Abstract
Background
‘Candidatus Liberibacter solanacearum’ (Lso) is a phloem-limited alphaproteobacterium associated with the devastating zebra chip disease of potato (Solanum tuberosum). Like other members of Liberibacter, Lso-ZC1 encodes a flagellin domain-containing protein (FlaLso) with a conserved 22 amino-acid peptide (flg22Lso). To understand the innate immune responses triggered by this unculturable intracellular bacterium, we studied the pathogen-associated molecular patterns (PAMPs) that triggered immunity in Nicotiana benthamiana, using the flg22Lso peptide and the full length flaLso gene.
Results
Our results showed that the expression of flaLso via Agrobacterium-mediated transient expression induced a slow necrotic cell death in the inoculated leaves of N. benthamiana, which was coupled with a burst of reactive oxygen species (ROS) production. Moreover, the expression of several representative genes involved in innate immunity was transiently up-regulated by the flg22Lso in N. benthamiana. The FlaLso, however, induced stronger up-regulation of these representative genes compared to the flg22Lso, especially that of flagellin receptor FLAGELLIN SENSING2 (FLS2) and respiratory burst oxidase (RbohB) in N. benthamiana. Although neither cell death nor ROS were induced by the synthetic flg22Lso, a weak callose deposition was observed in infiltrated leaves of tobacco, tomato, and potato plants.
Conclusion
The flagellin of Lso and its functional domain, flg22Lso share characteristics of pathogen-associated molecular patterns, and trigger unique innate immune responses in N. benthamiana. Slow and weak activation of the innate immune response in host plants by the flagellin of Lso may reflect the nature of its intracellular life cycle. Our findings provide new insights into the role of the Lso flagellin in the development of potato zebra chip disease and potential application in breeding for resistance.
Keywords: Candidatus Liberibacter solanacearum, Flagellin, Flg22, Cell death, Reactive oxygen species, Gene expression
Background
Zebra chip (ZC) is an important potato disease causing millions of dollars in losses to both potato producers and processors in the United States [1]. The disease was first discovered in potato fields near Saltillo, Mexico in 1994, and was reported in Texas, USA in 2000 [2]. Since 2000, the disease has spread to several other states and has been accompanied by serious economic impacts [1]. The disease symptoms are characterized by necrotic flecking and medullary ray discolorations in tubers, leaf chlorosis, twisted stems, swollen nodes, vascular discoloration, leaf scorching, and wilting [3],[4]. The putative causal agent of Zebra chip disease is ‘Candidatus Liberibacter solanacearum’ (Lso) (also known as Ca. Liberibacter psyllaurous), which is transmitted by the potato psyllid, Bactericera cockerelli[3],[5]. Lso has a significantly reduced genome and shares a high degree of similarity with another important plant pathogen, ‘Ca. Liberibacter asiaticus’ (Las) [6],[7]. Lso is also associated with diseases of other crops including tomato, carrot (Daucus carota L.) in Finland, and celery (Apium graveolens) in Spain [3]. Currently all commercial potato cultivars appear to be susceptible to Lso infection and the only strategy for controlling the spread of the disease is by managing the potato psyllid [1].
Plant immunity relies on two levels of defense response against pathogens: pathogen-associated molecular patterns (PAMP)-triggered immunity (PTI) that is a component of basal defense and effector-triggered immunity (ETI) that reflects immunity to specific strains [8],[9]. PTI is associated with PAMP recognition and activation of plant plasma membrane receptors [8],[10]. Typically, PTI induces several important signaling pathways including calcium ion (Ca2+) influx, reactive oxygen species (ROS) production, and mitogen-activated protein kinase (MAPK) pathway activation [10]. Interactions between the peptide flg22, which is located at the N-terminal domain of a flagellin, and host surface-localized pattern recognition receptors flagellin sensing 2 (FLS2) in Arabidopsis thaliana, are well studied. In A. thaliana, FLS2 interacts with the receptor kinase BAK1 by forming a functional FLS2/BAK1 complex, which is subsequently followed by a typical PTI response [8],[10]. Orthologs of the Arabidopsis FLS2 have been identified in many plant species including N. benthamiana[11], Lycopersicum esculentum[12] and Oryza japonica[13]. Recently, it has been shown that silencing the expression of NbFLS2 compromised the expression of downstream genes induced by flg22 [14]. In Arabidopsis, the ROS burst induced by the flg22 is regulated by RbohD[15]. In N. benthamiana, RbohB, a homolog of RbohD, is essential for ROS production and silencing of NbRbohB completely abolishes the ROS burst [14],[16]. In N. benthamiana, two mitogen-activated protein kinases (MAPKs), salicylic acid-induced protein kinase (SIPK), and wound-induced protein kinase (WIPK) are activated quickly after elicitation [17]. Furthermore, both SIPK and WIPK are essential for bacterial immunity in N. benthamiana[14].
It has recently been shown that another member of the Liberibacter genus, Ca. Liberibacter asiaticus (Las), encodes a functional flagellin that partially restored the motility of Sinorhizobium meliloti fla mutant and shares characteristics of pathogen-associated molecular patterns [18]. Like Las, the Lso genome contains the same number of open reading frames (30 ORFs) that are essential for the structure and assembly of a flagellum [6]. In this study, due to the inability to grow Lso in culture, we investigated the PAMP activity of Lso flagellin (FlaLso) and its peptide flg22Lsoin planta via infiltration or Agrobacterium-mediated transient expression. Our results demonstrate that transient expression of flaLso induces a delayed increase in ROS production and slow necrotic cell death in tobacco plants. Although the flg22 from P. aeruginosa (which was used as a control) induced the typical ROS bursts, the flg22Lso peptide did not induce ROS production in tobacco, tomato, or potato, but did induce callose deposition in these three species. We further demonstrated that the peptide flg22Lso and the flagellin, FlaLso induced expression of genes associated with PTI in N. benthamiana. These results provide new insights into the role of bacterial flagellin in the development of potato zebra chip disease.
Results
Lso encoding a flagellin with a conserved flg22Lso peptide
In the Lso-ZC1 genome, several clusters of flagellar biosynthesis related genes were identified by sequence analysis. CKC_02645 was characterized as encoding a flagellin domain-containing protein. This gene contains 1374 nucleotides and encodes a 457 amino-acid protein, designated as FlaLso. FlaLso shares 61% identity to the flagellin from Ca. Liberibacter asiaticus (Las), 59% identity to the flagellin from Ca. Liberibacter americanus (Lam) and 51% identity to the flagellin from L. crescens BT1. A conserved flagellin domain was identified consisting of 22 amino acids located at position 29 to 50 at the N terminus of FlaLso and was designated as flg22Lso. Flg22Lso shares high identity (86%) to the flg22 peptides from Las, Lam and L. crescens BT1and it shares 77% identity to the flg22 from the closely related species Agrobacterium tumefaciens and Sinorhizobium meliloti 1021, which do not induce plant immune responses [19],[20]. Flg22Lso shares 41% identity with the flg22 from Pseudomonas aeruginosa and 55% identity with the flg22 from Pseudomonas syringae pv. tabaci, which trigger a strong nonhost innate immune response [21]. In flg22, the amino acid residue D42 is critical for its PAMP activity [22],[23]. Although flg22Lso possesses amino acid D42, only two other amino acids are conserved in the central RINSAKDDA motif (Figure 1).
