Abstract
Objective
To present a case of a pediatric cervicofacial necrotizing fasciitis (NF), a rapidly progressive infection, and a review of a 10-year pediatric inpatient database.
Design
Case report and review.
Setting
Pediatric intensive care unit.
Patients
A healthy 5-year-old male who developed NF of the lower lip 36 hours following minor trauma. International Classification of Diseases, Ninth Revision, code 728.86 (NF), was the inclusion criteria for the Kids’ Inpatient Database (KID) in 1997 and 2006.
Results
A pediatric case is presented with a thorough photographic record demonstrating the need for rapid diagnosis and treatment. In a review of the KID from 1997 and 2006, the relative risk of being discharged with NF in 2006 vs 1997 was 1.4 (95% CI, 9.95-2.28). Age at diagnosis of NF was older in 2006 compared with 1997 (11.5 years vs 8.05 years; P<.001). Deaths with a diagnosis of NF increased from 1997 compared with 2006: from 3.9% to 5.4%. In 2006, the odds of death were 15.1 times higher in pediatric discharges with a diagnosis of NF compared with discharges without a diagnosis of NF (P<.001; 95% CI, 9.3-23.1).
Conclusions
Even with the advent of new treatments and antibiotics, the incidence and death rates of NF have changed little over the past 10 years. While it is still a rare diagnosis, knowledge and awareness of necrotizing fasciitis with aggressive medical and surgical treatment are still the foundation in disease survival.
Necrotizing fasciitis (NF) is a rapidly progressive, fulminant infection of the superficial fascia and subcutaneous tissue that rarely occurs in the head and neck. Early identification and aggressive treatment are critical to the preservation of life. A very high index of suspicion is needed for timely diagnosis. We describe our recent experience with cervicofacial NF in a pediatric patient to raise awareness of this devastating disease process.
METHODS
A case report of a pediatric patient with cervicofacial NF following minor trauma is presented. In addition, inpatient admissions for pediatric patients with a diagnosis of NF in 1997 and 2006 were examined using the Kids’ Inpatient Database (KID), Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality.1 The KID is an all-payer, inpatient care database for children in the United States, containing data from 2 to 3 million hospital discharges for children. International Classification of Disease, Ninth Revision, code 728.86, was used as the inclusion criteria. The database was queried for the years 1997 and 2006 to assess the incidence of NF over this 10-year period, and the relative risk and odds ratio for a discharge diagnosis of NF was calculated.
A previously healthy 5-year-old boy fell off his bike, skinned his chin, and bit his right lower lip. Twenty-four hours later he had mild swelling of the chin and lower lip, nausea, emesis, fever, and abdominal pain. The following morning he was seen at a local emergency department where he was noted to have a temperature of 101.6° F, with a blood pressure of 90/49 mm Hg, and heart rate of 138 bpm. He was then transferred to our institution because of concern for septic shock.
He was admitted to the pediatric intensive care unit. At the time of initial evaluation, he was not experiencing airway distress. Marked tense edema of the right face, cheek, and neck was seen, with poorly demarcated erythema. There was exquisite tenderness of the neck and anesthesia of the cheek. Minimal breakdown of skin lateral to the right lower lip was noted (Figure 1 and Figure 2). Results from laboratory tests demonstrated leukocytosis with leftward shift and a C-reactive protein level of 20 mg/dL. A computed tomographic scan was obtained and showed diffuse subcutaneous thickening and edema. Fat stranding was present along fascial planes without a distinct fluid collection (Figure 3).
Figure 1.
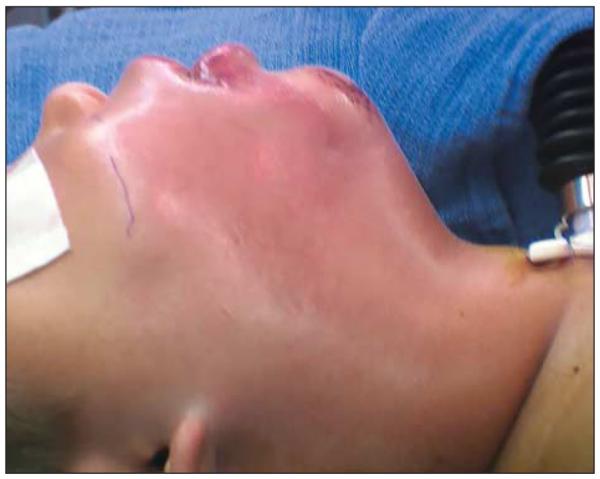
Tense edema prior to initial debridement.
