Abstract
Background and objectives
Peritoneal dialysis (PD) is associated with an increased risk of infection-related hospitalization (IRH) compared with hemodialysis. The objective of this study was to compare mortality and overall readmission after an IRH between PD and hemodialysis.
Design, setting, participants, & measurements
This propensity score–matched retrospective cohort study assessed patients undergoing long-term dialysis patients, derived from the Canadian Organ Replacement Register and Régie de l’assurance maladie du Québec, who had at least one IRH between January 2001 and December 2007. Patients were followed until death, kidney transplantation, or end of the study period. To estimate the probability of receiving PD versus hemodialysis, propensity scores were obtained using multivariable logistic regression. Mortality and overall readmission risks after the initial IRH were compared using a Cox survival model.
Results
A total of 354 pairs of patients who had at least one IRH were matched for propensity score. During follow-up (median, 1.25 years), 138 hemodialysis patients (24.7/100 patient-years; 95% confidence interval [95% CI], 20.7 to 29.1) and 130 PD patients (21.2/100 patient-years; 95% CI, 17.7 to 25.1) died; 265 hemodialysis patients (144.6/100 patient-years; 95% CI, 127.7 to 163.1) and 299 PD patients (173.2/100 patient-years; 95% CI, 154.1 to 194.0) were readmitted for any cause; and 121 hemodialysis patients (29.7/100 patient-years; 95% CI, 24.7 to 35.5) and 168 PD patients (44.7/100 patient-years; 95% CI, 38.2 to 52.0) were readmitted for an infection. Compared with hemodialysis, PD was not associated with a different mortality risk after an IRH (hazard ratio [HR], 0.87; 95% CI, 0.69 to 1.11). PD was associated with a higher risk of infection-related overall readmission compared with hemodialysis (HR, 1.44; 95% CI, 1.14 to 1.81), but not with the risk of all-cause overall readmission (HR, 1.15; 95% CI, 0.98 to 1.36).
Conclusions
PD was not associated with higher mortality or all-cause overall readmission following an IRH compared with hemodialysis, but PD patients were at higher risk of infection-related overall readmission after IRH. IRHs are associated with significant mortality and overall readmissions. Evaluation of strategies to reduce infections in both hemodialysis and PD recipients are needed to improve patient care and outcomes.
Keywords: chronic dialysis, epidemiology and outcomes, ESRD, mortality, peritoneal dialysis
Introduction
Infections are the second leading cause of death in patients undergoing long-term dialysis (1,2). Despite explaining more than half of deaths among dialysis patients, noncardiovascular causes of morbidity, such as infections, have rarely been studied in the past. Fortunately, the kidney community is now increasingly aware of their importance as a way to reduce overall mortality and morbidity among patients with CKD (3). Infections are also a common cause of hospitalizations in dialysis patients, and the proportion of admissions related to infections appears to have been increasing in the past years (1,4–9). In addition to imposing a high financial burden on health care systems, those admissions have a worse outcome in the dialysis population than in the general population (5,10). According to the US Renal Data System, 34% of discharges from infection-related hospitalization (IRH) were followed by a rehospitalization within 30 days, which is almost 2-fold the rate of rehospitalization in the general population (11). Moreover, IRH was reported as increasing the short-term risk of a cardiovascular event in older patients receiving dialysis (12).
When reaching ESRD, a patient will usually choose between two main dialysis modalities: peritoneal dialysis (PD) and hemodialysis (HD). While overall survival was similar between both modalities (13), PD was associated with a higher risk of death from infection compared with HD (14). Similarly, we have previously found that rates of all-cause hospitalization were equivalent between dialysis modalities but that PD was associated with a 52% increased risk of overall IRH compared with HD (15). This suggests that the higher risk of IRH was partly compensated for by a reduced risk of other causes of hospitalization. However, it remains unknown whether outcomes of these IRHs differ by dialysis modality. While IRHs are more frequent among PD patients, one can hypothesize that they are less severe, and therefore associated with better outcomes, in PD compared with HD.
To make the best informed decision, a patient should be informed of both the advantages and disadvantages of each dialysis modality. Better characterization of outcomes of the frequent complication of infections, and how these outcomes differ between modalities, may influence such a choice depending on comorbidities or other conditions predisposing to a higher risk of infection. The objective of this study was to compare mortality and overall readmission after an IRH between PD and HD.
