Abstract
Background and objectives
Fluid removal via ultrafiltration is a primary function of hemodialysis, and inadequate volume control is associated with significant morbidity and mortality among chronic dialysis patients. Treatment-to-treatment fluid removal goals are typically calculated on the basis of interdialytic weight gain and prescribed target weight. The clinical effect of frequent missed target weights is unclear. This study was designed to evaluate the associations of postdialysis weights above and below the prescribed target weight (separately) and outcomes.
Design, setting, participants, & measurements
Data were taken from a national cohort of 10,785 prevalent, thrice-weekly, in-center hemodialysis patients dialyzing from 2005 to 2008 (median time at risk, 2.1 [25th percentile, 75th percentile] years) at a single dialysis organization. Patients were characterized as having an above target weight miss if their postdialysis weight was >2 kg above target weight in at least 30% of baseline treatments (14.6% of cohort), or they were characterized as control otherwise. Below target weight miss characterization was analogous for patients with postdialysis weight >2 kg below target weight (6.6% of cohort). Coprimary endpoints were all-cause and cardiovascular mortality.
Results
Above target weight miss in at least 30% of treatments (versus not) was associated with greater all-cause mortality (adjusted hazard ratio, 1.28; 95% confidence interval, 1.15 to 1.43); and below target weight miss in at least 30% of treatments (versus not) was associated with greater all-cause mortality (adjusted hazard ratio, 1.22; 95% confidence interval, 1.05 to 1.40). Both above and below target weight misses were also significantly associated with greater cardiovascular mortality. Secondary analyses demonstrated dose-response relationships between target weight misses and mortality. Results from sensitivity analyses considering the difference in postdialysis and target weights as a proportion of body weight were analogous to the primary results.
Conclusions
Postdialysis weights >2 kg above and below target weight are associated with higher all-cause and cardiovascular mortality. Consistent target weight achievement is a viable target for improving fluid management.
Keywords: clinical epidemiology, cardiovascular, chronic hemodialysis
Introduction
Maintenance of fluid balance via ultrafiltration (UF) is one of the primary objectives of hemodialysis (HD). Inadequate volume control is associated with significant morbidity and mortality among chronic dialysis patients. Overaggressive UF can cause intradialytic hypotension (IDH) and cardiac ischemia (1,2), and under-aggressive UF can lead to volume expansion and cardiac hypertrophy (3,4). Both recurrent ischemia and ventricular hypertrophy are linked to heart failure and arrhythmia development (3,5,6).
UF volume is typically determined by the difference in the predialysis and prescribed target weights. Avoidance of over- and under-aggressive fluid removal therefore depends on accurate target weight establishment and consistent achievement of this weight postdialysis. Although both factors are important in optimizing volume control, we chose to investigate the latter because of its availability from registry data, and most importantly, the fact that it is immediately actionable. In fact, current United States ESRD facility regulations require documentation of treatment-to-treatment target weight attainment or documentation of the reason for missed target weight and corrective plan (7). However, achievement of target weight is challenging, and in one cohort, patients failed to achieve target weight in 63% of treatments (8).
Existing evidence supports an association between postdialysis weight above target weight and mortality. In an Italian cohort, Movilli et al. demonstrated that a postdialysis weight threshold of ≥0.3 kg above target weight best predicts mortality (8). However, the study design did not consider the effect of missed target weight frequency, and it did not distinguish between periodic, large postdialysis target weight differences and frequent, small postdialysis target weight differences. Occasional treatments with missed target weights are expected; they reflect appropriate response to acute health status change and target weight probing. Frequent treatments with missed target weights or those where the degree of miss is excessive suggest either incorrect target weight specification, suboptimal fluid management, or both. Finally, no data exist regarding outcome associations with postdialysis weights below target weight, but the harms of overly aggressive fluid removal and/or poor nutritional status suggest plausibility.
We undertook this study to investigate the associations of postdialysis weights above and below the prescribed target weight (separately) and outcomes. We hypothesized that postdialysis weight above target weight (and below, separately) would be associated with mortality and that greater frequencies of target weight misses would be associated with incrementally greater death hazards.
Materials and Methods
Study Population and Data Collection
Partners Health Care Institutional Review Board approved this study. Data were obtained from 12,417 randomly selected prevalent, thrice-weekly HD patients affiliated with a single large dialysis organization. Patients entered the cohort in January 2005 through December 2008 and dialyzed at one of 1263 facilities located across the United States. Patients were followed until death or censoring. We excluded patients who did not survive the 30-day baseline period (n=16). To ensure study of patients representative of the broader United States HD population, we excluded patients with prescribed treatment time <120 or >480 minutes and patients with weights <25 or >300 kg.
Data were collected according to standardized protocols and were obtained from the dialysis organization’s electronic medical record. Demographic information was documented at admission to one of the organization’s facilities. Comorbid illness was assessed by a nephrologist at time of organization entry and was updated on the basis of clinical course. Biochemical data were measured biweekly or monthly and were considered as the last value during the baseline period. Dialysis parameters (including target and postdialysis weights) were recorded on a treatment-to-treatment basis. Per routine practice, target weight was estimated and updated by the treating nephrologist on the basis of clinical course. Pre- and postdialysis weights were measured before and after each treatment in the standing position. Blood pressure was machine-measured in the seated position pre- and postdialysis and at least every 30 minutes during HD.
