Abstract
Background and objectives
Cystinuria is an autosomal recessive disorder affecting renal cystine reabsorption; it causes 1% and 8% of stones in adults and children, respectively. This study aimed to determine epidemiologic and clinical characteristics as well as comorbidities among cystinuric patients, focusing on CKD and high BP.
Design, setting, participants, & measurements
This retrospective study was conducted in France, and involved 47 adult and pediatric nephrology and urology centers from April 2010 to January 2012. Data were collected from 442 cystinuric patients.
Results
Median age at onset of symptoms was 16.7 (minimum to maximum, 0.3–72.1) years and median diagnosis delay was 1.3 (0–45.7) years. Urinary alkalinization and cystine-binding thiol were prescribed for 88.8% and 52.2% of patients, respectively, and 81.8% had at least one urological procedure. Five patients (1.1%, n=4 men) had to be treated by dialysis at a median age of 35.0 years (11.8–70.7). Among the 314 patients aged ≥16 years, using the last available plasma creatinine, 22.5% had an eGFR≥90 ml/min per 1.73 m2 (calculated by the Modification of Diet in Renal Disease equation), whereas 50.6%, 15.6%, 7.6%, 2.9%, and 0.6% had an eGFR of 60–89, 45–59, 30–44, 15–29, and <15, respectively. Among these 314 patients, 28.6% had high BP. In multivariate analysis, CKD was associated with age (odds ratio, 1.05 [95% confidence interval, 1.03 to 1.07]; P<0.001), hypertension (3.30 [1.54 to 7.10]; P=0.002), and severe damage of renal parenchyma defined as a past history of partial or total nephrectomy, a solitary congenital kidney, or at least one kidney with a size <10 cm in patients aged ≥16 years (4.39 [2.00 to 9.62]; P<0.001), whereas hypertension was associated with age (1.06 [1.04 to 1.08]; P<0.001), male sex (2.3 [1.3 to 4.1]; P=0.003), and an eGFR<60 ml/min per 1.73 m2 (2.7 [1.5 to 5.1]; P=0.001).
Conclusions
CKD and high BP occur frequently in patients with cystinuria and should be routinely screened.
Keywords: kidney stones, CKD, genetic renal disease, hypertension
Introduction
Cystinuria (OMIM 220100), an autosomal recessive hereditary disease, is the most frequent monogenic cause of renal calculi, and is responsible for 1% and 8% of nephrolithiasis in adults and children, respectively (1,2). Cystinuria affects dibasic amino acid and cystine reabsorption in the renal proximal tubule. Because of its poor solubility at a typical urine pH of <7, excess cystine results in urinary cystine stone recurrent formation. The final diagnosis mainly relies on stone analysis, urinary cystine crystal identification, or urinary cystine measurement. Alkaline hyperdiuresis is the cornerstone of the management. Sulfhydryl derivatives can sometimes be added to facilitate urinary cystine solubilization. In cases of stone formation, urological procedures must be performed. To date, few studies have evaluated comorbidities associated with cystinuria. This study aimed to determine epidemiologic and clinical characteristics, medical and surgical treatments, and comorbidities among cystinuric patients. To better characterize this pathology, we conducted a retrospective multicenter study in France. We focused on the prevalence of CKD and high blood pressure (HBP) among cystinuric patients and on their determinants.
Materials and Methods
Study Population
We conducted a national retrospective cohort study from April 2010 to January 2012. In April 2010, we contacted all 150 French departments of nephrology, urology, and pediatrics (likely to treat patients with kidney stones) by mail to ask physicians to complete a questionnaire. Thirty-one percent of the contacted departments participated in the study. All patients with a diagnosis of cystinuria confirmed by stone analysis, crystalluria, urinary cystine excretion, or Brand reaction (3) were eligible for inclusion. In France, the screening is proposed for at-risk siblings. Data were treated anonymously. The Advisory Committee on Information Processing Research in the Field of Health approved this study (approval no. 10.640bis).
Data Collection
The database included epidemiologic and clinical characteristics, laboratory data, medical and surgical treatments, and comorbidities. For the analysis, episodes of ARF were excluded. eGFR was calculated using the last available plasma creatinine (PCr) from the Modification of Diet in Renal Disease (MDRD; version 4) equation (4) in patients aged ≥16 years at that time or the Schwartz equation (5) in patients aged <16 years if the size was available concomitantly to creatinine measurement. In the absence of recorded albuminuria, we defined CKD as an eGFR<60 ml/min per 1.73 m2 according to the Kidney Disease Improving Global Outcomes (KDIGO) 2012 clinical practice guidelines (6). When available concomitantly to the last creatinine measurement, proteinuria was considered as significant if it was >0.5 g per 24 hours. Severe impairment of renal parenchyma was defined as a history of partial or total nephrectomy, a solitary congenital kidney, or at least one kidney with a size <10 cm in patients aged ≥16 years (7) during follow-up. Diagnosis of HBP was retained if the patient was declared by the physician to suffer from hypertension during follow-up, irrespective of antihypertensive treatment. No cut-off of BP was given to the physician. Diagnosis of obesity during follow-up relied on a body-mass index ≥30 kg/m2 or a positive answer to the question regarding obesity.
Statistical Analyses
Data are presented as medians (minimum to maximum), means (SD), and percentages. For continuous data, means were compared with a t test or ANOVA when more than two groups were studied. Correlations were assessed with the Pearson coefficient. Categorical data were compared with a chi-squared test or Fisher’s exact test when appropriate. We used univariate analysis to study the risk of CKD and HBP. Only variables found to be significant in univariate analysis were included in multivariate analysis using a logistic regression. Odds ratios (ORs) and 95% confidence intervals (95% CIs) for the probability of having CKD or HBP were calculated for each of these variables. A P value <0.05 was considered significant.
