Abstract
The detailed cytomorphologic appearance of circulating tumor cells (CTCs) in cancer patients is not well described, despite publication of multiple methods for enumerating these cells. In this case study, we present the cytomorphology of CTCs obtained from the blood of a woman with stage IIIB well-differentiated lung adenocarcinoma. Four years after she was diagnosed with her disease, 67 CTCs were identified in a blood sample using an immunofluorescent staining protocol and then subsequently stained with Wright-Giemsa. The cytomorphology of the CTCs was compared with the original tissue biopsy from 4 years prior. We found that CTCs and cells from the original biopsy had strikingly similar morphologic features, including large size in comparison to white blood cells and low nuclear to cytoplasmic ratios with voluminous cytoplasm. Careful cytomorphologic evaluation of CTCs will provide insights about the metastatic significance of these cells, which could yield widespread implications for the diagnosis, treatment, and management of cancer.
Most cancer-related deaths are caused by metastases. Circulating tumor cells (CTCs) that spread via the bloodstream to distant sites are presumed to be the cause of these lethal tumors. Circulating tumor cell detection and characterization could provide a valuable tool for stratifying cancer patients and aiding with individualized treatment strategies. Recent publications have described the prognostic significance of the number and/or change in number of detectable CTCs for patient outcome and response to therapy.1-4 Further, a recent article used CTCs to identify genetic alterations in tumor cells that impact therapy decisions.5 As our ability to find, quantitate, and qualitatively evaluate carcinoma cells within the blood-stream evolves, potential opportunities arise to impact the behavior of these cells while they are still en route to the metastatic site and thus to potentially alter patient outcome. Understanding the nature of this population of cells is essential.
Non–small cell lung cancer patients have a poor prognosis and most stage III and stage IV patients die within 2 years.6,7 Circulating tumor cells have been detected and enumerated in non–small cell lung cancer patients,5,8,9 and there is a growing body of literature on the potential utility of CTC detection in the clinic. However, most current technologies for identifying and enumerating carcinoma cells in the bloodstream do not allow for preservation of cytologic detail; thus, published cytomorphologic descriptions and images of tumor cells retrieved from the blood are scant. To our knowledge, there are no existing detailed cytomorphologic descriptions of lung cancer CTCs. The role of CTC cytomorphology in the evaluation of non-small cell lung cancer patients has received increased attention recently as 2 new agents, bevacizumab and pemetrexed, have received US Food and Drug Administration approvals for only those patients with nonsquamous histology.
In this case study we report on a nonsmoking 47-year-old woman who was diagnosed with stage IIIB non-small cell lung cancer in 2003. She was treated with chemora-diation and consolidative docetaxel, but the cancer recurred locally in January 2005, and the patient subsequently received multiple agents secondary to progression. In 2007, 67 CTCs were identified using an immunofluores-cent staining protocol and fiber-optic array scanning technology (FAST), then subsequently stained with Wright-Giemsa. Using this gallery of fluorescent and Wright-Giem-sa-stained CTCs isolated from the blood of this patient, and comparing the cells with those from the patient's archived primary tumor histology and cytology material, we evaluated the cytomorphology of her CTCs and compared them with cells found in her original primary tumor.
Materials and Methods
Isolation of CTCs
The immunofluorescent staining protocol used to isolate CTCs is described in detail elsewhere.10 Briefly, 10 ml of blood was collected in EDTA anticoagulated tubes and processed within 8 hours. Red blood cells were lysed with an ammonium chloride solution (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.4). The remaining nucleated cells were resuspended in phosphate buffered saline and plated as a monolayer on custom-made glass slides (Marienfeld, Lauda-Konigshofen, Germany) that hold 25 million cells. Cells were fixed with 2% paraformaldehyde for 20 minutes, washed in phosphate buffered saline, permeabilized with cold methanol for 5 minutes, and washed again with phosphate buffered saline. Cells were then incubated with 10% goat serum as a blocking reagent for 20 minutes. Subsequently cells were incubated with an epithelial cell-specific antibody, pancytokeratin (Sigma, St Louis, Missouri), and a preconjugated leukocyte-specific CD45-Alexa 647 (AbD Serotec, Raleigh, North Carolina) antibody for 40 minutes. Cells were then incubated with secondary antibody Alexa 555 (Invitrogen, Carlsbad, California) for 20 minutes. After another phosphate buffered saline wash, cells were incubated with 4′,6-diamidino-2-phenylindole (Invitrogen) for 10 minutes in the dark. Finally, slides were mounted with an aqueous mounting media.