Figure 2.
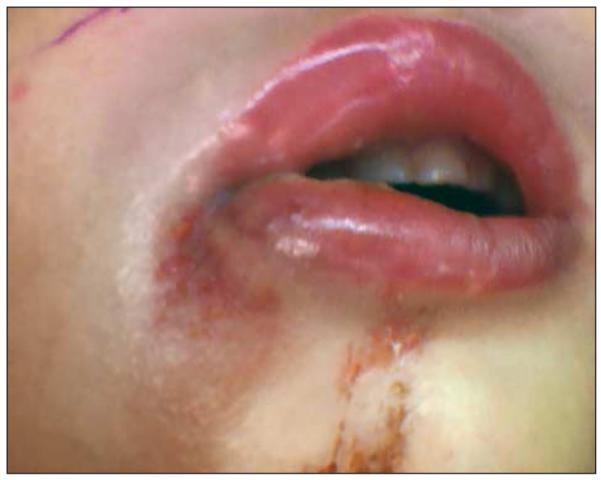
Erosion of the oral commissure.
Figure 3.
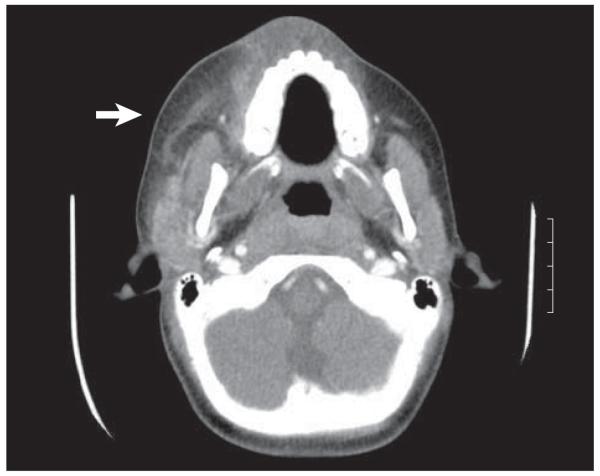
A computed tomographic image of the neck with contrast demonstrating increased soft-tissue edema with no discrete group A β-hemolytic streptococcus collection or abscess. The arrow indicates the soft-tissue edema seen with the infection. It should be noted that no air is seen in this case, but can be seen in other cases of necrotizing fasciitis.
A presumptive diagnosis of NF was made, and the patient was taken emergently to the operating room for debridement. A tracheotomy was placed, and the lip was debrided with jet irrigation until bleeding tissue was encountered. Submandibular and cervical fasciotomies were performed. He subsequently developed progressive necrosis of the right oral commissure, lower lip, and upper lip requiring a total of 5 operative debridements over 3 successive days. The final defect comprised one-half of the lower lip, one-fourth of the upper lip, the medial cheek, and mental skin (Figure 4). In addition, he underwent broad cervical fasciotomy.
Figure 4.
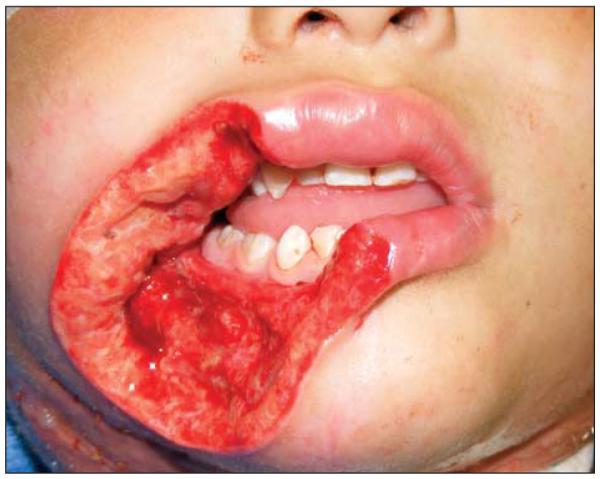
The defect following final debridement.
Owing to his progression of disease and sepsis, he was treated with broad spectrum antibiotics (Unasyn [ampicillin and sulbactam], clindamycin phosphate, vancomycin, and cefepime) and pressors. Intraoperative cultures grew group A β-hemolytic streptococcus (GAS); antibiotic coverage was then narrowed to Unasyn and clindamycin, which he received for a total of 3 weeks.