Results
Epidemiologic Characteristics of the Patients and Treatments
We collected data from 442 patients (220 male participants) through 47 clinical departments: 23 specialized in nephrology (n=250), eight in urology (n=101), and 16 in pediatrics (n=91). Epidemiologic characteristics are described in Table 1. Male and female patients had a similar median age at presentation (P=0.60). The median delay from first symptoms to diagnosis was the same in later years compared with earlier years. This delay was longer among patients who were older at first symptoms (median 0.33 [0–45.7] years if age at first symptoms was <16 years; median 3.04 [0–45.7] years if first symptoms occurred after 16 years; P<0.001).
Table 1.
Epidemiologic characteristics of the 442 patients and treatments
| Characteristic | Value |
|---|---|
| Median age at last follow-up (yr) | 32.5 (0.3–86.6) |
| Male sex | 49.9 |
| Age at diagnosis (yr) | |
| age <16 | 20.5 |
| 16≤ age <40 | 41.7 |
| 40≤ age <60 | 24.1 |
| age ≥60 | 13.7 |
| Ethnicity | |
| White | 74.3 |
| Black African | 0 |
| Other | 25.7 |
| Diabetes | 3.8 |
| Obesity | 10.4 |
| Hypertension | 21.4 |
| Median time from initial symptoms to last follow-up (yr) | 15.8 (0.1–65.6) |
| Median age at presentation (yr) | 16.7 (0.3–72.1) |
| Male participants | 16.5 (0.3–72.1) |
| Female participants | 17.1 (0.3–72.1) |
| Median age at diagnosis (yr) | 18.5 (0.0–74.3) |
| Median time from first symptoms to diagnosis (yr) | 1.3 (0.0–45.7) |
| Diagnostic tools | |
| Stone analysis | 57.7 |
| Cystinuria assessment | 28.5 |
| Crystalluria | 3 |
| Brand reaction | 1.9 |
| Several | 8.9 |
| Symptoms leading to diagnosis | |
| Renal colic | 60.6 |
| Family screening | 11.9 |
| Urinary tract infection | 5.0 |
| Hematuria | 2.4 |
| Bladder calculi | 2.1 |
| Staghorn calculi | 2.1 |
| Spontaneous expulsion of a stone | 0.8 |
| Combined symptoms | 7.9 |
| Other | 5.6 |
| Fortuitous discovery | 1.6 |
| Medical treatment (% of patients) | 90.6 |
| Urinary alkalinization | 88.8 |
| D-penicillamine or tiopronin | 52.2 |
| Captopril | 12.9 |
| Urological procedure among 402 symptomatic patients (% of patients) | |
| At least one urological procedure | 89.9 |
| At least one lumbotomya or ureterolithotomy | 42.6 |
| At least one minimally invasive procedure (endoscopy, shock wave lithotripsy or percutaneous surgery) | 80.1 |
| Median time from first symptoms to first surgical procedure (yr) | 0.5 (0.0–45.7) |
Data are given as medians (minimum to maximum) or percentages unless otherwise stated.
Lumbotomy is defined as extraperitoneal open surgery by lumbotomy (incision on the lumbar fossa).
Diagnosis modalities depended on the age at diagnosis: ≥16 years, diagnosis was mostly based on renal colic (79.6% versus 33.1% for patients aged <16 years; P<0.001); and <16 years, diagnosis came from screening in one third of patients (31.0% versus 0% ≥16 years; P<0.001) and after urinary tract infection in 10.3% of patients (versus 1.0% ≥16 years; P<0.001). Only 40 patients were symptom free at diagnosis and remained asymptomatic during follow-up. Most patients were treated with supportive measures (Table 1). The types of urological procedures per patient are described in Table 2.
Table 2.
Number and types of urological procedures per patient in the 442 cystinuric patients
| Urological Procedure | No. of Procedures | Patients Who Had Each Procedure (%) | ||
|---|---|---|---|---|
| Mean ± SD | Median (Minimum to Maximum) | 1 procedure | ≥2 procedures | |
| Lumbotomya | 0.58±1.01 | 0 (0–6) | 20.5 | 14.2 |
| Ureterolithotomy | 0.12±0.45 | 0 (0–4) | 6.1 | 2.3 |
| Partial nephrectomy | 0.02±0.13 | 0 (0–1) | 1.8 | 0 |
| Total nephrectomy | 0.07±0.26 | 0 (0–1) | 7.1 | 0 |
| Percutaneous nephrolithotomy | 1.17±1.87 | 0 (0–12) | 18.4 | 28.0 |
| Rigid ureteroscopy | 0.56±1.31 | 0 (0–10) | 17.2 | 11.6 |
| Flexible ureteroscopy | 0.67±1.48 | 0 (0–9) | 9.5 | 16.4 |
| Shock wave lithotripsy | 2.02±3.38 | 1 (0–30) | 14.8 | 38.8 |
| Vesical surgery | 0.05±0.23 | 0 (0–2) | 4.4 | 0.2 |
| Vesical endoscopy | 0.05±0.29 | 0 (0–4) | 2.8 | 0.7 |
| All procedures | 5.15±5.53 | 4 (0–36) | 9.6 | 71.4 |
Lumbotomy is defined as extraperitoneal open surgery by lumbotomy (incision on the lumbar fossa).
CKD
In the entire population of 442 patients, five (1.1%, n=four men) progressed to ESRD requiring RRT at a median age of 35.0 (11.8–70.7) years and only one was aged <16 years. The delay between onset of symptoms and RRT varied from 6.2 to 35.0 years. Four of these patients have received kidney transplants.