Materials and Methods
Study Population and Data Sources
For this study, we used linked datasets from the Canadian Organ Replacement Register (CORR) and provincial health services administrative databases (Régie de l’assurance maladie du Québec [RAMQ]). Data sources and derivation of the incident dialysis cohort were described previously (15,16). The CORR gathers information on ESRD in Canada. The RAMQ is the single payer of a provincial health insurance plan provided to all residents of Québec, Canada, that covers medical and hospital services. The Institut de la statistique du Québec (ISQ) holds official governmental vital statistic databases, which include dates and causes of death as reported on death certificates.
Patients initiating long-term HD or PD between January 1, 2001, and December 31, 2007, and identified in both CORR and RAMQ data sources were included in the cohort. Patients who had a kidney transplant before dialysis initiation or had less than 90 days of dialysis after the initiation of dialysis were excluded from the cohort. Because our objective was to evaluate outcomes of IRH in patients receiving long-term dialysis therapy, we restricted the cohort to patients who had at least one IRH after the first 90 days of dialysis. To consider IRH occurring in the first 90 days after dialysis initiation would have led to an immortal time bias because patients are required to survive at least 90 days after dialysis initiation to be included in the incident cohort as a chronic patient on a specific dialysis modality. IRHs were defined as an admission with an infection as primary diagnosis on the discharge sheet according to International Classification of Diseases (ICD), 9th or 10th Revision, codes (Supplemental Table 1). Categories and codes were based on US Renal Data System methods to allow comparison between populations (1). The first IRH episode was considered the index IRH. Because some patients may have discontinued dialysis after the initial 90 days, we then excluded patients who had no dialysis codes in the 90 days before the index IRH. Patients were then followed from their index IRH until death, kidney transplantation, or end of the study period (December 31, 2007).
Outcomes
Both mortality and overall readmission after the index IRH were evaluated. Mortality was measured at different time points: before discharge (in-hospital death), at 30 days after the admission date, and until end of follow-up (survival analysis). In addition to all-cause mortality, we also considered infection-related mortality using cause of death from the Institut de la statistique du Québec (Supplemental Table 1) in the survival analysis.
For 30-day rehospitalization and overall readmission (until end of follow-up), we identified both the first admission for any cause (all-cause readmission) and the next IRH (second IRH) that occurred after discharge of the index IRH.
Dialysis Modality
Dialysis modality (PD versus HD [home and in-center combined]) was assessed using the last physician claim (RAMQ) or procedure code (from a prior discharge summary) before the admission date of the index IRH. For the analyses, patients remained in their original group (HD or PD), similarly to an intent-to-treat analysis. However, dialysis modality switch was considered a secondary outcome and was defined as the first dialysis code change after discharge from index IRH.
Covariates
Covariates were measured at dialysis initiation and included the following: demographic variables, body mass index, race, smoking, visit to a nephrologist in the 6–12 months before dialysis initiation, hospitalization in the year prior, laboratory data. Comorbidities (listed in Table 1) were identified through ICD codes (from RAMQ) and CORR in the 2 years before dialysis initiation. The eGFR was calculated using the four-variable Modification of Diet in Renal Disease equation.
Table 1.
Characteristics of all patients with an infection-related hospitalization and the propensity-matched cohort
| Characteristics | All Patients with an IRH | Matched Cohort | ||||
|---|---|---|---|---|---|---|
| HD (n=1192) | PD (n=379) | P Value | HD (n=354) | PD (n=354) | P Value | |
| Age (yr) | 66.5±13.0 | 60.4±14.4 | <0.001 | 61.6±15.0 | 61.4±14.0 | 0.91 |
| Women (%) | 41.8 | 38.8 | 0.30 | 36.2 | 38.9 | 0.44 |
