Abstract
Background and objectives
Uncontrolled secondary hyperparathyroidism (sHPT) in patients with ESRD is a risk factor for calcific uremic arteriolopathy (CUA; calciphylaxis).
Design, setting, participants, & measurements
Adverse event reports collected during the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events trial were used to determine the frequency of CUA in patients receiving hemodialysis who had moderate to severe sHPT, as well as the effects of cinacalcet versus placebo. CUA events were collected while patients were receiving the study drug.
Results
Among the 3861 trial patients who received at least one dose of the study drug, 18 patients randomly assigned to placebo and six assigned to cinacalcet developed CUA (unadjusted relative hazard, 0.31; 95% confidence interval [95% CI], 0.13 to 0.79; P=0.014). Corresponding cumulative event rates (95% CI) at year 4 were 0.011% (0.006% to 0.018%) and 0.005% (0.002% to 0.010%). By multivariable analysis, other factors associated with CUA included female sex, higher body mass index, higher diastolic BP, and history of dyslipidemia or parathyroidectomy. Median (10%, 90% percentile) plasma parathyroid hormone concentrations proximal to the report of CUA were 796 (225, 2093) pg/ml and 410 (71, 4957) pg/ml in patients randomly assigned to placebo and cinacalcet, respectively. Active use of vitamin K antagonists was recorded in 11 of 24 patients with CUA, nine randomly assigned to placebo, and two to cinacalcet, in contrast to 5%–7% at any one time point in patients in whom CUA was not reported.
Conclusion
Cinacalcet appeared to reduce the incidence of CUA in hemodialysis recipients who have moderate to severe sHPT.
Keywords: calcium receptor, dialysis, hyperparathyroidism, hyperphosphatemia, mineral metabolism
Introduction
Calcific uremic arteriolopathy (CUA), often referred to as calciphylaxis, is a relatively rare syndrome, which carries a 1-year survival rate of only 45% (1–5). The central pathologic lesion in CUA is a calcified media of small and medium-sized arterial vessels of the skin and fat tissue, leading to progressive and extremely painful cutaneous ulcerations (1–5). Infections of such skin lesions represent the main reason for the high mortality of CUA (1–5). CUA rarely manifests in patients with normal kidney function (6), and most cases are confined to patients with advanced CKD and in particular ESRD (1). The exact prevalence of CUA in the dialysis population is unknown; estimates range from 1% to 4% of all patients with ESRD (7–9).
The pathogenesis of CUA is incompletely understood. Recent data suggest that CUA involves a cell-mediated, bone morphogenetic protein-2–driven osteogenic process with extensive subcutaneous extracellular matrix remodeling and deposition of hydroxyapatite (10). A cascade consisting of matrix remodeling, calcification, endothelial damage and thrombus formation, luminal obstruction, and finally development of full-blown CUA has been postulated (10). Risk factors for CUA identified in clinical studies include CKD/ESRD, female sex, diabetes mellitus, obesity, and vitamin K antagonism (3–5,11,12). With respect to mineral bone disorders in CKD/ESRD, CUA prevalence is higher in patients with hyperphosphatemia and those receiving calcium-containing phosphate binders (3–5). Both low-turnover bone disease in ESRD and high-turnover bone disease associated with severe secondary hyperparathyroidism (sHPT) have been associated with CUA (13,14).
At present it is unknown whether specific therapies that decrease excessive parathyroid hormone (PTH) concentrations raise or lower the incidence of CUA. In the current analysis, we aimed to confirm or refute known risk factors for CUA and to test the hypothesis that a reduction in PTH with cinacalcet reduces the frequency of CUA in the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial (www.ClinicalTrials.gov registration number: NCT00345839) (15,16).
Materials and Methods
Study Population and Design
In the EVOLVE trial, 3883 patients with sHPT receiving hemodialysis were randomly assigned 1:1 to receive cinacalcet (Sensipar or Mimpara, Amgen, Inc.) or placebo in addition to conventional therapies for CKD–mineral and bone disorder. Eligible participants were undergoing hemodialysis three times per week and had plasma PTH concentrations ≥300 pg/ml (31.8 pmol/L), serum calcium phosphate product ≥45 mg2/dl2 (3.63 mmol2/L2), and serum calcium ≥8.4 mg/dl (2.1 mmol/L). The dose of study drug was titrated every 4 weeks during the first 20 weeks and every 8 weeks during the subsequent follow-up period (from a starting dose of 30 mg to a maximum dose of 180 mg daily), depending on blood levels of PTH and calcium (17). The dialysis prescription, type/doses of phosphate binders, vitamin D sterols, calcium supplements and other medications, and other medical procedures were administered at the discretion of treating physicians. The trial assessed the effect of treatment with cinacalcet compared with placebo in addition to other conventional therapies (calcitriol or vitamin D sterols and phosphate binders) on the primary composite endpoint of all-cause mortality and major cardiovascular events (myocardial infarction, hospitalization for unstable angina, heart failure, or peripheral vascular event). The study design, baseline characteristics of the participants, primary results, and effect of cinacalcet on severe unremitting hyperparathyroidism have been previously published (15–18). The intervention lasted up to 64 months. The trial was sponsored by Amgen, Inc. An academic Executive Committee with direct oversight of all final analyses and publications led the trial. The ethics committees at all participating sites approved the study, and all patients provided informed consent.
