Abstract
Background and objectives
Thrombotic microangiopathy (TMA) in ANCA-associated vasculitis (AAV) has been mainly reported in isolated case reports. The aim of this study was to analyze clinical and pathologic characteristics and prognosis of patients with renal TMA in ANCA-associated GN in a large cohort of Chinese patients.
Design, setting, participants, & measurements
Clinical and renal histopathologic data of 220 patients with biopsy-proven ANCA-associated GN from 1996 to 2013 were retrospectively analyzed. Patients were followed up for a median period of 32 (interquartile range [IQR], 12–65) months, and outcomes of patients were analyzed.
Results
Among the 220 patients with ANCA-associated GN, 30 were identified having concomitant renal TMA by pathologic evaluation. Compared with the non-TMA group, patients with renal TMA presented with more severe renal injury, as evidenced clinically by a higher level of serum creatinine at diagnosis (5.0 [IQR, 3.5–9.0] versus 3.2 [IQR, 1.7–6.8] mg/dl; P=0.02) and pathologically by a higher percentage of cellular crescents (15.0% [IQR, 6.9%–34.9%] versus 6.9% [IQR, 0%–21.1%]; P=0.04) and more severe interstitial infiltration (2 [IQR, 2–2] versus 2 [IQR, 1–2]; P=0.03) in renal biopsies. Furthermore, multivariate analysis showed that renal TMA was independently associated with mortality of patients with AAV after adjusting for age, sex, initial serum creatinine, tubular atrophy, and interstitial fibrosis (hazard ratio, 1.92; 95% confidence interval, 1.08 to 3.41; P=0.03) or for age, sex, the histopathologic classification scheme proposed by Berden et al. (J Am Soc Nephrol 21: 1628–1636, 2010), tubular atrophy, and interstitial fibrosis (hazard ratio, 1.95; 95% confidence interval, 1.07 to 3.55; P=0.03).
Conclusions
Renal TMA in ANCA-associated GN is not rare and presents with more severe renal injury. Renal TMA is independently associated with all-cause mortality in patients with AAV.
Keywords: ANCA, vasculitis, thrombosis, outcomes
Introduction
ANCA-associated vasculitis (AAV) is a group of systemic autoimmune diseases characterized by pauci-immune necrotizing small-vessel vasculitis and circulating autoantibodies against neutrophil cytoplasmic constituents, especially proteinase 3 and myeloperoxidase. AAV comprises granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis (EGPA), and renal-limited vasculitis (RLV) (1). The kidney is one of the most vulnerable organs, often presenting with rapidly progressive GN. The diagnostic and prognostic value of the renal biopsy in ANCA-associated GN is widely recognized. Moreover, the histopathologic classification of ANCA-associated GN proposed by Berden et al. (2) has greatly prioritized the role of renal biopsy findings in the prognostication of patients at the time of diagnosis.
Thrombotic microangiopathy (TMA) comprises a group of clinical and pathologic syndromes that share a similar pathologic process, characterized by endothelial and blood cell damage and thrombotic microvascular occlusions. TMA comprises a spectrum of distinct disorders, including typical and atypical hemolytic uremic syndrome (HUS), congenital and acquired thrombotic thrombocytopenic purpura, malignant hypertension, pregnancy, organ transplantation, drugs, or systemic autoimmune diseases. Renal involvement is common in TMA.
To our knowledge, TMA in ANCA-associated GN has only been reported in isolated case reports (3–9), with two patients showing pathologic features of renal TMA. The clinicopathologic characteristics, especially the prognostic value of pathologic findings of renal TMA, in ANCA-associated GN are far from clear. The aim of this study was to analyze clinical and pathologic characteristics and the prognosis of patients with renal TMA in ANCA-associated GN in a large cohort of Chinese patients.
Materials and Methods
Patients
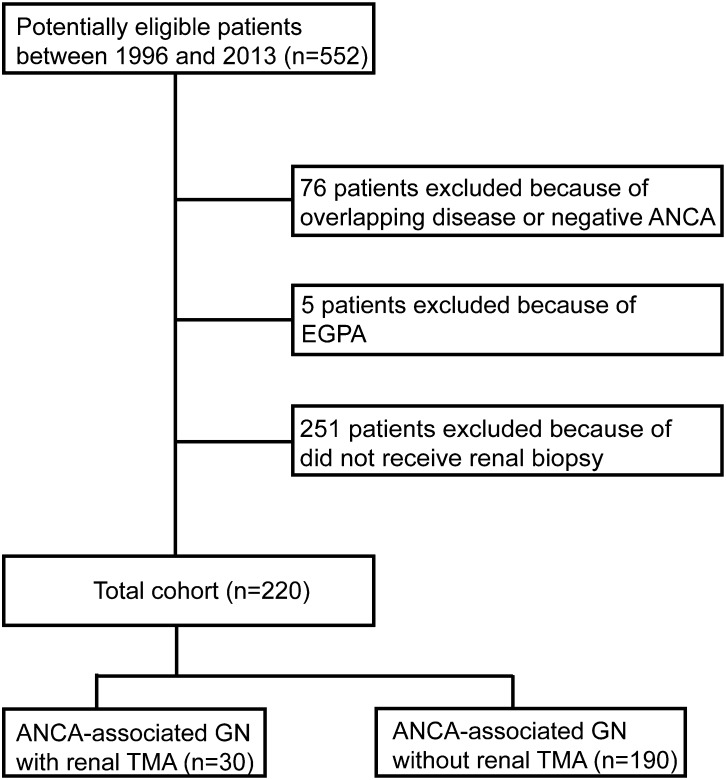
A total of 220 consecutive patients with ANCA-associated GN who received renal biopsy, diagnosed in the Department of Nephrology, Peking University First Hospital from 1996 to 2013, were analyzed retrospectively. Renal biopsy was performed at the time of diagnosis and before the initiation of immunosuppressive therapy. All patients met the criteria of the 2012 Chapel Hill Consensus Conference definition for AAV (1). Patients with comorbid renal disease or secondary vasculitis, such as membranous glomerulonephropathy, anti-glomerular basement membrane disease, drug-induced vasculitis, or lupus nephritis, were excluded. Patients with EGPA were also excluded because EGPA is increasingly considered a distinct type of AAV with different manifestations and outcomes compared with GPA, MPA, and RLV (10). The details of the recruitment process are shown in Figure 1.