Designation of Exposures and Outcomes and Time Sequence
Exposure was assessed over the first 30 days in study (baseline period). In coprimary analyses, we compared patients who had postdialysis weight >2 kg above target weight in at least 30% of baseline treatments versus patients who did not, and patients who had postdialysis weight >2 kg below target weight in at least 30% of baseline treatments versus patients who did not. The threshold of 2 kg above and below target weight was selected on the basis of data distribution and to minimize bias from instrument (scale) error (9). The choice to use the proportion of treatments affected rather than the number of treatments affected was made to account for differences among patients in the number of baseline treatments. The dichotomization threshold of 30% was selected on the basis of data distribution, literature precedent (10–12), and desire to investigate a clinically applicable cutpoint. Because, these exposures are not mutually exclusive, patients could have been considered exposed in both analyses (n=27). Because a 2-kg discrepancy between postdialysis and target weight varies in magnitude on the basis of body weight, we performed sensitivity analyses in which we considered the difference between postdialysis and target weights as a proportion of postdialysis weight. Results were analogous to those of the primary analyses and are presented in Supplemental Tables 1 and 2.
To evaluate for a dose response relationship between missed target weight and outcome, we conducted secondary analyses in which the proportion of treatments affected was considered as a multilevel categorical exposure (>2 kg above/below target weight in 0%, 1%–19%, 20%–39%, and ≥40% of baseline treatments). In other secondary analyses, we varied the threshold for defining target weight misses (1, 1.5, 2, 2.5, and 3 kg above/below target weight in ≥30% of baseline treatments).
Patients were considered at-risk for outcome starting day 31 and remained at-risk until death or censoring for care transfer, transplant, modality change, or study end. The coprimary endpoints were all-cause and cardiovascular mortality. Date of death and attributed cause of death were recorded by facility staff. Cardiovascular deaths were defined as those from ischemic heart disease, heart failure, pulmonary edema, volume overload, arrhythmia, valvular defect, or arterial embolism. A priori secondary endpoints included IDH (nadir intradialytic systolic BP <90 mmHg) (12), rapid UF rate (>13 ml/h per kg) (13), and large interdialytic weight gain (IDWG) (>3.5% body weight) (14).
Statistical Analyses
All analyses were performed using Stata 12.0 MP (College Station, TX). Baseline cohort characteristics were described as means and SDs for continuous variables and counts and proportions for categorical variables. Bivariable comparisons across target weight miss categories were made using chi-squared and ANOVA testing.
Time to event analyses were conducted using Cox proportional hazards models with proportionality assumption confirmation via Schoenfeld residual testing. Models contained terms for both exposure categories (above and below target weight) to account for patients who contributed to both. Adjusted model covariates were selected on the basis of their plausible associations with target weight miss and/or mortality. The final model covariates were age, sex, race, coronary artery disease, heart failure, diabetes, vascular access type, albumin (≤2.9, 3–3.9, and ≥4.0 g/dl), phosphorus (milligrams per deciliter), hemoglobin (grams per deciliter), equilibrated Kt/V, dialytic vintage (≤1.0, 1.1–1.9, 2.0–3.9, and ≥4.0 years), prescribed treatment time (minutes), IDWG (kilograms), postdialysis weight (quartiles), predialysis systolic BP (≤130, 131–150, and ≥151 mmHg), and missed treatments (0, 1, 2, and ≥3).
Effect modification of the target weight miss–mortality association on the basis of body weight was evaluated using likelihood ratio testing of nested models that did and did not include two-way cross-product terms. Because preterminal disease could alter target weight achievement in the perideath period (which could otherwise impart a reverse-causation bias), we repeated analyses with introductions of 30- and 60-day lag periods between the baseline period and the start of at-risk time. Findings from lagged analyses were similar to those from primary analyses and are not shown.
Analyses of target weight miss and secondary endpoints (selected because of pathway variable plausibility) were conducted with logistic regression models. The binary exposures (above and below target weight) and endpoints (IDH, rapid UF rate, and large IDWG) were specified as present in ≥30% of baseline treatments versus not. Covariate selection for adjusted models was analogous to the primary analyses. Logistic model fit testing was conducted via Hosmer–Lemeshow testing. Confirmatory analyses with outcomes considered on a treatment-to-treatment basis were conducted with generalized estimating equations (exchangeable model covariance).
Results
Baseline Characteristics of Cohort
Cohort characteristics are presented in Table 1. Compared with patients with postdialysis weight at and below target weight, patients with postdialysis weight >2 kg above target weight in at least 30% of treatments were more likely to be younger, male, black, heavier, have diabetes and heart failure, and have greater UF volume, treatment time, target weight adjustments, IDH, and serum phosphorus. Compared with patients with postdialysis weight at and above target weight, patients with postdialysis weight >2 kg below target weight in at least 30% of treatments were more likely to be female, lighter, of shorter dialytic vintage, dialyze via catheter, and have lower albumin.
Table 1.