Among the 128 patients aged <16 years, eGFR could be recorded for 58 (45%). Among them, 48 (82.2%), seven (12.1%), one (1.7%), 2 (3.4%), zero (0%), and zero (0%) had an eGFR≥90 ml/min per 1.73 m2, an eGFR of 60–89 ml/min per 1.73 m2, and CKD stage 3a, 3b, 4, and 5, respectively. Among these 58 patients, proteinuria could be recorded for 24. Only one child, whose eGFR was ≥90 ml/min per 1.73 m2, had significant proteinuria.
In order to standardize eGFR calculated using the last available PCr, we restricted analysis to the group of patients aged ≥16 years at that time (n=314). The median age of this adult cohort was 38.7 years (16.2–86.6); of these, 51.6% were aged 16–39 years, 30.6% were aged 40–59 years, and 17.8% were aged ≥60 years. Within this population of 314 patients, 14 (4.5%) suffered from diabetes, 40 (13.3%) were obese, and 88 (28.6%) had HBP.
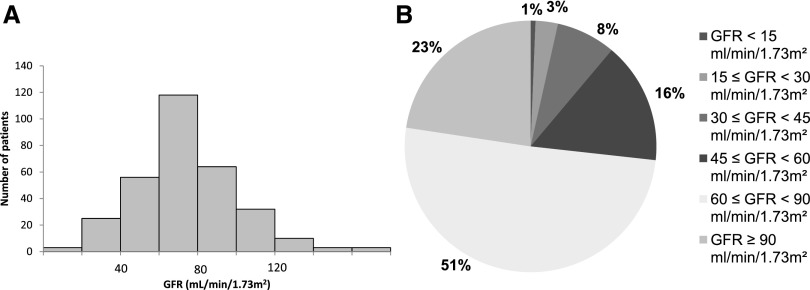
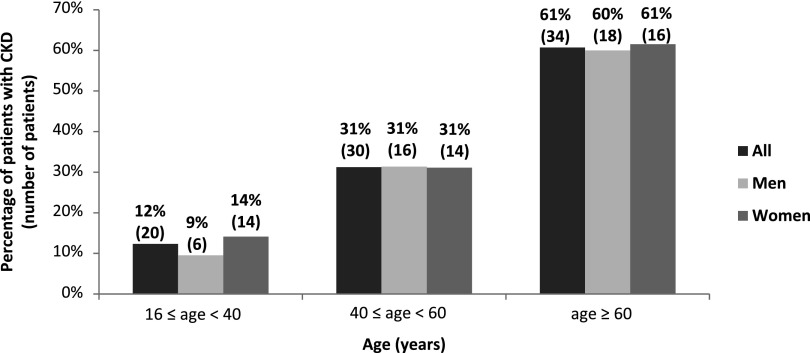
Last PCr was measured with a median delay of 19.0 (0–65.5) years after first symptoms. Median eGFR was 71.8 ml/min per 1.73 m2 (5.4–171.7). Eighty-four (26.8%) patients had impaired renal function as indicated by an eGFR<60 ml/min per 1.73 m2. The distribution of eGFR values is normal and is represented in Figure 1A. The distribution of eGFR according to the KDIGO 2012 clinical practice guidelines is represented in Figure 1B. For the study of renal parenchyma damage, data were available for 234 patients within the cohort of 314 adult patients. Among these, 117 (50%) had severe renal parenchyma damage: 82 had at least one kidney with a reduced size <10 cm, 34 had a history of partial/total nephrectomy (32 for lithiasis and two for tumor and tuberculosis), and one patient had a solitary congenital kidney. Among patients with at least one measurement of kidney size and/or a history of partial/total nephrectomy followed by PCr assessment (n=197), severe renal parenchyma damage was significantly associated with CKD (Table 3). The variables associated with CKD in univariate analysis are shown in Table 3. The distribution of CKD according to age and sex is represented in Figure 2. In multivariate analysis, CKD was associated with age (OR, 1.05; 95% CI, 1.03 to 1.07; P<0.001), hypertension (OR, 3.30; 95% CI, 1.54 to 7.10; P=0.002), and severe damage of renal parenchyma (OR, 4.39; 95% CI, 2.00 to 9.62; P<0.001). After excluding classic CKD risk factors (HBP, severe damage of renal parenchyma, diabetes mellitus, and obesity), the prevalence of CKD was 4.2%, 15.4%, and 20.0% among patients aged 16–39 years (n=48), 40–59 years (n=13), and ≥60 years, respectively (n=5).
Figure 1.
Distribution of GFR (eGFR using the MDRD equation, version 4) calculated from the last available plasma creatinine measurement in the 314 patients aged ≥16 years. (A) Overall distribution of GFR. (B) Distribution of GFR according to the Kidney Disease Improving Global Outcomes classification. MDRD, Modification of Diet in Renal Disease.
Table 3.