| Race (%) | ||||||
| Black | 4.5 | 2.6 | 0.28 | 5.1 | 2.3 | 0.13 |
| White | 86.3 | 87.3 | 86.7 | 88.4 | ||
| Other | 9.2 | 10.0 | 8.2 | 9.3 | ||
| BMI (kg/m2) | 27.5±6.5 | 26.9±5.3 | 0.05 | 27.3±6.0 | 26.7±5.3 | 0.39 |
| Smoking (%) | 14.9 | 15.0 | 0.93 | 13.3 | 15.3 | 0.45 |
| Visit to a nephrologist 6–12 mo earlier (%) | 59.0 | 63.1 | 0.16 | 62.4 | 61.6 | 0.82 |
| Hospitalization in prior year (%) | 64.1 | 77.3 | <0.001 | 78.3 | 76.0 | 0.47 |
| Comorbidities (%) | ||||||
| Cardiovascular disease | 58.5 | 46.4 | <0.001 | 50.6 | 48.0 | 0.50 |
| Cerebrovascular disease | 16.6 | 11.9 | 0.03 | 12.4 | 12.7 | 0.91 |
| COPD | 35.1 | 21.6 | <0.001 | 21.8 | 23.2 | 0.65 |
| Chronic liver disease | 3.4 | 3.4 | 0.94 | 4.0 | 3.7 | 0.84 |
| Congestive heart failure | 40.9 | 26.1 | <0.001 | 29.7 | 28.0 | 0.62 |
| Diabetes | 58.6 | 47.5 | <0.001 | 51.1 | 48.9 | 0.55 |
| Hyperlipidemia | 51.3 | 48.6 | 0.36 | 48.9 | 49.7 | 0.82 |
| Hypertension | 93.5 | 94.7 | 0.41 | 95.8 | 94.4 | 0.39 |
| Malignancy | 17.5 | 13.7 | 0.08 | 15.5 | 14.7 | 0.75 |
| PVD | 40.6 | 28.8 | <0.001 | 30.8 | 30.2 | 0.87 |
| Laboratory data | ||||||
| Albumin (g/dl) | 3.3±0.7 | 3.5±0.6 | <0.001 | 3.5±0.7 | 3.5±0.6 | 0.77 |
| eGFR (ml/min per 1.73 m2) | 9.2±4.1 | 8.8±3.8 | 0.08 | 8.9±4.0 | 8.8±3.9 | 0.90 |
| Hemoglobin (g/dl) | 10.4±1.8 | 10.8±1.7 | <0.001 | 10.9±1.9 | 10.8±1.7 | 0.73 |
Values expressed with a plus/minus sign are the mean±SD. Units conversion: albumin, multiply by 10 to convert g/dl to g/L; hemoglobin, multiply by 10 to convert g/dl to g/L. BMI, body mass index; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; IRH, infection-related hospitalization; HD, hemodialysis; PD, peritoneal dialysis.
Statistical Analyses
Descriptive baseline data are presented as mean±SD or median and interquartile range (IQR), as appropriate. Baseline characteristics in PD and HD patients were compared by t test, Mann–Whitney U test, or chi-squared proportion test, as appropriate.
Incidence rates of outcomes were calculated by dividing the number of events (deaths or admissions) by the total patient-years follow-up. We calculated 95% confidence intervals (95% CIs) for rates using a Poisson distribution.
Because patients receiving PD can be very different than patients receiving HD, we calculated propensity scores (probability of receiving PD versus HD) using multivariable logistic regression. All covariates described previously were included in the propensity-score model. Then, PD patients were matched to HD patients by propensity scores using a greedy nearest-neighbor matching algorithm (1:1 match) (17). Missing data for body mass index (BMI) and laboratory data were imputed by a multiple imputation technique (18).
To compare the in-hospital, 30-day death, and 30-day rehospitalization (all cause and IRH) risks between PD and HD after an IRH episode, we built unadjusted conditional logistic regression models using the propensity score–matched cohort. To compare the mortality risk during the whole follow-up and the overall readmission risk (all-cause and second IRH), we used unadjusted Cox proportional hazard survival models using the same propensity score–matched cohort (19).
Additional Analyses
We tested for significant statistical interactions by adding a multiplicative term for selected subgroups (age, sex, BMI, race, cardiovascular disease) in the models. We conducted three sensitivity analyses. First, we repeated the analyses for all outcomes using multivariable models adjusted for the above-mentioned covariates using all patients who had an IRH (unmatched sample). Second, we repeated all analyses without multiple imputation. Third, we restricted analyses to patients with septicemia or a dialysis-related infection as their index IRH instead of all-cause infections that were considered in the main analysis (Supplemental Table 1). This second analysis was motivated by our previous findings that the increased risk of IRH associated with PD appeared to be mostly explained by a higher risk of dialysis-related hospitalizations, including for peritonitis (15).
Ethical Considerations
This study was approved by the Government of Québec ethics committee (Commission d’accès à l’information) and Maisonneuve-Rosemont Hospital ethics committee. Informed consent was waived.