All adverse events, including CUA, were collected while patients were receiving the study drug. In contrast to the components of the primary composite outcome and selected secondary outcomes (i.e., stroke, parathyroidectomy, and clinical fracture), CUA adverse events were not adjudicated. The estimated incidence of CUA was based on the report of local investigators and was not necessarily confirmed by biopsy or other means. All demographic and laboratory data analyzed were collected as part of the primary study (15,16).
Statistical Analyses
All randomly assigned patients who received at least one dose of the study drug (n=3861; the safety analysis set) were included in these analyses. All CUA cases that occurred during the adverse event reporting period were reviewed and included. The observation period was from the first dose date of the study drug to initial CUA, excluding the time adverse events were not collected as per the protocol. A two-sided Gray test was used to compare the cumulative incidence function estimates of the survival time between the treatment groups. Fine–Gray subdistributional hazards regression models were used to calculate the relative hazard (cinacalcet versus placebo) and 95% confidence intervals (95% CIs). We performed unadjusted and multivariable analyses. The multivariable analysis was adjusted for baseline covariates using a backward selection procedure at a significance level of 0.10. Potential covariates included baseline patient and demographic characteristics, concomitant medication use, cardiovascular history, and laboratory measures.
Because findings in the primary analysis of the EVOLVE trial (determining the effect of cinacalcet versus placebo on the primary composite endpoint through use of an unadjusted intention-to-treat analysis) were not statistically significant, analysis results with a P value <0.05 were deemed nominally statistically significant. Statistical analyses were performed using SAS software version 9.3 (SAS Institute, Inc., Cary, NC).
Results
CUA Incidence
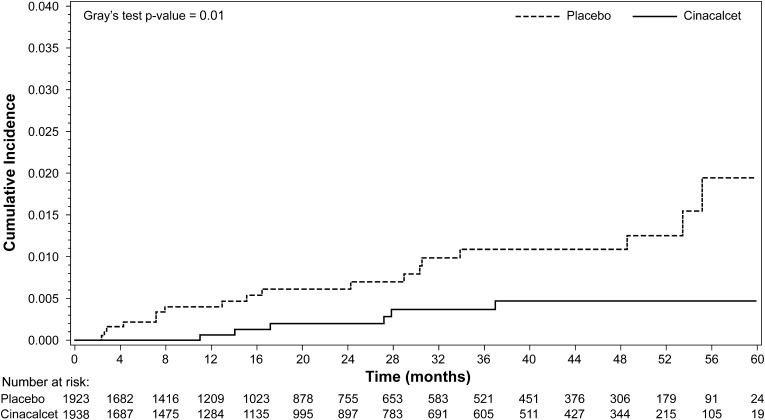
Among the 3861 trial patients who received at least one dose of study drug, 24 patients developed CUA: 18 patients randomly assigned to placebo and six patients assigned to cinacalcet (unadjusted relative hazard, 0.31; 95% CI, 0.13 to 0.79; P=0.014). The median (10%, 90% percentile) time to CUA was 1.3 (0.2, 4.5) years in patients randomly assigned to placebo and 1.8 (0.9, 3.1) years in patients assigned to cinacalcet. Corresponding cumulative event rates (95% CI) at year 4 were 0.011% (0.006% to 0.018%) and 0.005% (0.002% to 0.010%), respectively (Figure 1).
Figure 1.
Cumulative incidence plot of time to calcific uremic arteriolopathy adverse event (safety analysis set).
Baseline Characteristics
Table 1 shows baseline characteristics of patients who were reported to have developed CUA and those of all other trial participants. Compared with the latter, patients who developed CUA were more likely to be younger and female; to have a higher Quételet (body mass) index (BMI); to have hypertension, diabetes mellitus, and/or dyslipidemia; and to have had a history of heart failure, peripheral vascular disease, or prior parathyroidectomy (with severe enough recurrence of sHPT to meet the trial inclusion criteria). In contrast, ethnicity, region of origin, dialysis vintage, dialysis access, dialysate calcium, tobacco use, history of fractures or coronary artery disease, and baseline laboratory values were not associated with CUA (Table 2).