Figure 1.
Flowchart for inclusion/exclusion process. EGPA, eosinophilic granulomatosis with polyangiitis; TMA, thrombotic microangiopathy.
Renal TMA was defined as interlobular artery and arteriole and glomerular capillary lesions, including endothelial cell swelling, lumen narrowing, or obliteration and thrombi formation by light microscopy. Swelling of glomerular endothelial cells, detachment from the glomerular basement membrane, and widening of the subendothelial space were identified by electron microscopy (11). The lesions were further divided into acute and chronic changes. The acute lesion was characterized by swelling of the endothelial cells and subendothelial space; fibrin thrombi may also be seen in the afferent glomeruli, small arterioles, and/or arteries. Chronic changes were mucoid changes and onion skin lesions of arterioles and/or arteries (11). Known causes of renal TMA, including SLE, anti-phospholipid syndrome, scleroderma, pregnancy-associated TMA, malignant hypertension, transplantation-associated TMA, disseminated intravascular coagulation, drug-mediated TMA, and TMA associated with various infections, including HIV, hepatitis B virus, and hepatitis C virus, were further excluded. Informed consent for renal biopsy was obtained from each patient. The research was in compliance with the Declaration of Helsinki and was approved by the local ethical committees of Peking University First Hospital.
Follow-up was performed in outpatient clinics specific for AAV. The primary end point was defined as death, and the secondary end point was defined as ESRD. The combined end point was defined as a composite outcome of death or ESRD.
Detection of ANCA
ANCA tests were performed by both indirect immunofluorescence assay and antigen-specific ELISA for all patients at the time of presentation and before immunosuppressive treatment was instituted, according to the manufacturer’s instruction (Euroimmun, Lübeck, Germany).
Detection of Disintegrin and Metalloproteinase with a Thrombospondin Type 1 Motif, Member 13 Activity
The Disintegrin and Metalloproteinase with a Thrombospondin Type 1 Motif, Member 13 (ADAMTS-13) activity assay was assessed using a residual collagen-binding assay, slightly modified as previously described (12) (Supplemental Material). Data are reported as the percentage of collagen-binding activity remaining after dialysis compared with the collagen binding activity in the individual’s baseline sample. One hundred percent minus the residual collagen-binding activity was arbitrarily regarded as the ADAMTS-13 activity.
Renal Histopathology
Biopsies were separately scored by two pathologists blinded to the clinical data, according to the previously standardized protocol for scoring renal biopsies of patients with AAV (13–15). In short, each glomerulus was scored separately on the presence of crescents (cellular/fibrous), glomerulosclerosis (local/segmental/global), fibrinoid necrosis, and a number of other lesions. Interstitial and tubular lesions were scored semiquantitatively on the basis of the percentage of the tubulointerstitial compartment that was affected: tubular atrophy(– for 0%, + for 1%–50%, and ++ for>50%), interstitial infiltrates (– for 0%, + for 1%–20%, ++ for 21%–50%, and +++ for >50%), and interstitial fibrosis (– for 0%, + for 1%–50%, and ++ for >50%). Each biopsy was further classified as sclerotic, focal, crescentic, or mixed category, according to the histopathologic classification system of ANCA-associated GN proposed by Berden et al. (2).
Treatment and Response
The treatment protocols have been described previously (16,17). For a detailed description, see the Supplemental Material. Briefly, the induction therapy included corticosteroids in combination with cyclophosphamide. Patients with ARF or pulmonary hemorrhage received three pulses of intravenous methylprednisolone before the standard induction therapy. Patients with severe pulmonary hemorrhage additionally received plasma exchanges. For maintenance therapy, intravenous cyclophosphamide every 3 months or daily oral azathioprine was given, with a duration of at least 2 years. The response to the immunosuppressive treatment was defined as previously described (18) (detailed in the Supplemental Material). The renal response to treatment, evaluated at 6 months after initiation of immunosuppressive therapy, was judged according to the following criteria: (1) complete recovery of renal function was indicated by normalization of renal function and resolution of hematuria; (2) partial recovery of renal function was indicated by stabilization or improvement of renal function, with serum creatinine ≥1.5 mg/dl but dialysis independent; and (3) treatment failure was indicated by progressive decline in kidney function with persistence of active urinary sediment despite immunosuppressive therapy (19–21).
Statistical Analyses
The t test, nonparametric test, and chi-squared test were performed as appropriate. Kaplan–Meier curves were used to analyze the outcomes of patients. All of the clinicopathologic parameters and treatment regimens listed in Tables 1–3 were assessed as candidate predictors in the univariate survival analysis. If the P value was <0.05, this predictor was allowed to be included in multivariable Cox regression models. Tubular atrophy and interstitial fibrosis were also included in the multivariable models because they were potential histopathologic predictors according to previous study (22). Because of the close correlation between initial serum creatinine and the sequence of histopathologic categories proposed by Berden et al. (r=0.53, P<0.001), the two parameters were separately included in the multivariate analysis using models A and B, respectively. Interaction effect of age and renal TMA on all-cause mortality was also investigated. Differences were considered significant if the P value was <0.05. Analysis was performed with SPSS version 11.0 statistical software package (SPSS, Chicago, IL).
Table 1.