Hyperbaric oxygen (HBO) therapy was used adjunctively for a total of 7 treatments. The patient was successfully decannulated prior to hospital discharge. He underwent reconstruction with primary closure and local tissue rearrangement and to date is tolerating a normal diet without oral incompetence and has unimpaired speech (Figure 5). Further scar revision and commisuroplasty is planned in the future.
Figure 5.
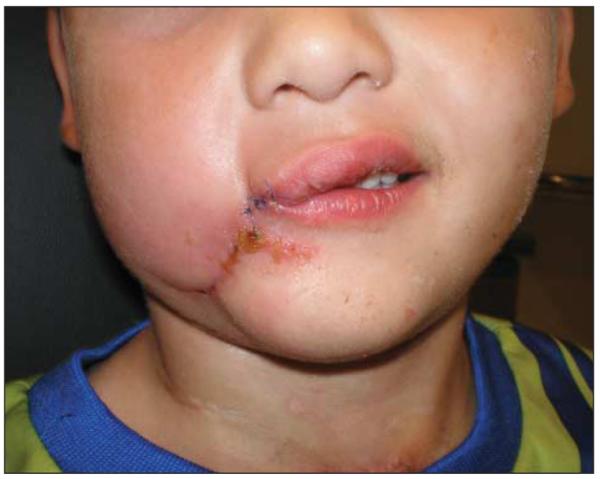
The patient following primary reconstruction.
RESULTS
The study examined inpatient admissions for pediatric patients with a diagnosis of NF in 1997 and 2006 using the HCUP KID.1 In a review of the KID from 1997 to 2006, the weighted relative risk of being discharged with NF in 2006 vs 1997 was 1.4 (95% CI, 0.95-2.28). Hospital deaths in children carrying a diagnosis of NF increased from 1997 to 2006 from 3.9% to 5.4%. In 2006, the odds of death were 15.1 times higher in pediatric discharges with a diagnosis of NF compared with discharges without a diagnosis of NF (P<.001; 95% CI, 9.3-23.1). Age at diagnosis of NF was older in 2006 than in 1997 (11.5 years vs 8.05 years; P<.001).
COMMENT
Necrotizing fasciitis is rare in children. In children, NF is usually monomicrobial, most commonly due to GAS.2,3 This infection is estimated to occur at a incidence of 0.08 per 100 000 hospitalized children.4 Infection may follow major and minor trauma, varicella lesions, intramuscular injections, and surgical wounds, and typically affects previously healthy children.3,5 In adults, NF occurs more often in those with diabetes mellitus, immunosuppression, peripheral vascular disease, chronic renal failure, and a history of intravenous drug abuse or alcoholism, and is more likely to result in polymicrobial infections, including anaerobic bacteria.2,6,7 Necrotizing fasciitis is most commonly seen in the abdomen, perineum, and extremities; head and neck involvement is rare.2 In a study of 128 adults with NF, the head and neck involvement occurred in only 3.9% of patients.8 The origin of cervicofacial NF in adults is most likely to be dental.7
Recent literature suggests that the incidence of pediatric NF may be increasing.8 Laupland et al4 found that the rate of invasive GAS infections in Ontario, Canada, increased from a median of 3 cases per year in the period 1985 to 1991 to a mean of 7.5 cases per year in the period 1992 to 1995 (P=.03). The current study used data from the HCUP KID and found that the relative risk of diagnosis of NF at discharge in 2006 vs 1997 was 1.40.
Necrotizing fasciitis is associated with a high mortality rate, with estimates in the literature ranging from 38% to 50% in adults7 and approximately 25% in children.5 Cervical infection can be particularly devastating owing to mediastinal extension, which is associated with a high mortality rate.3,7 In a study by Mathieu et al,7 10 of 45 patients (22%) died. Causes of death were multiple organ failure and septic shock in 4 patients each, and post-anoxic encephalopathy following upper airway obstruction and cardiac arrest in 2 patients.7 They found that risk factors for death included advanced age, diabetes mellitus, development of septic shock within 1 day of admission, prolonged prothrombin time, and mediastinal extension.7 In the present study, we found that the number of hospital deaths in children with a diagnosis of NF was 5.4% in 2006, an increase of 3.9% from 1997. Furthermore, the mean age for pediatric admission in 1997 for NF was 8.05 years, which was notably higher in 2006 at 11.5 years. This increase in hospital death and mean age at admission may also be attributed to the fact that the HCUP KID in 2006 included 38 hospitals with patients 20 years or younger vs the 1997 HCUP KID, which had 22 hospitals with patients 18 years or younger. In 2006, the odds of death were 15.1 times higher in pediatric discharges with a diagnosis of NF compared with discharges without a diagnosis of NF (P<.001; 95% CI, 9.3-23.1). To our knowledge, the death rate observed in the present review of the KID database is markedly lower than previously published case series. This likely reflects application of the NF diagnostic code to children less sick than those typically described in published case series. Another limitation of the database is the inability to select for patients with NF in the cervical region alone. The database includes patients with NF irrespective of anatomic location.
