Reliable measurement of internal bodily substances and structures is one of the cornerstones of modern medicine. Progress in cancer medicine, like that in many medical fields, must encompass and take advantage of progress in the physical sciences. Historically, the development and refinement of physical sciences-based detection of biological entities precedes periods of great advancements in therapies. To treat broken limbs and arthritis, we are indebted to Conrad Roentgen’s discovery of x-rays by which we can evaluate the bones; to apply gamma knife therapy for cancer, we are indebted to Marie Curie’s discoveries about radioactivity by which we can eradicate tumors; to manage the complications of diabetes, we are indebted to Tom Clemens, Ames Pharmaceuticals and Dick Bernstein’s refinement of direct blood glucose measurement technology by which we can count, hour-to-hour, the waxing and waning of blood sugar levels; to understand anything at all on the cellular level, we are indebted to Antonie van Leeuwenhoek’s microscope, by which we can see our cells. The application of physical sciences perspectives to biological and medical problems has a long and productive history. As of late, however, the increasing compartmentalization of science and exponential increases of knowledge in both arenas has resulted in a rift between the two. The NCI has initiated a research network establishing multiple centers of investigation, the Physical Sciences in Oncology Centers (http://physics.cancer.gov), which seek to mend the rift. Each headed by a pair of investigators, one in the physical sciences and one in the biological sciences, the centers seek to bring the advances and breakthroughs of the physical sciences world to bear on the question of cancer.
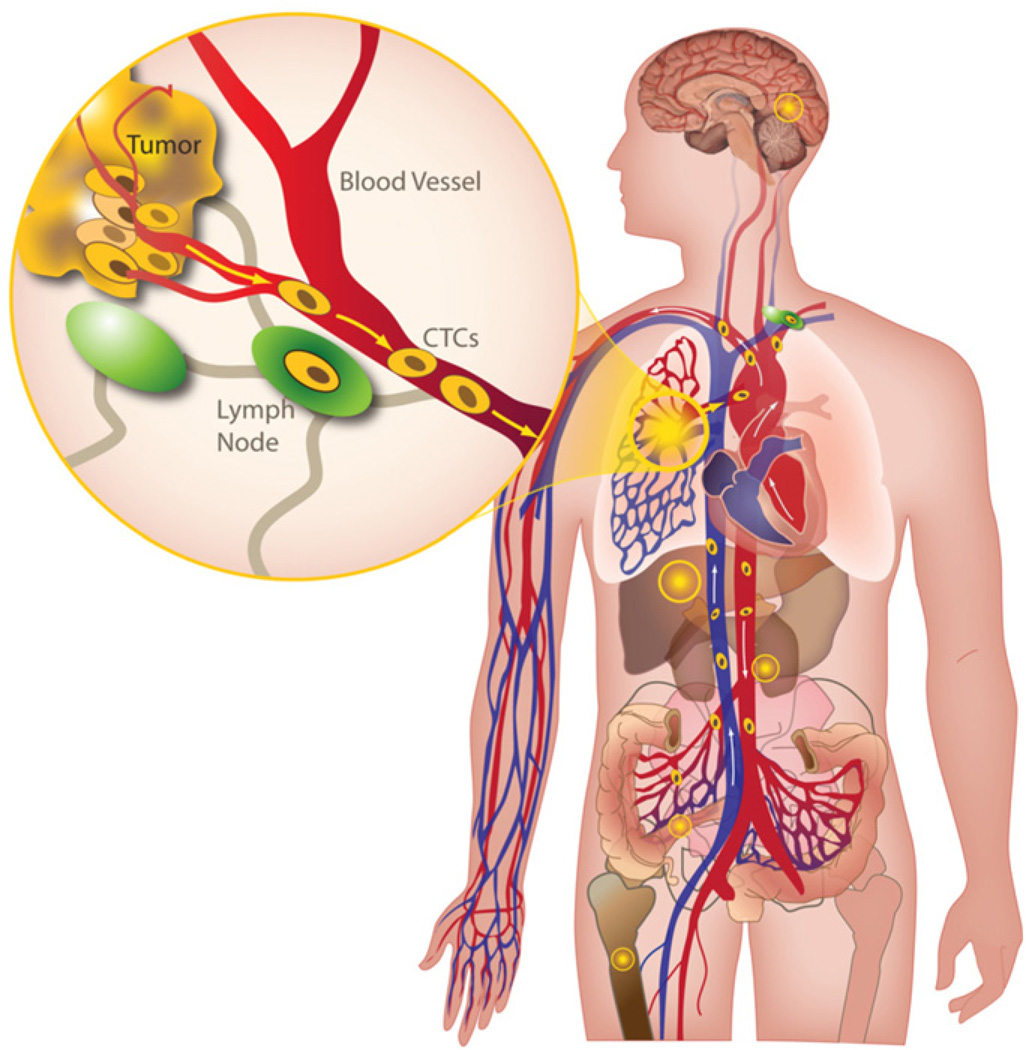
This issue of physical biology contains a series of articles exploring the utility and applicability of a new method for measuring cancer as it spreads, developed at the Scripps Physical Oncology Center. Although some progress has been made in fighting cancer, the victories are limited. Current medical and surgical strategies are very good at controlling local disease in cancer patients. We can successfully eradicate primary tumors in the sites where they arise and we can effectively control local recurrences in the nearby tissues. However, distant spread of cancer via the bloodstream (figure 1), known as hematogenous metastasis, has always been the fatal loophole. Like an evil humor, this process of hematogenous metastasis is mysterious to us. We do not understand its timing or its variability in terms of establishing new tumors at distant sites. It is invisible while it is happening, just as infectious transmission of Yersinia pestis is invisible. And like the resultant boils of bubonic plague in the pre-antibiotic era, by the time we see the ominous swellings, it is far too late.