Detection of CTCs
FAST was then used as described in previous publications to identify fluorescently labeled CTCs.10,11 FAST has been used to detect rare cytokeratin-positive cells with a sensitivity of 98% and a specificity of 99.99%, evaluating a background of 25 million total cells in 2 minutes. During a scan, the locations of fluorescent objects are recorded, enabling the ability to relocate CTCs in a fluorescent microscope for additional viewing or analysis. After FAST scanning, automated digital microscopy was used to take 60× fluorescent images of all FAST identified hits. Sixty-seven CTCs were identified, each qualifying as a CTC by demonstrating cytokeratin positivity, CD45 negativity, and a 4′,6-diamidino-2-phenylindole–positive nucleus.
Relocation and Characterization Using Wright-Giemsa Stain
After initial identification of CTCs via fluorescent images, subsequent Wright-Giemsa staining of these cells was performed. Coordinates obtained by FAST were used to relocate each Wright-Giemsa-stained cell using standard bright-field microscopy, and the cells were cytomorphologically evaluated.
Results
Cytomorphologic Features of CTCs by Fluorescence and Wright-Giemsa
Circulating tumor cells were identified in fluorescent images as cytokeratin positive, CD45 negative, and having a 4′,6-diamidino-2-phenylindole–positive nucleus. A representative gallery of CTCs identified is shown in Figure 1, A through F. Often, they were much larger than surrounding white blood cells (Figure 1, A and B). They demonstrated low to moderate nuclear to cytoplasmic (N:C) ratios and a generous cytoplasmic domain (Figure 1, A and B). Polygonal-shaped cells were common (Figure 1, C and D), and cell groups often appeared in clusters (Figure 1, C, E, and F).
Figure 1.
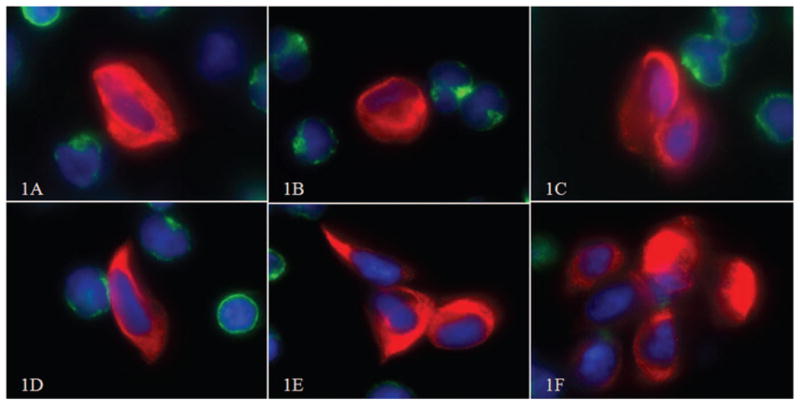
Typical circulating tumor cells (CTCs) identified in the blood of our patient. A, B, and D show single CTCs, and C, E, and F show clusters of CTCs (red, cytokeratin; blue, 4′,6-diamidino-2-phenylindole; green, CD45; original magnifications ×60).
Fluorescent CTCs (Figure 2, A and C) were restained with Wright-Giemsa (Figure 2, B and D) and revealed additional cytologic detail. The generous cytoplasm is seen clearly appears irregularly textured, and in some cells demonstrates a somewhat polygonal external cytoplasmic contour. The chromatin is variable, appearing condensed and hyperchromatic in some cells, whereas others show finer chromatin with variably prominent nucleoli. Fine irregularities of the nuclear membrane can be appreciated in some cells (Figure 2, D).
Figure 2.
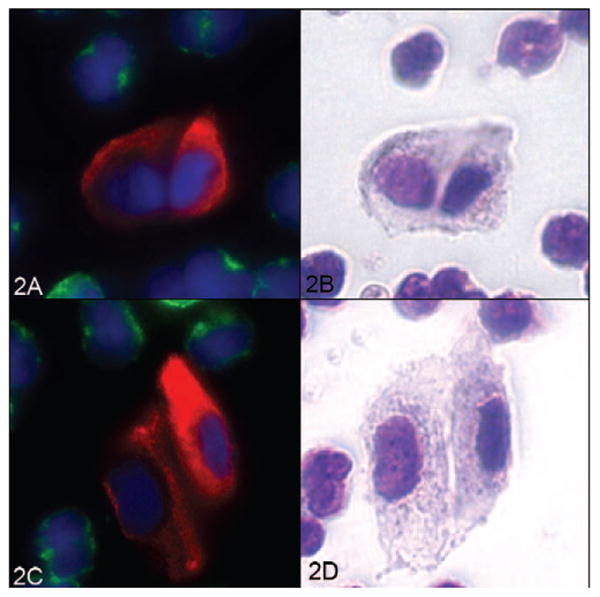
Two circulating tumor cell (CTC) clusters displayed in fluorescence and in the corresponding Wright-Giemsa stain. Left, Fluorescent CTCs (A and C). Right, The same cells after Wright-Giemsa staining (B and D) (red, cytokeratin; green, CD45; blue, 4′,6-diamidino-2-phenylindole; original magnifications ×60).