Figure 1.

Comparison of flg22 peptide sequence of ‘CandidatusLiberibacter solanacearum’ (GenBank accession number: YP_004062766.1) to the conserved flg22 from other bacteria. Las: ‘Ca. Liberibacter asiaticus’ (GenBank accession number: YP_003064944.1), Lam: Ca. Liberibacter americanus (GenBank accession number: YP_00008798658.1), Lcr: Liberibacter crescens BT1 (GenBank accession number: YP_007233200.1), Pa: Pseudomonas aeruginosa (GenBank accession number: ADZ56326.1), Ps: P. syringae (GenBank accession number: WP_005738283.1), Sm: Sinorhizobium meliloti Rm1021 (GenBank accession number: NP_384775.1), At: Agrobacterium tumefaciens C58 (GenBank accession number: NP_353572.1). The central domain is shown in box.
Slow necrotic cell death induced by transient expression of the flaLso in N. benthamiana
For the transient expression assay of flaLso in N. benthamiana, various concentrations of A. tumefaciens GV3101 with and without flaLso were tested. At an OD600 of 0.8 and 1.2, necrotic cell death was observed in the infiltration zone with pBin:flaLso at 8 days after inoculation; the control with pBin vector alone exhibited chlorosis but not necrosis, and GV3101 with no vector showed no visible response (Figure 2). In addition, cell death was measured by electrolyte leakage from leaf discs infiltrated with MgCl2, GV3101 carrying pBin vector and pBin:flaLso constructs. Overexpression of the flaLso in tobacco leaves increased electrolyte leakage on 4 and 5 dpi (Additional file 1: Figure S1). Taken together, this indicates that the expression of the flagellin gene, flaLso, causes necrotic cell death in the infiltrated zone of N. benthamiana. However, in tomato and potato inoculations, no obvious differences were observed after inoculation with various concentration of GV3101 containing the pBin vector alone or pBin:flaLso, respectively (data not shown).
Figure 2.
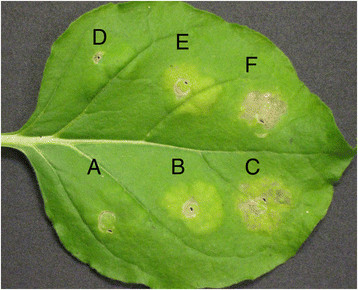
Cell death inN. benthamianaleaves induced by transient expression of theflaLso,‘Ca.Liberibacter solanacearum’ flagellin gene. Transient expression of Agrobacterium tumefaciens strain GV3101 alone, GV3101 containing the pBin vector, or GV3101 containing pBin:flaLso at an OD600 ~ 0.8 (A, B, C) and 1.2 (D, E, F), respectively. Photographs were taken ten days after infiltration. The experiments were performed in six independent replicates.
Callose deposition induced by flg22Lso and flg22Las
The conserved flagellin domain flg22Las from Ca. Liberibacter asiaticus induced callose deposition when infiltrated into tobacco [18]. Compared to flg22Las, flg22Lso has three amino acid substitutions including a serine (S) to alanine (A) at position 38, and alanine (A) to serine (S) substitutions at positions 40 and 41 (Figure 1). Both synthetic peptides flg22Lso and flg22Las at a concentration of 40 μM were infiltrated into leaves of N. benthamiana, tomato and potato. Neither peptide induced cell death in the infiltrated zone but callose deposition was observed in infiltrated N. benthamiana, tomato and potato, with more callose deposition from flg22Lso compared to flg22Las in both tobacco and tomato (Figure 3). Compared to tobacco and potato, a more robust callose deposition was observed in tomato after treatment with either one of the peptides (Figure 3E and F).
Figure 3.
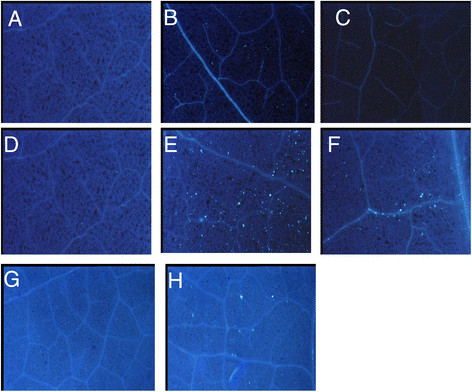
Microscopic detection of callose deposition.N. benthamiana(A, B and C) and tomato (D, E and F) infiltrated with the water control, flg22Lso and flg22Las, respectively. Potato (G and H) infiltrated with water and flg22Lso, respectively. Leaves were collected at 24 h post infiltration and then stained with aniline blue. Callose deposition was observed under UV epifluorescence microscope.
ROS production induced by the flaLso, but not by the flg22Lso in N. benthamiana
One of the important events of PTI response is a rapid and transient burst of ROS production. We examined whether ROS production is induced by flg22Lso or flg22Las. Neither flg22Lso nor flg22Las produced an ROS burst when infiltrated at 0.1 μM, 1 μM, 10 μM or 40 μM in tobacco, tomato or potato; while the control flg22 from P. aeruginosa shows a typical ROS production at a 0.1 μM concentration in the three plant species (Figure 4). In contrast, ROS production was detected in transient expression of N. benthamiana leaves infiltrated with A. tumefasciens containing pBin:flaLso and pBin:flaLas clones respectively (Figure 5). The strongest ROS response was observed on the third and fourth day after infiltration. However, no ROS response was detected in tomato or potato infiltrated with these peptides and constructs (data not shown), which is consistent with the observation that no obvious cell death was induced in these plants.
Figure 4.

ROS assay for flagellin peptide treated plants.N. benthamiana(A), potato (B) and tomato (C) treated with flg22 from P. aeruginosa showed typical ROS response. No ROS response was seen with flg22Las or flg22Lso treated plants. CPS: count per second.
Figure 5.