Univariate analysis of risk of CKD stages 3–5 (eGFR<60 ml/min per 1.73 m2) in patients aged ≥16 years (n=314)
| Considered Parameters | No. of Patientsa | Median eGFR (Minimum to Maximum) | Average eGFR (SD) or Correlation Coefficient | P Value | Patients with eGFR <60 (%) | OR (95% CI) | P Value |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Female | 170 | 72 (5–172) | 75 (27) | 0.79 | 25.9 | 1 | |
| Male | 144 | 72 (19–165) | 74 (26) | 27.9 | 0.91 (0.55 to 1.50) | 0.70 | |
| Age (yr) | |||||||
| 16≤ age <40 | 162 | 82 (5–172) | 87 (26) | <0.001 | 12.4 | 1 | |
| 40≤ age <60 | 96 | 66 (23–117) | 65 (17) | 31.3 | 3.23 (1.71 to 6.10) | <0.001 | |
| age ≥60 | 56 | 54 (14–108) | 54 (20) | 60.7 | 10.97 (5.38 to 22.36) | <0.001 | |
| Diabetes | |||||||
| No | 294 | 73 (5–172) | 75 (26) | 0.001 | 25.2 | 1 | |
| Yes | 14 | 62 (25–78) | 57 (18) | 50.0 | 2.97 (1.01 to 8.76) | 0.05 | |
| High BP | |||||||
| No | 220 | 75 (19–172) | 80 (26) | <0.001 | 17.7 | 1 | |
| Yes | 88 | 61 (5–121) | 60 (22) | 48.9 | 4.43 (2.58 to 7.63) | <0.001 | |
| Obesity | |||||||
| No | 260 | 74 (5–172) | 77 (27) | <0.001 | 23.1 | 1 | |
| Yes | 40 | 63 (19–121) | 61 (19) | 45.0 | 2.73 (1.37 to 5.42) | 0.004 | |
| Age at first symptoms (yr) | 282 | −0.19 | 0.001 | 1.02 (1.00 to 1.04) | 0.05 | ||
| <16 | 110 | 76 (23–172) | 81 (30) | 0.01 | 20.9 | 1 | |
| ≥16 | 172 | 71 (5–153) | 71 (24) | 29.7 | 1.59 (0.91 to 2.80) | 0.10 | |
| Median time: first symptoms—creatinine (yr) | 282 | −0.45 | <0.001 | 1.06 (1.04 to 1.08) | <0.001 | ||
| <10 | 79 | 86 (24–172) | 88 (27) | <0.001 | 11.4 | 1 | |
| 10≤–<20 | 69 | 76 (5–165) | 82 (29) | 15.9 | 1.48 (0.57 to 3.80) | 0.42 | |
| 20≤–<30 | 55 | 70 (31–117) | 69 (20) | 32.7 | 3.78 (1.55 to 9.25) | 0.003 | |
| ≥30 | 79 | 62 (14–110) | 60 (21) | 45.6 | 6.51 (2.86 to 14.83) | <0.001 | |
| Median time: first symptoms—treatment (yr) | 233 | −0.32 | <0.001 | 1.05 (1.03 to 1.08) | <0.001 | ||
| <5 | 113 | 78 (5–172) | 82 (30) | <0.001 | 18.6 | 1 | |
| 5≤–<10 | 31 | 74 (33–135) | 77 (26) | 22.6 | 1.28 (0.49 to 3.36) | 0.62 | |
| ≥10 | 89 | 68 (14–120) | 64 (21) | 39.3 | 2.84 (1.50 to 5.37) | 0.001 | |
| Severe impairment of renal parenchymab | |||||||
| No | 96 | 80 (31–165) | 83 (25) | <0.001 | 13.5 | 1 | |
| Yes | 101 | 62 (5–163) | 64 (27) | 42.6 | 4.73 (2.34 to 9.58) | <0.001 | |
| Cystine-binding thiol | |||||||
| No | 111 | 73 (14–152) | 76 (24) | 0.53 | 24.3 | 1 | |
| Yes | 195 | 72 (5–172) | 73 (27) | 27.7 | 1.20 (0.70 to 2.00) | 0.52 | |
| No. of urological procedures per patientc | 275 | −0.15 | 0.02 | 1.04 (0.90 to 1.10) | 0.08 |
eGFR was calculated with the Modification of Diet in Renal Disease equation (version 4). eGFR values are presented in milliliters per minute per 1.73 m2. OR, odds ratio; 95% CI, 95% confidence interval.
Number of patients with available data.
Severe impairment of renal parenchyma was defined as a past history of partial or total nephrectomy, a unique congenital kidney, or at least one kidney with a size <10 cm in patients aged ≥16 years.
Number of urological procedures per patient: mean 5.7±5.2; median 4 (0–31).
Figure 2.
Distribution of eGFR<60 ml/min per 1.73 m2 according to age and sex in the 314 patients aged ≥16 years. GFR was estimated from the last available plasma creatinine measurement using the MDRD equation (version 4). The given percentage is for men or women of the given age range. MDRD, Modification of Diet in Renal Disease.
Among adult patients, proteinuria could be recorded concomitantly to the last creatinine measurement for 113 (36%). Among them, one of 25 (4%) with an eGFR≥90 ml/min per 1.73 m2, two of 59 (3.4%) with an eGFR of 60–89 ml/min per 1.73 m2, two of 15 (13.3%) with CKD stage 3a, six of nine (66.7%) with CKD stage 3b, and three of five (60%) with CKD stage 4 had significant proteinuria (correlation coefficient between eGFR and proteinuria=−0.46; P<0.001). Patients with stage 5 CKD had no available proteinuria.