Comparison of clinicopathologic parameters between ANCA-associated GN patients with and without renal TMA
| Parameter | ANCA-Associated GN with Renal TMA (n=30) | ANCA-Associated GN without Renal TMA (n=190) | P Value |
|---|---|---|---|
| Age (y) | 63.9±11.3 | 57.0±14.6 | 0.004 |
| Sex (male/female) | 15/15 | 97/93 | 0.92 |
| MPO-ANCA/PR3-ANCA | 28/2 | 170/20 | 0.58 |
| Fever | 21 (70.0) | 101 (53.2) | 0.09 |
| Fatigue | 20 (66.7) | 126 (66.3) | 0.97 |
| Weight loss | 18 (60.0) | 106 (55.8) | 0.67 |
| Muscle pain | 9 (30.0) | 57 (30.0) | >0.99 |
| Arthralgia | 8 (26.7) | 71 (37.4) | 0.26 |
| Skin rash | 4 (13.3) | 31 (16.3) | 0.88 |
| Pulmonary involvement | 17 (56.7) | 112 (59.3) | 0.79 |
| Ophthalmic involvement | 9 (30.0) | 40 (21.1) | 0.27 |
| ENT involvement | 13 (43.3) | 91 (47.9) | 0.64 |
| Nervous system | 6 (20.0) | 33 (17.4) | 0.73 |
| Hematuria | 30 (100) | 182 (95.8) | 0.54 |
| Urinary protein (g/24 h)a | 1.24 (0.73–2.59) | 1.73 (0.90–3.05) | 0.24 |
| Initial Scr (mg/dl)a | 5.0 (3.5–9.0) | 3.2 (1.7–6.8) | 0.02 |
| eGFR (ml/min per 1.73 m2)a,b | 11.4 (5.2–14.4) | 17.3 (7.2–39.4) | 0.01 |
| Dialysis-dependent at presentation | 15 (50.0) | 58 (30.7) | 0.04 |
| Hemoglobin (g/dl) | 8.3±1.8 | 9.2±2.1 | 0.03 |
| Platelet (×103/mm3) | 296.4±135.7 | 287.5±136.4 | 0.74 |
| CRP (mg/L)a | 23.6 (14.5–96.1) | 18.0 (4.9–67.6) | 0.28 |
| ESR (mm/1 h) | 104.2±39.9 | 76.7±39.5 | <0.001 |
| BVAS | 21.5±4.6 | 20.6±5.8 | 0.38 |
| Normal glomeruli (%) | 26.1±23.1 | 34.9±31.2 | 0.07 |
| Crescent (%) | 54.8±24.2 | 56.8±33.5 | 0.69 |
| Cellular crescentsa | 15.0 (6.9–34.9) | 6.9 (0–21.1) | 0.04 |
| Fibrous crescentsa | 7.3 (0–18.3) | 3.6 (0–18.2) | 0.65 |
| Glomerular sclerosisa (%) | 0 (0–19.0) | 0 (0–5.0) | 0.20 |
| Fibrinoid necrosisa (%) | 3.4 (0–9.6) | 0 (0–4.6) | 0.02 |
MPO, myeloperoxidase; PR3, proteinase 3; Scr, serum creatinine; ENT, ear, nose, and throat; CRP, C-reaction protein; ESR, erythrocyte sedimentation rate; BVAS, Birmingham Vasculitis Activity Scores; TMA, thrombotic microangiopathy.
Values are median (interquartile range).
eGFR (ml/min per 1.73 m2)=175×(plasma creatinine)−1.234×age−0.179×0.79 (if female) (41).
Table 3.
Univariate analysis of patients’ survival in ANCA-associated GN
| Predictor | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age (per 10 y) | 1.60 | 1.29 to 1.98 | <0.001 |
| Sex (male versus female) | 1.97 | 1.21 to 3.18 | 0.01 |
| MPA/GPA/RLV | 0.15 | ||
| MPA | 2.10 | 0.76 to 5.82 | 0.15 |
| GPA | 1.38 | 0.46 to 4.10 | 0.57 |
| RLV | Reference group | ||
| ANCA specificity in ELISA (anti-MPO versus anti-PR3) | 2.20 | 0.79 to 6.09 | 0.09 |
| BVAS | 1.02 | 0.98 to 1.06 | 0.46 |
| Initial serum creatinine (per mg/dl) | 1.06 | 1.01 to 1.12 | 0.03 |
| Total crescents (%) | 0.95 | 0.49 to 1.85 | 0.87 |
| Berden classification | 0.02 | ||
| Focal | Reference group | ||
| Cresentic | 1.25 | 0.69 to 2.28 | 0.46 |
| Mixed | 1.74 | 0.94 to 3.20 | 0.08 |
| Sclerotic | 8.71 | 1.97 to 38.45 | 0.004 |
| Renal TMA | 2.41 | 1.39 to 4.16 | 0.004 |
| Tubular atrophy | 0.18 | ||
| 0% | Reference group | ||
| 1%–50% | 0.89 | 0.35 to 2.25 | 0.80 |
| >50% | 1.43 | 0.54 to 3.77 | 0.47 |
| Interstitial infiltrates | 0.42 | ||
| 0% | Reference group | ||
| 1%–20% | 1.58 | 0.46 to 5.40 | 0.47 |
| 21%–50% | 1.78 | 0.55 to 5.78 | 0.34 |
| >50% | 2.62 | 0.73 to 9.46 | 0.14 |
| Interstitial fibrosis | 0.26 | ||
| 0% | Reference group | ||
| 1%–50% | 1.01 | 0.60 to 1.72 | 0.96 |
| >50% | 1.77 | 0.83 to 3.78 | 0.14 |
| Cumulative CTX dose (per gram) | 0.19 | 0.01 to 3.85 | 0.28 |
MPA, microscopic polyangiitis; GPA, granulomatosis with polyangiitis; RLV, renal-limited vasculitis; BVAS, Birmingham Vasculitis Activity Scores; TMA, thrombotic microangiopathy.
Results
General Data of Patients
Among the 220 patients with ANCA-associated GN enrolled in this study, 30 (13.6%) were identified having concomitant renal TMA by pathologic evaluation (Figure 2). Renal TMA in ANCA-associated GN was equally distributed in time in our cohort during the study period, with a prevalence of five of 43 (11.6%), 13 of 86 (15.1%), and 12 of 91 (13.2%) in the period between 1996 and 2001, 2002 and 2007, and 2008 and 2013, respectively.
Figure 2.
