Abstract
Alterations in water homeostasis can disturb cell size and function. Although most cells can internally regulate cell volume in response to osmolar stress, neurons are particularly at risk given a combination of complex cell function and space restriction within the calvarium. Thus, regulating water balance is fundamental to survival. Through specialized neuronal “osmoreceptors” that sense changes in plasma osmolality, vasopressin release and thirst are titrated in order to achieve water balance. Fine-tuning of water absorption occurs along the collecting duct, and depends on unique structural modifications of renal tubular epithelium that confer a wide range of water permeability. In this article, we review the mechanisms that ensure water homeostasis as well as the fundamentals of disorders of water balance.
Keywords: renal physiology, water-electrolyte balance, hypernatremia, hyponatremia
Crawling out on dry land some millions of years later, terrestrial forms were faced with the diametrically opposite problems, as least with respect to water. Fluid conservation, rather than fluid elimination, was the major concern. Instead of discarding their now unnecessary pressure filters and redesigning their kidneys as efficient secretory organs, the terrestrial vertebrates modified and amplified their existing systems to salvage the precious water of the filtrate.
—Robert F. Pitts (1)
So wrote the great physiologist Robert F. Pitts describing the evolution of organisms from the ocean to land (1). Marine animals survive in the high tonicity of seawater (500–1000 mOsm/kg) through a variety of mechanisms. The shark maintains a high tonicity in its body fluids (2,3), whereas dolphins absorb water from foodstuffs while producing a highly concentrated urine through complex multilobed reniculate kidneys (4). For those of us on land, however, the challenge is not only water conservation but also water elimination, in our world of coffee shops, bottled water, and “hydration for health” philosophies.
Water is the most abundant component of the human body, constituting approximately 50%–60% of body weight. Cell membranes, which define the intracellular compartment, and the vascular endothelium, which defines the intravascular component, are both water permeable. Because the intracellular space constitutes the largest body compartment, holding approximately two thirds of body fluid, changes in water homeostasis predominantly affect cells; water excess leads to cellular swelling, and water deficit leads to cellular shrinkage. For every 1 liter of water change, approximately 666 ml affect the cellular space, with only about 110 ml affecting the vascular space.
Although cells have an innate capacity to respond to changes in cell volume when extracellular osmolality changes, the body protects cells primarily by tightly regulating extracellular osmolality. The amount of body water remains remarkably stable despite a huge range of water intake and a multitude of routes for water loss, including the respiratory and gastrointestinal tract, skin, and the kidneys. In this review, we explore the mechanisms that allow our bodies to respond to a wide range of external influences, fine-tuning the exact amount of urinary water excretion to match the body’s immediate needs.
Maintaining Brain Cell Size
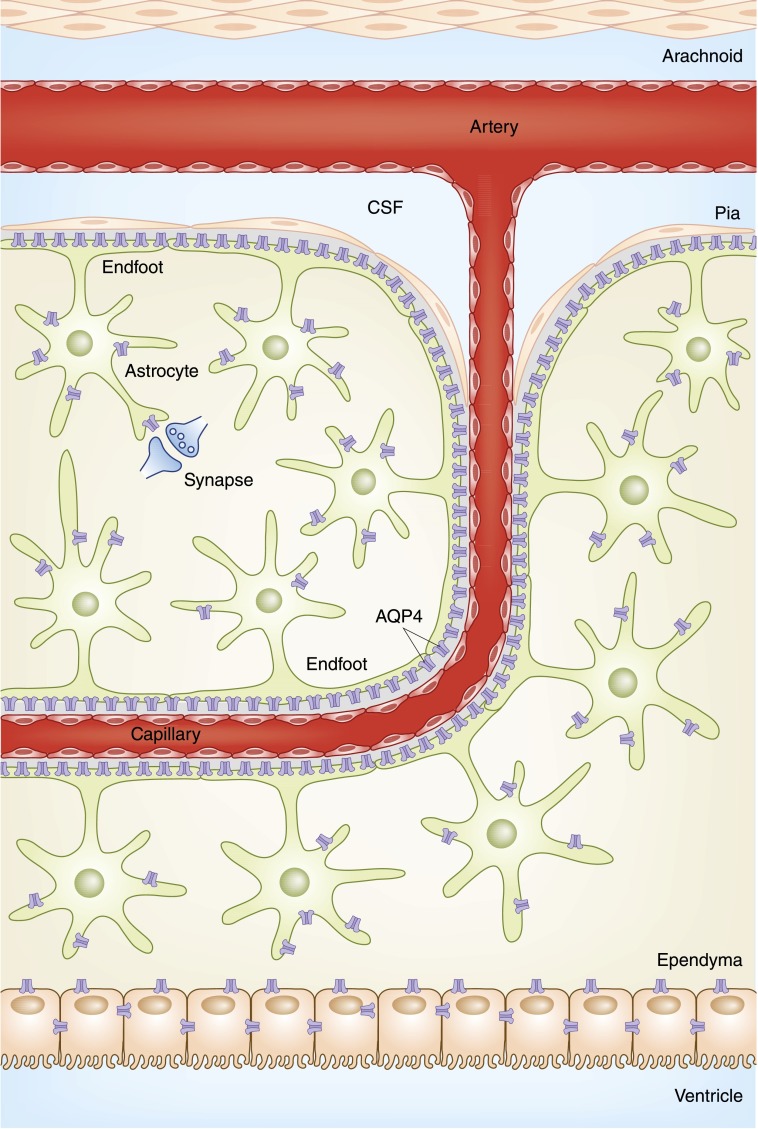
With a plethora of capillaries descending through the subarachnoid space into the parenchyma, the brain is remarkably vascular. Astrocytes, star-shaped neuronal cells, encapsulate the capillaries, forming a “blood-brain barrier” and controlling many important neurologic functions. Although previously thought to be impermeable (5,6), the discovery of aquaporin (AQP) channels within the astrocyte has elucidated the water permeability of this barrier (7) (Figure 1). AQP4 localizes to the perivascular and subpial aspects of astrocytes, and controls both water efflux and influx, as well as regulates potassium homeostasis, neuronal excitability, inflammation, and neuronal signaling (8). By controlling water movement from brain parenchyma into the systemic circulation, AQP4 regulates brain water content and volume (9). By controlling water influx, AQP4 plays a role in the signaling cascade that occurs in the setting of hypo-osmolar–induced cerebral edema (10).
Figure 1.
The blood-brain barrier. Penetrating capillaries descend through the subarachnoid space into the parenchyma, and are encased by astrocytes, which in addition to controlling important neurologic functions, form the blood-brain barrier. AQP4 water channels along the perivascular and subpial endfoot membranes confer water permeability to the blood-brain barrier. AQP, aquaporin; CSF, cerebrospinal fluid.