Table 1.
Key baseline demographic and medical history characteristics by occurrence of calcific uremic arteriolopathy adverse event during the study (safety analysis set)
| Characteristic | CUA (n=24) | No CUA (n=3837) | P Valuea | ||||
|---|---|---|---|---|---|---|---|
| Placebo (N1=18) | Cinacalcet (N1=6) | Total (N1=24) | Placebo (N1=1905) | Cinacalcet (N1=1932) | Total (N1=3837) | ||
| Median age (10%, 90% percentile) (yr) | 54.5 (43.0, 71.0) | 59.5 (32.0, 63.0) | 56.0 (43.0, 63.0) | 54.0 (35.0, 73.0) | 55.0 (35.0, 74.0) | 55.0 (35.0, 73.0) | 0.99 |
| Age category, n (%) | 0.05 | ||||||
| <65 yr | 16 (88.9) | 6 (100) | 22 (91.7) | 1435 (75.3) | 1403 (72.6) | 2838 (74.0) | |
| ≥65 yr | 2 (11.1) | 0 (0.0) | 2 (8.3) | 470 (24.7) | 529 (27.4) | 999 (26.0) | |
| Race group, n (%) | 0.28 | ||||||
| White | 11 (61.1) | 4 (66.7) | 15 (62.5) | 1100 (57.7) | 1115 (57.7) | 2215 (57.7) | |
| Black | 6 (33.3) | 1 (16.7) | 7 (29.2) | 417 (21.9) | 404 (20.9) | 821 (21.4) | |
| Other | 1 (5.6) | 1 (16.7) | 2 (8.3) | 388 (20.4) | 413 (21.4) | 801 (20.9) | |
| Women, n (%) | 11 (61.1) | 4 (66.7) | 15 (62.5) | 754 (39.6) | 802 (41.5) | 1556 (40.6) | 0.03 |
| Median BMI (10%, 90% percentile) (kg/m2) | 34.4 (24.7, 43.5) | 28.5 (21.3, 37.3) | 34.1 (23.1, 41.8) | 26.3 (20.6, 36.6) | 26.3 (20.4, 36.4) | 26.3 (20.5, 36.5) | <0.001 |
| BP (10%, 90% percentile) (mmHg) | |||||||
| Systolic | 163.0 (120.0, 184.0) | 153.5 (137.0, 209.0) | 162.5 (137.0, 184.0) | 140.0 (110.0, 177.0) | 140.0 (110.0, 176.0) | 140.0 (110.0, 176.0) | <0.001 |
| Diastolic | 85.0 (67.0, 100.0) | 80.0 (72.0, 130.0) | 83.0 (70.0, 100.0) | 80.0 (60.0, 100.0) | 80.0 (60.0, 100.0) | 80.0 (60.0, 100.0) | 0.02 |
| Tobacco use, n (%) | 0.11 | ||||||
| Never | 6 (33.3) | 3 (50.0) | 9 (37.5) | 1081 (56.7) | 1077 (55.7) | 2158 (56.2) | |
| Current | 3 (16.7) | 1 (16.7) | 4 (16.7) | 317 (16.6) | 310 (16.0) | 627 (16.3) | |
| Former | 9 (50.0) | 2 (33.3) | 11 (45.8) | 506 (26.6) | 545 (28.2) | 1051 (27.4) | |
| History of diabetes, n (%) | 11 (61.1) | 2 (33.3) | 13 (54.2) | 633 (33.2) | 650 (33.6) | 1283 (33.4) | 0.03 |
| Type 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 81 (4.3) | 73 (3.8) | 154 (4.0) | 0.32 |
| Type 2 | 11 (61.1) | 2 (33.3) | 13 (54.2) | 553 (29.0) | 577 (29.9) | 1130 (29.5) | <0.01 |
| History of cardiovascular disease, n (%) | 18 (100) | 6 (100) | 24 (100) | 1801 (94.5) | 1842 (95.3) | 3643 (94.9) | 0.26 |
| Hypertension | 18 (100) | 6 (100) | 24 (100) | 1746 (91.7) | 1786 (92.4) | 3532 (92.1) | 0.15 |
| Heart failure | 9 (50.0) | 2 (33.3) | 11 (45.8) | 443 (23.3) | 444 (23.0) | 887 (23.1) | <0.01 |
| Peripheral vascular disease | 8 (44.4) | 0 (0.0) | 8 (33.3) | 313 (16.4) | 313 (16.2) | 626 (16.3) | 0.03 |
| CABG | 1 (5.6) | 2 (33.3) | 3 (12.5) | 152 (8.0) | 133 (6.9) | 285 (7.4) | 0.35 |
| PCI | 1 (5.6) | 0 (0.0) | 1 (4.2) | 130 (6.8) | 130 (6.7) | 260 (6.8) | 0.61 |
| Myocardial infarction | 1 (5.6) | 0 (0.0) | 1 (4.2) | 242 (12.7) | 238 (12.3) | 480 (12.5) | 0.22 |
| Stroke | 1 (5.6) | 1 (16.7) | 2 (8.3) | 192 (10.1) | 160 (8.3) | 352 (9.2) | 0.89 |