Features of the Primary Tumor
The histologic sections from the 2003 needle biopsy of the lung primary showed infiltrating adenocarcinoma forming abnormally shaped glandular structures composed of columnar cells with moderate to low N:C ratios (Figure 3, A). No areas of solid sheets or single cell infiltrates were identified. The cells focally demonstrated a li-pidic growth pattern, and the tumor was characterized by the original pathologist as well-differentiated adenocarcinoma with bronchoalveolar features (Figure 3, A). The tumor cells were cytokeratin 7 and thyroid transcription factor 1 positive by immunostain (Figure 3, B and C). The Diff-Quick-stained cytologic touch preparation from the needle biopsy of the lung fine-needle aspirate showed large cells with a generous cytoplasmic domain, cells with lobated nuclei, and plasmacytoid cells with eccentrically located nuclei and variably prominent nucleoli (Figure 3, D).
Figure 3.

Original diagnostic lung biopsy from 2003. A, Core needle biopsy of the lung (hematoxylin-eosin, original magnification ×40). B, Cytokeratin 7 positive (original magnification ×40). C, Thyroid transcription factor 7 positive (original magnification ×40). D, Cytology from touch prep of the core needle biopsy (Diff-Quick stain, original magnification × 100 oil).
Cytomorphologic Comparison of CTCs to the Primary Tumor
Fluorescent and Wright–Giemsa-stained CTCs (Figure 4, A and B) showed cytologic features similar to those of the patient's original lung biopsy from 4 years prior (Figure 4, C). The features retained include large size, with the cancer cells being 2 to 4 times the size of neighboring white cells. As well, both sets of cells showed relatively low N:C ratios for malignant cells, with voluminous cytoplasm. The nuclei from both sites are round to oval, with occasional cells such as the ones in Figure 4 showing nuclear notching or lobation. A visible nucleolus is present in both the CTCs and the cells shown from the touch preparation of the original needle biopsy from 4 years prior.
Figure 4.

A, Fluorescently labeled circulating tumor cells (red, cytokeratin; green, CD45; blue, 4′, 6-diamidino-2-phenylindole; original magnification ×60, digitally enlarged). B, Same circulating tumor cell, re-stained with Wright-Giemsa (original magnification ×100 oil). C, A similar cell from the original biopsy 4 years prior with arrow pointing to lobated nucleus (Diff-Quick, original magnification ×100 oil).
Conclusions
In this case study of a representative selection of CTCs from a patient with a well-differentiated lung adenocarcinoma, we demonstrate that many of this patient's individual CTCs are cytomorphologically similar to cells from her primary tumor, biopsied several years prior. It has been theorized that only specific subsets of tissue tumor cells, for example, tumor stem cells, survive in the bloodstream. Furthermore, just as leukemic blast cells have a morphologic appearance distinct from their maturing progeny, one might expect tumor stem cells to have a morphologic appearance distinct from most cells composing a tumor mass. However, this evaluation demonstrates that cells similar in appearance to those of the primary tumor mass are at least present in the bloodstream relatively late in the course of this patient's metastatic cancer. Thus, there is no morphologic evidence, in this single sample in time, that the patient's circulating cells represent a morphologically distinct subset of highly metastable stem cells; rather, they appear to replicate the population of cells present in the primary tumor. Whether this observed population of daughter cells that so resemble the parent tumor cell population has the capacity to survive and implant elsewhere is unknown; it is possible these cells merely represent passively shed cells rather than actively metastasizing cells.12
Other methodologies are available that are useful for quantitation and molecular evaluation of CTCs; however, these methods generally incur marked disruption of cytologic detail. Our methodology allows us to retain cytologic features and thus produce these detailed images, which are among the first detailed cytomorphologic images of lung cancer cells in circulation. The adenocarcinoma in this case was well differentiated at the time of diagnosis, showing cells with only moderate nuclear enlargement and voluminous cytoplasm, yielding a relatively low N:C ratio. Based on the cytology of the CTCs years later, the tumor cells in circulation appear to have remained well differentiated without evidence of dedifferentiation over time.