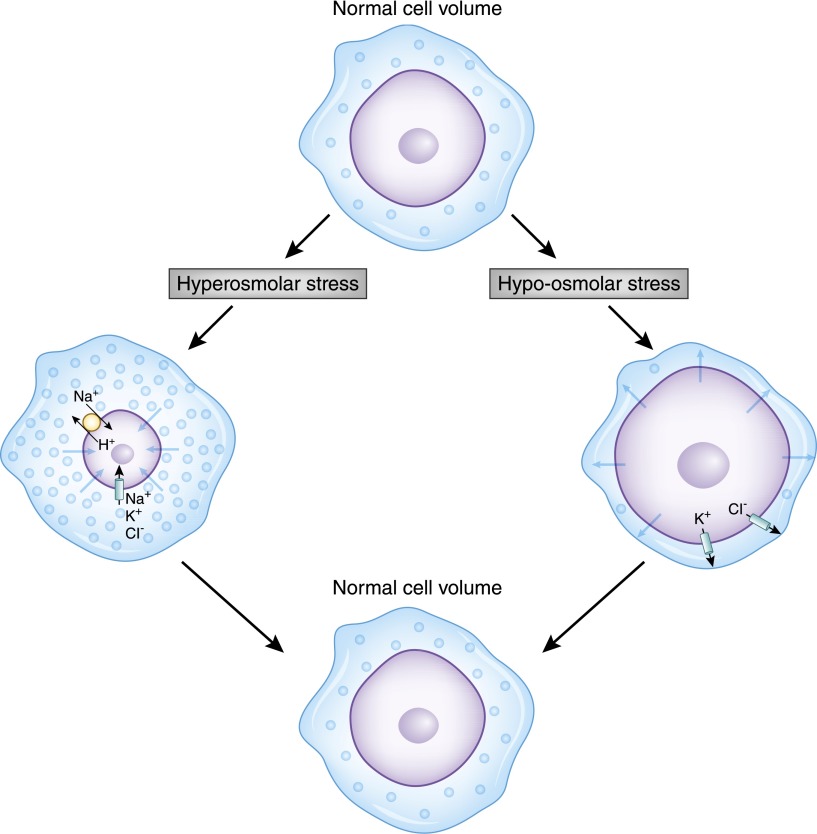
Because the amount of intracellular water affects the concentration of intracellular contents and cell size, changes in osmolality can disturb the complex signaling network that orchestrates cell function. Given the complexity of brain function, even minor changes in neuron ionic composition and size can have profound effects on the processing and transmission of neuronal signals. Consequently, the brain has developed complex osmoregulatory mechanisms to defend against changes in plasma osmolality. Within minutes of osmolar challenges, brain cells respond by either loss or accumulation of inorganic osmolytes, returning the cell size toward normal (11). In the setting of hypotonicity, as shown in Figure 2, the rapid swelling of the cell activates quiescent cell membrane channels and leads to immediate Cl−, K+, and attendant water loss, a process termed regulatory volume decrease. Over the subsequent 24 hours, the cells lose further organic solutes, such as myo-inositol, and amino acids, such as glutamine, glutamate, and taurine. With hyperosmolar-induced cell shrinkage, brain cells respond with immediate uptake of surrounding Na+, K+, and Cl−, correcting cell volume in a process termed regulatory volume increase (12). With more prolonged exposure, organic solute concentrations within the cells rise, replacing the high levels of ions.
Figure 2.
Cells regulate their internal volume in response to osmotic stress by activation of membrane carrier proteins and channels. In this figure, a normal cell is challenged by either a hyperosmolar (left) or hypo-osmolar (right) milieu. In the setting of hyperosmolar stress, whereby the cell shrinks with water egress, neurons then respond by rapidly accumulating Na+, K+, and Cl− ions, followed by the production of intracellular organic solutes. The increase of intracellular solute content then draws water in to normalize the concentrations across the cell membrane, thereby restoring cell size. In the setting of hypo-osmolar–induced swelling, activation of K+ and Cl− channels, as well as the K+-Cl− cotransporter, lead to solute and consequent water loss, thereby restoring cell volume.
Despite these important cell protective mechanisms, alterations in plasma osmolality can have disastrous consequences. The classic neurologic symptoms of hypo-osmolality, including headache, nausea, vomiting, and if severe enough, seizures, are generally thought to occur at a serum sodium of 125 mEq/L, although with a wide range of sensitivities that are greatly affected by the rate of osmolality change. More mild changes of plasma osmolality are also associated with neurologic symptoms, including gait instability, memory impairment, and cognitive decline. Certain groups have an increased sensitivity to changes in plasma osmolality. Children are considered at increased risk of hypo-osmolar encephalopathy, possibly because of the relatively larger brain to intracranial volume compared with adults (13). Conversely, because the brain begins to atrophy in the sixth decade, elderly individuals may be at a lower risk of severe complications from acute hyponatremia. In addition to age, sex is also considered an important determinant of neurologic sensitivity. The vast majority of reported cases of postoperative hyponatremia resulting in fatal outcomes have been in women (14), including postpartum and postmenopausal women (15).
Unlike the brain swelling associated with hypo-osmolality, the brain shrinks in hypertonic conditions. The protective reflex of intense thirst may disappear as hypertonicity worsens, replaced by somnolence, confusion, and muscle weakness (16). If severe enough, the shrinking brain will pull away from the calvarium, tearing the rich capillary plexus, and causing subarachnoid hemorrhage, cerebral bleeding, and death. The highest reported serum sodium in the adult literature remains 255 mEq/L, a consequence of drinking salty water as part of a fatal exorcism ritual (17). Presumably due to the use of table salt as a common antiemetic, fatal salt ingestion, either accidentally or voluntarily, is well reported (18), as is accidental iatrogenic administration (19). Seawater drowning has also been associated with profound hypernatremia (20). In summary, despite internal cellular mechanisms to protect cell volume, cells remain at risk with alterations of water balance; consequently, preventing significant changes in plasma osmolality is critical for survival.
Sensing Changes in Body Concentration: The Osmoreceptor
The ability to internally sense plasma osmolality is fundamental to the process of water homeostasis. Much progress in explaining the mechanisms of the “osmoreceptor” has been made, as reviewed by Sharif-Naeini et al. (21). Specialized neurons located in several brain areas, including the organum vasculosum laminae terminalis (OVLT) (22,23) and the supraoptic (24,25) and paraventricular nuclei of the hypothalamus, are able to sense changes in plasma osmolality, responding with complex neuronal commands. Electrophysiologic recordings from supraoptic nuclei of the hypothalamus in rats show an increasing rate of cellular depolarization in response to water deprivation (26), and a decreasing rate with water administration (27). More recent studies have shown that hyperosmolality causes osmoreceptor membrane depolarization via activation of nonselective calcium-permeable cation channels. It remains somewhat unresolved whether the exact stimulus is change of specific intracellular solutes associated with cell dehydration or a mechanical effect linked to cell membrane shrinkage. Identification of the transient receptor potential vanilloid (TRPV) family of cation channels as a potential “mechanic-stretch” receptor (28) has added support to the concept of osmosensing as a mechanical process (Figure 3), and polymorphisms have been linked to hyponatremia (29). Shrinking of OVLT neurons, either by dehydration or by negative suction pressure, stimulates cell activation via TRPV1 (30). The importance of cell volume in neuronal activation would explain why ineffective osmoles that cross the cell membrane, such as urea and glucose (in the presence of insulin), do not activate the osmoreceptor. The osmoreceptor, likely because of its role in orchestrating the pathways of water retention, has a blunted regulatory volume decrease response, whereby its own shrinkage due to hyperosmolality is maintained, allowing sustained stimulation of thirst and vasopressin release until the plasma osmolality can be corrected (30). In the following sections, we discuss how the osmoreceptor regulates thirst and vasopressin (synonymously known as antidiuretic hormone) release.
Figure 3.