| Transient ischemic attack | 1 (5.6) | 0 (0.0) | 1 (4.2) | 73 (3.8) | 98 (5.1) | 171 (4.5) | 0.95 |
| Amputation | 2 (11.1) | 0 (0.0) | 2 (8.3) | 127 (6.7) | 121 (6.3) | 248 (6.5) | 0.71 |
| Atrial fibrillation | 4 (22.2) | 0 (0.0) | 4 (16.7) | 221 (11.6) | 202 (10.5) | 423 (11.0) | 0.38 |
| History of parathyroidectomy, n (%) | 3 (16.7) | 1 (16.7) | 4 (16.7) | 84 (4.4) | 90 (4.7) | 174 (4.5) | <0.01 |
| History of dyslipidemia, n (%) | 12 (66.7) | 3 (50.0) | 15 (62.5) | 744 (39.1) | 768 (39.8) | 1512 (39.4) | 0.02 |
N1 refers to number of patients in the safety analysis set; n percentages are based on N1. CUA, calcific uremic arteriolopathy; BMI, body mass index; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention.
Wilcoxon rank sum test was used for continuous variables and chi-square test was used for categorical variables. Comparisons are between patients who experienced a CUA adverse event compared with those who did not.
Table 2.
Key baseline laboratory characteristics by occurrence of calcific uremic arteriolopathy adverse event during the study (safety analysis set)
| Characteristic | CUA (n=25) | No CUA (n=3858) | P Valuea | ||||
|---|---|---|---|---|---|---|---|
| Placebo (N1=19) | Cinacalcet (N1=6) | Total (N1=25) | Placebo (N1=1916) | Cinacalcet (N1=1942) | Total (N1=3858) | ||
| Intact PTH (pg/ml) | 515 (285, 1339) | 857 (344, 2755) | 579 (333, 2121) | 692 (364, 1693) | 694 (363, 1699) | 693 (364, 1695) | 0.18 |
| Corrected calcium (mg/dl) | 9.9 (9.0, 10.6) | 10.5 (9.7, 10.8) | 10.1 (9.5, 10.6) | 9.8 (9.0, 10.7) | 9.8 (9.0, 10.7) | 9.8 (9.0, 10.7) | 0.08 |
| Phosphorus (mg/dl) | 6.4 (4.6, 9.5) | 6.6 (4.8, 8.2) | 6.4 (4.8, 8.8) | 6.2 (4.9, 8.4) | 6.3 (4.9, 8.3) | 6.2 (4.9, 8.4) | 0.45 |
| 25(OH)D (ng/ml) | 14.5 (6, 38) | 20.5 (14, 26) | 15 (8, 28) | 18 (8, 39) | 17 (8, 37) | 17 (8, 37) | 0.44 |
| Alkaline phosphatase (U/L) | 91 (59, 151) | 129 (59, 500) | 91 (59, 166) | 108 (65, 232) | 107 (64, 248) | 108 (65, 24) | |
| Bone alkaline phosphatase (µg/L) | 17.53 (9.60, 32.89) | 31.51 (10.29, 139.66) | 20.96 (10.21, 38.21) | 22.92 (11.56, 66.81) | 23.09 (11.52, 70.52) | 23.02 (11.53, 68.11) | 0.12 |
| N-telopeptide (nmol/L) | 141.6 (45.6, 280.4) | 620.9 (134.4, 1414.1) | 196.2 (52.8, 692.8) | 249.6 (79.3, 821.0) | 261.0 (81.0, 925.4) | 255.3 (80.6, 884.9) | 0.13 |
| Hemoglobin (g/dl) | 11.9 (8.4, 13.2) | 11.7 (9.1, 12.7) | 11.7 (9.1, 12.7) | 11.8 (10.0, 13.8) | 11.7 (10.0, 13.5) | 11.8 (10.0, 13.6) | 0.62 |
| Albumin (g/dl) | 3.6 (3.1, 4.0) | 3.5 (3.3, 4.2) | 3.6 (3.1, 4.0) | 3.7 (3.2, 4.1) | 3.7 (3.2, 4.1) | 3.7 (3.2, 4.1) | 0.30 |
| Total protein (g/dl) | 7.0 (6.2, 7.7) | 6.8 (5.8, 7.1) | 6.9 (6.2, 7.5) | 7.0 (6.3, 7.7) | 6.9 (6.3, 7.7) | 6.9 (6.3, 7.7) | 0.44 |
| Bicarbonate (mEq/L) | 18.8 (14.4, 26.0) | 23.3 (18.9, 24.9) | 19.3 (14.7, 24.9) | 20.4 (15.8, 25.6) | 20.3 (15.7, 25.4) | 20.3 (15.7, 25.5) | 0.63 |
| Creatinine (mg/dl) | 9.7 (4.7, 13.6) | 10.9 (7.7, 12.8) | 10.0 (6.4, 13.2) | 9.9 (6.7, 13.9) | 9.9 (6.6, 13.9) | 9.9 (6.7, 13.9) | 0.77 |