Results
Among a cohort of 5858 incident long-term dialysis patients (15), we identified 1571 patients who had at least one IRH satisfying the inclusion criteria. Those selected patients were followed for a median time of 1.25 years (IQR, 0.47–2.47 years) after their index IRH. The mean age was 65.0±13.6 years; 41% were women, 87% were white, and 24% were undergoing PD. Table 1 compares patients’ characteristics by dialysis modality. As expected, the mean age was higher in the HD group than in the PD group (66.5 versus 60.4 years), and HD patients had higher proportions of comorbidities compared with PD patients. However, after matching by propensity scores, both groups were fairly balanced for all measured covariates. Among HD patients, the proportion of patients receiving home HD was 0.32% for the whole cohort and 0.28% for the matched cohort.
Initial IRH, Propensity-Matched Cohort
While for both HD and PD the principal cause of initial IRH was a dialysis-related infection or a septicemia, the proportion was higher in the PD group (34.5% in HD versus 66.9% in PD) (Table 2). Pneumonia was the second cause of infection (13.8% in HD and 5.4% in PD). The median length of stay (LOS) of the initial IRH was similar between dialysis modalities: 6 days (IQR, 3–13 days) for HD and 6 days (IQR, 3–12 days) for PD.
Table 2.
Type of infection for the initial infection-related hospitalization and the overall readmission infection-related hospitalization in the propensity-matched cohort
| Variable | Hemodialysis, n (%) | Peritoneal Dialysis, n (%) | ||
|---|---|---|---|---|
| Index IRH (n=354) | Overall Readmission (n=121) | Index IRH (n=354) | Overall Readmission (n=168) | |
| Abdominal | 37 (10.5) | 6 (5.0) | 16 (4.5) | 10 (6.0) |
| Dialysis-related and septicemia | 122 (34.5) | 44 (36.4) | 237 (66.9) | 111 (66.1) |
| Genitourinary | 28 (7.9) | 12 (9.9) | 13 (3.7) | 5 (3.0) |
| Musculoskeletal | 10 (2.8) | 6 (5.0) | 5 (1.4) | 8 (4.8) |
| Pneumonia | 49 (13.8) | 16 (13.2) | 19 (5.4) | 8 (4.8) |
| Skin | 27 (7.6) | 7 (5.8) | 17 (4.8) | 3 (1.8) |
| Other infection | 81 (22.9) | 30 (24.8) | 47 (13.3) | 23 (13.7) |
IRH, infection-related hospitalization.
Mortality
In the propensity-matched cohort, we identified 268 deaths during the whole follow-up (37.9%), 28 deaths during the initial hospitalization (4.0%), and 40 deaths in the first 30 days after being admitted (5.6%). Crude proportions of in-hospital deaths and 30-day mortality appeared higher among HD patients compared with PD patients (Table 3). Similarly, overall mortality rate after being admitted for an infection tended to be lower in PD recipients than HD recipients (21.2 versus 24.7/100 patient-years). While results from the regression analyses showed the same trend for a lower mortality risk for PD compared with HD, none were statistically significant (Table 4).
Table 3.
Mortality and readmission incidence after an infection-related hospitalization by dialysis modality
| Variable | Hemodialysis | Peritoneal dialysis | ||
|---|---|---|---|---|
| Participants, n (%) | Rate/100 Patient-Years (95% CI) | Participants, n (%) | Rate/100 Patient-Years (95% CI) | |
| Propensity score-matched cohort (n=708) | ||||
| In-hospital death | 17 (4.8) | – | 11 (3.2) | – |
| 30-dmortality | 24 (6.8) | – | 16 (4.5) | – |
| Overall mortality | 138 (40.0) | 24.7 (20.7 to 29.1) | 130 (36.7) | 21.2 (17.7 to 25.1) |
| Infection mortality | 20 (5.6) | 3.6 (2.2 to 5.5) | 17 (4.8) | 2.8 (1.6 to 4.4) |
| 30-d rehospitalization (all-causes) | 74 (20.9) | – | 70 (19.8) | – |
| 30-d rehospitalization (IRH) | 22 (6.2) | – | 26 (7.34) | – |
| Overall readmission (all-cause) | 265 (74.9) | 144.6 (127.7 to 163.1) | 299 (84.5) | 173.2 (154.1 to 194.0) |
| Overall readmission (IRH) | 121 (34.2) | 29.7 (24.7 to 35.5) | 168 (47.5) | 44.7 (38.2 to 52.0) |
| All patients with an IRH (n=1571) | ||||
| In-hospital death | 42 (3.5) | – | 11 (2.9) | – |
| 30-d mortality | 88 (7.4) | – | 16 (4.2) | – |
| Overall mortality | 550 (46.1) | 29.4 (27.0 to 31.9) | 132 (34.8) | 20.1 (16.8 to 23.8) |
| Infection mortality | 83 (7.0) | 4.4 (3.5 to 5.5) | 17 (4.5) | 2.6 (1.5 to 4.2) |
| 30-d rehospitalization (all-causes) | 256 (21.5) | – | 73 (19.3) | – |
| 30-day rehospitalization (IRH) | 79 (6.6) | – | 26 (6.9) | – |
| Overall readmission (all-cause) | 890 (74.7) | 143.4 (134.2 to 153.2) | 321 (84.7) | 173.1 (154.7 to 193.1) |
| Overall readmission (IRH) | 400 (33.6) | 29.5 (26.7 to 32.5) | 182 (48.0) | 44.6 (38.4 to 51.6) |
IRH, infection-related hospitalization; 95% CI, 95% confidence interval.