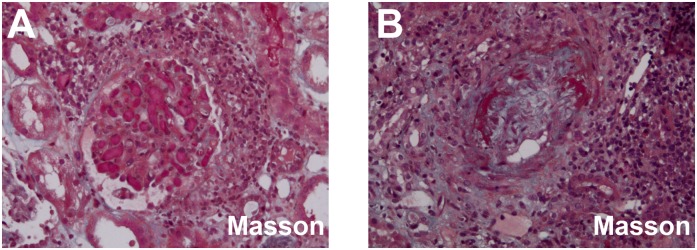
Representative renal TMA lesions in ANCA-associated GN. (A) Thrombus in glomerular endocapillary (Masson's trichrome ×200). (B) The thickened arteriole with mucoid intima edema and fibrinoid necrosis; arteriolar lumen was severely narrowed (Masson's trichrome ×200). TMA, thrombotic microangiopathy.
Among the 30 AAV patients with renal TMA, 15 (50.0%) were men and 15 (50.0%) were women, with an average age of 63.9±11.3 (range, 20–81) years at diagnosis. According to the 2012 Chapel Hill Consensus Conference definitions (1), 24 of 30 (80.0%), four of 30 (13.3%), and two of 30 (6.7%) were classified as MPA, GPA, and RLV, respectively. None of the 30 patients with TMA had concomitant infections with HIV, hepatitis B virus, or hepatitis C virus. According to the pathologic features previously described, seven patients presented with solely acute lesions, seven patients presented with solely chronic lesions, and 16 patients presented with both acute and chronic lesions.
Serum ADAMTS-13 Activity
None of the patients with renal TMA in our study presented with a deficiency of serum ADAMTS-13 activity, with a median activity of 98.6% (range, 97.1%–99.6%).
Clinical and Laboratory Parameters
The clinical and laboratory features of patients in the two groups are listed in Table 1. Patients with renal TMA were significantly older at diagnosis than those without renal TMA (63.9±11.3 versus 57.0±14.6 years, P=0.004). Compared with patients without TMA, patients with TMA had significantly higher levels of initial serum creatinine (5.0 (interquartile range (IQR), 3.5–9.0] versus 3.2 [IQR, 1.7–6.8] mg/dl; P=0.02) and a significantly higher proportion of patients was dialysis dependent at diagnosis (50.0% versus 30.7%, P=0.04). Additionally, patients with TMA presented with significantly lower levels of hemoglobin and increased levels of erythrocyte sedimentation rate than those without TMA (8.3±1.8 versus 9.2±2.1 g/dl, P=0.03; 104.2±39.9 versus 76.7±39.5 mm/1 h, P<0.001, respectively) (Table 1).
Renal Histopathology
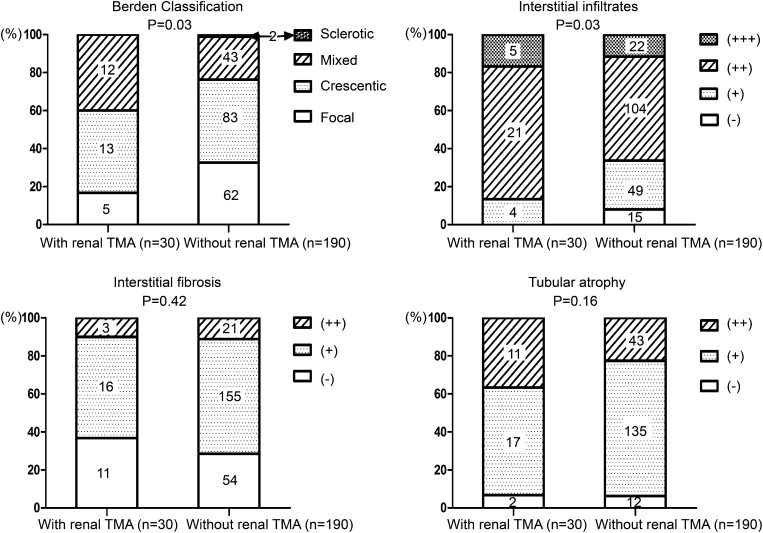
An average of 24.7±11.7 glomeruli were available for evaluation in the 220 renal biopsies. Parameters of renal histopathology of patients with and without renal TMA are listed in Figure 3 and Table 1.
Figure 3.
Comparison of tubulointerstitial lesions between patients with and without renal TMA. Tubulointerstitial lesions were scored semiquantitatively on the basis of the percentage of the affected compartment: interstitial infiltrates (− for 0%, + for 1%–20%, ++ for 21%–50%, and +++ for >50%), interstitial fibrosis (− for 0%, + for 1%–50%, and ++ for >50%), and tubular atrophy (− for 0%, + for 1%–50%, and ++ for >50%). TMA, thrombotic microangiopathy.
Compared with the 190 patients without renal TMA in renal histopathology, patients with renal TMA had a significantly higher percentage of cellular crescent formation (15.0% [IQR, 6.9%–34.9%] versus 6.9% [IQR, 0%–21.1%]; P=0.04) and a significantly higher percentage of fibrinoid necrosis (3.4% [IQR, 0%–9.6%] versus 0% [IQR, 0%–4.6%]; P=0.02) (Table 1). There was a significant difference in the classification scheme proposed by Berden et al. between patients with and without renal TMA (P=0.03) (Figure 3), with the proportion of focal category being much lower in the TMA group. Interstitial infiltrates were significantly more severe in patients with renal TMA than in those without (2 [IQR, 2–2] versus 2 [IQR, 1–2]; P=0.03), whereas the severity of interstitial fibrosis and tubular atrophy were comparable (1 [IQR, 0–1] versus 1 [IQR, 0–1]; P=0.42; 1 [IQR, 1–2] versus 1 [IQR, 1–1]; P=0.16, respectively) (Figure 3).