Figure 1.
Hematogenous metastasis from a primary lung tumor. The inset shows the spread of cancer cells via the bloodstream.
Consequently, despite furious effort to treat and cure cancer, we are frustrated at our poor ability to detect and predict the deadly phenomenon of hematogenous spread, and we are disheartened by the apparent impenetrability of the process. Persistent helplessness in the face of this randomness tempts us toward fatalistic acceptance of cancer deaths as a natural consequence of improved longevity, just as death from childbed fever was once accepted as a frequent natural consequence of delivering a child.
Finally, however, we are in the process of refining methods to reliably detect, measure and characterize this internal biological process. The cells that are spreading cancer are in the bloodstream and must therefore be found and studied in samples from the bloodstream. Such circulating tumor cells (CTCs) are the focus of the evolving field of CTC biology and have proved a wily target. Early intimations of their nature came with the application of the Cellsearch® methodology, which has shown predictive utility in several cancer types—the blurry outlines of things to come began to coalesce. The field of CTC research is in its adolescence; enumeration and characterization research effort is methodologically varied, vigorously individuated and rich in innovation. The high definition CTC (HD-CTC) assay described herein and applied to several currently relevant research questions about cancer spread represents a new measure of an internal biologic process that will hopefully lead to great advancements in cancer medicine.
First, the paper entitled ‘Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers’ by Marrinucci et al, represents the initial technical and clinical validation of an enrichment-free assay and demonstrates our ability to identify significant numbers of HD-CTCs in a majority of patients with prostate, breast and pancreatic cancers. It demonstrates very high rates of detection (>50%) in breast, prostate and pancreatic cancer patients with no CTCs found in normal control subjects. The assay detects significantly higher numbers of CTCs than the FDA-approved Cellsearch® assay and shows the presence of clusters of CTCs in many patients.
