Abstract
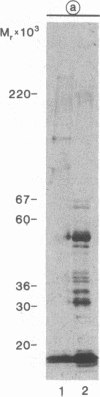
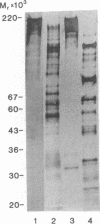
The comparison of measurements of fibronectin and lactoferrin in ejaculates from vasectomized men, subjects with functional deficiency or aplasia of the seminal vesicles, and reference subjects provided evidence that both the fibronectin and the lactoferrin in human seminal fluid originate from the seminal vesicles and the ampullae. The fibronectin is incorporated in the framework of the seminal gel formed during the immediate postejaculatory phase, whereas the lactoferrin remains in solution. In the seminal gel fibronectin is linked to its predominant structural protein, a high molecular weight seminal vesicle protein (semenogelin). Both the gel-bound fibronectin and semenogelin are progressively fragmented and solubilized by the abundant prostatic kallikrein-like protease (prostate-specific antigen) during and after seminal gel liquefaction. Lactoferrin remains essentially unaffected by the seminal proteases.
Full text
PDF


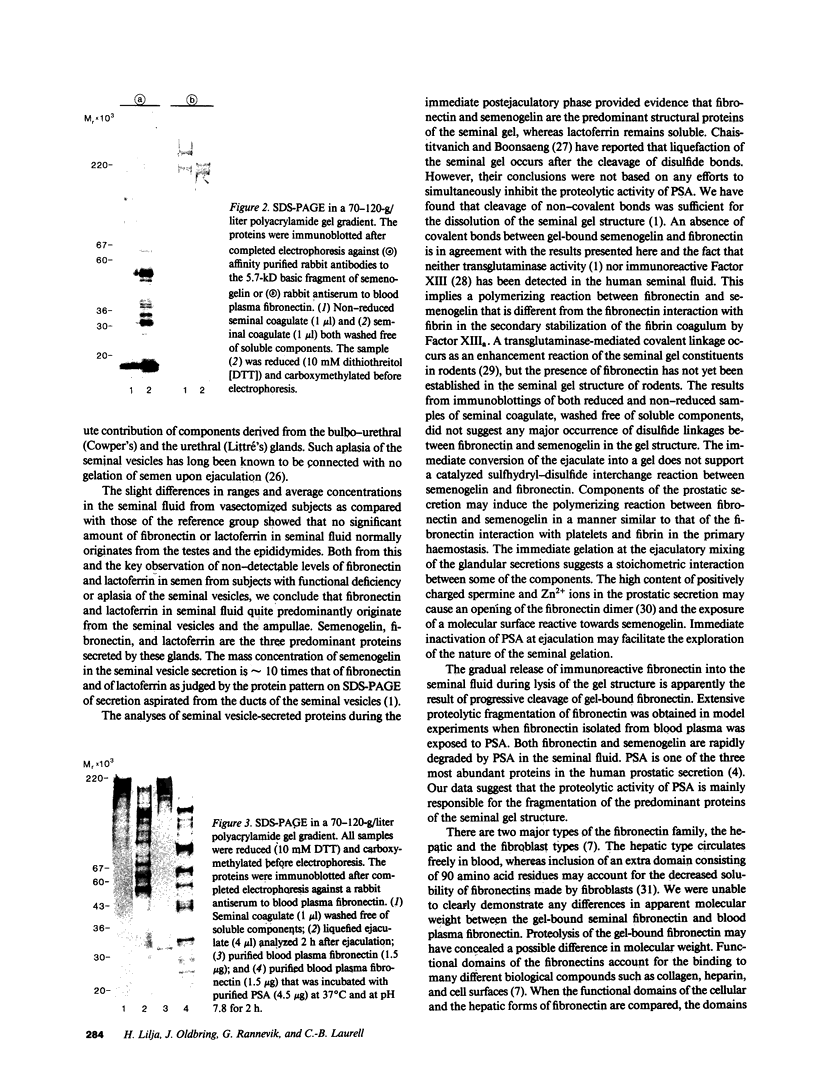

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMELAR R. D. Coagulation, liquefaction and viscosity of human semen. J Urol. 1962 Feb;87:187–190. doi: 10.1016/S0022-5347(17)64936-X. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chaistitvanich N., Boonsaeng V. Molecular structure of human seminal coagulum: the role of disulfide bonds. Andrologia. 1983 Sep-Oct;15(5):446–451. doi: 10.1111/j.1439-0272.1983.tb00167.x. [DOI] [PubMed] [Google Scholar]
- DE CARVALHO C. A., POGELL B. M. Modified skatole method for microdetermination of fructose and inulin. Biochim Biophys Acta. 1957 Oct;26(1):206–206. doi: 10.1016/0006-3002(57)90075-6. [DOI] [PubMed] [Google Scholar]
- GRAHAM R. C., Jr, LUNDHOLM U., KARNOVSKY M. J. CYTOCHEMICAL DEMONSTRATION OF PEROXIDASE ACTIVITY WITH 3-AMINO-9-ETHYLCARBAZOLE. J Histochem Cytochem. 1965 Feb;13:150–152. doi: 10.1177/13.2.150. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Differences in domain structures between plasma and cellular fibronectins. J Biol Chem. 1981 Nov 10;256(21):11292–11300. [PubMed] [Google Scholar]
- Jeppsson J. O., Laurell C. B., Franzén B. Agarose gel electrophoresis. Clin Chem. 1979 Apr;25(4):629–638. [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Human fibronectin: molecular cloning evidence for two mRNA species differing by an internal segment coding for a structural domain. EMBO J. 1984 Jan;3(1):221–226. doi: 10.1002/j.1460-2075.1984.tb01787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest. 1985 Nov;76(5):1899–1903. doi: 10.1172/JCI112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja H., Laurell C. B., Jeppsson J. O. Characterization of the predominant basic protein in human seminal plasma, one cleavage product of the major seminal vesicle protein. Scand J Clin Lab Invest. 1984 Sep;44(5):439–446. doi: 10.3109/00365518409083835. [DOI] [PubMed] [Google Scholar]
- Lilja H., Laurell C. B. Liquefaction of coagulated human semen. Scand J Clin Lab Invest. 1984 Sep;44(5):447–452. doi: 10.3109/00365518409083836. [DOI] [PubMed] [Google Scholar]
- Lilja H., Laurell C. B. The predominant protein in human seminal coagulate. Scand J Clin Lab Invest. 1985 Nov;45(7):635–641. doi: 10.3109/00365518509155271. [DOI] [PubMed] [Google Scholar]
- Lilja H., Weiber H. Synthetic protease inhibitors and post-ejaculatory degradation of human semen proteins. Scand J Clin Lab Invest. 1984 Sep;44(5):433–438. doi: 10.3109/00365518409083834. [DOI] [PubMed] [Google Scholar]