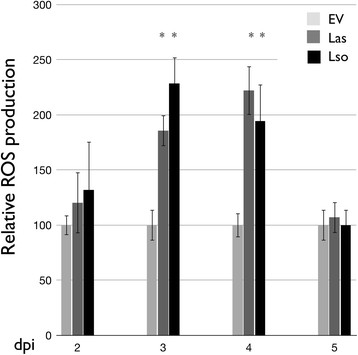
ROS assay for theflaLsoand theflaLasinfiltrated zones ofN. benthamianaleaves. Data shown are relative values (error bars represent standard error of the mean based on four samples from four different plants) at 2, 3, 4 and 5 dpi after infiltration with Agrobacterium tumefaciens strain GV3101 containing EV: Empty vector, pBin; Las: pBin:flaLas and Lso: pBin:flaLso. * marked as significant difference by student t-test.
PTI gene expression transiently up-regulated by flg22Lso in N. benthamiana
The expression of many plant genes is up-regulated in PAMP-triggered immunity [24],[25]. To investigate expression levels of PAMP-associated genes in N. benthamiana after flg22Lso infiltration, reverse transcriptase quantitative PCR (RT-qPCR) was performed. After N. benthamiana was infiltrated with the flg22Lso peptide, the transcript abundance was measured at 0.5, 1.0, 3.0, and 6.0 hours post infiltration (hpi). The expression of NbFLS2 was up-regulated approximately 2 fold at 0.5 hpi, more than 3 fold at 1.0 hpi, and then decreased at 3.0 hpi with flg22Lso treatment (Figure 6). Previously, three marker genes in N. benthamiana, NbCYP71D20, NbACRE31 and NbACRE32, were found to rapidly increase following flg22Psy treatment [14],[26]. These three PAMP-marker genes were transiently induced by flg22Lso at 1.0 hpi and then subsequently declined in our RT-qPCR assay, which indicates that flg22Lso induces a typical transient PAMP triggered immunity gene response. FLS2 directly binds bacterial flagellin and then interacts with BAK1 to form a recognition complex [8]. However, the expression level of NbBAK1 showed no obvious differences in our assay. Somatic embryogenesis receptor kinase 3 (NbSerk3)/BAK1 is required for PAMP-triggered immunity in N. benthamiana[26]. The expression pattern of NbSerk3/ NbBAK1 did not show an obvious change upon flg22Lso treatment. Two MAPKs, NbWIPK and NbSIPK, were transiently increased at 1.0 hpi in our experiment. NbRbohB expression was up-regulated about 2 fold except at 3.0 hpi. Notably, most of these PTI related genes are up-regulated at 0.5 hpi or 1.0 hpi, and then diminish at 3.0 hpi. Plastocyanin, which plays a key role in photosynthesis, was reported to be induced in the PTI response to nonpathogenic P. fluorescens[11]. In our experiment, transcript abundance of NbPlastocyanin increased 5 fold at 1.0 hpi and almost 10 fold at 3.0 hpi (Figure 6). Taken together, our results showed that flg22Lso transiently induced PAMP-triggered gene expression in N. benthamiana.
Figure 6.
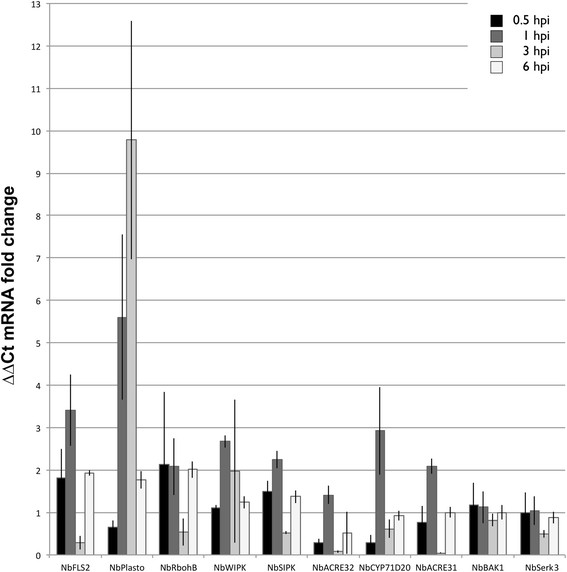
Flg22Lso-triggered changes in the expression of genes related to plant defense inN. benthamiana.N. benthamiana leaves were infiltrated with flg22Lso at 40 μM and water as a control. Leaf tissue was harvested from infiltrated spots at 0.5, 1, 3 and 6 h post-infiltration (hpi) for RNA isolation and cDNA preparation. RT-qPCR was performed to check gene expression of NbFLS2, NbWIPK, NbSIPK, NbBAK1, NbRbohB, NbCYP71D20, NbACRE31, NbACRE32, NbPlastocyanin and NbSerk3. The samples were normalized against NbEF1α. Data were shown as average fold gene induction in response to water infiltrated samples from three independent experiments.
Long lasting PTI gene expression induced by flaLso in N. benthamiana
The expression of flaLso and its elicitation of PAMP-associated gene expression in N. benthamiana were investigated after infiltration with A. tumefasciens containing pBin:flaLso or the empty pBin vector plasmid. A high level of expression of flaLso was indicated by a strong band from 1 to 4 days after inoculation (dpi). A very faint band was observed on 8 dpi (Figure 7A). RT-qPCR data further showed that the transcript abundance after inoculation increased to its highest level on 3 dpi and decreased to an undetectable level compared to the host reference gene NbEF1α (Figure 7B). Meanwhile, the gene expression levels of PAMP-associated genes were measured at 2, 3, 4, and 8 dpi (Figure 8). RT-qPCR data showed that the expression of NbFLS2 was up-regulated more than 9 fold at 4 dpi. The abundance of NbBAK1/NbSerk3 was increased about 2 fold on 4 dpi. The expression of three other PAMP-associated genes, NbCYP71D20, NbACRE31 and NbACRE32 were upregulated and varied only slightly at different time after infiltration. Two MAPKs, especially NbWIPK, were up-regulated more than 4 fold at 4 dpi. NbRbohB dramatically increased at 4 dpi, which agreed with our ROS assay results that the strongest ROS production was detected at 4 dpi. The expression level of plastocyanin gradually declined over the evaluation period, in contrast to the transient high induction at 1.0 and 3.0 hpi observed with flg22Lso. Collectively, the expression of PAMP-associated genes elicited by the flaLso was different from the transient up-regulation with peptide flg22Lso induction. Most of these genes were strongly induced at 4 dpi, and then decreased, which correlated with the expression pattern of the flaLso in N. benthamiana.
Figure 7.
