 1047 |
| 13–17 | 729 (58.9) | 1895 |
| Sex (%) | ||
| Male | 741 (59.9) | 2033 |
| Female | 496 (40.1) | 1325 |
| Treatment modality at start of RRT | ||
| Hemodialysis | 265 (21.4) | 619 |
| Peritoneal dialysis | 651 (52.7) | 2024 |
| Transplantation | 317 (25.6) | 709 |
| Unknown/missing | 4 (0.3) | 6 |
| Primary renal disease | ||
| Congenital anomalies of the kidney and the urinary tract | 549 (44.4) | 1354 |
| GN | 114 (9.2) | 240 |
| Cystic kidneys | 128 (10.4) | 363 |
| Hereditary nephropathy | 182 (14.7) | 757 |
| Ischemic renal failure | 26 (2.1) | 62 |
| Hemolytic-uremic syndrome | 36 (2.9) | 91 |
| Metabolic disorders | 39 (3.1) | 80 |
| Vasculitis | 14 (1.1) | 33 |
| Miscellaneous | 79 (6.4) | 217 |
| Unknown/missing | 70 (5.7) | 161 |
| Transplant type | ||
| Deceased donor | 688 (55.6) | 1971 |
| Living donor | 454 (36.7) | 1153 |
| Unknown donor | 95 (7.7) | 234 |
| eGFR (ml/min per 1.73 m2) | ||
| Median (interquartile range) | 62 (49, 78) | |
| ≥90 | 153 (12.4) | 388 |
| 60–89 | 529 (42.7) | 1370 |
| 45–59 | 313 (25.3) | 920 |
| 30–44 | 169 (13.7) | 487 |
| ≤29 | 73 (5.9) | 193 |
| Calcium (mg/dl) | ||
| Mean (SD) | 9.56 (0.37) | |
| SD score, mean (SD) | −0.07 (1.16) | |
| Phosphorus (mg/dl) | ||
| Mean (SD) | 4.03 (0.50) | |
| SD score, mean (SD) | −0.50 (0.98) | |
| PTH, mean (SD) (pg/ml) | 111 (162) | |
| Height SD score, mean (SD) | −1.87 (0.79) |
PTH, parathyroid hormone.
Mean height SD score in the total cohort was −1.87±0.79. The annual change in height SD score (e.g., longitudinal growth) could be calculated for 678 patients. We did not find any association between mineral levels and annual change in height SD score. Mean annual change in height SD score was −0.02 (95% confidence interval [95% CI], −0.04 to 0.00) per unit change in calcium SD score, 0.02 (95% CI, −0.01 to 0.05) per unit change in phosphorus SD score, and −0.001 (95% CI, −0.001 to 0.00) per unit change in PTH.
Abnormalities in Mineral Metabolism
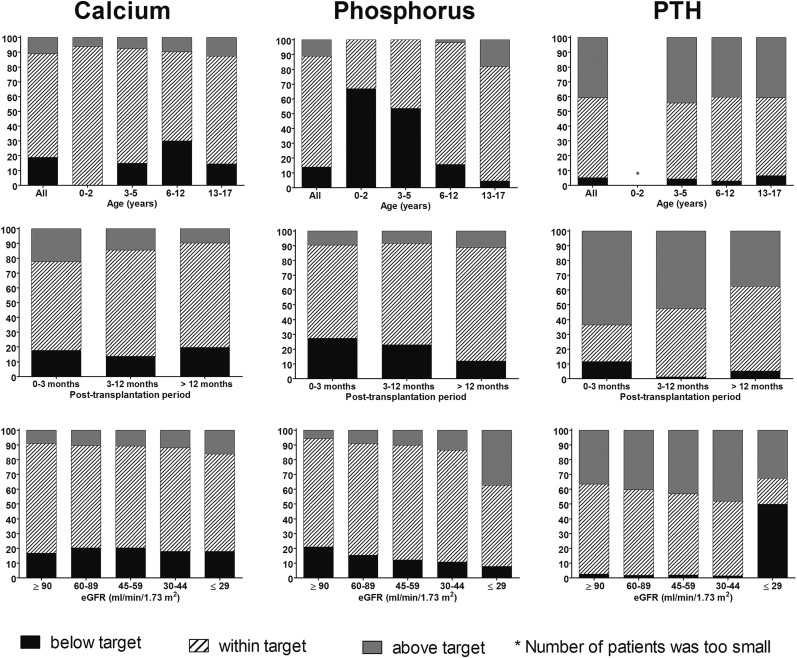
Hypocalcemia was found in 19.0% of the patients, with the highest prevalence observed among patients aged 6–12 years (30.1%), whereas 11.1% of the patients showed hypercalcemia and this prevalence was lower after a longer period since transplantation (Figure 1). Prevalence of hypophosphatemia was 13.9%, whereas prevalence of hyperphosphatemia was 11.0%. The rate of hypophosphatemia was higher among infants (66.9%) than in patients aged 13–17 years (4.6%). Furthermore, prevalence of hypophosphatemia was highest in the early post-transplant period and among patients with lower eGFR levels, whereas the reverse was true for hyperphosphatemia (Figure 1). PTH levels were below target range in 5.2% and above target range in 40.9% of the patients. This pattern was similar for all age groups. Results indicated that 63.6% of the patients showed elevated PTH levels in the immediate post-transplant period, whereas this was 37.6% after a post-transplant period of ≥12 months. The number of patients with PTH levels above the recommended target was higher with lower eGFR, except for the lowest eGFR group, in which 49.9% and 32.6% of the patients showed PTH levels below and above target range, respectively. However, this might partly be explained by the eGFR dependency of the PTH target levels (Figure 1).