- MAWSON C. A., FISCHER M. I. Zinc and carbonic anhydrase in human semen. Biochem J. 1953 Nov;55(4):696–700. doi: 10.1042/bj0550696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay E. J., Laurell C. B. The interaction of heparin with plasma proteins. Demonstration of different binding sites for antithrombin III complexes and antithrombin III. J Lab Clin Med. 1980 Jan;95(1):69–80. [PubMed] [Google Scholar]
- Mosesson M. W., Chen A. B., Huseby R. M. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Paul J. I., Schwarzbauer J. E., Tamkun J. W., Hynes R. O. Cell-type-specific fibronectin subunits generated by alternative splicing. J Biol Chem. 1986 Sep 15;261(26):12258–12265. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Richter H., Hörmann H. Early and late cathepsin D-derived fragments of fibronectin containing the C-terminal interchain disulfide cross-link. Hoppe Seylers Z Physiol Chem. 1982 Apr;363(4):351–364. doi: 10.1515/bchm2.1982.363.1.351. [DOI] [PubMed] [Google Scholar]
- Roberts T. K., Boettcher B. Identification of human sperm-coating antigen. J Reprod Fertil. 1969 Mar;18(2):347–350. doi: 10.1530/jrf.0.0180347. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Siri A., Zardi L., Hakomori S. Differences in domain structure between human fibronectins isolated from plasma and from culture supernatants of normal and transformed fibroblasts. Studies with domain-specific antibodies. J Biol Chem. 1985 Apr 25;260(8):5105–5114. [PubMed] [Google Scholar]
- Tauber P. F., Zaneveld L. J., Propping D., Schumacher G. F. Components of human split ejaculates. I. Spermatozoa, fructose, immunoglobulins, albumin, lactoferrin, transferrin and other plasma proteins. J Reprod Fertil. 1975 May;43(2):249–267. doi: 10.1530/jrf.0.0430249. [DOI] [PubMed] [Google Scholar]
- Tauber P. F., Zaneveld L. J., Propping D., Schumacher G. F. Components of human split ejaculates. II. Enzymes and proteinase inhibitors. J Reprod Fertil. 1976 Jan;46(1):165–171. doi: 10.1530/jrf.0.0460165. [DOI] [PubMed] [Google Scholar]
- Vuento M., Kuusela P., Virkki M., Koskimies A. Characterization of fibronectin on human spermatozoa. Hoppe Seylers Z Physiol Chem. 1984 Jul;365(7):757–762. doi: 10.1515/bchm2.1984.365.2.757. [DOI] [PubMed] [Google Scholar]
- Vuento M., Salonen E., Koskimies A., Stenman U. H. High concentrations of fibronectin-like antigens in human seminal plasma. Hoppe Seylers Z Physiol Chem. 1980 Sep;361(9):1453–1456. [PubMed] [Google Scholar]
- Vuento M., Vartio T., Saraste M., von Bonsdorff C. H., Vaheri A. Spontaneous and polyamine-induced formation of filamentous polymers from soluble fibronectin. Eur J Biochem. 1980 Mar;105(1):33–42. doi: 10.1111/j.1432-1033.1980.tb04471.x. [DOI] [PubMed] [Google Scholar]
- Wang M. C., Valenzuela L. A., Murphy G. P., Chu T. M. Purification of a human prostate specific antigen. Invest Urol. 1979 Sep;17(2):159–163. [PubMed] [Google Scholar]
- Watt K. W., Lee P. J., M'Timkulu T., Chan W. P., Loor R. Human prostate-specific antigen: structural and functional similarity with serine proteases. Proc Natl Acad Sci U S A. 1986 May;83(10):3166–3170. doi: 10.1073/pnas.83.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Ashman H. G. Transglutaminases and the clotting of mammalian seminal fluids. Mol Cell Biochem. 1984;58(1-2):51–61. doi: 10.1007/BF00240604. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]