Table 4.
Mortality and readmission risks
| Outcome | Risk Estimate in Propensity-Matched Cohort (95% CI) (n=708)a | Adjusted Risk Estimate in All Patients with an IRH (95% CI) (n=1571)b |
|---|---|---|
| In-hospital death | ||
| Hemodialysis | 1.00 (reference) | 1.00 (reference) |
| Peritoneal dialysis | 0.57 (0.24–1.36) | 1.02 (0.72–1.46) |
| 30-d mortality | ||
| Hemodialysis | 1.00 (reference) | 1.00 (reference) |
| Peritoneal dialysis | 0.65 (0.34 to 1.25) | 0.88 (0.66 to 1.18) |
| Overall mortality | ||
| Hemodialysis | 1.00 (reference) | 1.00 (reference) |
| Peritoneal dialysis | 0.87 (0.69 to 1.11) | 0.92 (0.75 to 1.12) |
| Infection mortality | ||
| Hemodialysis | 1.00 (reference) | 1.00 (reference) |
| Peritoneal dialysis | 0.81 (0.42 to 1.54) | 0.87 (0.50 to 1.49) |
| 30-d rehospitalization (all-cause) | ||
| Hemodialysis | 1.00 (reference) | 1.00 (reference) |
| Peritoneal dialysis | 0.93 (0.65 to 1.35) | 1.08 (0.93 to 1.26) |
| 30-d rehospitalization (IRH) | ||
| Hemodialysis | 1.00 (reference) | 1.00 (reference) |
| Peritoneal dialysis | 1.20 (0.66 to 2.15) | 0.97 (0.76 to 1.23) |
| Overall readmission (all-cause) | ||
| Hemodialysis | 1.00 (reference) | 1.00 (reference) |
| Peritoneal dialysis | 1.15 (0.98 to 1.36) | 1.19 (1.04 to 1.37) |
| Overall readmission (IRH) | ||
| Hemodialysis | 1.00 (reference) | 1.00 (reference) |
| Peritoneal dialysis | 1.44 (1.14 to 1.81) | 1.51 (1.25 to 1.83) |
Odds ratios are given for in-hospital and 30-day mortality, and hazard ratios are given for overall mortality and rehospitalization outcomes. 95% CI, 95% confidence interval; IRH, infection-related hospitalization.
All risk estimates are unadjusted.
All risk estimates are adjusted for covariates included in Table 1.
After considering all patients with an IRH (unmatched sample) as a sensitivity analysis, the gap in overall mortality between HD and PD appeared to be wider (crude rates: 20.1 versus 29.4/100 patient-years). However, results from the adjusted regression model almost completely removed this difference between HD and PD (hazard ratio [HR], 0.92; 95% CI, 0.75 to 1.12). In this adjusted model, age (HR, 1.51 by 10 years; 95% CI, 1.39 to 1.63), smoking (HR, 1.35; 95% CI, 1.09 to 1.68), cardiovascular disease (HR, 1.22; 95% CI, 1.02 to 1.47), congestive heart failure (HR, 1.24; 95% CI, 1.04 to 1.48), diabetes (HR, 1.28; 95% CI, 1.07 to 1.52), and peripheral vascular disease (HR, 1.25; 95% CI, 1.05 to 1.48) were associated with a higher risk of death.
The risk of death due to infection tended to be lower in PD patients than in HD patients, but this result was not statistically significant (HR, 0.81; 95% CI, 0.42 to 1.54).