Early identification and diagnosis is critical; unfortunately, the presentation of NF overlaps significantly with less serious infections, such as cellulitis. The infection initially spreads along fascial planes, with minimal involvement of the overlying skin; the plane commonly involved in cervicofacial NF is the superficial muscular apo-neurotic system.6 The most common initial skin manifestation is marked tissue edema, found in 100% of children with NF in 1 study; a peau d’orange appearance may also be present.5 Factors that are suggestive of NF, as opposed to cellulitis, include pain that is out of proportion to the findings on physical examination, rapid spread of edema, and bullae formation.3 The skin may appear smooth, shiny, and without well-demarcated erythema. Anesthesia of overlying skin is also suggestive.3 The patient generally appears to be experiencing toxic effects with altered sensorium, fever, hypotension, and tachycardia.3,5 As the subcutaneous tissues undergo necrosis, “dishwater pus” is seen on incision and drainage.2,6 Frank skin necrosis, crepitus, anesthesia, and ecchymosis are late findings.5
Imaging can be useful in identifying NF. Plain films may demonstrate gas within tissue in anaerobic infections; however, this is of limited use in pediatric patients, in whom aerobic infections predominate. Contrast-enhanced CT is particularly useful in identifying cervical disease and to delineate spread of infection. Becker et al9 identified diagnostic features found in a series of patients with head and neck NF; these included thickening of the skin and subcutis, reticular enhancement of the subcutaneous fat, thickening of the fascia, asymmetric thickening or enhancement of muscles, and fluid collections in multiple neck spaces. Less consistently, gas collections may be found within the neck and with involvement of the mediastinum. The initial CT for the study patient was consistent with these findings, with thickening of the superficial tissues with diffuse fat stranding.
Early surgical debridement is the mainstay of treatment. Moss et al5 found that in 20 patients with NF, the 5 patients who died all had inadequate initial surgical treatment, whereas the survivors had undergone aggressive surgical debridement within 3 hours of admission. Freischlag et al10 found that mortality doubled when surgical intervention was delayed by more than 24 hours. The goal of debridement is to remove all necrotic tissue, thus controlling progression and reducing the bacterial load and release of exotoxins.3 At surgery, subcutaneous tissues are noted to be gray, nonbleeding, with a watery discharge.5 Normally adherent fascia is dissected bluntly with a characteristic lack of resistance.2 Debridement continues until bleeding tissue is encountered.5 Some authors recommend mandatory reevaluation in the operating room within 24 hours to ensure adequate debridement.2 A mean of 3.8 surgical debridements were required for each patient in a study by Moss et al.5
Requisite medical therapy includes empirical antibiotic treatment to cover gram-positive, gram-negative, and anaerobic bacteria until cultures can be obtained. Several authors recommend a regimen of penicillin or a cephalosporin, in conjunction with an aminoglycoside and clindamycin or metronidazole for anaerobic coverage.2,5 Hyperbaric oxygen is an adjunctive measure. Physiologic effects of HBO have been shown to include increased killing ability of leukocytes, reduction of tissue edema, neoangiogenesis, and killing of anaerobic bacteria.2 Green et al2 reviewed the available literature regarding HBO in necrotizing infection and found that there is a trend toward improved survival in a few small retrospective studies. However, no prospective randomized controlled trials have been conducted. Green et al2 concluded that HBO should be considered a useful adjunct but should not preclude early surgery. Intravenous immunoglobulin is a controversial modality that has shown some effectiveness in limited studies of less severe GAS infections with toxic shock11 and could be considered for use in patients identified very early in the disease process, before fulminant infection, or in those patients too unstable to undergo surgery.3
In conclusion, NF is a thankfully rare but potentially fatal infection that must be diagnosed early and be treated aggressively to avoid mortality. A high index of suspicion is necessary to identify this disease process and differentiate it from more benign soft-tissue infections.