Figure 1.
Distribution of patients with calcium, phosphorus, and PTH levels according to target levels stratified by age, post-transplant period, and eGFR. The asterisk indicates that the number of patients was too small. PTH, parathyroid hormone.
Factors Associated with Mineral Levels Outside Target Range
Female sex was associated with a significantly lower risk of having serum calcium levels below target (odds ratio [OR], 0.76, 95% CI, 0.60 to 0.97) and serum phosphorus levels above target (OR, 0.60; 95% CI, 0.45 to 0.80) (Table 3). Among female adolescents (13–17 years), the lower risk of hyperphosphatemia was even stronger (OR, 0.51; 95% CI, 0.38 to 0.68). Longer time since transplantation was associated with a lower risk of having calcium and PTH levels above target range, and with a lower risk of subtarget phosphorus levels. Furthermore, patients who were transplanted preemptively had a lower risk of displaying mineral levels above target range compared with patients who had been on dialysis before receiving a renal transplant, but this was statistically significant only for serum calcium (OR, 0.43; 95% CI, 0.28 to 0.64) and PTH (OR, 0.64; 95% CI, 0.48 to 0.86). Spending a longer time on dialysis before transplantation was not associated with higher risks of having mineral levels outside the target range.
Table 3.
ORs for having mineral levels outside target range
| Variable | Calcium | Phosphate | PTH | |||
|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | |
| Age (yr)a | ||||||
| 0–2 | — | — | — | — | — | — |
| 3–5 | 0.47 (0.34 to 0.65)b | 0.68 (0.43 to 1.08) | — | — | 1.76 (0.67 to 4.63) | 1.11 (0.71 to 1.73) |
| 6–12 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 13–17 | 0.43 (0.34 to 0.53)b | 1.35 (1.04 to 1.76)b | 0.28 (0.20 to 0.38)b | 10.08 (6.15 to 16.53)b | 2.46 (1.29 to 4.68)b | 0.98 (0.75 to 1.28) |
| Sex | ||||||
| Female | 0.76 (0.60 to 0.97)b | 1.25 (0.95 to 1.65) | 0.86 (0.66 to 1.12) | 0.60 (0.45 to 0.80)b | 0.73 (0.40 to 1.34) | 1.05 (0.81 to 1.37) |
| Male | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Time on transplantation (yr)c | ||||||
| <0.5 | 0.39 (0.26 to 0.61)b | 3.05 (1.64 to 5.69)b | 1.80 (1.06 to 3.06)b | 1.45 (0.84 to 2.48) | 0.31 (0.06 to 1.78) | 3.26 (1.93 to 5.51)b |
| 0.5 < 1 | 0.50 (0.34 to 0.75)b | 2.65 (1.64 to 4.29)b | 0.96 (0.50 to 1.82) | 1.38 (0.77 to 2.43) | 0.25 (0.05 to 1.35) | 1.88 (1.15 to 3.06)b |
| 1 < 2.5 | 0.65 (0.50 to 0.87)b | 1.17 (0.70 to 1.96) | 0.73 (0.47 to 1.14) | 1.33 (0.89 to 1.98) | 0.42 (0.15 to 1.15) | 1.61 (1.08 to 2.39)b |