Baseline cohort characteristics by postdialysis weight group
| Characteristic | Below Target Weight (n=682) | At Target Weight (n=8527) | Above Target Weight (n=1549) | P Valuea |
|---|---|---|---|---|
| Age (y) | 62.3±14.9 | 61.8±14.9 | 58.9±14.5 | <0.001 |
| Female | 360 (52.8) | 3960 (46.4) | 630 (40.7) | <0.001 |
| Black | 272 (39.9) | 3337 (39.1) | 680 (43.9) | <0.01 |
| Diabetes | 406 (59.5) | 4959 (58.2) | 996 (64.3) | <0.001 |
| Heart failure | 274 (40.2) | 3573 (41.9) | 786 (50.7) | <0.001 |
| Coronary artery disease | 81 (11.9) | 1161 (13.6) | 199 (12.8) | 0.33 |
| Vintage (y) | <0.001 | |||
| ≤1.0 | 283 (41.5) | 2110 (24.7) | 350 (22.6) | |
| 1.1–1.9 | 67 (9.8) | 1238 (14.5) | 254 (16.4) | |
| 2.0–3.9 | 122 (17.9) | 2131 (25.0) | 421 (27.2) | |
| ≥4.0 | 210 (30.8) | 3048 (35.8) | 524 (33.8) | |
| Vascular access | <0.001 | |||
| Graft | 171 (25.2) | 2723 (32.2) | 468 (30.4) | |
| Fistula | 216 (31.8) | 3356 (39.6) | 617 (40.1) | |
| Catheter | 292 (43.0) | 2391 (28.2) | 454 (29.5) | |
| Post-HD weight (kg) | <0.001 | |||
| ≤62.7 | 236 (34.6) | 2264 (26.5) | 192 (12.4) | |
| 62.8–74.0 | 134 (19.6) | 2182 (25.6) | 370 (23.9) | |
| 74.1–88.9 | 149 (21.9) | 2124 (24.9) | 417 (26.9) | |
| ≥89.0 | 163 (23.9) | 1957 (23.0) | 570 (36.8) | |
| UF volume as a percentage of body weight (%) | 3.0±1.5 | 3.5±1.4 | 3.9±1.4 | <0.001 |
| UF rate (ml/h per kg) | 8.7±4.4 | 10.1±4.2 | 10.9±4.1 | <0.001 |
| IDWG as a percentage of body weight (%) | 2.7±1.5 | 3.5±1.5 | 3.9±1.5 | <0.001 |
| Prescribed treatment time (min) | 214.1±27.6 | 215.8±28.6 | 225.0±28.0 | <0.001 |
| Delivered treatment time (min) | 211.9±28.1 | 214.6±28.5 | 221.1±27.8 | <0.001 |
| Shortened treatment timeb | 50 (7.3) | 459 (5.4) | 182 (11.8) | <0.001 |
| Missed treatment | <0.001 | |||
| 0 | 443 (65.0) | 6276 (73.6) | 1018 (65.7) | |
| 1 | 63 (9.2) | 853 (10.0) | 159 (10.3) | |
| 2 | 79 (11.6) | 693 (8.1) | 155 (10.0) | |
| ≥3 | 97 (14.2) | 705 (8.3) | 217 (14.0) | |
| Target weight adjustment | <0.001 | |||
| 0 | 256 (37.5) | 4888 (57.3) | 737 (47.6) | |
| 1 | 248 (36.4) | 2434 (28.5) | 374 (24.1) | |
| 2 | 66 (9.7) | 789 (9.3) | 84 (5.4) | |
| ≥3 | 112 (16.4) | 416 (4.9) | 354 (22.9) | |
| Pre-HD SBP (mmHg) | 0.25 | |||
| ≤130 | 95 (13.9) | 1132 (13.3) | 212 (13.7) | |
| 131–150 | 236 (34.6) | 2727 (32.0) | 467 (30.1) | |
| ≥151 | 351 (51.5) | 4668 (54.7) | 870 (56.2) | |
| IDHc | 52 (7.6) | 781 (9.2) | 189 (12.2) | <0.001 |
| Equilibrated Kt/V | 1.3±0.3 (n=617) | 1.4±0.3 (n=7777) | 1.4±0.3 (n=1391) | <0.01 |
| Albumin (g/dl) | <0.001 | |||
| ≤2.9 | 85 (12.5) | 297 (3.5) | 58 (3.7) | |
| 3–3.9 | 362 (53.1) | 3598 (42.2) | 683 (44.1) | |
| ≥4.0 | 235 (34.4) | 4632 (54.3) | 808 (52.2) | |
| Hemoglobin (g/dl) | 12.0±1.6 | 12.2±1.4 | 12.1±1.4 | <0.001 |
| Phosphorus (mg/dl) | 5.1±1.6 | 5.4±1.6 | 5.8±1.8 | <0.001 |
Values are presented as mean±SD or n (%). Below target weight is defined as >2 kg below target weight in ≥30% of baseline period dialysis sessions, above target weight is defined as >2 kg above target weight in ≥30% of baseline period dialysis sessions, and at target weight is defined as neither. Table excludes patients who fell into both the above and below target weight exposure categories (n=27). HD, hemodialysis; UF, ultrafiltration; IDWG, interdialytic weight gain; SBP, systolic BP; IDH, intradialytic hypotension.
Across postdialysis weight groups, determined by ANOVA for continuous variables and chi-squared testing for categorical variables.