Acknowledgments
Funding/Support: The statistical support is supported, in part, by grant 1UL1RR031973 from the clinical and translational Science Award program of the national Center for Research Resources, National Institutes of Health.
Footnotes
Previous Presentation: This study was a poster presentation at the Annual Meeting of the American Society of Pediatric Otolaryngology; May 23, 2009; Seattle Washington.
Author Contributions: All authors had full access to all the data in the study and take responsibility for the in tegrity of the data and the accuracy of the data analysis. Study concept and design: King, Chun, and Sulman. Acquisition of data: Chun. Analysis and interpretation of data: King and Chun. Drafting of the manuscript: King and Chun. Critical revision of the manuscript for important intellectual content: King, Chun, and Sulman. Administrative, technical, and material support: Chun and Sulman. Study supervision: Chun and Sulman.
Financial Disclosure: None reported.
Additional Contribution: We thank Shi Zhao, MS, for biostatistics consultation.
REFERENCES
- 1.HCUP Kids’ Inpatient Database (KID) Agency for Healthcare Research and Quality. Rockville, MD: [Accessed January 23, 2012]. 1997 and 2006. Healthcare Cost and Utilization Project (HCUP) www.hcup-us.ahrq.gov/kidoverview.jsp. [Google Scholar]
- 2.Green RJ, Dafoe DC, Raffin TA. Necrotizing fasciitis. Chest. 1996;110(1):219–229. doi: 10.1378/chest.110.1.219. [DOI] [PubMed] [Google Scholar]
- 3.Bingöl-Kologğlu M, Yildiz RV, Alper B, et al. Necrotizing fasciitis in children: diagnostic and therapeutic aspects. J Pediatr Surg. 2007;42(11):1892–1897. doi: 10.1016/j.jpedsurg.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Laupland KB, Davies HD, Low DE, Schwartz B, Green K, McGeer A, Ontario Group A Streptococcal Study Group Invasive group A streptococcal disease in children and association with varicella-zoster virus infection. Pediatrics. 2000;105(5):E60. doi: 10.1542/peds.105.5.e60. [DOI] [PubMed] [Google Scholar]
- 5.Moss RL, Musemeche CA, Kosloske AM. Necrotizing fasciitis in children: prompt recognition and aggressive therapy improve survival. J Pediatr Surg. 1996;31(8):1142–1146. doi: 10.1016/s0022-3468(96)90104-9. [DOI] [PubMed] [Google Scholar]
- 6.Henrich DE, Smith TL, Mukherji S, Drake AF. Pediatric craniocervical necrotizing fasciitis. Ann Otol Rhinol Laryngol. 1996;105(1):72–74. doi: 10.1177/000348949610500114. [DOI] [PubMed] [Google Scholar]
- 7.Mathieu D, Neviere R, Teillon C, Chagnon JL, Lebleu N, Wattel F. Cervical necrotizing fasciitis: clinical manifestations and management. Clin Infect Dis. 1995;21(1):51–56. doi: 10.1093/clinids/21.1.51. [DOI] [PubMed] [Google Scholar]
- 8.Hsiao CT, Weng HH, Yuan YD, Chen CT, Chen IC. Predictors of mortality in patients with necrotizing fasciitis. Am J Emerg Med. 2008;26(2):170–175. doi: 10.1016/j.ajem.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 9.Becker M, Zbären P, Hermans R, et al. Necrotizing fasciitis of the head and neck: role of CT in diagnosis and management. Radiology. 1997;202(2):471–476. doi: 10.1148/radiology.202.2.9015076. [DOI] [PubMed] [Google Scholar]
- 10.Freischlag JA, Ajalat G, Busuttil RW. Treatment of necrotizing soft tissue infections: the need for a new approach. Am J Surg. 1985;149(6):751–755. doi: 10.1016/s0002-9610(85)80180-x. [DOI] [PubMed] [Google Scholar]
- 11.Norrby-Teglund A, Muller MP, Mcgeer A, et al. Successful management of severe group A streptococcal soft tissue infections using an aggressive medical regimen including intravenous polyspecific immunoglobulin together with a conservative surgical approach. Scand J Infect Dis. 2005;37(3):166–172. doi: 10.1080/00365540410020866. [DOI] [PubMed] [Google Scholar]


