Abstract
Extracellular matrix (ECM) proteins, including fibronectin, may contribute to the early development and progression of renal interstitial fibrosis associated with chronic renal disease. Recent studies showed that β1-integrin is associated with the development of renal fibrosis in a murine model of unilateral ureteral obstruction (UUO). However, the molecular events responsible for β1-integrin-mediated signaling, following UUO, have yet to be determined. In this study, we investigated the mechanism by which mechanical stretch, an in vitro model for chronic obstructive nephropathy, regulates fibronectin and transforming growth factor-β1 (TGF-β1) expression in cultured human proximal tubular epithelium (HK-2) cells. Mechanical stretch upregulated fibronectin and TGF-β1 expression and activated signal transducer and transcription factor 3 (STAT3) in a time-dependent manner. Stretch-induced fibronectin and TGF-β1 were suppressed by a STAT3 inhibitor, S3I-201, and by small interfering RNA (siRNA) targeting human STAT3 (STAT3 siRNA). Similarly, fibronectin and TGF-β1 expression and STAT3 activation induced by mechanical stretch were suppressed by the Src family kinase inhibitor PP2 and by transfection of HK-2 cells with a dominant-negative mutant of c-Src (DN-Src), whereas PP3, an inactive analog of PP2, had no significant effect. Furthermore, mechanical stretch resulted in increased β1-integrin mRNA and protein levels in HK-2 cells. Furthermore, neutralizing antibody against β1-integrin and silencing of β1-integrin expression with siRNAs resulted in decreased c-Src and STAT3 activation and TGF-β1 and fibronectin expression evoked by mechanical stretch. This work demonstrates, for the first time, a role for β1-integrin in stretch-induced renal fibrosis through the activation of c-Src and STAT3 signaling pathways.
Keywords: cyclic stretch, TGF-β1, obstructive nephropathy, STAT3, fibronectin, renal fibrosis, c-Src, β1-integrin
a hallmark of obstructive nephropathy and a major factor in the progressive loss of renal function in patients is excessive extracellular matrix (ECM) accumulation, especially fibronectin, of the tubulointerstitial compartment, leading to tubulointerstitial fibrosis. Mechanical stretching of tubular epithelium, caused by retrograde pressure shifts and urinary polling, is regarded as highly significant in the progression of obstructive nephropathy. In fact, cyclic stretch has been used in vitro to mimic the changes in intrarenal pressure in unilateral ureteral obstruction (UUO) (16, 17, 51, 52), a well-established experimental model of renal inflammation and fibrosis that encompasses many aspects of obstructive nephropathy. In this in vitro model, a number of signaling molecules, including cytosolic PLA2 (cPLA2), MAPK family members, ERK1/2, the EGF receptor (EGFR), and the nonreceptor tyrosine kinase c-Src, are activated by mechanical stretch (1). In addition, the activation of ERK1/2 is abrogated by the Src family kinase inhibitor 3-(4-chlorophenyl)-1-(1,1-dimethylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine (PP2) and by transfection of proximal tubule cells with a dominant negative mutant of c-Src (DN-Src), indicating that c-Src is critical for stretch-induced ERK1/2 activation in renal proximal tubular cells (1). Moreover, studies carried out in vitro have recently shown that mechanical stretch induces transforming growth factor (TGF)-β1 expression in renal proximal tubular cells (35, 41, 54, 57). However, the molecular mechanisms by which mechanical stretch contributes to TGF-β1 production leading to renal fibrosis are not yet completely understood.