Defined as delivered treatment time >10 minutes less than prescribed treatment time.
Defined as minimum intradialytic SBP<90 mmHg in ≥30% of exposure period dialysis sessions.
Target Weight Misses and Mortality
Overall, 10,785 patients underwent 131,541 treatments during the baseline period (mean, 12.5±1.7 treatments/patient). Of these, 1577 patients had postdialysis weight >2 kg above target in ≥30% of baseline treatments (versus 9208 controls), and 709 patients had postdialysis weight >2 kg below target in ≥30% of baseline treatments (versus 10,076 controls). Twenty-seven patients met criteria as being exposed in both analyses. The median follow-up time was 2.1 years, and there were 23,809 patient years of total follow-up time. Among the patients that survived the exposure period (10,769), there were 2954 deaths (27.4%). There were 1146 cardiovascular deaths (38.8% of deaths).
Above target weight miss in at least 30% of treatments (versus not) was associated with all-cause mortality (adjusted hazard ratio [HR], 1.28; 95% confidence interval [95% CI], 1.15 to 1.43) and cardiovascular mortality (adjusted HR, 1.26; 95% CI, 1.07 to 1.50). Below target weight miss in at least 30% of treatments (versus control) was also associated with all-cause mortality (adjusted HR, 1.22; 95% CI, 1.05 to 1.40) and cardiovascular mortality (adjusted HR, 1.56; 95% CI, 1.26 to 1.93).
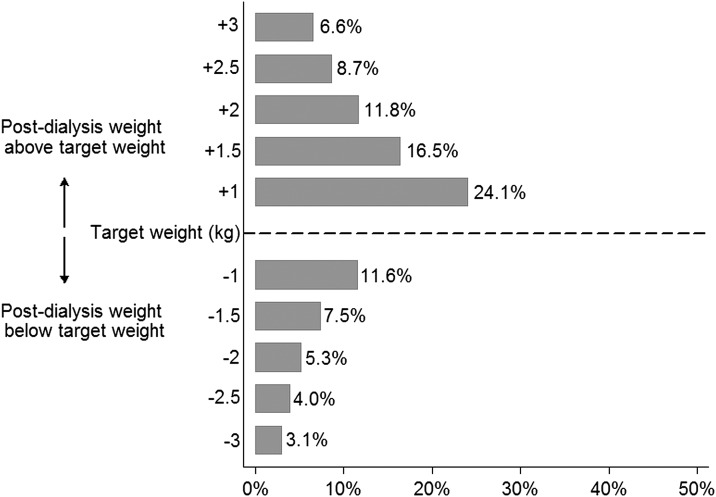
To examine dose response in the target weight miss–mortality associations, we categorized patients as having 0%, 1%–19%, 20%–39%, and ≥40% of baseline treatments with above and below target weight misses. Compared with 0% treatments affected, higher frequency of target weight misses (above and below) was incrementally associated with greater mortality (Figure 1). We also varied the weight threshold used to distinguish target weight miss from target weight attainment (1, 1.5, 2, 2.5, and 3 kg above and below target weight). Figure 2 displays the frequency of target weight miss by varying thresholds. As the threshold for categorization increased, the magnitude of the association between above target weight miss and all-cause mortality increased. The association between below target weight miss and all-cause mortality did not show a consistent dose-response pattern (Table 2).
Figure 1.
Higher frequency of target weight misses (above and below) was incrementally associated with greater mortality. Adjusted associations between baseline target weight misses (above and below) and all-cause (Panel 1) and cardiovascular (Panel 2) mortality. Multivariable models contained exposure terms for both above and below weight and were adjusted for age, sex, race (black or nonblack), coronary artery disease, heart failure, diabetes, vascular access type (fistula, graft, or catheter), albumin (≤2.9, 3–3.9, and ≥4.0 g/dl), phosphorus (milligrams per deciliter), hemoglobin (grams per deciliter), equilibrated Kt/V, dialytic vintage (≤1.0, 1.1–1.9, 2.0–3.9, and ≥4.0 years), prescribed treatment time (minutes), interdialytic weight gain (kilograms), postdialysis weight (≤62.7, 62.8–74.0, 74.1–88.9, and ≥89.0 kg), predialysis systolic blood pressure (≤130, 131–150, and ≥151 mmHg), and missed dialysis treatments (0, 1, 2, and ≥3). 95% CI, 95% confidence interval; HR, hazard ratio; ref., reference population.
Figure 2.
Frequency of target weight miss by varying thresholds. Frequency of missed target weight was defined by the number of dialysis sessions with postdialysis weight above (and below) the prescribed target weight by differing thresholds divided by the total number of dialysis treatments in the baseline period.
Table 2.