The nature of the clusters is further evaluated in ‘Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors’ by Cho et al, wherein CTC aggregates are identified in 43% of 86 patient samples. The fraction of CTC aggregation was investigated in blood draws from 24 breast, 14 non-small cell lung (NSCLC), 18 pancreatic, 15 prostate stage IV cancer patients and 15 normal blood donors (NBD). Cells contained in CTC aggregates had less area and length, on average, than single CTCs. The nuclear to cytoplasmic (N/C) ratio between single CTCs and CTC aggregates was similar.
To assist with translating into patients the substantial cell biology work done using cell line cells instead of human tumor tissue, the paper entitled ‘Cytometric comparisons between circulating tumor cells from prostate cancer patients and the prostate tumor derived LNCaP cell line’ by Lazar et al elucidates differences between the two sample types. In this study, immunocytochemistry is used to compare the protein expression levels of total cytokeratin (CK) and androgen receptor (AR) in CTCs from patients with prostate cancer versus those of cell line cells and to determine what translational insights might be gained through the use of cell line data. This paper demonstrates that LNCaP cells are phenotypically different from CTCs, both in terms of cytomorphic features and relative expression levels of proteins. The ability to perform comparisons between cell lines and actual CTCs from cancer patients furthers our ability to translate experimental cell line data into understanding cancer in the human body and, thus, into predicting and influencing clinical outcomes.
In the paper entitled ‘High definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: a longitudinal analysis’ by Nieva et al, the kinetics of CTCs over time is explored. Twenty eight patients with non-small cell lung cancer and hematogenously seeded metastasis were analyzed with multiple blood draws. We detected CTCs in 68% of analyzed samples and found a propensity for increased CTC detection as the disease progressed in individual patients. CTCs were present at a median concentration of 1.6 CTCs per milliliter of analyzed blood in the patient population. Higher numbers of detected CTCs were associated with an unfavorable prognosis.
The paper entitled ‘Fluid biopsy for circulating tumor cell identification in patients with early and late stage non-small cell lung cancer: a glimpse into lung cancer biology’ by Wendel et al, describes similar numbers of CTCs in the bloodstream of patients with widely differing extent of disease at the time they were first diagnosed. Whether the tumors were found when they were small and confined to the lung, or large and widespread in the body, the median count of CTCs did not differ in this set of lung cancer patients. HD-CTCs were analyzed in blood samples from 78 chemotherapy-naïve NSCLC patients. 73% of the total population had a positive HD-CTC count (>0 CTC in 1 mL of blood) with a median of 4.4 HD-CTCs/mL (range 0–515.6) and a mean of 44.7 (±95.2) HD-CTCs/mL. No significant difference in the medians of HD-CTC counts was detected between stage IV (n = 31, range 0–178.2), stage III (n = 34, range 0–515.6) and stages I/II (n = 13, range 0–442.3). Furthermore, HD-CTCs exhibited uniformity in terms of molecular and physical characteristics, such as fluorescent cytokeratin intensity, nuclear size, frequency of apoptosis and aggregate formation across the spectrum of staging.
Thus, this series of papers describes a new technology based on physical sciences and its first applications to several basic mysteries of cancer and its spread. As we extend the reach of this new physical sciences perspective to cancer, the invisible evil biological humors that often kill when cancer arises are coming within our sights. We aim to take aim.
Acknowledgments
We are indebted to our patients for their long-term commitment to making a difference in cancer care through their participation in research. We are grateful to the clinical staff and to the collaborators and colleagues that have worked with us over the years. We greatly appreciate the generous donations by the Borden Family and the many individual donations in memory of Sandy Thielicke who has infused in our group the motivation, drive and focus in our fight against cancer. The Scripps Physics Oncology Center (http://4db.us) is principally supported under grant number U54CA143906 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Contributor Information
Peter Kuhn, Department of Cell Biology, Scripps Research Institute, 10550 North Torrey Pines Road, GAC-1200, La Jolla, CA 92037, USA, pkuhn@scripps.edu.
Kelly Bethel, Department of Pathology, Scripps Clinic, 10666, North Torrey Pines Road, La Jolla, CA, 92037, USA, Bethel.Kelly@scrippshealth.org.