Treatment and Outcome
Therapy of patients with renal TMA and nonrenal TMA was comparable, with the exception of patients receiving methylprednisolone pulse therapy, the proportion of which was marginally higher in the renal TMA group (P=0.07) (Table 2). After the aforementioned induction therapy, 20 out of 30 (66.7%) patients with renal TMA achieved recovery of renal function, of which four patients achieved complete recovery and 16 patients achieved partial recovery; 10 of 30 (33.3%) patients had treatment failure. The proportion of recovery of renal function (complete or partial) was marginally higher in patients without renal TMA than in patients with renal TMA (82.1% versus 66.7%, P=0.05).
Table 2.
Comparison of treatment data between ANCA-associated GN patients with and without renal TMA
| Treatment | ANCA-Associated GN with Renal TMA (n=30) | ANCA-Associated GN without Renal TMA (n=190) | P Value |
|---|---|---|---|
| Induction therapy | 30 | 190 | |
| PE | 3 (10.0) | 14 (7.6) | 0.93 |
| MP pulse | 25 (83.3) | 123 (66.5) | 0.07 |
| CTX | 27 (90.0) | 177 (93.2) | 0.81 |
| iv | 24 (88.9) | 145 (81.9) | 0.54 |
| po | 3 (11.1) | 32 (18.3) | |
| Maintenance therapy | 16 | 121 | |
| CTX | 6 (37.5) | 48 (39.7) | 0.87 |
| AZA | 10 (62.5) | 73 (60.3) | |
| Treatment response | |||
| Remission | 27 (90.0) | 178 (93.7) | 0.72 |
| Treatment failure | 3 (10.0) | 12 (6.3) | |
| Renal response | |||
| Complete recovery | 4 (13.3) | 54 (28.4) | 0.05 |
| Partial recovery | 16 (53.3) | 102 (53.7) | |
| Treatment failure | 10 (33.3) | 34 (17.9) |
Data are given as n (%). TMA, thrombotic microangiopathy; PE, plasma exchange; MP, methylprednisolone; CTX, cyclophosphamide; iv, intravenous; po, per os; AZA, azathioprine.
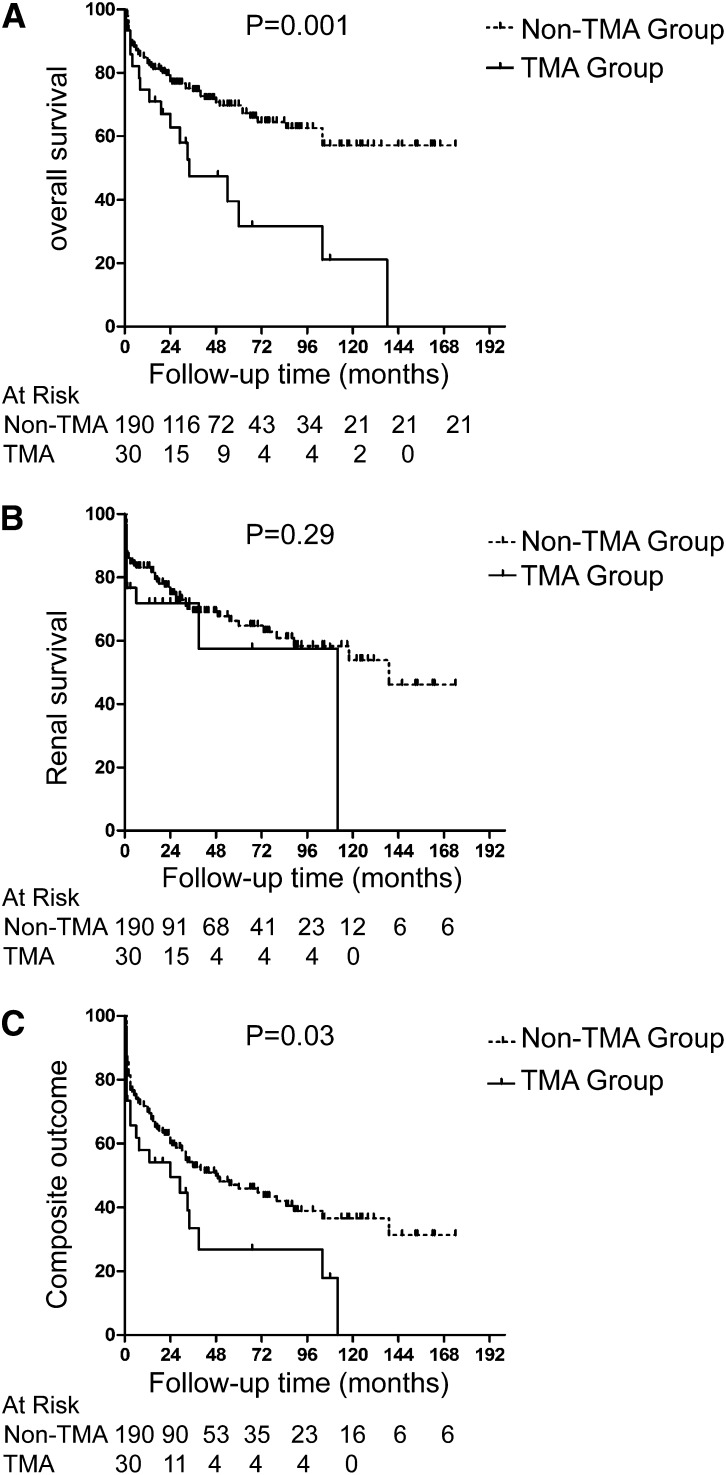
Patients were followed-up on for a median period of 32 (IQR, 12–65) months. Six out of 30 (20.0%) patients with renal TMA and 57 out of 190 (30.0%) patients without renal TMA experienced a disease relapse, respectively, but there was no significant difference between the two groups (P=0.60). In the TMA group, 17 (56.7%) patients died, and 10 (33.3%) patients reached ESRD. In the non-TMA group, 55 (28.9%) patients died, and 56 (29.5%) patients reached ESRD. Main causes of death are listed in Supplemental Table 1. The long-term survival was significantly poorer in the TMA group (P=0.001) (Figure 4A), whereas there was no significant difference in renal outcome between the two groups (P=0.29) (Figure 4B). Additionally, when comparing the combined end points (i.e., death, ESRD), patients with renal TMA also had significantly poorer outcomes (P=0.03) (Figure 4C). We further compared the prognosis of patients with renal TMA among different categories (solely acute versus acute and chronic versus solely chronic). The Kaplan–Meier survival analysis revealed that patients with acute TMA lesions only had a relatively favorable renal outcome, whereas patients with solely chronic TMA lesions had the highest risk for developing ESRD (P=0.01) (Supplemental Figure 1).