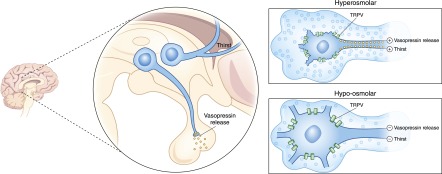
Osmoreceptor functions of the OVLT nuclei and SON control thirst and vasopressin release, respectively. In response to hyperosmolar-induced cell shrinkage, specialized mechanical-stretch TRPV cation channels are activated, allowing the influx of positive charges and consequent cell depolarization, provoking action potentials that stimulate thirst and vasopressin release. Conversely, hypo-osmolar cell swelling deactivates these channels, leading to cell hyperpolarization, extinguishing thirst and vasopressin release. Although the exact role of the TRPV channel remains under investigation, its presence is critical in this mechanism. OVLT, organum vasculosum laminae terminalis; SON, supraoptic nuclei; TRPV, transient receptor potential vanilloid.
Thirst
The sensation of thirst is the experiential component of the complex physiologic drive to drink. Neuroimaging studies have localized the anatomic origin of thirst, with hyperosmolality stimulating activity in the anterior wall of the third ventricle, the anterior cingulate, parahippocampal gyrus, insula, and the cerebellum (31). These brain regions are also associated with complex functions, including emotional behavior and thought, perhaps explaining why the perception of thirst, in addition to its physiologic basis, is so connected to social and behavioral mores.
Hypertonicity is a reproducible stimulus of thirst. The osmolar threshold for thirst has traditionally been considered to be approximately 5 mOsm/kg above the threshold for vasopressin release, although some suggest similar set points (32). A higher thirst threshold allows vasopressin titration of urinary water excretion without the need to be constantly drinking. Responding to increasing osmolality, OVLT osmoreceptors relay stimuli to the insula and cingulate cortices via several medially-located thalamic nuclei, stimulating thirst (33). Upon drinking, the sensation of thirst is quenched almost immediately, suggesting that a direct satiating effect of water on the tongue and buccal membrane as well as cognitive awareness of fluid intake might explain the resolution of thirst. In addition, the recent recognition of peripheral osmoreceptors located within the gastrointestinal tract and portal venous system suggest a local mechanism that directly senses gastric water absorption (34). TRPV-positive neurons within the thoracic ganglia innervating the liver detect changes in local osmolality, and can stimulate a wide array of physiologic responses, including modulation of BP (35), metabolism (36), and water homeostasis. Whether these peripheral osmoreceptors might contribute to the disorders of osmolality frequently seen in patients with cirrhosis remains unknown.
In addition to osmotic stimuli, there are important nonosmotic stimuli of thirst. The hemodynamics of hemorrhage are potently dipsogenic. Thirst on the battlefield is legendary, with exsanguinating soldiers asking for water. In animal models, hemorrhage stimulates intense water drinking (37), which is more easily extinguished by drinking saltwater than plain water (38,39). Angiotensin II, when injected into sensitive areas of the brain (40,41) or when injected systemically, is a powerful stimulus for water intake, as is activation of the renin-angiotensin axis (42), providing a mechanistic explanation for the association of thirst with abnormalities of body fluid volume. Thirst is a common complaint for patients with congestive heart failure (43,44), frequently plagues dialysis patients, and likely contributes to the prevalence of hyponatremia in these populations. Pharmacologic blockade of the renin-angiotensin axis, although theoretically attractive, does not seem to reduce thirst (45). In addition to disorders of fluid volume, thirst is also frequently encountered in patients with psychiatric disorders, reported in up to 25% of hospitalized patients with schizophrenia. Although this might be in part due to compulsive behavior or the anticholinergic side effects of psychotropic medications, studies have suggested an alteration of the sensation of thirst in patients with mental illness, with a lower osmolar threshold (46).
Vasopressin
Vasopressin is a potent endogenous peptide influencing a wide array of biologic functions, including regulation of water balance, BP, platelet function, and thermoregulation (47–49). It is synthesized as a prohormone in the magnocellular cell bodies of the paraventricular and supraoptic nuclei of the posterior hypothalamus, and by binding to the carrier protein neurohypophysin, it is transported along the supraoptic hypophyseal tract to the axonal terminals of magnocellular neurons in the posterior pituitary. Synthesis and storage take approximately 2 hours, with a t1/2 of 20–30 minutes, metabolized by vasopressinases in the liver and kidney. Vasopressin acts on V1, V2, V3, and oxytocin-type receptors. V1 receptors are located on the vasculature, myometrium, and platelets. V3 receptors are mainly found in the pituitary. V2 receptors are located along the distal tubule and collecting duct.
The most sensitive stimulus for vasopressin release is increasing plasma osmolality. Whereas normal vasopressin concentrations are 0.5–5 pg/ml in fasted, hydrated individuals (50), subtle increases in plasma osmolality, often in the range of <2% of body water, stimulate the osmoreceptor to release vasopressin, and serum concentrations rapidly increase 3-fold. The presence of stored vasopressin in the pituitary guarantees a rapid and effective mechanism of water regulation. As water is retained and the plasma osmolality returns to normal, the stimuli for vasopressin release is extinguished.
In addition, there are nonosmotic stimuli, including NE, dopamine, pain, hypoxia, and acidosis (51), and most importantly, circulatory hemodynamics. Cardiovascular collapse is associated with profound vasopressin release, with concentrations 100-fold greater than normal (52), presumably because greater vasopressin concentrations are needed to increase systolic BP than to regulate antidiuresis. Such high concentrations rapidly exhaust the pituitary vasopressin stores, and given the time-consuming nature of vasopressin production, vasopressin depletion is thought contributory to shock physiology (53). Subtle changes in body fluid volume modify the responsiveness of vasopressin release to osmolality. Early physiologic experiments on dogs using either hemorrhage or transfusion illustrated that circulatory blood volume modified the association between plasma osmolality and vasopressin (54). For any given plasma osmolality, hemorrhage was associated with a higher vasopressin concentration, whereas transfusion was associated with a lower vasopressin concentration. In these experiments, hemorrhage and transfusion were associated with a change in left atrial pressure, but not BP.
Although myriad terms, such as intravascular volume, effective arterial volume, or circulatory volume, have been used to describe the component of body fluid that effectively perfuses critical organs, these terms imply that the vascular compartment is readily measurable, a feat that is difficult in the laboratory and impossible at the bedside. Furthermore, because the vascular endothelium is freely permeable to water and sodium, the intravascular and interstitial compartments freely and dynamically communicate, further limiting the idea of a separate, quantifiable intravascular space. Instead, because pressure receptors located in the heart and carotid arteries and flow receptors in the juxtaglomerular apparatus are the sensors for body fluid volume, we favor the simple term sensed volume (55). Arterial baroreceptors, through cranial nerves IX and X, communicate with the hypothalamus and can modify vasopressin release. Sensed volume depletion, in the setting of true volume depletion (e.g., diarrhea or vomiting) or volume overload (e.g., heart failure and cirrhosis), both amplify the sensitivity to vasopressin so that for any given plasma osmolality, the urinary osmolality is greater.
In summary, the osmoreceptor is stimulated by both osmotic and nonosmotic stimuli to initiate thirst and to release vasopressin in order to maintain water balance.