Adjusted associations between baseline target weight misses and all-cause mortality using varied thresholds to distinguish missed versus achieved target weight
| Thresholds of Target Weight Miss | Above Target Weight | Below Target Weight | ||
|---|---|---|---|---|
| n (%) | Adjusted HRa (95% CI) | n (%) | Adjusted HRa (95% CI) | |
| >1 kg in <30% tmts | 7417 (68.8) | 1.00 (ref.) | 9222 (85.5) | 1.00 (ref.) |
| >1 kg in ≥30% tmts | 3368 (31.2) | 1.17 (1.07 to 1.27) | 1563 (14.5) | 1.14 (1.02 to 1.27) |
| >1.5 kg in <30% tmts | 7417 (68.8) | 1.00 (ref.) | 9222 (85.5) | 1.00 (ref.) |
| >1.5 kg in ≥30% tmts | 3368 (31.2) | 1.17 (1.07 to 1.27) | 1563 (14.5) | 1.14 (1.02 to 1.27) |
| >2 kg in <30% tmts | 9208 (85.4) | 1.00 (ref.) | 10,076 (93.4) | 1.00 (ref.) |
| >2 kg in ≥30% tmts | 1577 (14.6) | 1.28 (1.15 to 1.43) | 709 (6.6) | 1.22 (1.05 to 1.40) |
| >2.5 kg in <30% tmts | 9592 (88.9) | 1.00 (ref.) | 10,248 (95.0) | 1.00 (ref.) |
| >2.5 kg in ≥30% tmts | 1193 (11.1) | 1.28 (1.14 to 1.44) | 537 (5.0) | 1.21 (1.03 to 1.43) |
| >3 kg in <30% tmts | 9833 (91.2) | 1.00 (ref.) | 10,379 (96.2) | 1.00 (ref.) |
| >3 kg in ≥30% tmts | 952 (8.8) | 1.28 (1.12 to 1.45) | 406 (3.8) | 1.15 (0.96 to 1.39) |
tmts, dialysis treatments; HR, hazard ratio; 95% CI, 95% confidence ratio; ref., reference population.
Multivariable models contained exposure terms for both above and below weight and were adjusted for age, sex, race (black or nonblack), coronary artery disease, heart failure, diabetes, vascular access type (fistula, graft, or catheter), albumin (≤2.9, 3–3.9, and ≥4.0 g/dl), phosphorus (milligrams per deciliter), hemoglobin (grams per deciliter) equilibrated Kt/V, dialytic vintage (≤1.0, 1.1–1.9, 2.0–3.9, and ≥4.0 years), prescribed treatment time (minutes), interdialytic weight gain (kilograms), postdialysis weight (≤62.7, 62.8–74.0, 74.1–88.9, and ≥89.0 kg), predialysis systolic blood pressure (≤130, 131–150, and ≥151 mmHg), and missed dialysis treatments (0, 1, 2, and ≥3).
The data suggested effect modification of the target weight miss–mortality association on the basis of body weight (Table 3). Specifically, below target weight miss was associated with all-cause mortality in patients weighing ≥60 kg but was not among patients <60 kg. Above target weight miss was associated with mortality across all weights.
Table 3.
Effect modification of the association between baseline target weight misses and all-cause mortality on the basis of body weight
| Target Weight Miss | Postdialysis Weight <60 kg | Postdialysis Weight ≥60 kg | P Valueb | ||
|---|---|---|---|---|---|
| Crude Death Rate (Deaths/100 Patient Years) | Adjusted HRa (95% CI) | Crude Death Rate (Deaths/100 Patient Years) | Adjusted HRa (95% CI) | ||
| >2 kg above target in <30% tmts | 16.9 | 1.00 (ref.) | 11.0 | 1.00 (ref.) | 0.12 |
| >2 kg above target in ≥30% tmts | 24.6 | 1.40 (1.07 to 1.83) | 13.0 | 1.28 (1.14 to 1.43) | |
| >2 kg below target in <30% tmts | 17.3 | 1.00 (ref.) | 11.2 | 1.00 (ref.) | 0.01 |
| >2 kg below target in ≥30% tmts | 18.1 | 1.03 (0.80 to 1.32) | 13.9 | 1.30 (1.09 to 1.55) | |
tmts, dialysis treatments; HR, hazard ratio; 95% CI, 95% confidence ratio; ref., reference population.
Multivariable models contained exposure terms for both above and below weight and were adjusted for age, sex, race (black or nonblack), coronary artery disease, heart failure, diabetes, vascular access type (fistula, graft, or catheter), albumin (≤2.9, 3–3.9, and ≥4.0 g/dl), phosphorus (milligrams per deciliter), hemoglobin (grams per deciliter), equilibrated Kt/V, dialytic vintage (≤1.0, 1.1–1.9, 2.0–3.9, and ≥4.0 years), prescribed treatment time (minutes), interdialytic weight gain (kilograms), postdialysis weight (≤62.7, 62.8–74.0, 74.1–88.9, and ≥89.0 kg), predialysis systolic blood pressure (≤130, 131–150, and ≥151 mmHg), and missed dialysis treatments (0, 1, 2, and ≥3).
On the basis of likelihood ratio testing.
Target Weight Misses and Intermediate Endpoints
To explore possible mechanisms underlying the missed target weight–mortality association, we examined the associations of missed target weight and IDH, rapid UF rate, and large IDWG (separately). Of the 10,769 patients who survived the baseline period, 10.0% experienced IDH, 35.3% had delivered UF rates >13 ml/h per kg, and 70.8% had IDWG>3.5% of body weight in ≥30% of baseline treatments.