Figure 4.
Comparison of prognosis between ANCA patients with and without renal TMA (Kaplan–Meier analysis). (A) Comparison of patients’ survival. (B) Comparison of renal survival. (C) Comparison of composite outcomes. TMA, thrombotic microangiopathy.
Univariate survival analysis of long-term prognosis in all patients with ANCA-associated GN showed that renal TMA was associated with all-cause mortality (P=0.004). Besides TMA, variables including age, sex, initial serum creatinine, and the classification scheme proposed by Berden et al. were predictors of death in univariate analysis (Table 3). Multivariate analysis revealed that renal TMA was still an independent risk factor for patients’ survival after adjusting for age, sex, initial serum creatinine, interstitial fibrosis, and tubular atrophy (hazard ratio, 1.92; 95% confidence interval, 1.08 to 3.41; P=0.03) or for age, sex, the histopathologic classification scheme proposed by Berden et al., tubular atrophy, and interstitial fibrosis (hazard ratio, 1.95; 95% confidence interval, 1.07 to 3.55; P=0.03) (Table 4). Beceause patients with renal TMA were significantly older than those without renal TMA, we also investigated the effect of age and renal TMA on all-cause mortality in the multivariate regression analysis, but no significant interaction was observed (P=0.64).
Table 4.
Multivariate analysis of patients’ survival in ANCA-associated GN
| Predictor | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Model A | |||
| Age (per 10 y) | 1.68 | 1.34 to 2.12 | <0.001 |
| Sex (male versus female) | 2.08 | 1.26 to 3.43 | 0.004 |
| Initial serum creatinine (per mg/dl) | 1.05 | 0.99 to 1.11 | 0.11 |
| Tubular atrophy | 0.82 | ||
| 0% | Reference group | ||
| 1%–50% | 0.76 | 0.29 to 2.03 | 0.59 |
| >50% | 0.87 | 0.29 to 2.62 | 0.80 |
| Interstitial fibrosis | 0.14 | ||
| 0% | Reference group | ||
| 1%–50% | 1.27 | 0.72 to 2.22 | 0.41 |
| >50% | 2.61 | 1.01 to 6.71 | <0.05 |
| Renal TMA | 1.92 | 1.08 to 3.41 | 0.03 |
| Model B | |||
| Age (per 10 y) | 1.72 | 1.36 to 2.17 | <0.001 |
| Sex (male versus female) | 2.12 | 1.29 to 3.48 | 0.003 |
| Berden classification | 0.02 | ||
| Focal | Reference group | ||
| Cresentic | 1.31 | 0.68 to 2.52 | 0.42 |
| Mixed | 1.62 | 0.78 to 3.39 | 0.20 |
| Sclerotic | 13.52 | 2.55 to 71.78 | 0.002 |
| Tubular atrophy | 0.88 | ||
| 0% | Reference group | ||
| 1%–50% | 0.78 | 0.29 to 2.10 | 0.62 |
| >50% | 0.75 | 0.23 to 2.42 | 0.63 |
| Interstitial fibrosis | 0.10 | ||
| 0% | Reference group | ||
| 1%–50% | 1.16 | 0.66 to 2.02 | 0.62 |
| >50% | 2.88 | 1.08 to 7.69 | 0.04 |
| Renal TMA | 1.95 | 1.07 to 3.55 | 0.03 |
TMA, thrombotic microangiopathy.
Discussion
TMA comprises a group of disorders, including HUS, thrombocytopenic purpura, and disease-associated TMA. Renal TMA in AAV has mainly been reported in isolated case reports (3–9). Our study is, to our knowledge, the first to assess clinical and pathologic features and the prognosis of patients with renal biopsy-proven TMA in ANCA-associated GN in a large cohort.
Our study found that renal TMA in ANCA-associated GN is not rare, with a prevalence of 13.6% (30 of 220 patients). In the literature, epidemiologic series reporting the prevalence of renal TMA in total renal biopsies were insufficient. Recently, a large cohort study in our center reported that adult renal TMA accounted for 1.4% (109 of 7589 patients) of the total biopsied patients in the same period (23). However, the prevalence in other countries is unclear, especially for renal TMA in ANCA-associated GN. As reported in previous case reports, patients with concomitant renal TMA in ANCA-associated GN presented with more severe renal injury (3–9). Patients with renal TMA in our cohort also had a higher level of initial serum creatinine, higher percentage of cellular crescents and fibrinoid necrosis, and more severe interstitial infiltration compared with patients without TMA, which, by a large cohort study, further extends previous findings. Meanwhile, no significant differences were observed in chronic lesions, including fibrous crescents, glomerular sclerosis, interstitial fibrosis, and tubular atrophy between patients with and without renal TMA. Therefore, patients with renal TMA had more acute renal diseases. Furthermore, renal TMA was found to be independently associated with all-cause mortality of patients with AAV. It might suggest that patients with TMA in AAV should receive more intensive immunosuppressive therapy, such as plasma exchange, as described in previous case reports (3–8).
In this study, TMA was associated with mortality, rather than ESRD. Considering that TMA is a pathologic process with multiple organs involved, we speculate that the extrarenal disorder of TMA is a possible explanation for patients’ poor long-term survival. In our cohort, the higher proportion of crescents and the more severe interstitial inflammation observed in patients with renal TMA, compared with those without TMA, might be indicative of overall cellular activation. However, because this study is a retrospective one, direct evidence of extrarenal microvascular lesions was not available in some patients, especially during follow-up; the contribution of extrarenal TMA to the mortality in patients with AAV needs to be confirmed by prospective studies.