A principal mechanism whereby UUO induces renal fibrosis may involve induction of integrins. Integrins are a broad family of cell surface adhesion and signaling molecules, consisting of an α-subunit and a β-subunit, which link the ECM to the cytoskeleton. Among the members of the integrin family, β1-integrin is the most critical one given that β1-integrin can pair with different α-subunits, making it become a receptor for many types of stimuli, and it is expressed in renal tubular cells (4, 18, 20, 38, 61). Integrins can also function as force sensors, transducing mechanical stimuli into biochemical signals. Under normal conditions, integrins are critical for maintaining cellular homeostasis, triggering a number of signaling pathways, some of which are primarily related to cell migration and cell adhesion, whereas others provide signaling to the cells that regulate cellular differentiation, proliferation, survival, and apoptosis. Conversely, under pathological conditions, integrins are associated with a wide variety of renal pathologies including, but not limited to, obstructive nephropathy (4, 9, 28, 38, 53, 70, 72). In this context, Yeh et al. (70) recently demonstrated that β1-integrin gene and protein expression was significantly upregulated in UUO mice. This was accompanied by correspondingly elevated tubular expression of TGF-β1. Along these lines, blocking of β1-integrin signals by treatment with an antibody to β1-integrin reduced TGF-β1 levels and ameliorated fibrosis, demonstrating strong correlations between the expression of β1-integrin within the tubulointerstitium and the presence of tubulointerstitial fibrosis (70). However, little is known about the role of β1-integrin in the pathogenesis of renal fibrosis induced by cyclic mechanical stretch. Moreover, the molecular mechanism of β1-integrin-mediated renal fibrosis has not been studied. Because knockout of β1-integrin in mice is embryonically lethal (19, 58) and the limitation of a β1-integrin-blocking antibody in vivo, experiments on cultured proximal tubular cells will most likely provide more mechanistic information on the serial steps of β1-integrin-induced signal transduction. Given the increased expression of β1-integrin during tubulointerstitial fibrosis, and because it is the most prevalent β-chain of the heterodimers expressed in the kidney (32), we hypothesized that β1-integrin induction may contribute to fibrogenic renal disease. Using an in vitro model of UUO, we sought to determine: 1) whether cyclic mechanical stretch induced the expression of extracellular matrix (e.g., fibronectin) and profibrotic (e.g., TGF-β1) protein expression; 2) the effect of β1-integrin disruption and blockade on mechanical stretch-induced fibronectin and TGF-β1 protein expression; and 3) the potential mechanisms of β1-integrin-mediated renal fibrosis. In this study, we demonstrate for the first time that cyclic mechanical stretch induces the activation of signal transducer and transcription factor 3 (STAT3) and expression of TGF-β1 and fibronectin and that blocking or knockdown of STAT3 abrogated these effects. Similar results were obtained following pharmacological inhibition or overexpression of dominant-negative mutants of c-Src. Furthermore, we demonstrate for the first time that mechanical stretch upregulates β1-integrin gene and protein expression, and blocking or knockdown of β1-integrin signaling abrogated stretch-induced c-Src and STAT3 activation and TGF-β1 and fibronectin expression in HK-2 cells. These results suggest that β1-integrin is important for modulating renal fibrosis through c-Src/STAT3 signaling pathways and enhances our understanding of mechanotransduction pathways leading to fibrosis of proximal tubule epithelial cells.
EXPERIMENTAL PROCEDURES
Materials.
Recombinant human TGF-β1 and TGF-β1 ELISAs were purchased from R&D Systems (Minneapolis, MN). PP2 and 1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-amine (PP3) were purchased from Cayman Chemical (Ann Arbor, MI). Antibodies against p-c-Src, c-Src, p-SAT3, and STAT3 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against β1-integrin and β-actin were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-β1-integrin 4B4 was purchased from Coulter (Hialeah, FL). Anti-rabbit (goat) and anti-mouse (goat) horseradish peroxidase (HRP)-conjugated IgG antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Cell culture media and additives were purchased from Invitrogen (Grand Island, NY). All other chemicals were of best available quality, usually analytic grade.
Cell culture.
Human renal proximal epithelial (HK-2) cells were obtained from American Type Culture Collection (Manassas, VA) and cultured in DMEM/F-12 medium containing insulin (5 μg/ml), transferrin (5 μg/ml), hydrocortisone (0.5 μg/ml), penicillin (100 μg/ml), and streptomycin (100 μg/ml) supplemented with 10% fetal calf serum. Cells were used for experiments at passages 8–15 and rendered quiescent in media containing 0.5% FCS for 24 h before treatment with cyclic mechanical stretch or TGF-β1.
Cyclic mechanical stretch.
For studies involving mechanical stretch, differentiated HK-2 cells were seeded onto commercially available silastic six-well collagen I-coated stretch plates (Flexcell, Hillsborough, NC) for 3 days. After being serum-starved for 24 h, culture medium was replaced with new serum-free medium. The culture plates were placed on vacuum-based loading docks of the Flexcell FX-4000T apparatus (Flexcell) in the incubator and subjected to pulsatile mechanical stretch (10–20% of equibiaxial elongation) at a frequency of 0.1 Hz. Previous reports have shown that these parameters induce a significant difference in TGF-β1 secretion between nonstretched renal proximal tubule cells and stretched cells (41, 42, 54, 57). Nonstretched cells (control) were exposed to identical experimental conditions but without mechanical stretch. To assess the effects of the indicated inhibitors, drugs were added to cells 30 min before stimulation with cyclic mechanical stretch.