30-Day Rehospitalization and Overall Readmission
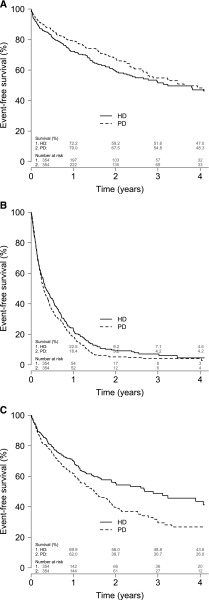
Among patients included in the propensity-matched cohort, rehospitalization risk after 30 days was 20.9% for all causes included and 6.2% for infections in the HD group. Similar results were observed in the PD group. Most patients (79.7%) were readmitted during overall follow-up (all-cause) at least once after being discharged from an IRH, and most overall readmissions occurred in the first year (Figure 1). Subsequent admissions for an infection were also frequent; they occurred in 289 (40.8%) patients. The distribution for the different types of infections explaining the infection-related readmission was similar to the initial IRH, where dialysis-related and septicemia explained most overall readmissions (Table 2). Both all-cause and infection-related readmissions tended to be more frequent in the PD group (Table 3). With hospitalization for the first time for dialysis-related infection, similar proportions of dialysis and nondialysis infection were reported for the subsequent hospitalization among HD patients. However, dialysis-related infection was the cause of overall readmission for 74.1% of patients initially hospitalized for the same reason (Table 5). Compared with HD, PD was not associated with 30-day rehospitalization risk for either all causes (HR, 0.93; 95% CI, 0.65 to 1.35) or IRH (HR, 1.20; 95% CI, 0.66 to 2.15). Using survival analysis, PD was associated with a higher risk of infection-related overall readmission compared with HD (HR, 1.44; 95% CI, 1.14 to 1.81), but not with the risk of all-cause overall readmission (HR, 1.15; 95% CI, 0.98 to 1.36).
Figure 1.
Kaplan–Meier survival curves for death, all-cause overall readmission, and infection-related overall readmission. (A) Death. (B) Readmission (all-cause). (C) Readmission (infection-related). HD, hemodialysis; PD, peritoneal dialysis.
Table 5.
Cause of infection-related overall readmission for hemodialysis and peritoneal dialysis based on cause of index infection-related hospitalization
| Variable | Propensity-Matched Cohort, Index IRH (n=708), n (%) | All Patients, Index IRH (n=1571), n (%) | ||
|---|---|---|---|---|
| Dialysis-Related and Septicemia | Non–Dialysis-Related | Dialysis-Related and Septicemia | Non–Dialysis-Related | |
| Hemodialysis | ||||
| Overall readmission, dialysis-related | 22 (46.8) | 22 (29.7) | 83 (74.1) | 28 (50.0) |
| Overall readmission, non–dialysis-related | 25 (53.2) | 52 (70.3) | 29 (25.9) | 28 (50.0) |
| Peritoneal dialysis | ||||
| Overall readmission, dialysis-related | 60 (46.8) | 63 (24.0) | 88 (72.1) | 30 (50.0) |
| Overall readmission, non–dialysis-related | 77 (56.2) | 200 (76.0) | 34 (27.9) | 30 (50.0) |
IRH, infection-related hospitalization.
When we used the unmatched sample and after adjustment for the various confounders, PD was not associated with a higher 30-day rehospitalization risk for either all causes (HR, 1.08; 95% CI, 0.93 to 1.26) or IRH (HR, 0.97; 95% CI, 0.76 to 1.23). PD was associated with a higher risk of both all-cause (HR, 1.19; 95% CI, 1.04 to 1.37) and infection-related overall readmission compared with HD (HR, 1.51; 95% CI, 1.25 to 1.83). Both smoking (HR, 1.22; 95% CI, 1.04 to 1.43) and peripheral vascular disease (HR, 1.30; 95% CI, 1.13 to 1.48) were associated with a higher risk of all-cause overall readmission in this adjusted model.
Among the PD group in the matched cohort, 20% switched to HD after the index IRH compared with 1% switching from HD to PD. Similar results were found for the whole cohort.
Additional Analyses
For all outcomes, evaluations of statistical interactions for all selected subgroups (age, sex, BMI, race, cardiovascular disease) were not statistically significant. All analyses were repeated without multiple imputation, and the results were similar.