The pathogenesis of renal TMA in ANCA-associated GN and why only a small subset of patients with ANCA GN developed TMA is far from clear. However, we observed that greater than one in eight patients with AAV combined with TMA, which suggests that, rather than a casual phenomenon, these two entities may share a common pathophysiologic process. Theoretically, there are several potential explanations. First, considering the pathologic features of TMA, endothelial damage has long been regarded as an important disease mechanism in TMAs (24). On the other hand, ANCA-mediated activation of neutrophils that results in endothelial injury is the basic pathophysiologic mechanism involved in AAV (25). It seems that TMA and AAV share the same target cells, namely, endothelial cells, which may contribute to the development of renal TMA in ANCA-associated GN. Second, the complement system might be another potential contributor. Although AAV has traditionally been characterized as pauci-immune, and decreased levels of circulating C3 are uncommon in AAV, recent studies have demonstrated that activation of the alternative complement pathway plays a critical role in the pathogenesis of AAV (26–30). Patients with active AAV have elevated levels of circulating C3a, C5a, soluble C5b-9, and Bb, which suggests activation of the complement system via the alternative pathway (30). As for TMA, a variety of hereditary and acquired disorders, which contribute to the loss of alternative pathway regulation on endothelial cells and on the surface of platelets, have been documented and predispose patients to TMA susceptibility (31–34). Indeed, it has been suggested that most TMAs are characterized by misdirected complement activation affecting endothelial cell and platelet integrity (34). Therefore, we speculate that systemic activation of complement, especially the alternative complement pathway, might play an important role in the development of renal TMA in AAV. Animal studies in AAV have suggested a potential therapeutic role of anti-C5 intervention (27,28,35), and clinical trials with CCX168, a small molecule antagonist of the human C5aR, are ongoing (36). Moreover, eculizumab, a human C5 inhibitor, has been found to be remarkably efficient for the treatment of atypical haemolytic uraemic syndrome (37). Whether anti-C5 therapy is beneficial in the treatment of patients with TMA in AAV could be of interest to study in the future. Third, neutrophil extracellular traps (NETs) have been shown to participate in the pathogenesis of AAV (38,39). Interestingly, a recent study suggested the formation of NETs as a second hit that precipitates acute disease in patients at risk for TMA (40). Therefore, NETs might be relevant to the occurrence of TMA in AAV. Finally, severe lesion of AAV might act as another potential trigger for mediating a secondary TMA reaction. Collectively, we speculate that the occurrence of TMA in AAV is a multifactorial process. The exact mechanism is of great interest for further investigation.
Our study has several limitations. Because it is a retrospective and observational study, it is difficult to sort out whether the TMA is secondary to more severe disease or whether the TMA is a causal factor of the more severe disease caused by a separate primary TMA process. In previous case reports (4–8), it was suggested that TMA in AAV is more likely to be a disorder secondary to ANCA-associated GN. However, this contention needs to be confirmed in future studies. Furthermore, limited by the relatively small sample size in each subgroup of TMA (i.e., solely acute lesions, acute and chronic lesions, solely chronic lesions), the results of renal outcomes of patients with renal TMA among different categories needs to be validated in larger cohorts.
In conclusion, renal TMA in ANCA-associated GN is not rare, and it presents with more severe renal injury. Renal TMA is independently associated with all-cause mortality of patients with AAV.
Disclosures
None.
Supplementary Material
Acknowledgments
We are very grateful to Professor Peter Heeringa for critically reading this manuscript and Professor Xue-Ying Li for the advice on statistical analysis.
This study was supported by a grant of Chinese 973 project (no. 2012CB517700) and four grants of the National Natural Science Fund (nos. 81425008, 81370829, 81321064, and 8140040085).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07910814/-/DCSupplemental.
References
- 1.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, Hoffman GS, Jayne DR, Kallenberg CG, Lamprecht P, Langford CA, Luqmani RA, Mahr AD, Matteson EL, Merkel PA, Ozen S, Pusey CD, Rasmussen N, Rees AJ, Scott DG, Specks U, Stone JH, Takahashi K, Watts RA: 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65: 1–11, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Berden AE, Ferrario F, Hagen EC, Jayne DR, Jennette JC, Joh K, Neumann I, Noël LH, Pusey CD, Waldherr R, Bruijn JA, Bajema IM: Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 21: 1628–1636, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Hirsch DJ, Jindal KK, Trillo AA: Antineutrophil cytoplasmic antibody-positive crescentic glomerulonephritis and thrombotic microangiopathy. Am J Kidney Dis 26: 385–386, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Lim HE, Jo SK, Kim SW, Choi HK, Suh IB, Yoon SY, Moon JS, Won NH, Kwon YJ, Pyo HJ: A case of Wegener’s granulomatosis complicated by diffuse pulmonary hemorrhage and thrombotic thrombocytopenic purpura. Korean J Intern Med 13: 68–71, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefanidis I, Helmchen U, Schmitt H, Maurin N, Sieberth HG: Coincidence of haemolytic uraemic syndrome and c-ANCA-associated rapidly progressive glomerulonephritis. Nephrol Dial Transplant 13: 1818–1821, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Agrawal V, Vaidya CK, Ye J, Freeman J, McKiernan C, Blier PR, Andrzejewski C, Jr, Germain M, Braden GL: Concomitant thrombotic thrombocytopenic purpura and ANCA-associated vasculitis in an adolescent. Pediatr Nephrol 26: 1317–1320, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Nagai K, Kotani T, Takeuchi T, Shoda T, Hata-Kobayashi A, Wakura D, Kagitani M, Makino S, Hanafusa T: Successful treatment of thrombotic thrombocytopenic purpura with repeated plasma exchange in a patient with microscopic polyangitis. Mod Rheumatol 18: 643–646, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi Y, Nagatoya K,Okuno A, Fujii N, Inoue T: Successful treatment for thrombotic thrombocytopenic purpura complicated with myeloperoxidase anti-neutrophil cytoplasmic autoantibody-associated vasculitis. NDT Plus 3: 279–281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asamiya Y, Moriyama T, Takano M, Iwasaki C, Kimura K, Ando Y, Aoki A, Kikuchi K, Takei T, Uchida K, Nitta K: Successful treatment with rituximab in a patient with TTP secondary to severe ANCA-associated vasculitis. Intern Med 49: 1587–1591, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Abril A, Calamia KT, Cohen MD: The Churg Strauss syndrome (allergic granulomatous angiitis): Review and update. Semin Arthritis Rheum 33: 106–114, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Zoltan GL, Fred GS: Hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, and other thrombotic microangiopathies. In: Hepinstall's Pathology of the Kidney, 6th Ed., edited by Jennette JC, Olson JL, Schwartz MM, Silva FG, Philadelphia, Lippincott Williams & Wilkins, 2007, pp 711–717 [Google Scholar]