Hypertension
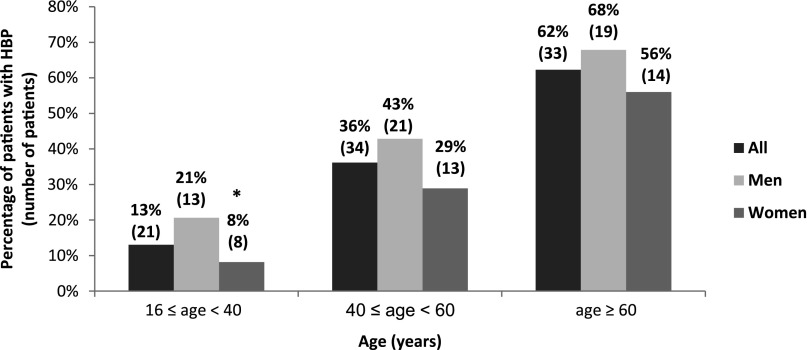
Among pediatric patients, only one (girl, aged 10 years at diagnosis with an eGFR of 63 ml/min per 1.73 m2 and no overt proteinuria) had HBP. Eighty-eight (28.6%) of the 314 patients aged ≥16 years suffered from HBP during follow-up. In adult patients, Table 4 summarizes the univariate analysis of HBP risk and Figure 3 represents HBP distribution according to age and sex. In multivariate analysis, hypertension was associated with male sex (OR, 2.3; 95% CI, 1.3 to 4.1; P=0.003), age (OR, 1.06; 95% CI, 1.04 to 1.08; P<0.001), and an eGFR<60 ml/min per 1.73 m2 (OR, 2.7; 95% CI, 1.5 to 5.1; P=0.001). After excluding comorbidities usually associated with HBP (CKD, renal parenchymal damage, and obesity), the prevalence of HBP was 9.8%, 34.8%, and 33.3% among patients aged 16–39 years (n=61), 40–59 years (n=23), and ≥60 years (n=6), respectively.
Table 4.
Univariate analysis of risk of high BP in patients aged ≥16 years (n=314)
| Considered Parameters | No. of Patientsa | Patients with High BP, % | OR (95% CI) | P Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 168 | 20.8 | 1 | |
| Male | 140 | 37.9 | 2.3 (1.4 to 3.8) | 0.001 |
| Age (yr) | ||||
| 16≤ age <40 | 158 | 13.3 | 1 | |
| 40≤ age <60 | 95 | 34.7 | 3.5 (1.9 to 6.5) | <0.001 |
| age ≥60 | 55 | 61.8 | 10.6 (5.2 to 21.5) | <0.001 |
| CKD | ||||
| No | 226 | 19.9 | 1 | |
| Yes | 82 | 52.4 | 4.4 (2.6 to 7.6) | <0.001 |
| Obesity | ||||
| No | 257 | 24.1 | 1 | |
| Yes | 40 | 47.5 | 2.8 (1.4 to 5.6) | |
| Median time: first symptoms—treatment (yr) | ||||
| <5 | 112 | 16.1 | 1 | |
| 5≤–<10 | 31 | 32.3 | 2.5 (1.0 to 6.2) | 0.05 |
| ≥10 | 88 | 40.9 | 3.6 (1.9 to 7.0) | <0.001 |
| Severe impairment of renal parenchymab | ||||
| No | 117 | 24.8 | 1 | |
| Yes | 114 | 38.6 | 1.9 (1.1 to 3.4) | |
| Cystine-binding thiol | ||||
| No | 110 | 22.7 | 1 | |
| Yes | 192 | 31.3 | 1.5 (0.9 to 2.7) | 0.11 |
| No. of urological procedures per patientc | 275 | 1.0 (0.97 to 1.07) | 0.44 |
OR, odds ratio; 95% CI, 95% confidence interval.
Number of patients with available data.
Severe impairment of renal parenchyma is defined as a past history of partial or total nephrectomy, a unique congenital kidney, or at least one kidney with a size <10 cm in patients aged ≥16 years.
Number of urological procedures per patient: mean 5.7±5.2; median 4 (0–31).
Figure 3.
Distribution of high BP according to age and sex in the 314 patients aged ≥16 years. The given percentage is for men or women of the given age range. *P<0.05 (significant difference between men and women).
Discussion
We have herein analyzed data on a large series of cystinuric patients, the largest one to our knowledge. Our results highlight a high prevalence of CKD, based on MDRD eGFR, and a high prevalence of hypertension among cystinuric patients.
The median age at diagnosis in our population was 18.5 years, but about 20% of patients were diagnosed after age 35 years. Although there is agreement that cystinuria should be diagnosed as soon as possible in order to better prevent nephrolithiasis recurrence, the median delay from first symptoms to diagnosis was 1.3 years (average 5.95±8.99), but the diagnosis was delayed ≥5 years in more than one third of patients. The average diagnosis delay was 6.9 years (0–48) in a previous retrospective study (8).
In our study, we show that 27% of the 314 cystinuric patients aged ≥16 years had impaired renal function as indicated by an eGFR<60 ml/min per 1.73 m2 and only 22.5% had an eGFR≥90 ml/min per 1.73 m2, so considered as strictly normal. A study of British patients estimated the prevalence of CKD stages 3–5 at 8.5% of the general population aged >18 years (mean age 58.1±18.1 years), with a prevalence <1% in individuals aged 18–44 years, around 5% for those aged 45–64 years, and around 32% for those aged >64 years (9). CKD stages 3–4 did not exceed 2%, 4%, and 15% in individuals aged 20–39 years, 40–59 years, and 60–69 years, respectively, in the US National Health and Nutrition Examination Survey (10). The prevalence of CKD in our population was far higher than in these studies, although our patients were younger and had fewer comorbidities. The association between CKD and stones has been debated in many studies. The incidence and prevalence of CKD is significantly higher in patients with nephrolithiasis (11). However, the risk of CKD depends on the type of stones. Patients with hereditary stone diseases, including cystinuria, have been shown to be at higher risk of CKD than patients with other stone disease (12–14), and it has been reported that patients with cystine stones had a lower GFR than those with other kinds of stones (15–17). In the field of cystinuria, depending on the definition of renal impairment and duration of follow-up, the prevalence of CKD ranges from 5.8% (n=40) (15) to 30% (n=40) (18) in limited series. There are only two other large series of cystinuric patients published >12 years ago. In the study by Dello Strologo et al. (19), 146 cystinuric patients (of 176 with reported data) had a PCr<120 μmol/L (1.36 mg/dl), and 6 patients had a PCr>200 μmol/L (2.27 mg/dl). In the series of 116 French cystinuric patients reported by Kirsch-Noir et al., 12% had a PCr>150 µmol/L (1.71 mg/dl) (8). Contrary to our study, none of these studies provided information on the delay between first symptoms or diagnosis and PCr measurement nor whether patients with ARF episodes were excluded, and most studies have evaluated renal failure considering only PCr concentration and not eGFR. In our study, 1.1% of patients required RRT. In France, there is a prevalence of RRT of 1091 per million inhabitants (approximately 0.1%) (20), suggesting that the prevalence of ESRD is higher in cystinuric patients than in the general population. In the previously reported series of cystinuric patients, the prevalence of ESRD varied from 0.4% to 4.3% (8,19,21). Moreover, among the 1391 consecutive patients who started dialysis in a single French center, the overall proportion of nephrolithiasis-related ESRD was 3.2% (n=45), and 0.14% had cystinuria (n=2). These two cystinuric patients had never received appropriate medical treatment, suggesting that ESRD should have been avoided (22).