Among the unmatched 1571 patients identified with an IRH, 533 (HD, 254; PD, 279) were patients with septicemia or a dialysis-related infection. Compared with HD, PD was associated with a similar adjusted risk of in-hospital death (odds ratio, 1.01; 95% CI, 0.44 to 2.32), 30-day mortality (odds ratio, 1.13; 95% CI, 0.43 to 2.89), and overall mortality (HR, 0.96; 95% CI, 0.70 to 1.31) after being admitted for septicemia or a dialysis-related infection. However, PD was associated with a higher risk of infection-related overall readmission (HR, 1.37; 95% CI, 1.02 to 1.85) and dialysis-related infection overall readmission (HR, 2.52; 95% CI, 1.76 to 3.82).
Discussion
This population-based study unveils the likeness in mortality risk inherent to an IRH between dialysis modalities. It enlightens the differences in readmission between PD and HD patients. Indeed, even though the risk of a subsequent IRH was higher among patients receiving PD compared with HD, the risk of all-cause overall readmission was similar between the two groups.
Whether PD was associated with a greater risk of death from infection has been a matter of debate in the literature for a long time. A large registry study showed a higher risk of death from infection among PD patients compared with HD patients after 6 months of treatment; this "excess risk" was attributed to a higher likeliness of fatal peritonitis in PD patients compared with those undergoing HD (14). Restricting the comparison to septicemia has led to diverging results between PD and HD patients. Comparison of overall mortality between PD and HD also shows conflicting results, but recent studies suggest that mortality is similar between both modalities (13,20). Evidence on mortality after IRH remains scarce for dialysis patients receiving either modality. Indeed, to our knowledge, among patients who experienced IRH, no studies have directly compared relevant outcomes between patients receiving HD and PD. Similar outcomes between modalities would be of interest in everyday clinical practice (14).
Using the same cohort, we have previously shown a similar risk of all-cause hospitalization between PD and HD patients (15). However, PD was related to a 52% increased risk of IRH compared with HD. An interesting finding pertains to the consequences of such an elevated risk of IRH among PD patients, which would be useful to clinicians. The dialysis modality does not appear to influence the overall hospitalization or mortality risk. It seems to be a trade-off between different complications, wherein PD is associated with an increased risk of infections, which would then result in a lower risk of other complications. This higher risk of infection-related readmission did not manifest within 30 days after discharge, which may reflect post-IRH exposure to antibiotics. Although a 30-day mortality rate of 4.5% after an IRH is not negligible, our results are reassuring by showing that outcomes from these infections are not worse than those seen with HD.
The fact that those PD-related infections do not translate into a higher mortality is very pertinent to improving patient care. Because infections are often preventable, frequency of IRH and its associated mortality and morbidity may be used in education and training of PD candidates to emphasize the importance of preventing peritoneal catheter infections and peritonitis.
We assembled a relatively large cohort of incident ESRD patients by including all centers from an entire province, reflecting the source population. Moreover, systematic care to dialysis patients through a universal health care system greatly limits selection bias. It also allows the inclusion of all age groups, an important confounder in the relationship between dialysis modality and outcomes. Use of linked datasets allowed us to enter considerably more covariates to our models; this would not be the case with registry data alone and allowed us to minimize confounding.
Our study has some limitations. We could not identify the initial IRH by chart reviews because of the multicenter setting of this study; thus, we had to rely on ICD codes alone. Under- or overidentification of IRH from ICD codes should not differ from one dialysis modality to another, and therefore results should not be affected. Both HD and PD patients are equally represented in our health care system, and reports of infectious episodes necessitating hospitalization should not be dissimilar between the two. The proportion of patients undergoing either continuous ambulatory peritoneal dialysis and assisted peritoneal dialysis was not available to us. Residual confounding remains possible even though we used a propensity-matched cohort adjusting for numerous confounders between dialysis modalities. We could not account for the type of vascular access in the HD group without introducing an informative bias. This information is obviously very relevant for analyzing infection rates. Although our cohort is one of the largest cohorts of incident PD patients, statistical power was limited for mortality analyses. A larger cohort may also allow comparisons of outcomes by modality for each type of infection.
In conclusion, this study elucidates the risk of death and all-cause overall readmission after an IRH between HD and PD patients. PD was not associated with a higher mortality rate or all-cause overall readmission after an IRH compared with HD, but PD patients were at higher risk of infection-related overall readmission after IRH. Findings from this retrospective study do not emphasize the superiority of one dialysis modality over the other but rather provide evidence to make an informed medical decision that considers patient and clinical parameters. IRHs are associated with significant mortality and readmissions. Evaluation of strategies to reduce infections for both HD and PD is needed to improve patient care and outcomes.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by a Fonds de recherche du Québec-Santé operating grant. J.P.-L. was supported by a Kidney Research Scientist Core Education and National Training New Investigator Award.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09210914/-/DCSupplemental.