Values are expressed as median (10%, 90% percentile). N1 refers to number of patients in the safety analysis set; n percentages are based on N1. CUA, calcific uremic arteriolopathy; PTH, parathyroid hormone; 25(OH)D, 1,25-dihydroxyvitamin D.
Wilcoxon rank-sum test was used for continuous variables and chi-square test was used for categorical variables. Comparisons are between patients who experienced a CUA adverse event compared with those who did not.
Laboratory Findings Immediately Before CUA Manifestation
Laboratory findings most proximal to the reporting of CUA are shown in Table 3. Median (10%, 90% percentiles) plasma intact PTH at the time of CUA was 796 (225, 2093) pg/ml and 410 (71, 4957) pg/ml in patients randomly assigned to placebo and cinacalcet, respectively. Corresponding total serum calcium concentrations were 9.8 (8.5, 10.6) mg/dl and 9.7 (8.0, 11.6) mg/dl, respectively. Corresponding serum phosphate concentrations were 5.8 (4.5, 8.1) mg/dl and 6.3 (3.4, 10.2) mg/dl, and alkaline phosphatase values were 98 (67, 148) U/L and 190 (65, 648) U/L.
Table 3.
Laboratory values before onset of calcific uremic arteriolopathy adverse event
| Variable | Placebo (n=18) | Cinacalcet (n=6) |
|---|---|---|
| Intact PTH (pg/ml) | 796 (225, 2093) | 410 (71, 4957) |
| Serum calcium (mg/dl) | 9.8 (8.5, 10.6) | 9.7 (8.0, 11.6) |
| Serum phosphorus (mg/dl) | 5.8 (4.5, 8.1) | 6.3 (3.4, 10.2) |
| Alkaline phosphatase (U/L) | 98 (67, 148) | 190 (65, 648) |
| Bone alkaline phosphatase (µg/L) | 15.56 (7.97, 33.62) | 44.01 (15.97, 188.97) |
| N-telopeptide (nmol/L) | 205.2 (36.8, 490.5) | 491.4 (109.9, 1471.4) |
| Hemoglobin (g/dl) | 11.4 (8.3, 13.2) | 11.6 (6.7, 13.1) |
| Albumin (g/dl) | 3.4 (2.7, 3.8) | 3.2 (2.8, 4.1) |
| Total protein (g/dl) | 6.9 (6.4, 7.9) | 6.9 (5.9, 7.3) |
Values are expressed as median (10%, 90% percentile). PTH, parathyroid hormone.
Concomitant Medications Immediately before CUA Manifestation
Table 4 shows the use of concomitant medications in patients reported to have developed CUA. Only one of 18 patients with CUA in the placebo group had received commercial cinacalcet (single 30-mg dose at 5 months before the onset of CUA). Vitamin K antagonists were actively prescribed in 11 of 24 (46%) patients with CUA: nine among those assigned to placebo and two among those assigned to cinacalcet. Four of 24 (17%) patients were receiving vitamin K antagonists at baseline; others started vitamin K antagonists during the trial. In contrast, among patients not developing CUA, vitamin K antagonist prescription ranged from 7.4% (284 of 3837 patients) at study year 1 to 5.1% (196 of 3837 patients) at study year 3. Because we did not collect complete data on concomitant medication use throughout the trial, we cannot be certain which patients were prescribed vitamin K antagonists and, if so, when during the trial. Thus, we could not include this variable in the regression model. Among patients diagnosed with CUA, data on vitamin K antagonist use were obtained from direct review of medical records.