- 12.Rick ME, Moll S, Taylor MA, Krizek DM, White GC, 2nd, Aronson DL: Clinical use of a rapid collagen binding assay for von Willebrand factor cleaving protease in patients with thrombotic thrombocytopenic purpura. Thromb Haemost 88: 598–604, 2002 [PubMed] [Google Scholar]
- 13.Bajema IM, Hagen EC, Hansen BE, Hermans J, Noël LH, Waldherr R, Ferrario F, van der Woude FJ, Bruijn JA: The renal histopathology in systemic vasculitis: An international survey study of inter- and intra-observer agreement. Nephrol Dial Transplant 11: 1989–1995, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Bajema IM, Hagen EC, Hermans J, Noël LH, Waldherr R, Ferrario F, Van Der Woude FJ, Bruijn JA: Kidney biopsy as a predictor for renal outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int 56: 1751–1758, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Hauer HA, Bajema IM, van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, Jayne DR, Rasmussen N, Bruijn JA, Hagen EC, European Vasculitis Study Group (EUVAS) : Renal histology in ANCA-associated vasculitis: Differences between diagnostic and serologic subgroups. Kidney Int 61: 80–89, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Li ZY,Chang DY, Zhao MH, Chen M: Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated vasculitis: A study of 439 cases in a single Chinese center. Arthritis Rheumatol 66: 1920–1926, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Jennette JC: Rapidly progressive crescentic glomerulonephritis. Kidney Int 63: 1164–1177, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Hellmich B, Flossmann O, Gross WL, Bacon P, Cohen-Tervaert JW, Guillevin L, Jayne D, Mahr A, Merkel PA, Raspe H, Scott DG, Witter J, Yazici H, Luqmani RA: EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: Focus on anti-neutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis 66: 605–617, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC, Nachman PH: Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med 143: 621–631, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ: Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 7: 23–32, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Nachman PH, Hogan SL, Jennette JC, Falk RJ: Treatment response and relapse in antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 7: 33–39, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Hauer HA, Bajema IM, Van Houwelingen HC, Ferrario F, Noël LH, Waldherr R, Jayne DR, Rasmussen N, Bruijn JA, Hagen EC, European Vasculitis Study Group (EUVAS) : Determinants of outcome in ANCA-associated glomerulonephritis: A prospective clinico-histopathological analysis of 96 patients. Kidney Int 62: 1732–1742, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Yu XJ, Yu F, Song D, Wang SX, Song Y, Liu G, Zhao MH: Clinical and renal biopsy findings predicting outcome in renal thrombotic microangiopathy: A large cohort study from a single institute in China. ScientificWorldJournal 2014: 680502, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosove MH: Thrombotic microangiopathies. Semin Arthritis Rheum 43: 797–805, 2014 [DOI] [PubMed] [Google Scholar]
- 25.van Timmeren MM, Heeringa P: Pathogenesis of ANCA-associated vasculitis: Recent insights from animal models. Curr Opin Rheumatol 24: 8–14, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC: Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol 170: 52–64, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huugen D, van Esch A, Xiao H, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, Jennette JC, Heeringa P: Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int 71: 646–654, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R: C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol 20: 289–298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xing GQ, Chen M, Liu G, Heeringa P, Zhang JJ, Zheng X, E J, Kallenberg CG, Zhao MH: Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J Clin Immunol 29: 282–291, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Gou SJ, Yuan J, Chen M, Yu F, Zhao MH: Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int 83: 129–137, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Noris M, Remuzzi G: Atypical hemolytic-uremic syndrome. N Engl J Med 361: 1676–1687, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Zipfel PF, Heinen S, Skerka C: Thrombotic microangiopathies: New insights and new challenges. Curr Opin Nephrol Hypertens 19: 372–378, 2010 [DOI] [PubMed] [Google Scholar]
- 33.George JN, Nester CM: Syndromes of thrombotic microangiopathy. N Engl J Med 371: 1847–1848, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Meri S: Complement activation in diseases presenting with thrombotic microangiopathy. Eur J Intern Med 24: 496–502, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Xiao H, Dairaghi DJ, Powers JP, Ertl LS, Baumgart T, Wang Y, Seitz LC, Penfold ME, Gan L, Hu P, Lu B, Gerard NP, Gerard C, Schall TJ, Jaen JC, Falk RJ, Jennette JC: C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol 25: 225–231, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ClinicalTrials.gov: A Study to Evaluate the Safety and Efficacy of CCX168 in Subjects With ANCA-Associated Vasculitis, 2014. Available at: http://clinicaltrials.gov/ct2/show/NCT01363388. Accessed September 27, 2014
- 37.Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C: Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med 368: 2169–2181, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Kessenbrock K, Krumbholz M, Schönermarck U, Back W, Gross WL, Werb Z, Gröne HJ, Brinkmann V, Jenne DE: Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15: 623–625, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakazawa D, Shida H, Tomaru U, Yoshida M, Nishio S, Atsumi T, Ishizu A: Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA-associated microscopic polyangiitis. J Am Soc Nephrol 25: 990–997, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fuchs TA, Kremer Hovinga JA, Schatzberg D, Wagner DD, Lämmle B: Circulating DNA and myeloperoxidase indicate disease activity in patients with thrombotic microangiopathies. Blood 120: 1157–1164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17: 2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.