| ≥2.5 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Preemptive transplantc | ||||||
| Yes | 1.54 (1.17 to 2.02)b | 0.43 (0.28 to 0.64)b | 0.88 (0.62 to 1.25) | 0.72 (0.50 to 1.04) | 1.37 (0.71 to 2.63) | 0.64 (0.48 to 0.86)b |
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Time on dialysis before transplant (yr)c | ||||||
| <0.25 | 1.03 (0.51 to 2.05) | 1.92 (0.97 to 3.77) | 0.24 (0.06 to 0.92)b | 0.93 (0.50 to 1.71) | — | — |
| 0.25 < 1 | 0.80 (0.56 to 1.14) | 0.96 (0.65 to 1.41) | 1.08 (0.70 to 1.68) | 1.03 (0.72 to 1.49) | 1.06 (0.44 to 2.54) | 1.02 (0.68 to 1.50) |
| 1 < 2 | 0.87 (0.61 to 1.23) | 1.29 (0.88 to 1.88) | 1.10 (0.70 to 1.72) | 0.69 (0.45 to 1.05) | 0.94 (0.40 to 2.24) | 0.79 (0.55 to 1.13) |
| ≥2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Steroidsd | ||||||
| Yes | 1.00 | 1.00 | 1.00 | 1.00 | — | — |
| No | 0.41 (0.20 to 0.83)b | 1.43 (0.83 to 2.46) | 0.60 (0.26 to 1.35) | 1.56 (0.86 to 2.81) | — | — |
| Calcineurin inhibitorsd | ||||||
| Cyclosporin A | 1.00 | 1.00 | 1.00 | 1.00 | — | — |
| Tacrolimus | 2.34 (1.54 to 3.55)b | 0.35 (0.22 to 0.56)b | 1.07 (0.73 to 1.57) | 1.09 (0.67 to 1.77) | — | — |
| None | 5.71 (2.85 to 11.42)b | 0.16 (0.05 to 0.54)b | 1.09 (0.33 to 3.66) | 1.53 (0.74 to 3.16) | ||
ORs are presented with 95% CIs. Dashes indicate that the number of patients with levels below or above target range in this category was too low to obtain an effect estimate. OR, odds ratio; 95% CI, 95% confidence interval; PTH, parathyroid hormone.
Adjusted for sex.
Significantly different from 1.
Adjusted for age, sex, and year of transplantation.
Adjusted for age, sex, time since transplantation, year of transplantation, and eGFR.
Patients who were off steroids showed a significantly lower risk of hypocalcemia compared with patients using steroids (OR, 0.41; 95% CI, 0.21 to 0.83), whereas patients using tacrolimus (OR, 3.37; 95% CI, 1.78 to 6.38) or patients not using calcineurin inhibitors as part of their immunosuppressive regimen (OR, 5.71; 95% CI, 2.85 to 11.42) showed a significantly higher risk of hypocalcemia compared with patients using cyclosporine.
Association between Mineral Levels and eGFR
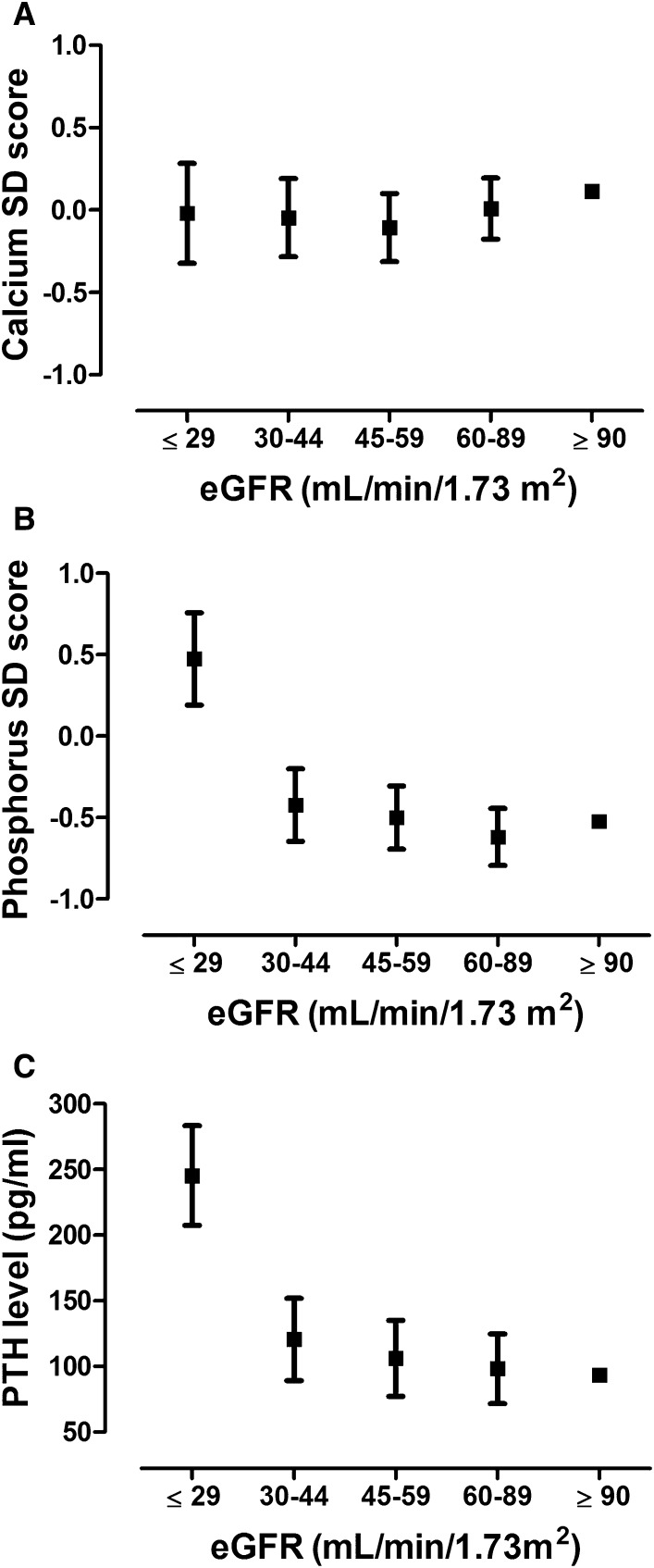
Calcium SD score was not associated with eGFR, whereas phosphorus SD score and PTH levels were significantly higher in patients in the lowest eGFR group (eGFR≤29 ml/min per 1.73 m2) compared with patients with an eGFR>90 ml/min per 1.73m2 (Figure 2).