Table 4.
Concomitant medication use before onset of calcific uremic arteriolopathy adverse event
| Variable | Placebo (n=18) | Cinacalcet (n=6) |
|---|---|---|
| Vitamin D sterol use (%) | 72 | 100 |
| Median IV paricalcitol-equivalent dose (10%, 90% percentile) (μg/wk) | 15 (6, 24) | 10 (5, 45) |
| Phosphate binder use (%) | 100 | 100 |
| Calcium-containing phosphate binder use (%) | 67 | 83 |
| Study drug dose | ||
| Patients (n) | N/A | 5 |
| Median dose (10%, 90% percentile) (mg/d) | 30 (30, 180) | |
| Commercial cinacalcet dose | ||
| Patients (n) | 1 | NA |
| Median dose (10%, 90% percentile) (mg/d) | 30a | |
| Statin use (%) | 100 | 100 |
| Warfarin or other oral anti-coagulant use (%) | 50 | 33 |
| Erythropoietin agents (%) | 100 | 100 |
| Iron use (%) | 78 | 83 |
n refers to number of patients in the safety analysis set. Percentages are based on n. IV, intravenous; N/A, not available.
Single dose at 5 months before calcific uremic arteriolopathy onset.
Risk Factors for CUA
After adjustment for baseline characteristics, the relative hazard (cinacalcet versus placebo) was 0.25 (95% CI, 0.10 to 0.67). Baseline factors independently associated with a higher rate of CUA included random assignment to placebo, female sex, higher BMI, higher diastolic BP, history of dyslipidemia, history of parathyroidectomy, and former tobacco use (Table 5).
Table 5.
Multivariate regression using Fine-Gray subdistributional hazards model of calcific uremic arteriolopathy adverse events (safety analysis set)
| Multivariate Model | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Treatment (cinacalcet versus placebo) | 0.25 (0.10 to 0.67) | <0.01 |
| Male sex (reference, female) | 0.33 (0.14 to 0.75) | <0.01 |
| Body mass index (per kg/m2) | 1.09 (1.05 to 1.13) | <0.001 |
| Diastolic BP (per 10 mmHg) | 1.50 (1.19 to 1.90) | <0.001 |
| History of dyslipidemia | 2.15 (0.90 to 5.15) | 0.09 |
| History of parathyroidectomy | 5.79 (1.79 to 18.70) | 0.003 |
| Baseline tobacco use (reference, never use) | ||
| Current | 1.79 (0.54 to 5.89) | 0.34 |
| Former | 3.04 (1.19 to 7.74) | 0.02 |
Baseline variables were included using backward elimination. Heart failure and diabetes mellitus were removed during the backward elimination procedure at a significance level of 0.10. Additional baseline variables of age, vitamin K antagonist use, vitamin D sterol use, and peripheral vascular disease were evaluated but not included in the multivariate regression model using the backward elimination procedure because they did not meet the entry criteria, P<0.25 in a univariate model. 95% CI, 95% confidence interval.
Discussion
Within the EVOLVE trial, 24 of 3861 enrolled patients who received at least one dose of the study drug developed CUA. The overall exposure-adjusted rate of 0.3 per 100 patient-years in our total population is relatively low. In the literature, a prevalence of 1%–4% was reported among nonselected patients receiving maintenance dialysis (1,3,19). It is unlikely that CUA was substantially underreported, given the severity of the diagnosis. Rather, our data suggest that the CUA risk in our selected population of relatively young patients with advanced sHPT may be lower than that of a nonselected maintenance hemodialysis population.
Our central observation was that a cinacalcet-based therapeutic regimen in hemodialysis patients with advanced sHPT reduced CUA incidence by 69%–75%, considering unadjusted or adjusted data, respectively. This observation makes EVOLVE the first randomized controlled of this intervention to show a reduced risk of CUA. To date, the therapeutic approach undertaken to prevent or ameliorate CUA has been deduced from case-control studies that identified risk factors for CUA in patients with ESRD, including hyperphosphatemia, peritoneal dialysis (rather than hemodialysis), diabetes mellitus, obesity, and the use of vitamin K antagonists (3–5,11,12). The role of sHPT in the pathogenesis of CUA is not well understood. In some cases with extremely high plasma PTH concentrations, subtotal or total parathyroidectomy was effective in ameliorating CUA (1). In other reports, lower levels of PTH (“oversuppression”) which are associated with low bone turnover, were also associated with CUA (1). In the EVOLVE population, a relatively young group of patients with moderate to severe sHPT, cinacalcet therapy appeared to reduce the risk of CUA. Among patients randomly assigned to placebo (in other words, patients generally treated with vitamin D sterols and phosphate binders), the median plasma PTH concentrations were higher in the weeks and months proximal to the recognition of CUA than at baseline. The number of patients randomly assigned to cinacalcet who developed CUA was very small. However, in the latter, CUA developed in the setting of very high and very low plasma PTH concentrations, suggesting that factors other than PTH alone play a role in CUA pathogenesis.