In multivariate analysis, we showed that CKD was associated with age, HBP, and severe damage of renal parenchyma. Age is also a risk factor for CKD in the general population (23,24). In a cohort of cystinuric patients, PCr appeared to increase with age (19). HBP and diabetes mellitus are risk factors for CKD in the general population (23) as well as in patients with nephrolithiasis (11,14,25,26). However, in a small series of cystinuric patients, it was reported that age and HBP were not significant risk factors for CKD (15). Fifty percent of our patients had severe renal parenchyma damage. It is well known that cystinuric patients are overrepresented among nephrolithiasis patients with an acquired solitary kidney (27,28), and that they have significantly more nephrectomy than patients with calcium oxalate nephrolithiasis (15). The prevalence of nephrectomy varies from 7.7% to 20% among cystinuric patients (8,12,15,17,18,29). Stone-former patients with a single functioning kidney have a lower GFR than those with two functioning kidneys (28) and nephrectomy was shown to be a risk factor for elevated PCr in cystinuric patients (15). By contrast, cystinuric patients who had severe renal parenchyma damage did not have a faster decrease in eGFR (29). After exclusion of known risk factors for CKD, we were able to study only patients aged <40 years because data in older patients were too sparse. However, CKD prevalence remained higher in cystinuric patients in our cohort than in people of similar age in the general population, suggesting that other risk factors for CKD due to other comorbidities or to cystinuria itself may exist, such as urinary tract infections (14) and nephrotoxic treatments. Another pathophysiologic hypothesis for CKD occurrence in cystinuric patients could be the obstruction of Bellini ducts by cystine crystals (27).
We observed a surprisingly high prevalence (28.6%) of hypertension in adult cystinuric patients. In multivariate analysis, HBP was significantly associated with male sex, age, and CKD. In a French study conducted in the general population during the same period with the same declarative diagnostic criteria for HBP, the prevalence of HBP was 16.5% and varied according to age from 1.2% for those aged 20–29 years, 3.4% for the group aged 30–39 years, 8.4% for those aged 40–49 years, 20.4% for the group aged 50–59 years, 35.3% for individuals aged 60–69 years, 46.2% for the group aged 70–79, with a maximum of 49.9% in patients aged >80 years (30). Taking into account the results of this study, the prevalence of HBP is higher in cystinuric patients than in the general population. Other studies in the general population reported a higher prevalence of HBP that ranged from 26.4% to 31% of adults (31,32) with diagnostic criteria based on BP measurement and not on declarative data, which probably underestimate the prevalence of HBP.
Patients with nephrolithiasis develop HBP more frequently than those without nephrolithiasis (33,34). The prevalence of HBP among patients with nephrolithiasis varies from 19.4% to 71.7% (11,14,35). HBP is poorly studied in cystinuric patients. BP>160/100 mmHg was reported in 25.8% of cystinuric patients (17) and Dahlberg et al. reported that 12.4% of cystinuric patients had HBP (36). It has also been reported that HBP was not more common in cystinuric patients than in patients with stones of other causes (17). After excluding risk factors known to be associated with HBP, the available data were too sparse to evaluate the prevalence of HBP in patients aged ≥40 years. However, in patients aged 16–39 years, HBP prevalence was higher in our cohort than in the general population (30). Therefore, HBP could be independent of CKD in younger cystinuric patients. Consequently, cystinuric patients should be routinely screened for HBP, which is a modifiable risk factor for CKD, and potential underlying mechanisms of hypertension in cystinuria should guide further studies.
In our study, the prevalence of obesity in adults was 13.3% and was comparable to the prevalence reported in French adults (11.9%, 13.1%, and 14.5% in 2003, 2006, and 2009, respectively) (37).
Our study has several limitations. There is no registry of cystinuric patients in France, so we were not able to study the real prevalence of cystinuria and of its renal consequences. Given its retrospective design, we were not able to study GFR decline in our population because too few patients had at least three PCr measurements sufficiently spaced in time outside episodes of ARF, which we took care to exclude. Unfortunately, we were not able to systematically study proteinuria that would have allowed studying prevalence of CKD stages 1 and 2 nor to characterize proteinuria (albuminuria or low molecular weight protein). These missing data justify the need for a consistent and uniform approach to monitor CKD in cystinuric patients.