Figure 2.
Mineral levels according to eGFR. Data represent levels of calcium SD score (A), phosphorus SD score (B), and PTH (C) with 95% confidence intervals. Adjustments were made for age, sex, time since transplantation, and year of transplantation. PTH, parathyroid hormone.
Mineral Levels and Risk of Graft Failure
HRs for the association between mineral levels and risk of graft failure are shown in Table 4. After a median follow-up of 3.0 years, 45 grafts were lost (including seven deaths). Although not significantly different, higher calcium levels (and calcium SD score) were associated with a lower risk of graft failure. Calcium levels below EPDWG target range were independently associated with a higher risk of graft failure compared with levels within target range (HR, 2.45; 95% CI, 1.10 to 5.47); however, after additional adjustment for eGFR, this association was no longer statistically significant. Phosphorus levels were independently associated with a higher risk of graft failure (adjusted HR per 0.30 mg/dl, 1.19; 95% CI, 1.07 to 1.33), but this association lost its statistical significance after further adjustment for eGFR. Because phosphorus levels within target range are possibly safe, we studied the effect of phosphorus levels above target. They were associated with a higher risk of graft failure compared with having phosphorus levels within target range, and this association remained after adjustment for eGFR (adjusted HR, 2.18; 95% CI, 1.10 to 4.32). To test the confounding effect of low eGFR levels in this association, we performed two sensitivity analyses. Our first sensitivity analysis, excluding eGFR measurements below 20 ml/min per 1.73 m2, showed that after adjustment for age, sex, year of transplantation, and eGFR phosphorus levels above EPDWG target range were associated with a higher risk of graft failure, but this association was not statistically significant (HR, 1.68; 95% CI, 0.78 to 3.60). The second sensitivity analysis, using marginal structural models and thereby adjusting for the effect of eGFR on phosphorus levels but not for the effect of phosphorus levels on eGFR, showed a significantly higher risk of graft failure for phosphorus levels above target range (HR, 2.04; 95% CI, 1.19 to 3.49).
Table 4.
HRs for mineral levels and graft failure
| Mineral Level | Unadjusted HR (95% CI) | Adjusted HR (95% CI)a | Adjusted HR (95% CI)b |
|---|---|---|---|
| Calcium | |||
| Continuous per 0.40 mg/dl | 0.85 (0.71 to 1.03) | 0.83 (0.68 to 1.02) | 0.90 (0.74 to 1.09) |
| SD score, per SD score | 0.85 (0.70 to 1.03) | 0.83 (0.67 to 1.01) | 0.92 (0.76 to 1.12) |
| EPDWG targets | |||
| Below target | 2.25 (1.02 to 4.96)c | 2.45 (1.10 to 5.47)c | 1.50 (0.66 to 3.41) |
| Within target | 1.00 | 1.00 | 1.00 |
| Above target | 0.66 (0.26 to 1.67) | 0.63 (0.25 to 1.61) | 0.73 (0.29 to 1.85) |
| Phosphorus | |||
| Continuous per 0.30 mg/dl | 1.19 (1.07 to 1.32)c | 1.19 (1.07 to 1.33)c | 1.08 (0.96 to 1.20) |
| SD score, per SD score | 1.33 (1.12 to 1.60)c | 1.34 (1.12 to 1.60)c | 1.11 (0.93 to 1.33) |
| EPDWG targets | |||
| Below target | 0.76 (0.16 to 3.65) | 0.75 (0.16 to 3.61) | 0.67 (0.14 to 3.24) |
| Within target | 1.00 | 1.00 | 1.00 |
| Above target | 3.79 (1.96 to 7.33)c | 3.82 (1.97 to 7.42)c | 2.18 (1.10 to 4.32)c |
| PTH | |||
| Continuous per 10 pg/ml | 1.01 (1.00 to 1.02) | 1.01 (0.99 to 1.02) | 1.00 (0.98 to 1.01) |
| EPDWG targets | |||
| Below target | 7.76 (2.20 to 27.4)c | 10.3 (2.77 to 38.13)c | 2.14 (0.49 to 9.34) |
| Within target | 1.00 | 1.00 | 1.00 |
| Above target | 1.45 (0.52 to 4.01) | 1.44 (0.51 to 4.07) | 0.87 (0.27 to 2.74) |
EPDWG guidelines on prevention and treatment of renal osteodystrophy in children on chronic renal failure. HR, hazard ratio; 95% CI, 95% confidence interval; EPDWG, European Pediatric Dialysis Working Group; PTH, parathyroid hormone.