Predisposing factors, which occurred more often in our patients who experienced CUA than in the overall EVOLVE population, were consistent with the literature and included female sex (3,4), obesity (5), and diabetes mellitus (3). Recent case-control studies also reported that administration of warfarin was associated with a 4- to 11-fold risk for CUA in patients receiving dialysis (11,12). Our study further supports a role of vitamin K antagonism in precipitating CUA given that the frequency of vitamin K antagonist use was about 7- to 8-fold higher in patients who developed CUA than in those not developing CUA; about one third of the patients who developed CUA started vitamin K antagonists during the trial. Similarly, in a CUA registry (http://www.calciphylaxis.net), almost 50% of the patients were receiving vitamin K antagonists at CUA manifestation (1). The mechanism may involve the inactivation of the major vascular wall calcification inhibitor, matrix Gla protein, which depends on vitamin K for its bioactivity (20). Our observations thus further support a restrictive policy of vitamin K antagonism in patients receiving dialysis (21).
The role of other comedications in serving as “triggers” for CUA is less well established. A case-control study has suggested that statins reduce CUA risk and that calcitriol, but not paricalcitol, increases this risk (3,12). In EVOLVE, all 24 patients who developed CUA were receiving statins in the weeks and months before CUA was reported. This may reflect the severity of diabetes, atherosclerotic vascular disease, or other conditions in these patients; there is insufficient evidence to link statin use with CUA on the basis of these findings. Likewise, almost all patients with CUA were receiving vitamin D sterols, but these were used very commonly among all patients, and only a small fraction developed CUA.
Strengths of our analysis include data obtained from a large, prospective, randomized trial, as well as a diverse population assessed for a broad range of baseline clinical characteristics and serial laboratory determinations. Limitations include the relatively small number of CUA events despite the large trial size, which makes it more difficult to precisely determine the magnitude of the treatment effect or the relative and absolute importance of clinical factors other than cinacalcet treatment that influence CUA risk. Another limitation is that the diagnoses of CUA events were based on physician’s assessment, without biopsy confirmation in all cases. However, the advisability of skin biopsy in CUA is controversial, given concerns that the biopsy may precipitate further lesions (1). Because of relatively poor adherence with cinacalcet, we may have underestimated the therapeutic effect on CUA (however, adverse events were no longer captured after discontinuation of study drug, and thus we have no information on how many CUA events have developed in such patients). Most important, the trial was not designed to detect a reduction in the rate of CUA, and the power to detect such a difference, using reasonable assumptions, was low. Thus, the findings might be “false positive” despite relative hazards that were quite low and reached levels of nominal statistical significance.
In summary, upon carefully examining safety/adverse event data from the EVOLVE trial, the largest and longest randomized clinical trial conducted in the hemodialysis population, we confirmed important associations among a variety of known risk factors for CUA, including diabetes mellitus, obesity, and the use of vitamin K antagonists. We also found a 69%–75% risk reduction in CUA among patients randomly assigned to cinacalcet, the first suggestion from a prospective trial that any therapeutic strategy could reduce the risk of CUA.
Disclosures
As Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events executive committee members, J.F., G.M.C., and P.S.P. have received consulting fees from Amgen. Y.K. is employed by and owns stock in Amgen. In addition, J.F. has received consulting fees, travel support, or lecture fees from Amgen, Abbott, Fresenius Medical Care, Genzyme, Chugai Pharmaceuticals, and Boehringer Ingelheim and royalties from Elsevier. P.S.P. has received lecture fees from Amgen. G.M.C. has received grant support and travel support from Amgen; is a board member of Satellite Healthcare, Inc.; has provided consultation to Reata Pharmaceuticals; and owns stock or stock options in Ardelyx, Home Dialysis Plus, PuraCath, and Thrasos.
Acknowledgments
This study was supported by Amgen, Inc., Thousand Oaks, CA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Evolving Calciphylaxis—What Randomized, Controlled Trials Can Contribute to the Capture of Rare Diseases,” on pages 726–728.