Above target weight miss was associated with greater odds of IDH (adjusted odds ratio [OR], 1.43; 95% CI, 1.18 to 1.73), but below target weight miss was not. Similarly, above target weight miss was associated with greater odds of large IDWG (adjusted OR 1.68; 95% CI, 1.32 to 2.13), but below target weight miss was not. Below target weight miss was associated with greater odds of rapid UF rates (adjusted OR, 1.58; 95% CI, 1.24 to 2.02), and above target weight miss had a less potent but significant association with greater odds of rapid UF rates. Confirmatory analyses considering outcomes on a treatment-to-treatment basis were similar (Table 4).
Table 4.
Associations of target weight misses and secondary outcomes during the 30-day baseline period
| Secondary Outcome | No. of treatments (%) with Outcome/No. of treatments without Outcome (n=131,333) | Logistic Adjusted ORa (95% CI) | Generalized Estimating Equation Adjusted ORb (95% CI) |
|---|---|---|---|
| IDH (nadir SBP<90 mmHg) | |||
| >2 kg above target in <30% tmts | 10,179 (9.1)/102,126 (90.9) | 1.00 (ref.) | 1.00 (ref.) |
| >2 kg above target in ≥30% tmts | 2,281 (12.0)/16,747 (88.0) | 1.43 (1.18 to 1.73) | 1.32 (1.19 to 1.47) |
| >2 kg below target in <30% tmts | 11,772 (9.6)/111,325 (90.4) | 1.00 (ref.) | 1.00 (ref.) |
| >2 kg below target in ≥30% tmts | 688 (8.4)/7548 (91.6) | 0.83 (0.61 to 1.14) | 0.96 (0.82 to 1.46) |
| Rapid UF rate (>13 ml/h per kg) | |||
| >2 kg above target in <30% tmts | 29,129 (25.9)/83,176 (74.1) | 1.00 (ref.) | 1.00 (ref.) |
| >2 kg above target in ≥30% tmts | 5902 (31.0)/13,126 (69.0) | 1.29 (1.08 to 1.54) | 1.17 (1.11 to 1.25) |
| >2 kg below target in <30% tmts | 33,224 (27.0)/89,873 (73.0) | 1.00 (ref.) | 1.00 (ref.) |
| >2 kg below target in ≥30% tmts | 1807 (21.9)/6429 (78.1) | 1.58 (1.24 to 2.02) | 1.14 (1.05 to 1.25) |
| Large IDWG (>3.5% body weight) | |||
| >2 kg above target in <30% tmts | 52,775 (47.0)/59,530 (53.0) | 1.00 (ref.) | 1.00 (ref.) |
| >2 kg above target in ≥30% tmts | 10,903 (57.3)/8125 (42.7) | 1.68 (1.32 to 2.13) | 1.09 (1.04 to 1.15) |
| >2 kg below target in <30% tmts | 60,675 (49.3)/62,422 (50.7) | 1.00 (ref.) | 1.00 (ref.) |
| >2 kg below target in ≥30% tmts | 3003 (36.5)/5233 (63.5) | 0.86 (0.64 to 1.15) | 0.92 (0.85 to 0.99) |
OR, odds ratio; 95% CI, 95% confidence interval; IDH, intradialytic hypotension; SBP, systolic BP; tmts, dialysis treatment; ref., reference population; UF, ultrafiltration; IDWG, interdialytic weight gain.
Separate logistic multivariable models considered the outcome of interest as binary (present in ≥30% of baseline treatments versus not) and contained exposure terms for both above and below weight and were adjusted for age, sex, race (black or nonblack), coronary artery disease, heart failure, diabetes, vascular access type (fistula, graft, or catheter), albumin (≤2.9, 3–3.9, and ≥4.0 g/dl), phosphorus (milligrams per deciliter), hemoglobin (grams per deciliter), equilibrated Kt/V, dialytic vintage (≤1.0, 1.1–1.9, 2.0–3.9, and ≥4.0 years), prescribed treatment time (minutes), IDWG (kilograms), postdialysis weight (≤62.7, 62.8–74.0, 74.1–88.9, and ≥89.0 kg), predialysis systolic blood pressure (≤130, 131–150, and ≥151 mmHg), and missed dialysis treatments (0, 1, 2, and ≥3). The model for IDWG did not include adjustment for postweight or IDWG.
Separate generalized estimating equations considered the outcome of interest on a treatment-to-treatment basis in the baseline period and within-subject correlation was accounted for via an exchangeable covariance. Model exposures and covariates were analogous to logistic models.
Discussion
Prior analysis of the target weight miss–outcomes association did not consider target weight miss frequency and did not account for patients with dialysis treatments with postweight above and below target weight. In this analysis, we demonstrate that postdialysis weight >2 kg above (and below) target weight is associated with higher all-cause and cardiovascular mortality. Additionally, the data suggest a dose-response relationship between target weight misses and outcomes.
Our findings support and expand the existing evidence regarding the risks of failed target weight achievement. In a prospective study of 182 prevalent HD patients, Movilli et al. demonstrated an association between above target weight misses and greater all-cause and cardiovascular mortality (8). However, study interpretation is limited by use of 30-day mean values for the exposure. Arithmetic means are sensitive to extreme values and may obscure outcome associations particularly in the settings of integer opposites and small samples (15). In contrast, our exposure definition required that patients meet the target weight miss definition in at least 30% of baseline treatments to qualify as (+) target weight miss. Frequency-based definitions lend themselves to intuitive clinical application: thrice-weekly HD patients with ≥1 weekly treatment postweight >2 kg above (or below) target weight are at heightened risk for adverse outcomes.