A Highly Concentrated Medulla
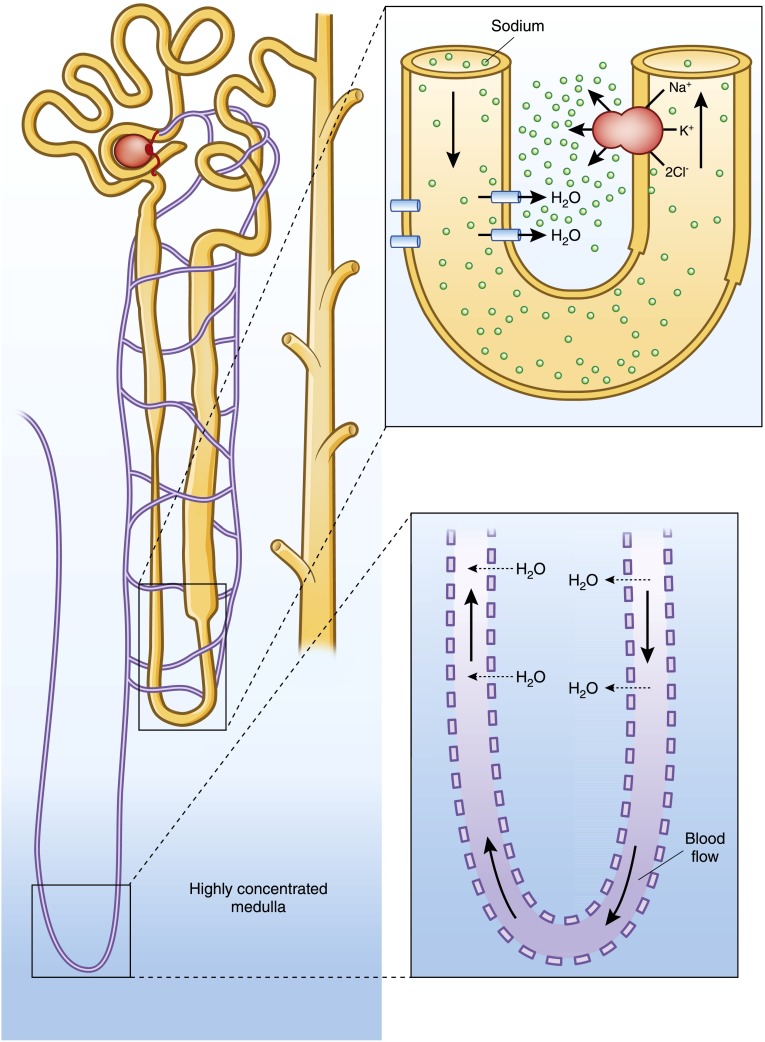
We previously described how the body senses and responds to changes in plasma osmolality. Next we turn to the final steps of osmotic homeostasis: renal water retention or excretion. Having a highly concentrated medullary interstitium is essential for water conservation, providing the osmotic force for water egress from filtered renal tubular fluid. The medulla, reaching up to four times the concentration of the surrounding interstitial fluid, is like a concentration oasis or a pocket of hypertonic fluid within a deeply vascular organ unprotected by a barrier epithelium. The generation and maintenance of the medullary interstitial gradient is one of the fundamental teachings of renal physiology (Figure 4).
Figure 4.
The medullary interstitium has a concentration >4 times that of its surrounding fluid, and must be both generated and maintained. The countercurrent multiplier, composed of a hairpin tubule loop with a water-permeable descending limb juxtaposed against an impermeable ascending limb with a highly active Na-K-2Cl pump, generates the concentration gradient. A separate hairpin loop within the tubular capillary system allows shunting of water from the descending limb to the ascending limb preventing the dilution of the medullary gradient. This process, countercurrent exchange, maintains the medullary concentration.
Generating the medullary concentration depends on three important structural modifications of the renal tubule. First, a hairpin loop in the renal tubule allows solute and water exchange between the descending thin limb and the ascending thick limb. Second, the combination of the highly energy-dependent Na/K-ATPase and the NaK2Cl cotransporter, along with the apical water impermeability of the thick ascending limb, drive solute without water egress from the medulla. Third, because the descending limb is water permeable, the exiting sodium from the thick ascending limb creates a concentration gradient that pulls water from the descending limb, and as that tubular fluid then moves into the ascending limb, the NaK2Cl cotransporter is presented with increasingly concentrated tubular fluid, further generating more of an interstitial concentration. This process, termed countercurrent multiplication, is responsible for generation of approximately one half (600 mOsm/kg) of the maximal medullary concentration gradient (1200 mOsm/kg), with the remainder being generated by urea recycling (56).
Given that the kidneys receive approximately 25% of cardiac output, with the potential to rapidly wash away any area of hyperosmolarity, maintaining the medullary concentration is fundamental. There are two major mechanisms to prevent medullary washout. First, the majority of renal blood flow is directed to superficial glomeruli limited to the outer cortex, with <2% perfusing the deep medullary glomeruli (57,58). Second, for the vasa rectae that descend into the medulla, a hairpin loop prevents medullary dilution, a process known as countercurrent exchange. In a manner similar to the vascular structure of a penguin’s webbed foot that allows heat conservation despite walking on ice, whereby the warmth of descending blood shuttles to the ascending limb and bypasses the colder distal loop, the vasa recta’s hairpin loop prevents water from reaching the distal aspects of the circuit, preventing medullary washout (59). In essence, these mechanisms shunt water away from the highly concentrated deep medulla, protecting it as a pocket of highly concentrated fluid. This combination of building and maintaining a concentrated medulla provides the force for tubular water egress and allows the fine-tuning of water balance, discussed next.
Fine-Tuning Water Balance in the Collecting Duct
The ability of the nephron to excrete a urine that is more concentrated than the plasma (water reabsorption) or more dilute than the plasma (water excretion) relies on the presence of nephron segments that are extremely permeable to water, as well as segments that are nearly impermeable. To excrete dilute urine, the collecting duct must be able to maintain an almost 30-fold concentration gradient between the dilute urinary filtrate and the surrounding highly concentrated medullary interstitium. Conversely, in order to conserve water, the collecting duct must alter its water permeability, allowing the egress of filtrate water into the more concentrated interstitium. The water permeabilities of the different sections of the tubule are determined by the presence or absence of important structural modifications that control both the paracellular and transcellular routes of flow.
Tight junction proteins, including cytoplasmic scaffolding proteins, transmembrane proteins, and signaling proteins, act like a biologic zipper, controlling movement of water and solutes in the intercellular passageway (60). Zona occludens protein-1 functions as a scaffold protein, anchoring to other transmembrane proteins and the actin cytoskeleton, helping to seal the intercellular space. The expression of zona occludens protein-1 may respond directly to changes in medullary tonicity (61), suggesting a local level of permeability regulation. Claudins are key integral membrane proteins that function as high-conductance cation pores, regulating the transcellular movement of sodium, magnesium, and calcium (62). In addition to their role in the impermeability of the renal tubule, the tight junctions control gastrointestinal permeability (63) and have been associated with a wide range of diarrheal illness, including Crohn’s disease (64,65). The expression of tight junction proteins increases along the length of the tubule, particularly along the thick ascending limb and the collecting duct (66,67).
In addition to controlling the paracellular route, the collecting duct must prevent the transcellular movement of water. Recent work has provided a mechanistic explanation for how barrier epithelial cells achieve this transcellular impermeability (68–70). Although once thought to be simply due to the depth of the cell barrier, important modifications within the apical cell membrane are likely responsible for barrier impermeability (71,72). Barrier epithelia segregate high levels of glycosphingolipid, which entraps cholesterol, as well as long, relatively saturated fatty acid–laden triglycerides, in their outer leaflets. This composition leads to tight packing of the triglycerides, so that nearly all of the surface is composed of phosphate headgroups, which impede water flow. Water that does find the surface and penetrates has difficulty diffusing across the space between the chains because of tight packing caused by cholesterol (73,74).