In conclusion, considering the young age of our population, we reported a surprisingly high prevalence of CKD in cystinuric patients. Of course, the role of age, HBP, and severe damage of renal parenchyma has been shown to be associated with CKD in many kidney diseases. However, HBP and severe impairment of renal parenchyma are overrepresented in cystinuric patients and are modifiable risk factors of CKD. In order to improve the renal prognosis, severe impairment of renal parenchyma should be avoided on the basis of a well managed preventive medical treatment and noninvasive urological procedures and HBP should be especially well detected. Finally, CKD should be systematically screened in cystinuric patients outside episodes of ARF and, if present, should be managed according to the KDIGO 2012 clinical practice guidelines.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Drs. Ballanger, Cazin, Dobremez, Ferrière, Harper, Lapouge, Lasseur, Pasticier, de Précigout, Robert, and Valentin (Bordeaux); Dr. Drelonand Delepaul (Boulogne sur Mer); Drs. Francannet and Palcoux (Clermont-Ferrand); Drs. Aljalaby, Cardineau, Chatelet, Eckart, Hurault de Ligny, Legal, Le Tuquin, and Potier (Caen); Drs. Debras, Jung, and Schneider (Colmar); Drs. Ferry, Motte, Strieffling, and Zanetta (Dijon); Drs. Fourcade, LesurMauroy, Novo, and Priso (Lille); Drs. Berger, Charmes, Colombeau, Jeaneau, Paulhac, and Pfeiffer (Limoges); Drs. Landru and Levesque (Lisieux); Dr. Comlombel (Lyon); Drs. Berland, Bruno, Coulange, Delarue, Deturmeny, Grisoni, Jaubert, Moal, Sabiani, and Vidal (Marseille); Drs. Atassi, Cardey, Chabanne, Debiere, Girardot, Mourad, and Vautrin (Montbeliard); Dr. D'Albignac (Montluçon); Drs. Coudert and Lemelle (Nancy); Dr. Amiel (Nice); Drs. Branger and De Graeve (Nîmes); Drs. Bonfils, Colau, Desgrandchamps, Dubosq, Gaudez, Girard, Klifa, Leduc, and Simon (Saint-Louis Hospital, Public Assistance Hospitals of Paris [APHP], Paris); Drs. Dumonceau and Karras (Georges Pompidou European Hospital, APHP, Paris); Drs. Baudouin, Deschenes, Garnier, Lottmann, and Paye-Jaouen (Robert Debré Hospital, APHP, Paris); Drs. Doublet, Haymann, and Rondeau (Tenon Hospital, APHP, Paris, France); Drs. Boccon-Gibod, Daugas, Delmas, and Ravery (Bichat Hospital, APHP, Paris); Drs. Charbit, Biebuyck, Chrétien, Grünfeld, Hummel, Landais, Lottman, and Naret (Necker Hospital for Sick Children, APHP, Paris); Drs. Deligne, Fournier, and Sarret (Val de Grâce Hospital, Paris); Drs. Ballanger, Bellegarde, Ferchaud, Nony, Phillipot, and Valentin (Pessac); Dr. Touchard (Poitiers); Drs. Collard, Faulon, Liard, Pavard, and Rouache (Rouen); Drs. Armand and Lavocat (Saint-Etienne); Drs. Brignon and Krummel (Strasbourg); Drs. Allard and Tack (Toulouse); Drs. Brun and Shendel (Troyes); and Drs. Bacri, Fleury, Lemaitre, Riquet, Sauvage, and Wahidy (Valenciennes).
Members of the French Cystinuria Group are as follows: P. Chauveau and B. Llanas (Bordeaux); H. Bensadoun and J.P. Ryckelynck (Caen); J. Biserte (Lille); A. Descazeaud, V. Guigonis, and M. Rince (Limoges); D. Fouque (Lyon); M. Cailliez and E. Le Chevalier (Marseille); C. Lopez (Montpellier); G. Favre (Nice); T. Kwon and C. Loirat (Robert Debré Hospital, APHP, Paris); M. Tligui (Tenon Hospital, APHP, Paris); M. Flamant (Bichat Hospital, APHP, Paris); R. Salomon, M.F. Gagnadoux (Necker Hospital for Sick Children, APHP, Paris); M.O. Timsit (Georges Pompidou European Hospital, APHP, Paris); F. Broux, M. Godin, and G. Landthaler (Rouen); M. Fischbach and B. Moulin (Strasbourg); and P. Vanhille (Valenciennes).
This work was presented previously in abstract form at the 14th Joint Meeting of the Society of Nephrology and the Francophone Society for Dialysis, held October 2–5, 2012, in Geneva Switzerland; the 49th European Renal Association–European Dialysis and Transplant Association Congress, held May 24–27, 2012, in Paris, France; and the 2012 Annual Meeting of the American Society of Nephrology, held October 30–November 4, 2012, in San Diego, California.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.06680714/-/DCSupplemental.