Transient transfections.
Commercially available small interfering RNA (siRNA) of Smartpool siRNA for human β1-integrin, human STAT3, and negative control (scramble) siRNA were purchased from Santa Cruz Biotechnology. Briefly, HK-2 cells were transfected with either 100 mol/l of siRNA targeting human β1-integrin (siRNA β1), human STAT3 (siRNA STAT3), or with the same amount of control (scramble) siRNA (siRNA scramble), or with 10 μg of dominant-negative plasmid of c-Src or with the same amount of empty vector using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Forty-eight hours posttransfection, cells were stimulated with indicated stimuli or vehicle, lysed, and analyzed for β1-integrin expression by Western blotting with a rabbit anti-β1-integrin polyclonal antibody, and with phospho-specific anti-rabbit polyclonal antibodies for c-Src and STAT3. Blots were stripped and reprobed with a mouse monoclonal c-Src, STAT3, or β-actin antibody to control for protein loading and for silencing efficiency and specificity.
Western blot analysis.
Western blot analysis was carried out as previously described (1, 2). Briefly, proteins were extracted with buffer containing 50 mM Tris, pH 7.2, 1% (vol/vol) Triton X-100, 1 mM Na3VO4, 1 mM EGTA, 0.2 mM phenylmethanesulfonyl fluoride, 25 μg/ml leupeptin, and 10 μg/ml aprotinin. Whole-cell lysate of treated cells was subjected to 4–20% SDS-PAGE. The fractionated proteins were transferred onto nitrocellulose membranes, which were then incubated with various primary antibodies, and target proteins were detected by enhanced chemiluminescence (ECL) and exposed to X-ray films. All experiments had at least one membrane reprobed with antibodies recognizing nonphosphorylated kinases to confirm equal protein loading. The exposure autoradiograph was analyzed by Un-Scan-It gel, version 5.1, to obtain densitometry data. Protein contents were determined by BCA assay (Pierce).
Real-time RT-PCR.
Total RNA was extracted from cells using TRIzol reagent (Invitrogen), treated with DNase I (Ambion) to remove potential genomic DNA contamination, and purified using an RNeasy Mini Kit (Qiagen). Total RNA concentration was measured, and the purity of the samples was estimated by the OD ratios (A260/A280, ranging within 1.8–2.2). cDNA was synthesized from 2 μg of DNA-free total RNA in a 25-μl volume using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega). cDNA samples were diluted 10-fold for real-time PCR reactions. Gene-specific transcriptional levels were determined in a 20-μl reaction volume, in duplicate, using SYBR Green and an ABI 7500 real-time PCR system (Applied Biosystems). The sense primer for human β1-integrin was 5′-GCAAGTTGCAGTTTGTGGATCA-3′; and the antisense primer was 5′-TGCCACCAAGTTTCCCATCT-3′. The sense for the human GAPDH was 5′-GAAGGTGAAGGTCGGAGTC-3′; and the antisense primer was 5′-GAAGATGGTGATGGGATTTC-3′. A quantitative analysis was performed to evaluate the expression of β1-integrin and normalized to GAPDH. The comparative Ct method (ΔΔCt) was used to quantify gene expression, and the relative quantification was calculated as 2−ΔΔCt. Melting curve analysis was performed to check for any presence of nonspecific application products.
Determination of TGF-β1.
HK-2 cells were passaged onto commercially available silastic membranes coated with collagen type I (Flexcell, McKeesport, PA) and grown to confluence. After an incubation period of 12–72 h with the treatment, the medium was removed and kept at −80°C for ELISA. TGF-β1 in the media of stretch and nonstretched HK-2 cells was measured using ELISA kits (Quantikine; R&D Systems). To activate latent TGF-β1 to immunoreactive TGF-β1 detectable by the immunoassay, samples were acidified with 1 N HCl, incubated at room temperature for 10 min, and neutralized with 1.2 N NaOH. The remainder of the assay was carried out according to the manufacturer's instructions with absorbance read at 450 nm.
Statistical analyses.