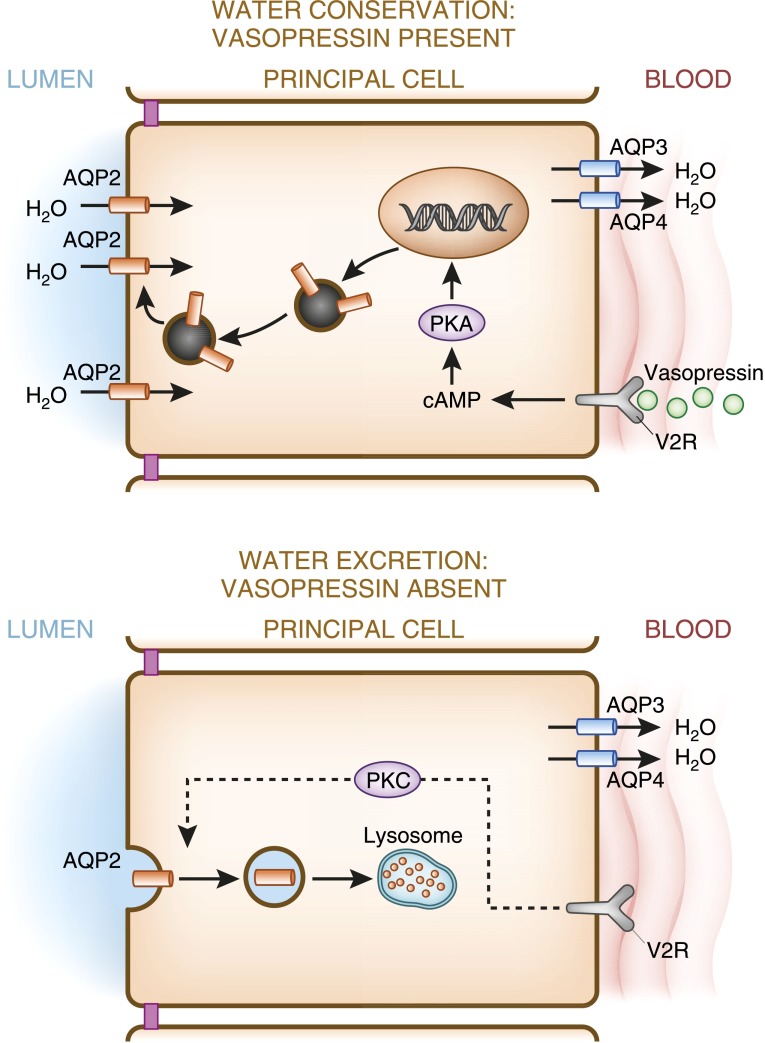
Finally, water movement across the renal tubule also depends on the presence of AQP channels, as reviewed by Agre (75). AQP1 is constitutively present in the apical and the basolateral membranes of the proximal tubule and descending limb, providing a route for transcellular movement, but is absent in the thick ascending limb. AQP2, which is expressed along the apical membrane of collecting duct principal cells, is regulated by vasopressin. Upon binding to its receptor in the basolateral membrane, vasopressin initiates a complex cascade of signals that ultimately result in the movement of AQP2 channels to the apical membrane, rendering the cell water permeable. The biologic details of this complex mechanism have largely been elucidated. As seen in Figure 5, binding of vasopressin to the vasopressin V2 receptor on the basolateral membrane activates adenylate cyclase, increasing intracellular cAMP levels, activating protein kinase A, and leading to the translocation of AQP2 bearing vesicles to the apical membrane. Upon withdrawal of vasopressin, AQP2 is internalized into intracellular storage vesicles. In addition to the short-term regulation of AQP2 trafficking, vasopressin also influences the long-term expression of AQP2 in collecting ducts, increasing their abundance. AQP2 expression is also thought to be controlled by vasopressin-independent mechanisms, including other transcription factors (76), oxytocin (77), and possibly the novel hormone secretin (78).
Figure 5.
Vasopressin regulates AQP2 expression. In the presence of vasopressin, increased production of cAMP activates PKA, which in turn phosphorylates stored AQP-containing vesicles, and targets them to the apical membrane, increasing its water permeability, and facilitating water reclamation from the lumen. In the absence of vasopressin, AQP2 is endocytosed and internally degraded, conferring water impermeability to the apical membrane, thereby maximizing water excretion. AQP3 and AQP4, constitutively expressed on the basolateral membrane, allow water egress from the cell. PKA, protein kinase A; V2R, vasopressin 2 receptor.
As seen in Figure 6, the distribution of tight junctions and AQP channels, along with unique “barrier” qualities of renal tubular epithelium, determine the water permeability of the renal tubule. The collecting duct is unique in its capacity to rapidly alter its water permeability under the tutelage of vasopressin, allowing fine-tuning of water excretion and guarding water homeostasis.
Figure 6.
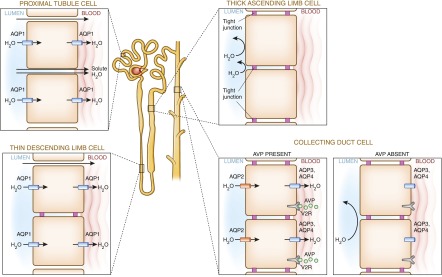
Water permeability along the tubule is determined by the presence or absence of intracellular tight junctions and AQP water channels. AQP1, along the proximal tubule and thin descending limb, is constitutively expressed, whereas AQP2, in the collecting duct, is under the control of vasopressin. The presence of AQP1 and the absence of tight junctions render the proximal tubule permeable, facilitating filtered solute and water reclamation (91). In the thin descending limb, the presence of AQP1 and tight junctions (claudin 2) render it water permeable but solute impermeable (92). Conversely, the impermeability of the thick ascending limb results from extensive tight junctions and absent AQP channels. The collecting duct is unique in its homeostatic responsiveness. In times of water conservation, vasopressin (AVP) binds to vasopressin 2 receptors (V2R), inducing AQP2 channel expression and consequent water retention, and in times of water excess, AQP2 retreats from the apical membrane due to vasopressin’s absence.
Clinical Correlation
Diabetes insipidus, a failure of water conservation resulting in hyperosmolarity and compensatory polydipsia, is frequently encountered in clinical practice. Central diabetes insipidus can result from traumatic, surgical, or ischemic injury at any site of vasopressin production, but is most often idiopathic, possibly due to autoimmune destruction of vasopressin (79). Hereditary forms, termed familial neurohypophyseal diabetes insipidus, are caused by mutations in the vasopressin gene, resulting in protein misfolding and degeneration of the vasopressin-producing magnocellular neurons. Genetic abnormalities are also associated with nephrogenic diabetes insipidus (80), with mutations in the vasopressin 2 receptor gene as the most common cause. Protein misfoldings trap the vasopressin 2 receptor gene within the cell’s endoplasmic reticulum, preventing it from docking with circulating vasopressin (81). These mutations are inherited in an X-linked pattern; hence, male individuals tended to have more pronounced concentration defects, whereas female individuals are usually asymptomatic. Mutations in the AQP2 gene, which can be inherited in a recessive or dominant fashion, are associated with defects in trafficking of the water channel to the apical membrane. In addition to these genetic causes, lithium use frequently causes diabetes insipidus, occurring in approximately 40% of chronic lithium users (82). It is associated with downregulation of AQP2 and cellular remodeling of the collecting duct. The route of lithium toxicity is thought to be due to cellular uptake via the epithelial Na channel (83), and although experimental data suggest that amiloride administration may prevent lithium nephrotoxicity (84), clinical data are lacking.