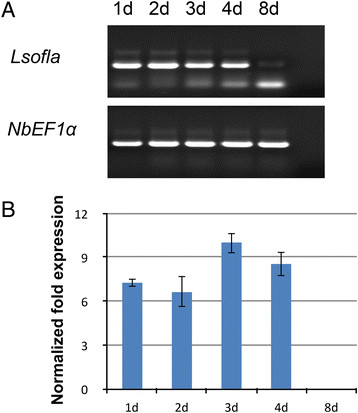
Transient expression of theflaLsoinN. benthamiana.N. benthamiana leaves were inoculated with A. tumefaciens GV3101 containing pBin:flaLso. The infiltrated tissues were harvested at 1, 2, 3, 4 and 8 days post-infiltration (dpi) for RNA isolation and cDNA preparation. RT-PCR (A) and RT-qPCR (B) were performed to determine gene expression of the flaLso at different time after infiltration. For RT-qPCR, the samples were normalized against NbEF1α. Data were shown as average fold change from three independent experiments.
Figure 8.
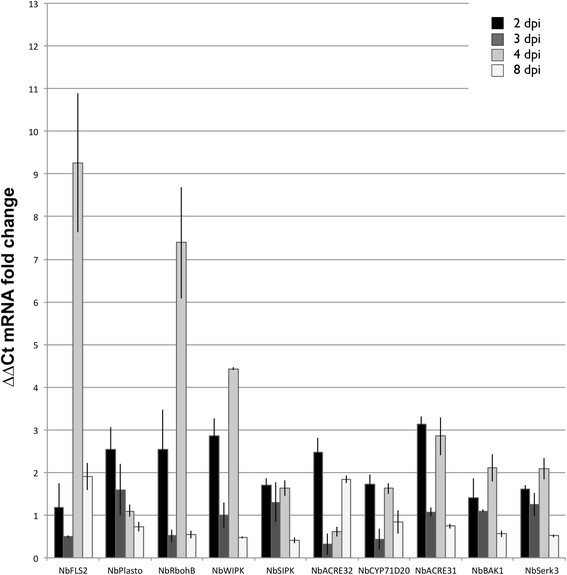
Transient expression of theflaLsotriggered changes in the expression of genes related to plant defense inN. benthamiana.N. benthamiana leaves were inoculated with A. tumefaciens GV3101 containing pBin:flaLso and GV3101 containing empty vector pBin as a control. The infiltrated spots were harvested at 2, 3, 4 and 8 days post-infiltration (dpi) for RNA isolation and cDNA preparation. RT-qPCR was performed to check gene expression of NbFLS2, NbWIPK, NbSIPK, NbBAK1, NbRbohB, NbCYP71D20, NbACRE31, NbACRE32, NbPlastocyanin and NbSerk3. The samples were normalized against NbEF1α. Data were shown as average fold gene induction in response to vector infiltrated samples from three independent experiments.
Discussion
Our results revealed that transient expression of the Lso flagellin gene (flaLso) induced a burst of ROS and slow necrotic cell death in N. benthamiana plants but not in the tomato or potato plants tested. Although the peptide flg22Lso did not induce cell death or a ROS reaction, it did induce callose deposition in all three plant species. We determined that the expression of PAMP-triggered genes was transiently up-regulated with synthesized peptide flg22Lso treatment. We further showed that these genes were more strongly affected by Agrobacterium-mediated transient expression of the full length gene, flaLso. Our results demonstrate that Lso flagellin and its short peptide, flg22Lso have a PAMP activity, and both of them trigger PTI response.
Callose deposition induced by Lso and Las peptides
Many reports document that flg22Lso peptides can induce plant defense responses, including reactive oxygen species (ROS), pathogenesis related gene expression and callose deposition [27]. Amino acid D42 of flg22Lso has been demonstrated to be essential for the elicitor activity of Xanthomonas and P. syringe pv. tabaci in non-host species [22],[23]. In our study, flg22Lso, which contains D42, did not induce visible cell death or ROS production in the plants tested, however, it was found to induce callose deposition in tobacco, tomato and potato plants. This suggests that flg22Lso may interact with the FLS2 receptors of all three plant species. In addition, we screened over one hundred potato genotypes using flg22Lso and flg22Las. We found that flg22Lso and flg22Las induced ROS response in several potato cultivars and the full flagellin gene has the ability to interact with the potato FLS2 receptor in yeast two hybridization assay (Duan, unpublished data). The flg22 peptides from A. tumefaciens and S. meliloti posses the D42 amino acid but did not show PAMP activity [27]. This indicates that other amino acids are also important for flg22 elicitor activity in addition to D42. Amino acid changes made at S38 and A39 completely abolished the PAMP activity of flg22Las, indicating that serine and aspartate at these positions are essential for flg22Las recognition by tobacco plants [18]. Compared to flg22Las, flg22Lso has three amino acid substitutions and induces more callose deposition than flg22Las in tobacco and tomato. It is worth noting that callose deposition was observed although no ROS production was detected in the infiltrated plants with either flg22Lso or flg22Las. However, callose deposition is sometimes seen in a host plant’s response to pathogen infection as well as being a component of non-host resistance, and high levels of callose are formed in response to Las infection in citrus [28].
Necrotic cell death induced by the flaLso in N. benthamiana
Flagellin is a bacterial elicitor associated with the induction of plant and animal defense responses. In our study, we discovered that transient expression of the flaLso induced necrotic cell death in tobacco, which was much slower than that reported for a typical hypersensitive response (HR) [29]. Transient expression of P. syringae flagellin FliC gene with or without signal peptide induced ROS and FLS2-dependent immunity in a non-host, but did not induce cell death within five days [30]. In our infiltration experiments, the expression of flaLso reached the highest level on 3 dpi and decreased to a very low level on 8 dpi, however necrotic cell death was not observed until 8–10 days after infiltration. This resembles cell death in a compatible pathogen-host interaction, which is much slower than non-host responses. Recently it has been reported that tobacco (N. tabacum) is a host for Lso [31]: response of a weak host rather than a non-host may explain our observation of necrotic cell death rather than a HR, which is characterized as rapid, localized plant cell death [29]. In contrast, perception of PAMPs leads to small metabolic changes during PTI so that energy production can be sustained in the presence of PAMPs [32]. It is intriguing that transient expression of the full length flaLso inside plant cells could also induce up-regulation of PAMP-associated gene expression, ROS response and slow necrotic cell death. FLS2 is a receptor that is known to recognize extracellular flagellin. In mammals, PAMPs such as flagellin in the cytosol induce a localized cell-death-associated immune response to pathogens. However, no cytoplasmic nucleotide-binding leucine-rich (NLR) receptor or other cytoplasmic receptor was identified in plants [31]. Most studies on plant FLS2 have focused on interaction with flg22 of flagellin administered extracellularly [27], as would occur with infection by Pseudomonas and many other bacterial pathogens. Candidatus Liberibacter species are strictly intracellular, and therefore, in order to function as a PAMP, their flagellins would require recognition within the plant cell. It has been demonstrated that transgenic expression of the flagellin from a rice-incompatible strain of Acidovorax avenae induced immune responses, expression of defense related genes, production of hydrogen peroxide and cell death in rice plants [33]. In another study, when FLS2-GFP was transgenically expressed, the FLS2 was primarily localized on the cell membrane before binding to flg22. After binding, the FLS2 accumulated into intracellular mobile vesicles and degraded upon flg22 activation [34]. In addition to being localized on cell membranes, FLS2 was also observed in intracellular vesicles of various sizes and shapes in a protoplast assay [35]. All of these together indicate that there is a unique pathway involving the interaction between FLS2 and Lso flagellin inside a plant cell. Further research is necessary to understand this pathway and downstream host response.