References
- 1.Eggermann T, Venghaus A, Zerres K: Cystinuria: An inborn cause of urolithiasis. Orphanet J Rare Dis 7: 19, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palacín M, Borsani G, Sebastio G: The molecular bases of cystinuria and lysinuric protein intolerance. Curr Opin Genet Dev 11: 328–335, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Smith A: Evaluation of the nitroprusside test for the diagnosis of cystinuria. Med J Aust 2: 153–155, 1977 [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 6.Levin A, Stevens PE: Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 85: 49–61, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Pruijm M, Ponte B, Ackermann D, Vuistiner P, Paccaud F, Guessous I, Ehret G, Eisenberger U, Mohaupt M, Burnier M, Martin PY, Bochud M: Heritability, determinants and reference values of renal length: A family-based population study. Eur Radiol 23: 2899–2905, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Kirsch-Noir F, Thomas J, Fompeydie D, Debré B, Zerbib M, Arvis G: [Cystine lithiasis: Study of a series of 116 cases]. Prog Urol 10: 1135–1144, 2000 [PubMed] [Google Scholar]
- 9.Stevens PE, O’Donoghue DJ, de Lusignan S, Van Vlymen J, Klebe B, Middleton R, Hague N, New J, Farmer CK: Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int 72: 92–99, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Rule AD, Bergstralh EJ, Melton LJ, 3rd, Li X, Weaver AL, Lieske JC: Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 4: 804–811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambaro G, Favaro S, D’Angelo A: Risk for renal failure in nephrolithiasis. Am J Kidney Dis 37: 233–243, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Rule AD, Krambeck AE, Lieske JC: Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol 6: 2069–2075, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saucier NA, Sinha MK, Liang KV, Krambeck AE, Weaver AL, Bergstralh EJ, Li X, Rule AD, Lieske JC: Risk factors for CKD in persons with kidney stones: A case-control study in Olmsted County, Minnesota. Am J Kidney Dis 55: 61–68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assimos DG, Leslie SW, Ng C, Streem SB, Hart LJ: The impact of cystinuria on renal function. J Urol 168: 27–30, 2002 [PubMed] [Google Scholar]
- 16.Worcester EM, Parks JH, Evan AP, Coe FL: Renal function in patients with nephrolithiasis. J Urol 176: 600–603, discussion 603, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Worcester EM, Coe FL, Evan AP, Parks JH: Reduced renal function and benefits of treatment in cystinuria vs other forms of nephrolithiasis. BJU Int 97: 1285–1290, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Lindell A, Denneberg T, Granerus G: Studies on renal function in patients with cystinuria. Nephron 77: 76–85, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Dello Strologo L, Pras E, Pontesilli C, Beccia E, Ricci-Barbini V, de Sanctis L, Ponzone A, Gallucci M, Bisceglia L, Zelante L, Jimenez-Vidal M, Font M, Zorzano A, Rousaud F, Nunes V, Gasparini P, Palacín M, Rizzoni G: Comparison between SLC3A1 and SLC7A9 cystinuria patients and carriers: A need for a new classification. J Am Soc Nephrol 13: 2547–2553, 2002 [DOI] [PubMed] [Google Scholar]
- 20.French Biomedicine Agency: Epidemiological Network and Information in Nephrology 2011 Annual Report, 2011. Available at: http://www.agence-biomedecine.fr/IMG/pdf/rapport_reinvdef.pdf?bcsi_scan_43167910db6ab4d9=0&bcsi_scan_filename=rapport_reinvdef.pdf. Accessed November 12, 2013
- 21.Bouzidi H, Daudon M: [Cystinuria: From diagnosis to follow-up]. Ann Biol Clin (Paris) 65: 473–481, 2007 [PubMed] [Google Scholar]
- 22.Jungers P, Joly D, Barbey F, Choukroun G, Daudon M: ESRD caused by nephrolithiasis: Prevalence, mechanisms, and prevention. Am J Kidney Dis 44: 799–805, 2004 [PubMed] [Google Scholar]
- 23.Hippisley-Cox J, Coupland C: Predicting the risk of chronic kidney disease in men and women in England and Wales: Prospective derivation and external validation of the QKidney Scores. BMC Fam Pract 11: 49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Lieske JC, de la Vega LS, Gettman MT, Slezak JM, Bergstralh EJ, Melton LJ, 3rd, Leibson CL: Diabetes mellitus and the risk of urinary tract stones: A population-based case-control study. Am J Kidney Dis 48: 897–904, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Taylor EN, Stampfer MJ, Curhan GC: Diabetes mellitus and the risk of nephrolithiasis. Kidney Int 68: 1230–1235, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Evan AP, Coe FL, Lingeman JE, Shao Y, Matlaga BR, Kim SC, Bledsoe SB, Sommer AJ, Grynpas M, Phillips CL, Worcester EM: Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int 69: 2227–2235, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Worcester E, Parks JH, Josephson MA, Thisted RA, Coe FL: Causes and consequences of kidney loss in patients with nephrolithiasis. Kidney Int 64: 2204–2213, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Barbey F, Joly D, Rieu P, Méjean A, Daudon M, Jungers P: Medical treatment of cystinuria: Critical reappraisal of long-term results. J Urol 163: 1419–1423, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Frérot L, Le Fur P, Le Pape A, Sermet C: L’hypertension artérielle en France: Prévalence et prise en charge thérapeutique, CREDES Biblio no. 1276, 1999. Available at: http://www.irdes.net/Publications/Rapports1999/rap1276.pdf. Accessed December 9, 2013
- 31.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J: Global burden of hypertension: Analysis of worldwide data. Lancet 365: 217–223, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Godet-Thobie H,Vernay M, Noukpouape A, Salanave B, Malon A, Castetbon K, de Peretti C: Niveau tensionnel moyen et prévalence de l'hypertension artérielle chez les adultes de 18 à 74 ans, ENNS 2006-2007. BEH Thématique 49–50: 478–483, 2008 [Google Scholar]
- 33.Madore F, Stampfer MJ, Rimm EB, Curhan GC: Nephrolithiasis and risk of hypertension. Am J Hypertens 11: 46–53, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Madore F, Stampfer MJ, Willett WC, Speizer FE, Curhan GC: Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis 32: 802–807, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Elves AW, Tilling K, Menezes P, Wills M, Rao PN, Feneley RC: Early observations of the effect of extracorporeal shockwave lithotripsy on blood pressure: A prospective randomized control clinical trial. BJU Int 85: 611–615, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Dahlberg PJ, van den Berg, Kurtz SB, Wilson DM, Smith LH: Clinical features and management of cystinuria. Mayo Clin Proc 52: 533–542, 1977 [PubMed] [Google Scholar]
- 37.French National Authority for Health: Recommandation de bonne pratique. Surpoids et obésité de l'adulte: prise en charge médicale de premier recours, 2011. Available at: http://www.has-sante.fr. Accessed June 6, 2013
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.