Adjusted for age, sex, and year of transplantation.
Adjusted for age, sex, eGFR, and year of transplantation.
Significantly different from 1.
PTH levels below target range were significantly associated with graft failure (adjusted HR, 10.3; 95% CI, 2.77 to 38.13). However, this seemed mainly the effect of the eGFR dependency of PTH target levels, because the effect was no longer statistically significant after additional adjustment for eGFR.
Discussion
In this study, we demonstrate that disturbances in mineral metabolism are frequent in pediatric renal graft recipients. Furthermore, we found significant inverse associations between eGFR and phosphorus and PTH levels, as well as a higher risk of graft failure in patients with levels of serum phosphorus above the recommended targets.
We found a high prevalence of abnormalities in calcium, phosphorus, and PTH levels. Abnormalities in phosphorus levels were largely age dependent. Confirming previous reports (5,28,29), we observed that 25% of the patients had phosphorus levels outside target range. Hypophosphatemia was more prevalent than hyperphosphatemia, ranging from 67% in infants to 5% in adolescents. A high prevalence of hypophosphatemia (41%) has also been noted in infants on dialysis (4) and has been related to insufficient dietary supply. Hence, many patients may already be in a phosphate-depleted state when undergoing transplantation. In the early post-transplant period, impaired phosphorus reabsorption due to tubular dysfunction of the allograft may aggravate hypophosphatemia. Persistently elevated PTH and possibly fibroblast growth factor-23 (FGF-23) secretion may also contribute to urinary phosphate losses after transplantation (5). Although serum FGF-23 and vitamin D levels are not reported to our registry, our data show lower rates of hyperparathyroidism after a period of transplantation. Because hypophosphatemia may affect the mineralization of the growing bone, our findings point to a need for regular monitoring of serum phosphorus levels and consequent phosphate supplementation of hypophosphatemic patients.
Overall, 41% of our patients had elevated PTH levels. The prevalence of hyperparathyroidism was lower after a longer time since transplantation. When applying the same guidelines, the prevalence of hyperparathyroidism in our study is lower compared with North America (5) and Iran (28) (both 57%), and might reflect regional differences in PTH control, in keeping with data from pediatric patients on peritoneal dialysis (4).
Children who were on dialysis before transplantation tended to have higher mineral levels than those transplanted preemptively, possibly due to the persistence of hyperparathyroidism (5,12,30). However, we did not find an association between dialysis vintage and mineral disturbances. Furthermore, a longer time since transplantation was associated with a lower risk of having mineral levels above target range, suggesting that mineral levels improve over time, in keeping with others (30). This potential improvement might also be explained by a more optimal management over time. With a mean height SD score of −1.87, growth failure was highly prevalent after pediatric renal transplantation. However, there was no association between mineral levels and annual change in height SD score, suggesting that other factors play a more dominant role in growth after transplantation.
Surprisingly, we found a lower risk of hyperphosphatemia among female patients, especially among adolescents. Data from a recent study in adults suggest that women are more susceptible to hyperactivity of the parathyroid gland due to estrogen action (30). Unfortunately, information regarding estrogen levels or menstrual history was not available. Immunosuppressive drugs can induce mineral effects. Corticosteroids can modulate calcium homeostasis, leading to decreased intestinal absorption and increased urinary excretion (31). Indeed, patients off steroids had a significantly lower risk of hypocalcemia compared with patients using steroids.
We found inverse relationships of serum phosphorus and PTH levels with renal function. Levels were found relatively stable up to 30 ml/min per 1.73 m2 eGFR and were significantly higher at lower eGFR. By contrast, we did not find any association with global renal function for calcium. This is in line with other studies, as Bachetta et al. did not find any relationship between GFR and phosphorus and calcium among patients with GFRs>30 ml/min per 1.73 m2 (32). In studies in native kidneys, normal phosphorus levels, phosphorus SD score, and PTH were maintained until GFR dropped below 30 (33), whereas serum calcium was independent of GFR.