PTI gene expression responses induced by Lso flagellin and peptide
As a complement to the observations discussed above, we investigated flg22Lso-induced gene expression following infiltration into N. benthamiana. NbFLS2 and three other PAMP-marker genes, NbCYP71D20, NbACRE31 and NbACRE32, were shown to be rapidly and transiently up-regulated (Figure 6) as was previously reported with the flg22 from Pseudomonas[14],[26]. We also assessed several other genes which are reported to be associated with different host-defense systems. ACRE (Avr9/Cf9 rapidly elicited) genes associated with race-specific defense responses were found to be up-regulated by the infiltration of flg22Lso in N. benthamiana, similar to the result seen when resistant-race tobacco cells (Cf9 genotype) are treated with the fungal elicitor Avr9 [24].
When P. fluorescens was infiltrated into non-host N. benthamiana, the plastocyanin gene was markedly up-regulated, and silencing this gene compromised PTI [11]. Plastocyanin is a small Cu-containing protein which acts as an electron carrier between the cytochrome b6f and photosystem 1 complexes in the photosynthetic electron-transfer chain [11]. Surprisingly, our results showed that the expression of NbPlastocyanin was the most highly induced gene in the PTI response by flg22Lso treatment. However, the up-regulation of NbPlastocyanin expression gradually decreased over several days with Agrobacterium-mediated expression of full length flaLso, which may have resulted from impaired chloroplast function as cell death was slowly induced. It has been reported that there is cross-talk between PAMP-triggered immunity and photosynthesis [32]. Though a typical pattern of PAMP-triggered gene expression was observed with flg22Lso infiltration, no ROS production was detected. This may be explained by the fact that two different signaling pathways were separated after calcium influx initiation: one leading to the ROS burst and the other to MAPKs and other gene activation [14]. It was reported that domains other than flg22 contribute very little to the elicitation of the FLS2-mediated Arabidopsis defense response [22]. However, a second region designated as flgII-28 within flagellin apart from flg22 was recently identified in P. syringae pv. tomato and flgII-28 was shown to induce ROS in N. benthamiana not in Arabidopsis or bean [36]. Furthermore, the allelic variation of flg22 and flgII-28 were reported to affect the plant immune response significantly, and to have no effect on bacterial motility [36]. This may explain our findings that the full flagellin gene, flaLso, not the peptide flg22Lso, has the ability to induce ROS production and slow cell death in tobacco, which suggests other flagellin domains such as flgII-28 or post-translational modifications may also be associated with plant defense response. It is important to point out that transient expression of the flaLso only induced ROS production in tobacco but did not lead to ROS reaction in tested tomato and potato plants. This could be explained by the sequence variations within the FLS2 genes among species/varieties of host plants (Duan unpublished data).
It is established that FLS2 directly binds to flagellin in the Pseudomonas non-host system [27] and then interacts with BAK1 to form the FLS2/BAK1 complex, which activates the downstream signaling such as MAPK pathway. In our experiments, the expression of two MAPKs, NbWIPK and NbSIPK were indeed up-regulated in N. benthamiana by the flaLso (Figure 8). The prolonged activation of a MAPK pathway in cells may cause a redox imbalance and generate ROS, eventually leading to cell death [37]. Since MAP kinases are primarily regulated at protein levels, the levels of these proteins in N. benthamiana after inoculation with flg22Lso and or flaLso need a further investigation.
PAMP-triggered immunity is important for plants to limit pathogen growth or generate signals for adaptation to secondary infections [38]. PAMPs, including flg22, activate components of the salicylic acid and jasmonic acid defense pathways, which protect against potential pathogenic bacteria [39]. The molecular events that occur during PTI and elicitor-triggered immunity (ETI) partially overlap including SA, ROS, and activation of MAPK cascades [40]. However, ETI elicits a much stronger response than PTI, indicating a quantitative difference between these two immunity responses [8]. The discovery that the FlaLso and flg22Lso have PAMP activity and trigger PTI reveals that the flagellin from intracellular bacteria can initiate plant defense responses. The identification of a compatible FLS2 is critical for the development of potato plants with increased resistance against Ca. L. solanacearum via marker-assisted conventional breeding and genetic engineering.
Conclusion
Zebra chip (ZC) is an important potato disease associated with the phloem-limited intracellular bacterium ‘Candidatus Liberibacter solanacearum’ (Lso). In this study, we examined the PAMP activity of the flagellin of Lso and its functional domain, flg22Lsoin planta. We found that flg22Lso has the ability to induce callose deposition and it also triggers transient up-regulation of PTI associated genes in N. benthamiana. We determined that the expression of NbFLS2 and three marker genes, NbCYP71D20, NbACRE31 and NbACRE32, are rapidly upregulated. Surprisingly we found the expression of NbPlastocyanin increased dramatically, rising by 10 fold at 3 hpi. However, neither cell death nor ROS were induced by the flg22Lso. We also determined that expression of the full length flagellin gene induces a much stronger PTI response compared to peptide flg22Lso, especially upregulation of the PAMP-associated genes NbFLS2 and NbRbohB. In addition, the expression of flaLso induced ROS production and necrotic cell death in N. benthamiana via Agrobacterium-mediated transient expression. The discovery that the FlaLso and its short peptide have PAMP activity and the ability to trigger PTI provide new insights into the role of bacterial flagellin in the development of potato zebra chip disease and a potential application in breeding for resistance.
Methods
Plants and bacteria cultivation
Seeds of N. benthamiana and tomato were germinated in chambers with cycles of 16 h light and 8 h dark at 26°C. The seedlings were then transferred into Fafard® 4P Mix soil in plastic containers and grown in greenhouses. Potato cultivar (Atlantic) was planted directly in Fafard® 4P Mix soil in plastic containers and grown in greenhouses.