Hyponatremia is the most common electrolyte disturbance (85) and results from water intake, either orally or intravenously, in excess of excretion. For normal individuals, a water load will extinguish the osmoreceptor stimulation of thirst and vasopressin release, allowing for dilution of the urine down to <50 mOsm/kg, and rapid water excretion. Given that the average solute load of average diets is approximately 800 mOsm, primarily in the form of protein and sodium, most individuals can excrete up to 16 liters of water, and thus can drink similar amounts before becoming hyponatremic. The classic disorders of “tea and toast” or “beer potomania” occur in the setting of low-solute diets (i.e., carbohydrates that are rapidly converted to water without providing solute) combined with high water intake, thus allowing hyponatremia to develop at much more modest amounts of water intake. For true psychogenic polydipsia, as defined by an ability to overwhelm the kidney’s capacity to excrete water through dilute urine, patients must drink huge amounts of fluid. Hyponatremia with a urine osmolality>100 mOsm/kg signifies the presence and action of vasopressin. Because serum osmolality is the normal driver for vasopressin release, its presence at low serum osmolality suggests concentration-independent mechanisms of vasopressin release. As noted above, sensed volume depletion can stimulate vasopressin release. This can occur in volume depletion (e.g., diarrhea or vomiting) or volume overload (e.g., cirrhosis or congestive heart failure). Conversely, the syndrome of inappropriate antidiuretic hormone (SIADH) secretion manifests as an inability to excrete water due to insuppressible vasopressin activity. The diagnosis of SIADH requires the absence of sensed volume depletion, and an inappropriately concentrated urine in the setting of hypo-osmolality, and occurs in a wide range of settings, including neurologic and pulmonary disease, medications, pain, and nausea (86). Recent gain-of-functions mutations in the vasopressin gene have been described, causing a “SIADH-like” clinical picture with undetectable vasopressin levels, termed nephrogenic syndrome of inappropriate antidiuresis (87).
Multiple studies have linked hyponatremia to increased mortality, with an increased risk ranging from 2-fold (88) to as much as 60-fold (89). Given the wide range of underlying pathologies potentially associated with hyponatremia, and the difficulty in adequately controlling for residual confounding, these observational studies should be interpreted with some caution. Although most studies have shown a linear inverse effect of decreasing sodium with mortality, recent studies have suggested a parabolic phenomenon, whereby the increased mortality associated with serum sodium in the mid-120 mEq/l range dissipates at concentrations <120 mEq/l (90). Given the risks associated with correcting hyponatremia, including central pontine myelinosis and volume overload, prospective studies are needed to further clarify the relationship of hyponatremia to outcomes.
In summary, water homeostasis depends on a functional and sensitive osmoreceptor, intact vasopressin and thirst mechanisms, and a renal tubule that can respond to the tightly orchestrated commands that dictate water retention or excretion.
Disclosures
None.
Acknowledgments
J.D. is supported by a Normon S. Coplon Extramural Grant from Satellite Healthcare.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Pitts RF: Physiology of the Kidney and Body Fluids, Chicago, Year Book Medical Publishers, 1963 [Google Scholar]
- 2.Epstein FH: The shark rectal gland: A model for the active transport of chloride. Yale J Biol Med 52: 517–523, 1979 [PMC free article] [PubMed] [Google Scholar]
- 3.Silva P, Stoff J, Field M, Fine L, Forrest JN, Epstein FH: Mechanism of active chloride secretion by shark rectal gland: Role of Na-K-ATPase in chloride transport. Am J Physiol 233: F298–F306, 1977 [DOI] [PubMed] [Google Scholar]
- 4.Ortiz RM: Osmoregulation in marine mammals. J Exp Biol 204: 1831–1844, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Crone C, Olesen SP: Electrical resistance of brain microvascular endothelium. Brain Res 241: 49–55, 1982 [DOI] [PubMed] [Google Scholar]
- 6.Crone C, Christensen O: Electrical resistance of a capillary endothelium. J Gen Physiol 77: 349–371, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP: Specialized membrane domains for water transport in glial cells: High-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci 17: 171–180, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagelhus EA, Ottersen OP: Physiological roles of aquaporin-4 in brain. Physiol Rev 93: 1543–1562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haj-Yasein NN, Vindedal GF, Eilert-Olsen M, Gundersen GA, Skare Ø, Laake P, Klungland A, Thorén AE, Burkhardt JM, Ottersen OP, Nagelhus EA: Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc Natl Acad Sci U S A 108: 17815–17820, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thrane AS, Rappold PM, Fujita T, Torres A, Bekar LK, Takano T, Peng W, Wang F, Rangroo Thrane V, Enger R, Haj-Yasein NN, Skare Ø, Holen T, Klungland A, Ottersen OP, Nedergaard M, Nagelhus EA: Critical role of aquaporin-4 (AQP4) in astrocytic Ca2+ signaling events elicited by cerebral edema. Proc Natl Acad Sci U S A 108: 846–851, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamberlin ME, Strange K: Anisosmotic cell volume regulation: A comparative view. Am J Physiol 257: C159–C173, 1989 [DOI] [PubMed] [Google Scholar]
- 12.Cserr HF, DePasquale M, Nicholson C, Patlak CS, Pettigrew KD, Rice ME: Extracellular volume decreases while cell volume is maintained by ion uptake in rat brain during acute hypernatremia. J Physiol 442: 277–295, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arieff AI, Ayus JC, Fraser CL: Hyponatraemia and death or permanent brain damage in healthy children. BMJ 304: 1218–1222, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayus JC, Arieff AI: Chronic hyponatremic encephalopathy in postmenopausal women: Association of therapies with morbidity and mortality. JAMA 281: 2299–2304, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Ayus JC, Wheeler JM, Arieff AI: Postoperative hyponatremic encephalopathy in menstruant women. Ann Intern Med 117: 891–897, 1992 [DOI] [PubMed] [Google Scholar]
- 16.Snyder NA, Feigal DW, Arieff AI: Hypernatremia in elderly patients. A heterogeneous, morbid, and iatrogenic entity. Ann Intern Med 107: 309–319, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Ofran Y, Lavi D, Opher D, Weiss TA, Elinav E: Fatal voluntary salt intake resulting in the highest ever documented sodium plasma level in adults (255 mmol L-1): A disorder linked to female gender and psychiatric disorders. J Intern Med 256: 525–528, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Addleman M, Pollard A, Grossman RF: Survival after severe hypernatremia due to salt ingestion by an adult. Am J Med 78: 176–178, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Calvin ME, Knepper R, Robertson WO: Hazards to health. Salt poisoning. N Engl J Med 270: 625–626, 1964 [DOI] [PubMed] [Google Scholar]
- 20.Ellis RJ: Severe hypernatremia from sea water ingestion during near-drowning in a hurricane. West J Med 167: 430–433, 1997 [PMC free article] [PubMed] [Google Scholar]