References
- 1.Brandenburg VM, Sinha S, Specht P, Ketteler M: Calcific uraemic arteriolopathy: A rare disease with a potentially high impact on chronic kidney disease-mineral and bone disorder. Pediatr Nephrol 29: 2289–2298, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Coates T, Kirkland GS, Dymock RB, Murphy BF, Brealey JK, Mathew TH, Disney AP: Cutaneous necrosis from calcific uremic arteriolopathy. Am J Kidney Dis 32: 384–391, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Fine A, Zacharias J: Calciphylaxis is usually non-ulcerating: Risk factors, outcome and therapy. Kidney Int 61: 2210–2217, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Mazhar AR, Johnson RJ, Gillen D, Stivelman JC, Ryan MJ, Davis CL, Stehman-Breen CO: Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int 60: 324–332, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR: Calciphylaxis: Natural history, risk factor analysis, and outcome. J Am Acad Dermatol 56: 569–579, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Nigwekar SU, Wolf M, Sterns RH, Hix JK: Calciphylaxis from nonuremic causes: A systematic review. Clin J Am Soc Nephrol 3: 1139–1143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandenburg VM, Cozzolino M, Ketteler M: Calciphylaxis: A still unmet challenge. J Nephrol 24: 142–148, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Don BR, Chin AI: A strategy for the treatment of calcific uremic arteriolopathy (calciphylaxis) employing a combination of therapies. Clin Nephrol 59: 463–470, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Hayden MR, Goldsmith D, Sowers JR, Khanna R: Calciphylaxis: Calcific uremic arteriolopathy and the emerging role of sodium thiosulfate. Int Urol Nephrol 40: 443–451, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Kramann R, Brandenburg VM, Schurgers LJ, Ketteler M, Westphal S, Leisten I, Bovi M, Jahnen-Dechent W, Knüchel R, Floege J, Schneider RK: Novel insights into osteogenesis and matrix remodelling associated with calcific uraemic arteriolopathy. Nephrol Dial Transplant 28: 856–868, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Hayashi M, Takamatsu I, Kanno Y, Yoshida T, Abe T, Sato Y, Japanese Calciphylaxis Study Group : A case-control study of calciphylaxis in Japanese end-stage renal disease patients. Nephrol Dial Transplant 27: 1580–1584, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Nigwekar SU, Bhan I, Turchin A, Skentzos SC, Hajhosseiny R, Steele D, Nazarian RM, Wenger J, Parikh S, Karumanchi A, Thadhani R: Statin use and calcific uremic arteriolopathy: A matched case-control study. Am J Nephrol 37: 325–332, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mawad HW, Sawaya BP, Sarin R, Malluche HH: Calcific uremic arteriolopathy in association with low turnover uremic bone disease. Clin Nephrol 52: 160–166, 1999 [PubMed] [Google Scholar]
- 14.Katikaneni M, Lwin L, Villanueva H, Yoo J: Calciphylaxis and subtotal parathyroidectomy: a double-edged sword. Hemodial Int 17[Suppl 1]: S33–S36, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Chertow GM, Correa-Rotter R, Block GA, Drueke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Wheeler DC, Parfrey PS: Baseline characteristics of subjects enrolled in the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Nephrol Dial Transplant 27: 2872–2879, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Trotman ML, Wheeler DC, Parfrey PS, Parfrey PS: Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 367: 2482–2494, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Chertow GM, Pupim LB, Block GA, Correa-Rotter R, Drueke TB, Floege J, Goodman WG, London GM, Mahaffey KW, Moe SM, Wheeler DC, Albizem M, Olson K, Klassen P, Parfrey P: Evaluation of Cinacalcet Therapy to Lower Cardiovascular Events (EVOLVE): Rationale and design overview. Clin J Am Soc Nephrol 2: 898–905, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Parfrey PS, Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Herzog CA, London GM, Mahaffey KW, Moe SM, Wheeler DC, Dehmel B, Trotman ML, Modafferi DM, Goodman WG: The clinical course of treated hyperparathyroidism among patients receiving hemodialysis and the effect of cinacalcet: the EVOLVE trial. J Clin Endocrinol Metab 98: 4834–4844, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Angelis M, Wong LL, Myers SA, Wong LM: Calciphylaxis in patients on hemodialysis: A prevalence study. Surgery 122: 1083–1089, discussion 1089–1090, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Ketteler M, Wanner C, Metzger T, Bongartz P, Westenfeld R, Gladziwa U, Schurgers LJ, Vermeer C, Jahnen-Dechent W, Floege J: Deficiencies of calcium-regulatory proteins in dialysis patients: A novel concept of cardiovascular calcification in uremia. Kidney Int Suppl (84 Suppl): S84–S87, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Krüger T, Floege J: Vitamin K antagonists: Beyond bleeding. Semin Dial 27: 37–41, 2014 [DOI] [PubMed] [Google Scholar]