E. coli was grown at 37°C and A. tumefaciens was grown at 28°C in Luria-Bertani (LB) medium. Kanamycin (kan) was added to the medium at a concentration of 50 μg/mL.
flaLso construction for transient expression in plants
The full length gene of flaLso was amplified using genomic DNA as a template with primers flaLsoF and flaLsoR (5′-AAACCCGGGTGTCATTTCGATTTTTAAGGATA-3′; 5′- AAACCCGGGCTAACCACGGAAAAGAGATAGAATT-3′). Italicized bases are SmaΙ restriction sites included for cloning. The PCR product was ligated into PCR2.1-TOPO vector and transformed chemically into E. coli TOPO10 cells following the manufacture’s instruction (Invitrogen, Carlsbad, CA). The positive clones were used for plasmid isolation and sequence verification. The consensus clone was digested, gel purified and cloned into a binary vector pBINPLUS/ARS-2x35S (pBin) generating pBin:flaLso. The recombinant vector was then transformed into A. tumefaciens GV3101 by a freeze-thaw method [41].
Peptide infiltration and callose deposition assay
The peptides of flg22Lso (DRVSSGLRVADSSDNAAYWSIA) and flg22Las (DRVSSGLRVSDAADNAAYWSIA) were synthesized by Life Tein Company (South Plainfield, NJ). Synthetic peptides were diluted with double distilled water to a final concentration of 40 μM and infiltrated into plant leaves with a 1 mL needleless syringe. At the second day post-infiltration (dpi), callose deposition was detected with aniline staining as described previously [39]. Briefly, the tissue was cleared and dehydrated in 100% ethanol. Cleared leaves were washed with distilled water and then stained overnight at room temperature in 0.01% aniline blue in 67 mM K2HPO4 (pH 12). Stained material was mounted in 50% glycerol and observed under ultraviolet of epifluorescence (Leitz DMR microscope, Leica Microsystems, Buffalo Grove, IL).
Plant infiltration and RNA isolation
A. tumefaciens GV3101 and A. tumefaciens GV3101 containing vector pBin or pBin:flaLso were cultured over night in 2 mL of LB medium with the addition of 50 μg/mL kan. Fifty microliter of the overnight cultures were inoculated into fresh 5 mL LB medium for another 24 hr with the addition of 50 μg/mL kan, 10 mM MES (2-(N-morpholino)-ethanesulfonic acid), and 100 μM acetosyringone. The overnight cultures were centrifuged, washed, and re-suspended in Agromix (10 mM MgCl2, 10 mM MES, and 100 μM acetosyringone). The suspension was adjusted to different OD600 values with Agromix and kept at room temperature for at least 3 hr. The final cell suspension was used to inoculate plant leaves with a 1 ml needleless syringe.
N. benthamiana leaves were infiltrated with flg22Lso at 40 μM and water as a control. As flg22Lso induced rapid and transient PAMP-triggered gene expression [14], the infiltrated zones were harvested at 0.5, 1, 3 and 6 h post-infiltration (hpi) for RNA isolation. For full flaLso transient expression, A. tumefaciens GV3101 containing vector pBin or pBin:flaLso was used to infiltrate N. benthamiana leaves at OD600 ~ 0.5. Similarly the infiltrated zones were harvested at 2, 3, 4 and 8 d post-infiltration (dpi) for RNA isolation because cell death was observed after 8 to 10 dpi. Trizol reagent was used for RNA extraction according to the manufacture’s instructions (Sigma-Aldrich, St. Louis, MO). Total RNA was quantified using the Nanodrop and treated with RQ1 RNase-free DNase from Promega Corp (Madison, WI).
ROS assay and ion-leakage assay
For the flg22Lso and flg22Las peptides, leaf discs of N. benthamiana, tomato and potato were floated on water overnight. Prior to ROS measurement, water was replaced with 100 μl of assay solution (17 mM lumino, 1 μM horseradish peroxidase, flg22Lso or flg22Las at concentrations of 0.1 μM, 1 μM, 10 μM and 40 μM). Luminescence was measured using the Perkin Elmer Victor3 V 1420 Multilabel Plate Counter (Waltham, Massachusetts). The flg22 peptide from P. aeruginosa was used at 0.1 μM as a positive control for the ROS assay.
For the FlaLso assay, A. tumefaciens GV3101 containing vector pBin or pBin:flaLso with an OD600 at 0.5 was used to infiltrate leaves of N. benthamiana, tomato and potato as described above. Leaf discs from infiltrated zones were taken on 2, 3, 4, and 5 dpi. ROS assay was performed as described above.
The ion-leakage assay was performed as described [42]. Briefly six leaf discs (5 mm in diameter) were collected at 2, 3, 4, 5 and 8 d with a sharp cork borer after infiltration with 10 mM MgCl2, A. tumefaciens containing pBin:flaLso or pBin vector alone as a control, and then washed with 10 mL distilled water for 30 min. Then they were transferred to fresh distilled water. Conductance was measured with an OAKTON electrical conductivity meter (Singapore).
Reverse transcription quantitative PCR (RT-qPCR)
DNase-treated RNA (~2 μg) was used to synthesize first-strand cDNA with 0.5 μg of oligo (dT) primer and 1 μL of SuperScript® III reverse transcriptase in a 20 μL reaction (Invitrogen). A negative control without the reverse transcriptase was performed to verify the absence of genomic DNA contamination. RT-qPCR was performed with SYBR in triplicate using an Eppendorf Mastercycler® Realplex thermal cycler. The 15 μL amplification reactions contained the following: 7.5 μL of SYBR® Green PCR Master Mix system (PERFECTA SYBR FASTMX LRX, VWR), 250 nM of each forward and reverse primer, and 2.0 μL of diluted cDNA template. The following protocol was used: 95°C for 5 min, 40 cycles of 30 s for denaturation at 95°C and 30 s for extension at 60°C. Primers for NbFLS2 were designed as 5′-TCAAATGGTGGATGACTGGA-3′ and 5′-ATGATATGCTGCTCCCATCC-3′. The N. benthamiana elongation factor 1 alpha (NbEF1α) was amplified and used to normalize the values as an internal control with primers (5′-GACCACTGAAGTTGGATCTGTTG-3′; 5′-TAGCACCAGTTGGGTCCTTCTT-3′). All other primers were used as previously reported (NbWIPK, NbSIPK, NbRbohB, NbACRE31, NbACRE32 and NbCYP71D20 as in publication [14]; NbPlastocyanin as in [11]; NbBAK1 as in [18] and NbSerk3 as in [26]). For evaluation of transient expression of the flaLso, tissue collection, RNA isolation and cDNA amplification were carried out as described above. RT-PCR and RT-qPCR were performed to detect the flaLso expression levels at various times after infiltration. The primers for flaLso were designed as 5′-TTGCGTGTTGCTGATTCTTC-3′ and 5′-TCTGCCTGAACAGAATGTGC-3′. The expression level was normalized against internal control NbEF1α. Meanwhile, the 2_△△CT method was used to calculate the changes in relative copy number of the target genes under treated conditions, 2_△△CT method was taken [43] where CT is the point at which the fluorescence signal crosses the threshold and △CT = CT (target gene) - CT (internal control) and △△CT = (CT, Target – CT, internal control) Time x - (CT, Target – CT, internal control) Time x.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GH and YPD conceived and designed the experiments. GH and MP performed the experiments. GH, DF and MP analyzed the data. GH, MP and LH contributed reagents/materials/analysis tools. GH, ES and YPD wrote the manuscript. All authors read and approved the final manuscript.