- 21.Sharif-Naeini R, Ciura S, Zhang Z, Bourque CW: Contribution of TRPV channels to osmosensory transduction, thirst, and vasopressin release. Kidney Int 73: 811–815, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Vivas L, Chiaraviglio E, Carrer HF: Rat organum vasculosum laminae terminalis in vitro: Responses to changes in sodium concentration. Brain Res 519: 294–300, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Ciura S, Bourque CW: Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. J Neurosci 26: 9069–9075, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leng G, Mason WT, Dyer RG: The supraoptic nucleus as an osmoreceptor. Neuroendocrinology 34: 75–82, 1982 [DOI] [PubMed] [Google Scholar]
- 25.Mason WT: Supraoptic neurones of rat hypothalamus are osmosensitive. Nature 287: 154–157, 1980 [DOI] [PubMed] [Google Scholar]
- 26.Walters JK, Hatton GI: Supraoptic neuronal activity in rats during five days of water deprivation. Physiol Behav 13: 661–667, 1974 [DOI] [PubMed] [Google Scholar]
- 27.Brimble MJ, Dyball RE: Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol 271: 253–271, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liedtke W, Choe Y, Martí-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S: Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103: 525–535, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tian W, Fu Y, Garcia-Elias A, Fernández-Fernández JM, Vicente R, Kramer PL, Klein RF, Hitzemann R, Orwoll ES, Wilmot B, McWeeney S, Valverde MA, Cohen DM: A loss-of-function nonsynonymous polymorphism in the osmoregulatory TRPV4 gene is associated with human hyponatremia. Proc Natl Acad Sci U S A 106: 14034–14039, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciura S, Liedtke W, Bourque CW: Hypertonicity sensing in organum vasculosum lamina terminalis neurons: A mechanical process involving TRPV1 but not TRPV4. J Neurosci 31: 14669–14676, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egan G, Silk T, Zamarripa F, Williams J, Federico P, Cunnington R, Carabott L, Blair-West J, Shade R, McKinley M, Farrell M, Lancaster J, Jackson G, Fox P, Denton D: Neural correlates of the emergence of consciousness of thirst. Proc Natl Acad Sci U S A 100: 15241–15246, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson CJ, Bland J, Burd J, Baylis PH: The osmotic thresholds for thirst and vasopressin release are similar in healthy man. Clin Sci (Lond) 71: 651–656, 1986 [DOI] [PubMed] [Google Scholar]
- 33.Hollis JH, McKinley MJ, D’Souza M, Kampe J, Oldfield BJ: The trajectory of sensory pathways from the lamina terminalis to the insular and cingulate cortex: A neuroanatomical framework for the generation of thirst. Am J Physiol Regul Integr Comp Physiol 294: R1390–R1401, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Lechner SG, Markworth S, Poole K, Smith ES, Lapatsina L, Frahm S, May M, Pischke S, Suzuki M, Ibañez-Tallon I, Luft FC, Jordan J, Lewin GR: The molecular and cellular identity of peripheral osmoreceptors. Neuron 69: 332–344, 2011 [DOI] [PubMed] [Google Scholar]
- 35.McHugh J, Keller NR, Appalsamy M, Thomas SA, Raj SR, Diedrich A, Biaggioni I, Jordan J, Robertson D: Portal osmopressor mechanism linked to transient receptor potential vanilloid 4 and blood pressure control. Hypertension 55: 1438–1443, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boschmann M, Steiniger J, Franke G, Birkenfeld AL, Luft FC, Jordan J: Water drinking induces thermogenesis through osmosensitive mechanisms. J Clin Endocrinol Metab 92: 3334–3337, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Russell PJ, Abdelaal AE, Mogenson GJ: Graded levels of hemorrhage, thirst and angiotensin II in the rat. Physiol Behav 15: 117–119, 1975 [DOI] [PubMed] [Google Scholar]
- 38.Stricker EM: Inhibition of thirst in rats following hypovolemia and-or caval ligation. Physiol Behav 6: 293–298, 1971 [DOI] [PubMed] [Google Scholar]
- 39.Stricker EM: Osmoregulation and volume regulation in rats: Inhibition of hypovolemic thirst by water. Am J Physiol 217: 98–105, 1969 [DOI] [PubMed] [Google Scholar]
- 40.el Ghissassi M, Thornton SN, Nicolaïdis S: Angiotensin II-induced thirst, but not sodium appetite, via AT1 receptors in organum cavum prelamina terminalis. Am J Physiol 268: R1401–R1405, 1995 [DOI] [PubMed] [Google Scholar]
- 41.Weisinger RS, Blair-West JR, Burns P, Denton DA, Tarjan E: Role of brain angiotensin in thirst and sodium appetite of rats. Peptides 18: 977–984, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Leenen FH, Stricker EM: Plasma renin activity and thirst following hypovolemia or caval ligation in rats. Am J Physiol 226: 1238–1242, 1974 [DOI] [PubMed] [Google Scholar]
- 43.Waldréus N, Sjöstrand F, Hahn RG: Thirst in the elderly with and without heart failure. Arch Gerontol Geriatr 53: 174–178, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Waldréus N, Hahn RG, Jaarsma T: Thirst in heart failure: A systematic literature review. Eur J Heart Fail 15: 141–149, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Masajtis-Zagajewska A, Nowicki M: Influence of dual blockade of the renin-angiotensin system on thirst in hemodialysis patients. Nephron Clin Pract 112: c242–c247, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Goldman MB, Luchins DJ, Robertson GL: Mechanisms of altered water metabolism in psychotic patients with polydipsia and hyponatremia. N Engl J Med 318: 397–403, 1988 [DOI] [PubMed] [Google Scholar]
- 47.Ishikawa SE, Schrier RW: Pathophysiological roles of arginine vasopressin and aquaporin-2 in impaired water excretion. Clin Endocrinol (Oxf) 58: 1–17, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Martin PY, Abraham WT, Lieming X, Olson BR, Oren RM, Ohara M, Schrier RW: Selective V2-receptor vasopressin antagonism decreases urinary aquaporin-2 excretion in patients with chronic heart failure. J Am Soc Nephrol 10: 2165–2170, 1999 [DOI] [PubMed] [Google Scholar]
- 49.Schrier RW: Vasopressin and aquaporin 2 in clinical disorders of water homeostasis. Semin Nephrol 28: 289–296, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cowley AW, Jr, Cushman WC, Quillen EW, Jr, Skelton MM, Langford HG: Vasopressin elevation in essential hypertension and increased responsiveness to sodium intake. Hypertension 3: I93–I100, 1981 [DOI] [PubMed] [Google Scholar]
- 51.Leng G, Brown CH, Russell JA: Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog Neurobiol 57: 625–655, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Cowley AW, Jr, Switzer SJ, Guinn MM: Evidence and quantification of the vasopressin arterial pressure control system in the dog. Circ Res 46: 58–67, 1980 [DOI] [PubMed] [Google Scholar]
- 53.Sharshar T, Carlier R, Blanchard A, Feydy A, Gray F, Paillard M, Raphael JC, Gajdos P, Annane D: Depletion of neurohypophyseal content of vasopressin in septic shock. Crit Care Med 30: 497–500, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Quillen EW, Jr, Cowley AW, Jr: Influence of volume changes on osmolality-vasopressin relationships in conscious dogs. Am J Physiol 244: H73–H79, 1983 [DOI] [PubMed] [Google Scholar]
- 55.Danziger J, Zeidel M, Parker MJ: Renal Physiology: A Clinical Approach, Baltimore, Lippincott Williams and Wilkins, 2012 [Google Scholar]
- 56.Epstein FH, Kleeman CR, Pursel S, Hendrikx A: The effect of feeding protein and urea on the renal concentrating process. J Clin Invest 36: 635–641, 1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moffat DB, Fourman J: The vascular pattern of the rat kidney. J Anat 97: 543–553, 1963 [PMC free article] [PubMed] [Google Scholar]