We found an association between serum phosphorus and risk of graft failure. Several studies have shown associations between serum phosphorus levels and decline in renal function in pediatric and adult patients with CKD (8–10), and graft failure in adult renal transplant patients (11,13–15). Serum phosphorus levels above recommended targets were associated with a higher risk of graft loss, even after adjustment for eGFR. Because a decreasing eGFR affects overall patient well-being, increasing serum phosphorus levels might also be a marker of disease severity, and elevated phosphorus levels are thus rather the consequence of the failing transplant. It should be noted that the patient group (5.9% of total population) with the lowest eGFR (<29 ml/min per 1.73 m2) is responsible for the statistical weight of this association. When excluding this group, the association between serum phosphorus and graft failure lost its significance. This is not surprising, because statistical power is markedly reduced when the patient group with the highest risk of graft loss is excluded. Similarly, in our sensitivity analysis excluding eGFR levels below 20 ml/min per 1.73 m2, the association between elevated phosphorus levels and graft failure lost its significance. The effect in this sensitivity analysis is likely to be a long-term effect as the mean time between the measurement of high phosphorus and graft failure was 2.6 years. Our other sensitivity analysis (the marginal structural model), likewise identified a higher risk of graft failure for elevated phosphorus levels, suggesting that phosphorus levels may be the cause of graft failure. However, our study is observational and can therefore not prove causation. By contrast, two studies among adult (16) and pediatric (5) graft recipients did not show consistent associations between elevated phosphorus levels and decrease of renal function, time to first rejection, graft loss, or mortality. They did, however, find independent associations of elevated FGF-23 with these outcomes, even in a subgroup of patients with eGFR between 30–90 (16). This difference might be due to the longer follow-up time in our study, because recent data in children with predialysis CKD have shown that FGF-23 concentrations increased early and progressively as GFR declined, and preceded any increase in serum phosphorus (34). Unfortunately, FGF-23 is not reported in our registry, so we were not able to test whether this hormone was associated with graft failure. More research is needed to elucidate whether, and by which pathophysiological mechanisms, serum phosphorus levels may lead to a higher risk of graft failure.
Although we studied a large population with a long follow-up, and used repeatedly measured mineral levels for estimating the risk of graft failure instead of using only a single baseline value, some limitations of our work need to be acknowledged. These include the lacking availability of serum vitamin D or FGF-23 levels, which might have provided greater insight into potential etiological mechanisms. In addition, the reported medication use (e.g., phosphate or vitamin D supplementation) or mineral intake was very limited in our registry; therefore, we were not able to adjust the associations for medication use. Phosphorus levels seem to follow the normal circadian rhythm (35) and fluctuate during the day. In this large patient population, the variation in measurements is likely to be leveled out. Furthermore, the use of different analytical methods for determining mineral levels may have influenced our results. PTH measurements in particular are subject to high intermethod variability (36). However, the association between mineral levels and outcomes is likely unrelated to varying mineral levels due to timing of measurement or use of different analytical methods (nondifferential misclassification), which, if anything, would most likely have led to an underestimation or dilution of the reported effects.
To summarize, abnormalities in mineral metabolism after pediatric kidney transplantation are common. In infants, hypophosphatemia is highly prevalent, reflecting persistently impaired bone mineralization, even after transplantation, during this critical period of growth and development. Conversely, adolescent graft recipients are largely hyperphosphatemic. Hyperphosphatemia predicts the loss of graft function, although cause-effect relationships are unclear.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank the patients, their parents, and the staff of all of the dialysis and transplant units who have contributed data via their national registries and contact persons. We also thank R. Coppo, D. Haffner, J. Harambat, and C. Stefanidis for being members of the European Society for Paediatric Nephrology/European Renal Association–European Dialysis and Transplant Association (ESPN/ERA-EDTA) Registry Committee. In addition, we thank D. Shtiza, R. Kramar, R. Oberbauer, S. Baiko, A. Sukalo, K. van Hoeck, F. Collart, J.M. des Grottes, D. Pokrajac, D. Roussinov, D. Batinić, M. Lemac, J. Slavicek, T. Seeman, K. Vondrak, J.G. Heaf, U. Toots, P. Finne, C. Grönhagen-Riska, C. Couchoud, M. Lasalle, E. Sahpazova, N. Abazi, N. Ristoka Bojkovska, G. von Gersdorff, C. Scholz, B. Tönshoff, N. Afentakis, A. Kapogiannis, N. Printza, G. Reusz, C.S. Berecki, A. Szabó, T. Szabó, Z.S. Györke, E. Kis, R. Palsson, V. Edvardsson, B. Gianoglio, S. Maringhini, C. Pecoraro, S. Picca, S. Testa, E. Vidal, A. Jankauskiene, B. Pundziene, V. Said-Conti, S. Gatcan, O. Berbeca, N. Zaikova, S. Pavićević, T. Leivestad, A. Bjerre, A. Zurowska, I. Zagozdzon, C. Mota, M. Almeida, C. Afonso, G. Mircescu, L. Garneata, E.A. Molchanova, N.A. Tomilina, B.T. Bikbov, M. Kostic, A. Peco-Antic, B. Spasojevic-Dimitrijeva, G. Milosevski-Lomic, D. Paripovic, S. Puric, D. Kruscic, L. Podracka, G. Kolvek, J. Buturovic-Ponikvar, G. Novljan, N. Battelino, A. Alonso Melgar, and the Spanish Pediatric Registry. Finally, we thank S. Schön, K.G. Prütz, L. Backman, M. Stendahl, M. Evans, B. Rippe, C.E. Kuenhi, S. Rossi, E. Maurer, B. Schnarwyler, C.E. Kuehni, G. Laube, A. Hoitsma, A. Hemke, and all centers participating in the RichQ study, as well as R. Topaloglu, D. Ivanov, R. Pruthi, F. Braddon, S. Mannings, A. Cassula, and M.D. Sinha for contributing data to the ESPN/ERA-EDTA Registry.