Values are means ± SE. For statistical significance, multiple comparisons among three or more groups were performed using one-way ANOVA followed by a Student-Newman-Keuls multiple comparison post hoc test when appropriate. For comparing two groups, an unpaired t-test was used. Differences with values of P < 0.05 were considered significant.
RESULTS
Fibronectin expression is upregulated in response to cyclic mechanical stretch in HK-2 cells.
We first examined the time course of cyclic stretch-dependent fibronectin expression in HK-2 cells. Nonstretched cells were used as a control. We found that cyclic stretch at 20% elongation increased fibronectin expression, which was significant at 12 h, with a maximum 2.6-fold increase at 48 h, and then declining after 72 h to 2.5-fold (Fig. 1A). In addition, cyclic stretch-induced fibronectin protein expression was found to be intensity dependent. When exposed for 48 h to increasing intensities of cyclic stretch (10–20% stretch), fibronectin protein expression was significant at 15% stretch with a maximal effect at 20% stretch, compared with control nonstretched cells (Fig. 1B). For subsequent studies, cells were treated for 48 h at 20% stretch to detect maximal fibronectin protein expression, unless otherwise indicated.
Fig. 1.
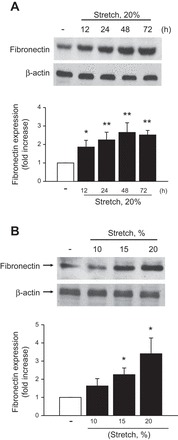
Mechanical stretch increased the expression of fibronectin in HK-2 cells. A: HK-2 cells are treated with cyclic stretch (20% elongation, 6 cpm) for the indicated time period (A) or with indicated levels of stretch for 48 h (B). Expression of fibronectin was determined by Western blotting, and respective quantitation analysis for expression fibronectin was performed (A and B). β-Actin was used to verify equivalent loading. Bar graphs depict the quantitative densitometry analysis for Western blot densitometry data. Values are means ± SE of 6 independent experiments. *P < 0.05, **P < 0.01 vs. no stretch (control) cells.
Cyclic stretch upregulates fibronectin and TGF-β1 expression via STAT3 in HK-2 cells.
In a recent study, Pang et al. (46) has shown that the STAT3 inhibitor S3I-201 alters fibronectin expression in a mouse model of renal interstitial fibrosis induced by UUO. Therefore, we examined the effect of S3I-201 in stretch-induced fibronectin expression. We first tested the effects of cyclic stretch on STAT3 phosphorylation by using an antibody recognizing the tyrosine 705 phosphorylated form of STAT3. As shown in Fig. 2A, cyclic stretch induces STAT3 phosphorylation in a time-dependent manner, reaching a maximum value after 15 min of stimulation. Pretreatment of proximal tubule cells with S3I-201 (50 μmol/l) significantly reduced the phosphorylation of STAT3 and expression of fibronectin in the presence of cyclic stretch (Fig. 2, B and C, respectively). In addition, transfecting proximal tubule cells with siRNA targeting human STAT3 (siRNA STAT3) abrogated stretch-induced STAT3 and fibronectin expression (Fig. 3, A and B, respectively). Moreover, stimulation of proximal tubule cells with cyclic mechanical stretch induced expression of TGF-β1 time dependently (Fig. 4A). In addition, application of S3I-201 or overexpression of STAT3 siRNA effectively suppressed stretch-induced TGF-β1 expression (Fig. 4, B and C, respectively). Furthermore, treatment of proximal tubule cells with an anti-TGF-β antibody attenuated stretch-induced fibronectin expression in HK-2 cells whereas nonspecific IgG had no effect (Fig. 4D). These results show that STAT3 is involved in stretch-induced fibronectin and TGF-β1 expression and that stretch-stimulated fibronectin expression in HK-2 cells was mediated by TGF-β1.
Fig. 2.