- 58.Moffat DB, Fourman J: A vascular pattern of the rat kidney. 1963. J Am Soc Nephrol 12: 624–632, 2001 [DOI] [PubMed] [Google Scholar]
- 59.Pallone TL, Edwards A, Mattson DL: Renal medullary circulation. Compr Physiol 2: 97–140, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Denker BM, Sabath E: The biology of epithelial cell tight junctions in the kidney. J Am Soc Nephrol 22: 622–625, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Then C, Bergler T, Jeblick R, Jung B, Banas B, Krämer BK: Hypertonic stress promotes the upregulation and phosphorylation of zonula occludens 1. Nephron, Physiol 119: 11–21, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Hou J, Rajagopal M, Yu AS: Claudins and the kidney. Annu Rev Physiol 75: 479–501, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shen L, Turner JR: Role of epithelial cells in initiation and propagation of intestinal inflammation. Eliminating the static: Tight junction dynamics exposed. Am J Physiol Gastrointest Liver Physiol 290: G577–G582, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Howden CW, Gillanders I, Morris AJ, Duncan A, Danesh B, Russell RI: Intestinal permeability in patients with Crohn’s disease and their first-degree relatives. Am J Gastroenterol 89: 1175–1176, 1994 [PubMed] [Google Scholar]
- 65.Katz KD, Hollander D, Vadheim CM, McElree C, Delahunty T, Dadufalza VD, Krugliak P, Rotter JI: Intestinal permeability in patients with Crohn’s disease and their healthy relatives. Gastroenterology 97: 927–931, 1989 [DOI] [PubMed] [Google Scholar]
- 66.Gonzalez-Mariscal L, Namorado MC, Martin D, Luna J, Alarcon L, Islas S, Valencia L, Muriel P, Ponce L, Reyes JL: Tight junction proteins ZO-1, ZO-2, and occludin along isolated renal tubules. Kidney Int 57: 2386–2402, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Gonzalez-Mariscal L, Namorado Mdel C, Martin D, Sierra G, Reyes JL: The tight junction proteins claudin-7 and -8 display a different subcellular localization at Henle's loops and collecting ducts of rabbit kidney. Nephrol Dial Transplant 21: 2391–2398, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Nagle JF, Mathai JC, Zeidel ML, Tristram-Nagle S: Theory of passive permeability through lipid bilayers. J Gen Physiol 131: 77–85, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mathai JC, Tristram-Nagle S, Nagle JF, Zeidel ML: Structural determinants of water permeability through the lipid membrane. J Gen Physiol 131: 69–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mathai JC, Zeidel ML: Measurement of water and solute permeability by stopped-flow fluorimetry. Methods Mol Biol 400: 323–332, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Zeidel ML: Low permeabilities of apical membranes of barrier epithelia: What makes watertight membranes watertight? Am J Physiol 271: F243–F245, 1996 [DOI] [PubMed] [Google Scholar]
- 72.Rivers R, Blanchard A, Eladari D, Leviel F, Paillard M, Podevin RA, Zeidel ML: Water and solute permeabilities of medullary thick ascending limb apical and basolateral membranes. Am J Physiol 274: F453–F462, 1998 [DOI] [PubMed] [Google Scholar]
- 73.Gensure RH, Zeidel ML, Hill WG: Lipid raft components cholesterol and sphingomyelin increase H+/OH- permeability of phosphatidylcholine membranes. Biochem J 398: 485–495, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tristram-Nagle S, Kim DJ, Akhunzada N, Kucerka N, Mathai JC, Katsaras J, Zeidel M, Nagle JF: Structure and water permeability of fully hydrated diphytanoylPC. Chem Phys Lipids 163: 630–637, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Agre P: Homer W. Smith award lecture. Aquaporin water channels in kidney. J Am Soc Nephrol 11: 764–777, 2000 [DOI] [PubMed] [Google Scholar]
- 76.Hasler U, Jeon US, Kim JA, Mordasini D, Kwon HM, Féraille E, Martin PY: Tonicity-responsive enhancer binding protein is an essential regulator of aquaporin-2 expression in renal collecting duct principal cells. J Am Soc Nephrol 17: 1521–1531, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Jeon US, Joo KW, Na KY, Kim YS, Lee JS, Kim J, Kim GH, Nielsen S, Knepper MA, Han JS: Oxytocin induces apical and basolateral redistribution of aquaporin-2 in rat kidney. Nephron, Exp Nephrol 93: e36–e45, 2003 [DOI] [PubMed] [Google Scholar]
- 78.Chu JY, Chung SC, Lam AK, Tam S, Chung SK, Chow BK: Phenotypes developed in secretin receptor-null mice indicated a role for secretin in regulating renal water reabsorption. Mol Cell Biol 27: 2499–2511, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pivonello R, De Bellis A, Faggiano A, Di Salle F, Petretta M, Di Somma C, Perrino S, Altucci P, Bizzarro A, Bellastella A, Lombardi G, Colao A: Central diabetes insipidus and autoimmunity: Relationship between the occurrence of antibodies to arginine vasopressin-secreting cells and clinical, immunological, and radiological features in a large cohort of patients with central diabetes insipidus of known and unknown etiology. J Clin Endocrinol Metab 88: 1629–1636, 2003 [DOI] [PubMed] [Google Scholar]
- 80.Fujiwara TM, Bichet DG: Molecular biology of hereditary diabetes insipidus. J Am Soc Nephrol 16: 2836–2846, 2005 [DOI] [PubMed] [Google Scholar]
- 81.Morello JP, Salahpour A, Laperrière A, Bernier V, Arthus MF, Lonergan M, Petäjä-Repo U, Angers S, Morin D, Bichet DG, Bouvier M: Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest 105: 887–895, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stone KA: Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract 12: 43–47, 1999 [DOI] [PubMed] [Google Scholar]
- 83.Christensen BM, Zuber AM, Loffing J, Stehle JC, Deen PM, Rossier BC, Hummler E: alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol 22: 253–261, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kortenoeven ML, Li Y, Shaw S, Gaeggeler HP, Rossier BC, Wetzels JF, Deen PM: Amiloride blocks lithium entry through the sodium channel thereby attenuating the resultant nephrogenic diabetes insipidus. Kidney Int 76: 44–53, 2009 [DOI] [PubMed] [Google Scholar]
- 85.Upadhyay A, Jaber BL, Madias NE: Incidence and prevalence of hyponatremia. Am J Med 119[Suppl 1]: S30–S35, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Robertson GL: Regulation of arginine vasopressin in the syndrome of inappropriate antidiuresis. Am J Med 119[Suppl 1]: S36–S42, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Feldman BJ, Rosenthal SM, Vargas GA, Fenwick RG, Huang EA, Matsuda-Abedini M, Lustig RH, Mathias RS, Portale AA, Miller WL, Gitelman SE: Nephrogenic syndrome of inappropriate antidiuresis. N Engl J Med 352: 1884–1890, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Waikar SS, Mount DB, Curhan GC: Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 122: 857–865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anderson RJ, Chung HM, Kluge R, Schrier RW: Hyponatremia: A prospective analysis of its epidemiology and the pathogenetic role of vasopressin. Ann Intern Med 102: 164–168, 1985 [DOI] [PubMed] [Google Scholar]
- 90.Chawla A, Sterns RH, Nigwekar SU, Cappuccio JD: Mortality and serum sodium: Do patients die from or with hyponatremia? Clin J Am Soc Nephrol 6: 960–965, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S: Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002 [DOI] [PubMed] [Google Scholar]
- 92.Maunsbach AB, Marples D, Chin E, Ning G, Bondy C, Agre P, Nielsen S: Aquaporin-1 water channel expression in human kidney. J Am Soc Nephrol 8: 1–14, 1997 [DOI] [PubMed] [Google Scholar]