The ESPN/ERA-EDTA Registry is funded by the European Society of Pediatric Nephrology and the European Renal Association and European Dialysis and Transplant Association. This publication arises from the project of the ESPN/ERA-EDTA Registry (ESPN_FY2013), which has received funding from the European Union, in the Health Framework Programme.
Preliminary results from this study were presented at the 51st ERA-EDTA Congress, held May 31–June 3, 2014, in Amsterdam, The Netherlands.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06200614/-/DCSupplemental.
References
- 1.Schmitt CP, Mehls O: Mineral and bone disorders in children with chronic kidney disease. Nat Rev Nephrol 7: 624–634, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Bacchetta J, Harambat J, Cochat P, Salusky IB, Wesseling-Perry K: The consequences of chronic kidney disease on bone metabolism and growth in children. Nephrol Dial Transplant 27: 3063–3071, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitsnefes MM: Cardiovascular disease in children with chronic kidney disease. J Am Soc Nephrol 23: 578–585, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borzych D, Rees L, Ha IS, Chua A, Valles PG, Lipka M, Zambrano P, Ahlenstiel T, Bakkaloglu SA, Spizzirri AP, Lopez L, Ozaltin F, Printza N, Hari P, Klaus G, Bak M, Vogel A, Ariceta G, Yap HK, Warady BA, Schaefer F, International Pediatric PD Network (IPPN) : The bone and mineral disorder of children undergoing chronic peritoneal dialysis. Kidney Int 78: 1295–1304, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Wesseling-Perry K, Tsai EW, Ettenger RB, Jüppner H, Salusky IB: Mineral abnormalities and long-term graft function in pediatric renal transplant recipients: A role for FGF-23? Nephrol Dial Transplant 26: 3779–3784, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamminen IS, Valta H, Jalanko H, Salminen S, Mäyränpää MK, Isaksson H, Kröger H, Mäkitie O: Pediatric solid organ transplantation and osteoporosis: A descriptive study on bone histomorphometric findings. Pediatr Nephrol 29: 1431–1440, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Evenepoel P, Lerut E, Naesens M, Bammens B, Claes K, Kuypers D, Vermeersch P, Meijers B, Van Damme B, Vanrenterghem Y: Localization, etiology and impact of calcium phosphate deposits in renal allografts. Am J Transplant 9: 2470–2478, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Staples AO, Greenbaum LA, Smith JM, Gipson DS, Filler G, Warady BA, Martz K, Wong CS: Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol 5: 2172–2179, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chue CD, Edwards NC, Davis LJ, Steeds RP, Townend JN, Ferro CJ: Serum phosphate but not pulse wave velocity predicts decline in renal function in patients with early chronic kidney disease. Nephrol Dial Transplant 26: 2576–2582, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Voormolen N, Noordzij M, Grootendorst DC, Beetz I, Sijpkens YW, van Manen JG, Boeschoten EW, Huisman RM, Krediet RT, Dekker FW, PREPARE Study Group : High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 22: 2909–2916, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Chang PC, Saha S, Gomes AM, Padiyar A, Bodziak KA, Poggio ED, Hricik DE, Augustine JJ: Donor phosphorus levels and recipient outcomes in living-donor kidney transplantation. Clin J Am Soc Nephrol 6: 1179–1184, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roodnat JI, van Gurp EA, Mulder PG, van Gelder T, de Rijke YB, de Herder WW, Kal-van Gestel JA, Pols HA, Ijzermans JN, Weimar W: High pretransplant parathyroid hormone levels increase the risk for graft failure after renal transplantation. Transplantation 82: 362–367, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Sampaio MS, Molnar MZ, Kovesdy CP, Mehrotra R, Mucsi I, Sim JJ, Krishnan M, Nissenson AR, Kalantar-Zadeh K: Association of pretransplant serum phosphorus with posttransplant outcomes. Clin J Am Soc Nephrol 6: 2712–2721, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egbuna OI, Taylor JG, Bushinsky DA, Zand MS: Elevated calcium phosphate product after renal transplantation is a risk factor for graft failure. Clin Transplant 21: 558–566, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Moore J, Tomson CR, Tessa Savage M, Borrows R, Ferro CJ: Serum phosphate and calcium concentrations are associated with reduced patient survival following kidney transplantation. Clin Transplant 25: 406–416, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I: Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 22: 956–966, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garabedian M, Silve C, Levy-Bentolila D, Bourdeau A, Ulmann A, Nguyen TM, Lieberherr M, Broyer M, Balsan S: Changes in plasma 1,25 and 24,25-dihydroxyvitamin D after renal transplantation in children. Kidney Int 20: 403–410, 1981 [DOI] [PubMed] [Google Scholar]