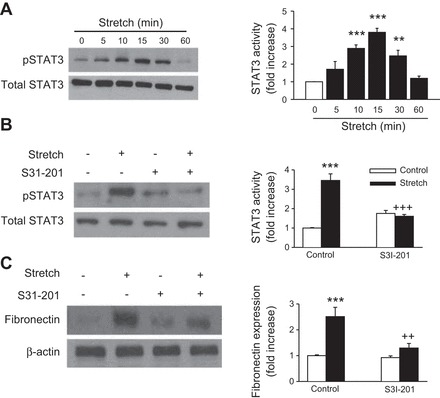
Effects of S3I-201 on mechanical stretch-induced signal transducer and transcription factor 3 (STAT3) activation and fibronectin expression. HK-2 cells serum-starved for 24 h were exposed to mechanical stretch (20% elongation, 6 cpm) for the indicated times (A) or pretreated without or with the STAT3 inhibitor S3I-201 (50 μmol/l) for 1 h before treatment with mechanical stretch (20% elongation, 6 cpm) for 15 min (B) or for 48 h (C). Immunoblotting with specific antibodies against p-STAT3 (Tyr705), STAT3, fibronectin, or β-actin and respective quantitation analysis for expression STAT3 and fibronectin were performed. Bar graphs depict the quantitative densitometry analysis for Western blot densitometry data. Values are means ± SE of 5 independent experiments. **P < 0.01, ***P < 0.001 vs. no stretch (control) cells. ++P < 0.01, +++P < 0.001 vs. S3I-201-treated cells with stretch.
Fig. 3.
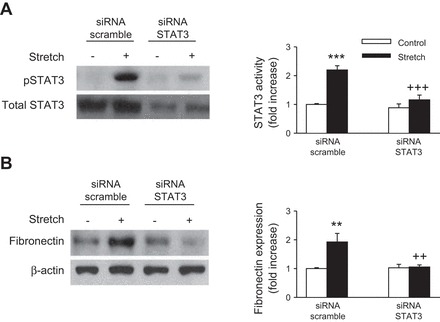
Ablation of STAT3 decreases cyclic stretch-induced fibronectin expression. HK-2 cells were transfected with 100 nM of small interfering (si) RNA specific for human STAT3 (siRNA STAT3) or with the same amount of scrambled siRNA (siRNA scramble) followed by mechanical stretch stimulation (20% elongation, 6 cpm) for 15 min (A) or for 48 h (B). Immunoblotting with specific antibodies against p-STAT3 (Tyr705), STAT3, fibronectin, or β-actin and respective quantitation analysis for expression of SAT3 and fibronectin were performed. Activated STAT3 was normalized to STAT3 (A). Fibronectin protein expression levels were normalized to β-actin (B). Bar graphs depict the quantitative densitometry analysis for Western blot densitometry data. Values are means ± SE of 5 different experiments. **P < 0.01, ***P < 0.001, vs. no stretch (control) cells. ++P < 0.01, +++P < 0.001, vs. STAT3 siRNA-treated cells with stretch.
Fig. 4.
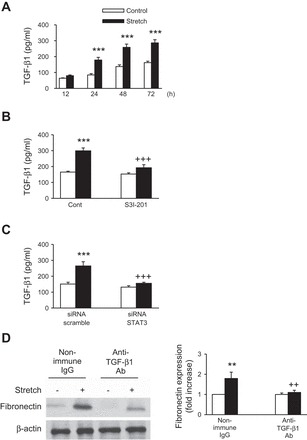
Role of STAT3 in stretch-induced TGF-β1 expression. A: HK-2 cells were treated with cyclic stretch (20% elongation, 6 cpm) for indicated time intervals, after which TGF-β1 was analyzed by ELISA. Values are means ± SE of 6 independent experiments. ***P < 0.001 vs. time-matched control. B: HK-2 cells serum-starved for 24 h were pretreated without or with the STAT3 inhibitor S3I-201 (50 μmol/l) for 1 h before treatment with mechanical stretch (20% elongation, 6 cpm) for 48 h, after which TGF-β1 was analyzed by ELISA. Values are means ± SE of 5 independent experiments. ***P < 0.001 vs. no stretch (control) cells. +++P < 0.001 vs. S3I-201-treated cells with stretch. C: siRNA scramble or siRNA STAT3 was transfected in HK-2 cells followed by mechanical stretch (20% elongation, 6 cpm) stimulation for 48 h, after which TGF-β1 was analyzed by ELISA. Values are means ± SE of 6 independent experiments. ***P < 0.001 vs. no stretch (control) cells. +++P < 0.001 vs. siRNA STAT3-treated cells with stretch. D: HK-2 cells were stimulated in the presence or absence of blocking antibodies (10 μg/ml) to TGF-β1 for 48 h in serum-free medium. Immunoblotting with specific antibodies against fibronectin or β-actin and respective quantitation analysis for expression of fibronectin was performed. Fibronectin protein expression levels were normalized to β-actin. Bar graphs depict the quantitative densitometry analysis for Western blot densitometry data. Values are means ± SE of 5 different experiments. **P < 0.01 vs. no stretch (control) cells. ++P < 0.01 vs. anti-TGF-β1 antibody-treated cells with stretch.