We have previously characterized adenocarcinoma cells from other tumor types, both of which were less well differentiated than this case; specifically, moderately to poorly differentiated breast cancer13 and colon cancer. The striking cytomorphologic difference between the CTCs in this patient with a well-differentiated lung adenocarcinoma, versus previously studied patients with less well-differentiated adenocarcinomas of breast and colon, suggests that cytomorphologic features of primary tumors may be retained when cells enter the bloodstream. For example, a case study of circulating breast cancer cells demonstrated a preponderance of round, high N:C ratio cells, with an even circumferential distribution of cytoplasm. The primary tumor in that case was a moderately differentiated breast adenocarcinoma, composed of predominantly cu-boidal cells with scant cytoplasm, growing in ill-formed glands. That a cell with a cuboidal appearance in tissue should assume a round appearance with evenly distributed circumferential cytoplasm, when separated from its neighbors, replicates the cytopathologic features of such cells in other body fluid compartments, such as effusions. Interestingly, we have observed that cells from a colonic adenocarcinoma, which in the primary tumor tissue were very columnar and elongated, appeared in the peripheral blood as elongated ovals, with marked eccentricity of cytoplasm that concentrated at one end of the CTC (K. B., D. M., unpublished data, December 2008). Thus, although blood is a very different tissue compartment from a pleural effusion or ascites, it may be that, from a morphologic perspective, tumor cells behave in blood much as they do in other body fluids.
In summary, there is little cytomorphologic knowledge about CTCs in patients with cancer. Although pathologists are intimately familiar with the appearance of tumor cells in their primary site, in their metastatic sites, and in various body fluids such as malignant effusions, there is scant literature and no atlas on the cytomorphologic appearance of these cells during their travel through the bloodstream. They are technically challenging to find during this critical and likely brief phase of their life cycle, and because finding and counting them often alters them significantly, they are even more challenging to then characterize. As the CTC field advances, we are beginning to investigate the composition of the CTC population, their mechanisms of entry into and departure from the bloodstream, the metastatic potential of various subsets of CTCs, and most importantly their significance for patients with early- and late-stage cancers. As a foundation for these advances, a careful cytomorphologic evaluation of CTCs from various cancer types will provide a backdrop against which to conduct the genetic and functional studies of these cells.
Acknowledgments
This work was supported by grant 5R01CA125653-02 from the National Cancer Institute, National Institutes of Health, Bethesda, Maryland. This is TSRI manuscript number 19826.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
Contributor Information
Miss Dena Marrinucci, Department of Cell Biology, The Scripps Research Institute.
Dr Kelly Bethel, Department of Pathology, Scripps Clinic La Jolla, California.
Miss Madelyn Luttgen, Department of Cell Biology, The Scripps Research Institute.
Dr Richard H. Bruce, Department for Biotechnology, The Palo Alto Research Center, Palo Alto, California.
Dr Jorge Nieva, Department of Oncology and Hematology, Billings Clinic, North, Billings, Montana.
Dr Peter Kuhn, Department of Cell Biology, The Scripps Research Institute.
References
- 1.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 2.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 3.Sastre J, Maestro ML, Puente J, et al. Circulating tumor cells in colorecta cancer: correlation with clinical and pathological variables. Ann Oncol. 2008;19:935–938. doi: 10.1093/annonc/mdm583. [DOI] [PubMed] [Google Scholar]
- 4.Pachmann K, Camara O, Kavallaris A, et al. Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer al-lows detection of patients at risk of early relapse. J Clin Oncol. 2008;26:1208–1215. doi: 10.1200/JCO.2007.13.6523. [DOI] [PubMed] [Google Scholar]
- 5.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359(4):366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belani CP, Ramalingam S, Perry MC, et al. Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:468–473. doi: 10.1200/JCO.2007.13.1912. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 8.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with non-malignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 9.Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh HB, Marrinucci D, Bethel K, et al. High speed detection of circulating tumor cells. Biosens Bioelectron. 2006;21:1893–1899. doi: 10.1016/j.bios.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 11.Krivacic RT, Ladanyi A, Curry DN, et al. A rare-cell detector for cancer. Proc Natl Acad Sci U S A. 2004;101:10501–10504. doi: 10.1073/pnas.0404036101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bockhorn M, Jain RK, Munn LL. Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007;8:444–448. doi: 10.1016/S1470-2045(07)70140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrinucci D, Bethel K, Bruce RH, et al. Case study of the morphologic variation of circulating tumor cells. Hum Pathol. 2007;38:514–519. doi: 10.1016/j.humpath.2006.08.027. [DOI] [PubMed] [Google Scholar]