References
- 1.U.S. Renal Data System : USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 2.Lafrance J, Rahme E, Iqbal S, Leblanc M, Pichette V, Elftouh N, Vallee M: Magnitude of discordance between registry data and death certificate when evaluating leading causes of death in dialysis patients BMC Med Res Methodol 13: 51, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James MT, Laupland KB: Examining noncardiovascular morbidity in CKD: Estimated GFR and the risk of infection. Am J Kidney Dis 59: 327–329, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Allon M, Depner TA, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, Gassman JJ, Kaufman AM, Kaysen GA, Lewis JA, Schwab SJ, HEMO Study Group : Impact of dialysis dose and membrane on infection-related hospitalization and death: Results of the HEMO Study. J Am Soc Nephrol 14: 1863–1870, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Allon M, Radeva M, Bailey J, Beddhu S, Butterly D, Coyne DW, Depner TA, Gassman JJ, Kaufman AM, Kaysen GA, Lewis JA, Schwab SJ, HEMO Study Group : The spectrum of infection-related morbidity in hospitalized haemodialysis patients. Nephrol Dial Transplant 20: 1180–1186, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Chavers BM, Solid CA, Gilbertson DT, Collins AJ: Infection-related hospitalization rates in pediatric versus adult patients with end-stage renal disease in the United States. J Am Soc Nephrol 18: 952–959, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Rayner HC, Pisoni RL, Bommer J, Canaud B, Hecking E, Locatelli F, Piera L, Bragg-Gresham JL, Feldman HI, Goodkin DA, Gillespie B, Wolfe RA, Held PJ, Port FK: Mortality and hospitalization in haemodialysis patients in five European countries: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 19: 108–120, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Dalrymple LS, Johansen KL, Chertow GM, Cheng SC, Grimes B, Gold EB, Kaysen GA: Infection-related hospitalizations in older patients with ESRD. Am J Kidney Dis 56: 522–530, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lafrance J, Rahme E, Iqbal S, Elftouh N, Laurin LP, Vallée M: Trends of infection-related hospitalization rates in a large Canadian retrospective cohort of chronic dialysis patients accounting for length of time on dialysis. Open CMAJ 2: E109–E114, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Renal Data System : USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007 [Google Scholar]
- 11.U.S. Renal Data System : USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2012 [Google Scholar]
- 12.Dalrymple LS, Mohammed SM, Mu Y, Johansen KL, Chertow GM, Grimes B, Kaysen GA, Nguyen DV: Risk of cardiovascular events after infection-related hospitalizations in older patients on dialysis. Clin J Am Soc Nephrol 6: 1708–1713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E: Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 171: 110–118, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Johnson DW, Dent H, Hawley CM, McDonald SP, Rosman JB, Brown FG, Bannister KM, Wiggins KJ: Associations of dialysis modality and infectious mortality in incident dialysis patients in Australia and New Zealand. Am J Kidney Dis 53: 290–297, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Lafrance JP, Rahme E, Iqbal S, Elftouh N, Vallée M, Laurin LP, Ouimet D: Association of dialysis modality with risk for infection-related hospitalization: A propensity score-matched cohort analysis. Clin J Am Soc Nephrol 7: 1598–1605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lafrance JP, Rahme E, Iqbal S, Leblanc M, Pichette V, Elftouh N, Vallée M: Magnitude of discordance between registry data and death certificate when evaluating leading causes of death in dialysis patients. BMC Med Res Methodol 13: 51, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC: The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 32: 2837–2849, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ake CF, Carpenter AL: Survival analysis with PREG: using MI and MIANALYZE to accomodate missing data. In: Proceedings of the 10th Annual Western Users of SAS Software Regional Users Group Conference (2002). Cary, NC, SAS Institute, Inc., 2002, pp 102–107 [Google Scholar]
- 19.Stuart EA: Matching methods for causal inference: A review and a look forward. Stat Sci 25: 1–21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vonesh EF, Snyder JJ, Foley RN, Collins AJ: Mortality studies comparing peritoneal dialysis and hemodialysis: What do they tell us? Kidney Int Suppl (103, Suppl): S3–S11, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.