Our report of an association between above target weight miss and outcomes is consistent with evidence linking volume expansion and adverse outcomes. In the setting of a correctly specified target weight, above target weight miss renders patients volume-expanded and at risk for the development of hypertension and ventricular hypertrophy and their downstream consequences of structural remodeling, heart failure, arrhythmia, and death (3,4,16–18). Our finding of an association between above target weight miss and greater IDWG supports this pathway. Often times, providers are reluctant to expose patients with high IDWG to the rapid UF rates required to reach target weight (presuming static treatment times). This tendency is reflected in our finding that above target weight miss had a less potent association with greater UF rates than below target weight miss. Therefore, high weight gainers may incur risk from both greater IDWG and more frequent missed target weight. Research to determine the relative harm of higher UF rates and achievement of target weight versus lower UF rates and chronic fluid overload is needed.
Similarly, our finding of an association between below target weight miss and outcomes is not surprising because below target weight misses may reflect overaggressive UF and associated cardiac consequences (13). However, we did not demonstrate an association between below target weight and IDH, suggesting that overly rapid UF and associated cardiac ischemia (19) may not drive the demonstrated association. There may be two alternative explanations of the observation. First, an overestimated target weight may partially explain the association between below target weight and outcomes. When target weights are overestimated, patients incur risk from volume expansion when target weight is achieved. In such patients, target and postweight discrepancies represent systems error because the prescribed target weight is not updated in accordance with actual treatment course. Second, below target weight may identify individuals with poorer overall health status. For example, below target weight patients were more likely to be dialyzed via a catheter, have lower postdialysis weight, and have lower albumin. However, our finding that the association between below target weight and outcome was attenuated in patients weighing <60 kg argues against ambient health status as the only explanation.
Our results suggest the need for greater attention to target weight attainment and titration. Although existing standards for target weight achievement exist (7), they are not routinely met. One potential intervention is weekly nursing or dietician review of facility patients with postdialysis weights not within some threshold value of their target weights. Additionally, more frequent provider visits with greater attention to volume may be beneficial. More frequent provider visits have been shown to decrease hospitalizations (20) and 30-day rehospitalizations (21). Better volume management associated with increased oversight may contribute to these improved outcomes.
Although consistent target weight attainment may offer one avenue to lessen risk associated with inadequate fluid management, it should not detract from efforts to improve target weight estimation. If the prescribed target weight does not accurately reflect total-body euvolemia, consistent achievement of this misestimated target weight will not fully mitigate fluid-associated risk. Practitioners commonly rely on history and physical examination to guide target weight estimation, but accuracy is limited. Bioimpedance data suggest that >30% of HD patients remain volume-expanded at their target weights (22). Technologies that aid in target weight estimation (e.g., bioimpedance, blood volume monitors, lung ultrasound) exist (23), but adoption has been limited. Further outcomes and practice-based research to define the utilities of these tools and to inform their incorporation into clinical practice is needed.
Our study does have limitations. Confounding is a limitation in all observational studies. We attempted to minimize confounding by including factors plausibly associated with exposure and/or outcome in our models. Specifically, we lacked data on residual renal function and treatment nonadherence. To minimize confounding from these factors, we considered vintage as a partial surrogate for urine output and missed treatments as a surrogate for treatment nonadherence. We cannot exclude the possibility of residual confounding from these or other variables. Second, target weights were prescribed by treating nephrologists per routine practice, and clinical approach to target weight estimation may have varied across providers. Prescribed target weights may not have reflected true intravascular euvolemia, therefore introducing misclassification bias. However, such misclassification bias would be nondifferential and bias results to the null, lending credence to our findings. Additionally, because misclassification in this regard is inherent to contemporary practice, demonstration of meaningful associations despite potential misclassification bias suggests that targeted intervention may be beneficial even as processes aimed at empirically estimated euvolemic target weight are being worked out. Third, inadequately calibrated scales or other equipment flaws may have introduced instrument bias. We selected a higher threshold (2 kg) to define missed target weight to ensure adequate separation between missed versus achieved target weight in efforts to minimize the influence of such bias (9). Fourth, we were unable to consider body mass index as a measure of body size because of missing height data. Fifth, cardiovascular-specific mortality was determined from the medical record on the basis of death certificate and hospital data review. Lack of formal cause of death adjudication may have introduced misclassification bias into cardiovascular mortality findings. Finally, we studied thrice-weekly chronic HD patients and excluded patients at the extremes of treatment times and body weights. Our results should not be extrapolated to populations not represented in our analyses.
In summary, this study demonstrates that postdialysis weights >2 kg above and below target weight are associated with higher all-cause and cardiovascular mortality. Research to confirm findings and further specify missed target weight thresholds is needed. Finally, concurrent focus on improved target weight estimation is imperative if we are to reduce volume-related complications among dialysis patients.