Additional file
Supplementary Material
Electrolyte leakage from leaf discs of N. benthamiana leaves inoculated with 10 mM MgCl2, Agrobacterium tumefaciens strain GV3101 containing the vector control pBin and the pBin:flaLso constructs, respectively. * marked as significant change by student t-test.
Contributor Information
Guixia Hao, Email: guixia.hao@ars.usda.gov.
Marco Pitino, Email: marco.pitino@ars.usda.gov.
Fang Ding, Email: dingfang2008@gmail.com.
Hong Lin, Email: hong.lin@ars.usda.gov.
Ed Stover, Email: ed.stover@ars.usda.gov.
Yongping Duan, Email: yongping.duan@ars.usda.gov.
Acknowledgements
We thank Dr. Cheryl Armstrong for critical review of the manuscript, and Dr. Scott Adkins and Carrie Vanderspool for providing N. benthamiana and tomato plants. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U. S. Department of Agriculture.
References
- Munyaneza JE. Zebra chip disease of potato: biology, epidemiology, and management. Am J Potato Res. 2012;89(5):329–350. doi: 10.1007/s12230-012-9262-3. [DOI] [Google Scholar]
- Sector GA, Rivera VV. Emerging diseases of cultivated potato and their impact on Latin America. Rev Latinoam Papa (Suppl) 2004;1:1–8. [Google Scholar]
- Sector GA, Rivera VV, Abad JA, Lee IM, Clover GRG, Liefting LW, Li X, De Boer SH. Association of ‘Candidatus Liberibacter solanacearum’ with zebra chip disease of potato established by graft and psyllid transmission, electron microscopy, and PCR. Plant Dis. 2009;93(6):574–583. doi: 10.1094/PDIS-93-6-0574. [DOI] [PubMed] [Google Scholar]
- Wen A, Mallik I, Alvarado VY, Pasche JS, Wang X, Li W, Levy L, Scholthof HB, Mirkov TE, Rush CM, Gudmestad NC. Detection, distribution, and genetic variability of ‘Candidatus Liberibacter’ species associated with zebra complex disease of potato in North America. Plant Dis. 2009;93(11):1102–1115. doi: 10.1094/PDIS-93-11-1102. [DOI] [PubMed] [Google Scholar]
- Hansen AK, Trumble JT, Stouthamer R, Paine TD. A new huanglongbing species, ‘Candidatus Liberibacter psyllaurous’ found to infect tomato and potato, is vectored by the psyllid Bactericera cockerelli (Sulc) Appl Environ Microbiol. 2008;74(18):5862–5865. doi: 10.1128/AEM.01268-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan YP, Zhou LJ, Hall D, Li WB, Doddapaneni H, Lin H, Liu L, Vahling CM, Gabriel DW, Williams KP, Dickerman A, Sun Y, Gottwald T. Complete genome sequence of citrus huanglongbing bacterium, ‘Candidatus Liberibacter asiaticus’ obtained through metagenomics. Mol Plant Microbe Interact. 2009;22(8):1011–1120. doi: 10.1094/MPMI-22-8-1011. [DOI] [PubMed] [Google Scholar]
- Lin H, Lou BH, Glynn JM, Doddapaneni H, Civerolo EL, Chen CW, Duan YP, Zhou LJ, Vahling CM. The complete genome sequence of ‘Candidatus Liberibacter solanacearum’, the bacterium associated with potato zebra chip disease. PLoS ONE. 2011;6(4):e19135. doi: 10.1371/journal.pone.0019135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444(7117):323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Nicaise V, Roux M, Zipfel C. Recent advances in PAMP-triggered immunity against bacteria: pattern recognition receptors watch over and raise the alarm. Plant Physiol. 2009;150(4):1638–1647. doi: 10.1104/pp.109.139709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gómez L, Bauer Z, Boller T. Both the extracellular leucine-rich repeat domain and the kinase activity of FLS2 are required for flagellin binding and signaling in Arabidopsis. Plant Cell. 2001;13(5):1155–1163. doi: 10.1105/tpc.13.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy S, Velásquez AC, Ekengre SK, Collmer A, Martin GB. Identification of Nicotiana benthamiana genes involved in pathogen-associated molecular pattern-triggered immunity. Mol Plant Microbe Interact. 2010;23(6):715–726. doi: 10.1094/MPMI-23-6-0715. [DOI] [PubMed] [Google Scholar]
- Robatzek S, Bittel P, Chinchilla D, Köchner P, Felix G, Shiu SH, Boller T. Molecular identification and characterization of the tomato flagellin receptor LeFLS2, an orthologue of Arabidopsis FLS2 exhibiting characteristically different perception specificities. Plant Mol Biol. 2007;64(5):539–547. doi: 10.1007/s11103-007-9173-8. [DOI] [PubMed] [Google Scholar]
- Takai R, Isogai A, Takayama S, Che FS. Analysis of flagellin perception mediated by flg22 receptor OsFLS2 in rice. Mol Plant Microbe Interact. 2008;21(12):1635–1642. doi: 10.1094/MPMI-21-12-1635. [DOI] [PubMed] [Google Scholar]
- Segonzac C, Feike D, Gimenez-Ibanez S, Hann DR, Zipfel C, Rathjen JP. Hierarchy and roles of pathogen-associated molecular pattern-induced responses in Nicotiana benthamiana. Plant Physiol. 2011;156(2):687–699. doi: 10.1104/pp.110.171249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Peck SC, Hirt H, Boller T. Microbial elicitors induce activation and dual phosphorylation of the Arabidopsis thaliana MAPK6. J Biol Chem. 2000;275(11):7521–7526. doi: 10.1074/jbc.275.11.7521. [DOI] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JD, Doke N. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell. 2003;15(3):706–718. doi: 10.1105/tpc.008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan J, Pichereaux C, Rossignol M, Blanc S, Wendehenne D, Pugin A, Bourque S. Activation of a nuclear-localized SIPK in tobacco cells challenged by cryptogein, an elicitor of plant defense reactions. Biochem J. 2009;418(1):191–200. doi: 10.1042/BJ20081465. [DOI] [PubMed] [Google Scholar]