- 18.Wesseling-Perry K, Pereira RC, Tsai E, Ettenger R, Jüppner H, Salusky IB: FGF23 and mineral metabolism in the early post-renal transplantation period. Pediatr Nephrol 28: 2207–2215, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klaus G, Watson A, Edefonti A, Fischbach M, Rönnholm K, Schaefer F, Simkova E, Stefanidis CJ, Strazdins V, Vande Walle J, Schröder C, Zurowska A, Ekim M, European Pediatric Dialysis Working Group (EPDWG) : Prevention and treatment of renal osteodystrophy in children on chronic renal failure: European guidelines. Pediatr Nephrol 21: 151–159, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lockitch G, Halstead AC, Albersheim S, MacCallum C, Quigley G: Age- and sex-specific pediatric reference intervals for biochemistry analytes as measured with the Ektachem-700 analyzer. Clin Chem 34: 1622–1625, 1988 [PubMed] [Google Scholar]
- 22.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR: Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 338: b2393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative : The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 370: 1453–1457, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Pottel H, Hoste L, Martens F: A simple height-independent equation for estimating glomerular filtration rate in children. Pediatr Nephrol 27: 973–979, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Kuss O, McLerran D: A note on the estimation of the multinomial logistic model with correlated responses in SAS. Comput Methods Programs Biomed 87: 262–269, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Robins JM, Hernán MA, Brumback B: Marginal structural models and causal inference in epidemiology. Epidemiology 11: 550–560, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Jager KJ, Zoccali C, Macleod A, Dekker FW: Confounding: What it is and how to deal with it. Kidney Int 73: 256–260, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Derakhshan A, Behbahan AG, Lotfi M, Omrani GH, Fallahzadeh MH, Basiratnia M, Al-Hashemi GH: Bone mineral disorders in pediatric and adolescent renal transplant recipients. Pediatr Transplant 15: 367–375, 2011 [DOI] [PubMed] [Google Scholar]
- 29.van Husen M, Lehnhardt A, Fischer AK, Brinkert F, Loos S, Oh J, Kemper MJ: Fibroblast growth factor 23 and calcium phosphate homeostasis after pediatric renal transplantation. Pediatr Transplant 16: 443–450, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Kim YJ, Kim MG, Jeon HJ, Ro H, Park HC, Jeong JC, Oh KH, Ha J, Yang J, Ahn C: Clinical manifestations of hypercalcemia and hypophosphatemia after kidney transplantation. Transplant Proc 44: 651–656, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Bacchetta J, Ranchin B, Demède D, Allard L: The consequences of pediatric renal transplantation on bone metabolism and growth [published online ahead of print August 29, 2013]. Curr Opin Organ Transplant 10.1097/MOT.0b013e3283651b21 [DOI] [PubMed] [Google Scholar]
- 32.Bacchetta J, Dubourg L, Harambat J, Ranchin B, Abou-Jaoude P, Arnaud S, Carlier MC, Richard M, Cochat P: The influence of glomerular filtration rate and age on fibroblast growth factor 23 serum levels in pediatric chronic kidney disease. J Clin Endocrinol Metab 95: 1741–1748, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL: Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: Results of the study to evaluate early kidney disease. Kidney Int 71: 31–38, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Portale AA, Wolf M, Jüppner H, Messinger S, Kumar J, Wesseling-Perry K, Schwartz GJ, Furth SL, Warady BA, Salusky IB: Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol 9: 344–353, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portale AA, Halloran BP, Morris RC, Jr: Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25-dihydroxyvitamin D. J Clin Invest 80: 1147–1154, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Souberbielle JC, Boutten A, Carlier MC, Chevenne D, Coumaros G, Lawson-Body E, Massart C, Monge M, Myara J, Parent X, Plouvier E, Houillier P: Inter-method variability in PTH measurement: Implication for the care of CKD patients. Kidney Int 70: 345–350, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.