Disclosures
Dr. Flythe has received speaking honoraria from Dialysis Clinic Inc. When the study began, Dr. Brunelli was employed by Brigham and Women’s Hospital. As of September 2012, Dr. Brunelli’s employment shifted to DaVita Clinical Research, the company that provided the data for this research. His continuation on the study is as a DaVita Clinical Research employee. Additionally, Dr. Brunelli has received speaking honoraria from Fresenius Medical Care North America, has served on advisory boards for Amgen and C.B. Fleet, Keryx, and Otsuka and his spouse is employed by Astra Zeneca.
Supplementary Material
Acknowledgments
We thank DaVita Clinical Research for providing data for this study.
DaVita Clinical Research had no role in the design or implementation of this study or on the decision to publish. DaVita Clinical Research is committed to advancing the knowledge and practice of kidney care.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10201014/-/DCSupplemental.
See related editorial, “Posthemodialysis Weights and Mortality: Another Narrow Range Target?,” on pages 729–731.
References
- 1.Saran R, Bragg-Gresham JL, Levin NW, Twardowski ZJ, Wizemann V, Saito A, Kimata N, Gillespie BW, Combe C, Bommer J, Akiba T, Mapes DL, Young EW, Port FK: Longer treatment time and slower ultrafiltration in hemodialysis: Associations with reduced mortality in the DOPPS. Kidney Int 69: 1222–1228, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zoccali C, Benedetto FA, Tripepi G, Mallamaci F: Cardiac consequences of hypertension in hemodialysis patients. Semin Dial 17: 299–303, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Sharpe N: Left ventricular remodeling: Pathophysiology and treatment. Heart Fail Monit 4: 55–61, 2003 [PubMed] [Google Scholar]
- 5.Burton JO, Korsheed S, Grundy BJ, McIntyre CW: Hemodialysis-induced left ventricular dysfunction is associated with an increase in ventricular arrhythmias. Ren Fail 30: 701–709, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Chen PS, Chou CC, Tan AY, Zhou S, Fishbein MC, Hwang C, Karagueuzian HS, Lin SF: The mechanisms of atrial fibrillation. J Cardiovasc Electrophysiol 17[Suppl 3]: S2–S7, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Medicare & Medicaid Services (CMS), HHS : Medicare and Medicaid programs; conditions for coverage for end-stage renal disease facilities. Final rule. Fed Regist 73: 20369–20484, 2008 [PubMed] [Google Scholar]
- 8.Movilli E, Camerini C, Gaggia P, Zubani R, Feller P, Poiatti P, Pola A, Carli O, Cancarini G: Magnitude of end-dialysis overweight is associated with all-cause and cardiovascular mortality: A 3-year prospective study. Am J Nephrol 37: 370–377, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Biehl A, Hovengen R, Meyer HE, Hjelmesaeth J, Meisfjord J, Grøholt EK, Roelants M, Strand BH: Impact of instrument error on the estimated prevalence of overweight and obesity in population-based surveys. BMC Public Health 13: 146, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang TI, Paik J, Greene T, Desai M, Bech F, Cheung AK, Chertow GM: Intradialytic hypotension and vascular access thrombosis. J Am Soc Nephrol 22: 1526–1533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chesterton LJ, Selby NM, Burton JO, McIntyre CW: Cool dialysate reduces asymptomatic intradialytic hypotension and increases baroreflex variability. Hemodial Int 13: 189–196, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Flythe J, Xue H,Lynch K, Curhan G, Brunelli S: Association of mortality risk with various definitions of intradialytic hypotension [published online ahead of print September 30, 2014]. J Am Soc Nephrol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flythe JE, Kimmel SE, Brunelli SM: Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int 79: 250–257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-Gómez JM, Villaverde M, Jofre R, Rodriguez-Benítez P, Pérez-García R: Interdialytic weight gain as a marker of blood pressure, nutrition, and survival in hemodialysis patients. Kidney Int Suppl (93): S63–S68, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Manikandan S: Measures of central tendency: The mean. J Pharmacol Pharmacother 2: 140–142, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMullen JR, Sherwood MC, Tarnavski O, Zhang L, Dorfman AL, Shioi T, Izumo S: Inhibition of mTOR signaling with rapamycin regresses established cardiac hypertrophy induced by pressure overload. Circulation 109: 3050–3055, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Cotter G, Felker GM, Adams KF, Milo-Cotter O, O’Connor CM: The pathophysiology of acute heart failure--is it all about fluid accumulation? Am Heart J 155: 9–18, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Ritz E, Wanner C: The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol 3: 920–929, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 4: 1925–1931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slinin Y, Guo H, Li S, Liu J, Ensrud K, Gilbertson DT, Collins AJ, Ishani A: Association of provider-patient visit frequency and patient outcomes on hemodialysis. J Am Soc Nephrol 23: 1560–1567, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson KF, Winkelmayer WC, Chertow GM, Bhattacharya J: Physician visits and 30-day hospital readmissions in patients receiving hemodialysis. J Am Soc Nephrol 25: 2079–2087, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passauer J, Petrov H, Schleser A, Leicht J, Pucalka K: Evaluation of clinical dry weight assessment in haemodialysis patients using bioimpedance spectroscopy: A cross-sectional study. Nephrol Dial Transplant 25: 545–551, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Davies SJ, Davenport A: The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int 86: 489–496, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.