- Zou HS, Gowda S, Zhou LJ, Hajeri S, Chen GY, Duan YP. The destructive citrus pathogen, ‘Candidatus Liberibacter asiaticus’ encodes a functional flagellin characteristic of a pathogen-associated molecular pattern. PLoS ONE. 2012;7:e46447. doi: 10.1371/journal.pone.0046447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18(3):265–276. doi: 10.1046/j.1365-313X.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Bastianel C, Garnier-Semancik M, Renaudin J, Bové JM, Eveillard S. Diversity of “Candidatus Liberibacter asiaticus”, based on the omp gene sequence. Appl Environ Microbiol. 2005;71(11):6473–6478. doi: 10.1128/AEM.71.11.6473-6478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XY, Lin HQ, Zhang WG, Zou Y, Zhang J, Tang XY, Zhou JM. Flagellin induces innate immunity in nonhost interactions that is suppressed by Pseudomonas syringae effectors. Proc Natl Acad Sci U S A. 2005;102(36):12990–12995. doi: 10.1073/pnas.0502425102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Dunning FM, Pfund C, Weingarten R, Bent AF. Within-species flagellin polymorphism in Xanthomonas campestris pv. campestris and its impact on elicitation of Arabidopsis FLAGELLIN SENSING2-dependent defenses. Plant Cell. 2006;18(3):764–779. doi: 10.1105/tpc.105.037648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito K, Taguchi F, Suzuki T, Inagaki Y, Toyoda K, Shiraishi T, Ichinose Y. Amino acid sequence of bacterial microbe-associated molecular pattern flg22 is required for virulence. Mol Plant Microbe Interact. 2008;21(9):1165–1174. doi: 10.1094/MPMI-21-9-1165. [DOI] [PubMed] [Google Scholar]
- Navarro L, Zipfel C, Rowland O, Keller I, Robatzek S, Boller T, Jones JD. The transcriptional innate immune response to flg22: interplay and overlap with Avr gene-dependent defense responses and bacterial pathogenesis. Plant Physiol. 2004;135(2):1113–1128. doi: 10.1104/pp.103.036749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JD, Felix G, Boller T. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 2004;428(6948):764–767. doi: 10.1038/nature02485. [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci U S A. 2007;104(29):12217–12222. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. doi: 10.1146/annurev.arplant.57.032905.105346. [DOI] [PubMed] [Google Scholar]
- Achor DS, Etxeberria E, Wang N, Folimonova SY, Chung KR, Albrigo LG. Citrus affected with huanglongbing disease. Plant Pathol J. 2010;9:56–64. doi: 10.3923/ppj.2010.56.64. [DOI] [Google Scholar]
- Alfano JR, Collmer A. Type III secretion system effector proteins: double agents in bacterial disease and plant defense. Annu Rev Phytopathol. 2004;42:385–414. doi: 10.1146/annurev.phyto.42.040103.110731. [DOI] [PubMed] [Google Scholar]
- Wei HL, Chakravarthy S, Worley JN, Collmer A: Consequences of flagellin export through the type III secretion system ofPseudomonas syringaereveal a major difference in the innate immune systems of mammals and the model plantNicotiana benthamiana.Cell Microbiol 2012, doi:10.1111/cmi.12059. [DOI] [PubMed]
- Munyaneza JE, Aguilar E, Bextine B, McCue KF. First Report of “Candidatus Liberibacter solanacearum” associated with psyllid-infested tobacco in Nicaragua. Plant Dis. 2013;97(9):1244. doi: 10.1094/PDIS-03-13-0247-PDN. [DOI] [PubMed] [Google Scholar]
- Göhre V, Jones AME, Sklenář J, Robatzek S, Weber PM. Molecular crosstalk between PAMP-triggered immunity and photosynthesis. Mol Plant Microbe Interact. 2012;25(8):1083–1092. doi: 10.1094/MPMI-11-11-0301. [DOI] [PubMed] [Google Scholar]
- Takakura Y, Che F, Ishida Y, Tsutsumi F, Kurotani K, Usami S, Isogai A, Imaseki H. Expression of a bacterial flagellin gene triggers plant immune responses and confers disease resistance in transgenic rice plants. Mol Plant Pathol. 2008;9(4):525–529. doi: 10.1111/j.1364-3703.2008.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S, Chinchilla D, Boller T. Ligand –induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev. 2006;20(5):537–542. doi: 10.1101/gad.366506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali GS, Prasad KVSK, Day I, Reddy ASN. Ligand-dependent reduction in the membrane mobility of flagellin sensing2, an Arabidopsis receptor-like kinase. Plant Cell Physiol. 2007;48(11):1601–1611. doi: 10.1093/pcp/pcm132. [DOI] [PubMed] [Google Scholar]
- Cai R, Lewis J, Yan S, Liu H, Clarke CR, Campanile F, Almeida NF, Studholme DJ, Lindeberg M, Schneider D, Zaccardelli M, Setubal JC, Morales-Lizcano NP, Bernal A, Coaker G, Baker C, Bender CL, Leman S, Vinatzer BA. The plant pathogen Pseudomonas syringae pv. tomato is genetically monomorphic and under strong selection to evade tomato immunity. PLoS Pathog. 2011;7(8):e1002130. doi: 10.1371/journal.ppat.1002130. doi:10.1371/journal.ppat.1002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Yang H, Zhang S. Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis. J Biol Chem. 2002;277(1):559–565. doi: 10.1074/jbc.M109495200. [DOI] [PubMed] [Google Scholar]
- Coll NS, Epple P, Dangl JL. Programmed cell death in the plant immune system. Cell Death Differ. 2011;18(8):1247–1256. doi: 10.1038/cdd.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JDG, Felix G, Boller T. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defense. Nature. 2007;448(7152):497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- Denoux C, Galletti R, Mammarella N, Gopalan S, Werck D, De Lorenzo G, Ferrari S, Ausubel FM, Dewdney J. Activation of defense response pathways by OGs and flg22 elicitors in Arabidopsis seedlings. Mol Plant. 2008;1(3):423–445. doi: 10.1093/mp/ssn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Nelson RS, Sherwood JL. Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze-thaw transformation and drug selection. Biotechniques. 1994;16(4):664–670. [PubMed] [Google Scholar]
- Choi DS, Hwang BK. Proteomics and functional analyses of pepper abscisic acid–responsive 1 (ABR1), which is involved in cell death and defense signaling. Plant Cell. 2011;23:823–842. doi: 10.1105/tpc.110.082081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2_△△CT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electrolyte leakage from leaf discs of N. benthamiana leaves inoculated with 10 mM MgCl2, Agrobacterium tumefaciens strain GV3101 containing the vector control pBin and the pBin:flaLso constructs, respectively. * marked as significant change by student t-test.