Inhibition of c-Src reduces STAT3 activation and fibronectin and TGF-β1 expression in HK-2 cells.
c-Src is a well-known regulator of STAT3 (11, 24, 50) and fibronectin (2, 59, 60). Moreover, previous studies from this laboratory have shown that mechanical stretch activates c-Src in renal tubular cells (1). To examine the role of c-Src in STAT3 activation and fibronectin and TGF-β1 expression by mechanical stretch, cells were pretreated either with the Src family kinase inhibitor PP2, its inactive analog PP3, or transiently transfected with a dominant-negative mutant of c-Src (DN-Src). Pretreatment of proximal tubular cells with PP2 (10 μmol/l) significantly decreased stretch-induced STAT3 activation and fibronectin and TGF-β1 expression, whereas it's inactive analog PP3 (10 mmol/l) had no significant effect (Fig. 5, A, B, and C, respectively). Similarly, transfection of proximal tubule cells with DN-Src significantly reduced stretch-induced STAT3 activation and fibronectin and TGF-β1 expression (Fig. 6, A, B, and C, respectively). By analogy, pretreatment with the selective STAT3 inhibitor S3I-201 had no effect on stretch-induced c-Src activation (data not shown). These data strongly implicate c-Src in the activation of STAT3 and the expression of fibrogenic protein expression in human proximal tubular cells exposed to cyclic mechanical stretch.
Fig. 5.
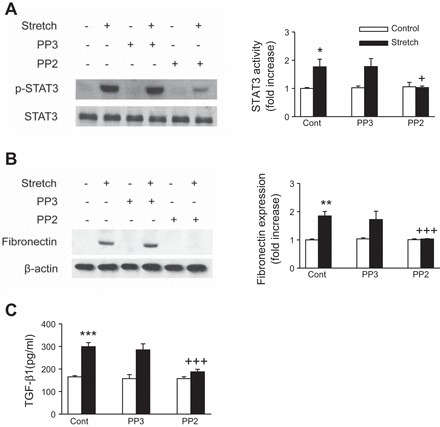
Effect of c-Src inhibition on mechanical stretch-induced STAT3 activation and TGF-β1 and fibronectin expression. HK-2 cells were preincubated for 30 min with vehicle (Cont), Src family kinase inhibitor PP2 (10 μmol/l), or its analog PP3 (10 μmol/l) and further stimulated with cyclic mechanical stretch (20% elongation, 6 cpm) for 15 min (A) or 48 h (B and C). Phosphorylation of STAT3 and expression of fibronectin were determined by Western blotting (A and B). STAT3 and β-actin was used to verify equivalent loading. Bar graphs depict the quantitative densitometry analysis for Western blot densitometry data. TGF-β1 was analyzed by ELISA (C). Values are means ± SE of at least 5 independent experiments. *P < 0.05, **P < 0.01 vs. no stretch (control) cells. +P < 0.05, +++P < 0.001 vs. stretch with inhibitor.
Fig. 6.
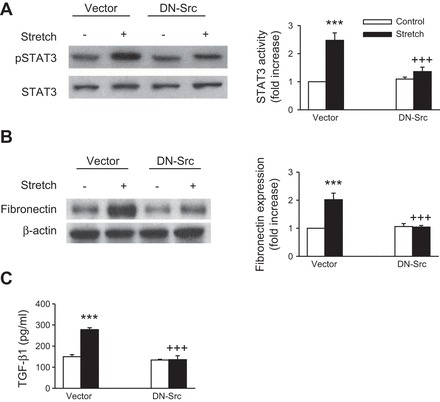
c-Src is required for stretch-induced STAT3 activation and TGF-β1 and fibronectin expression. HK-2 cells were transiently transfected with expression vectors encoding dominant-negative c-Src (DN-Src) or with vector alone. Phosphorylation and expression of STAT3 and fibronectin were analyzed by Western blotting, and the equal loading of the respective protein samples was confirmed by Western blotting of STAT3 and β-actin. Bar graphs depict the quantitative densitometry analysis for Western blot densitometry data. Values are means ± SE of 4 independent experiments. ***P < 0.001 vs. empty vector with no stretch (control) cells. +++P < 0.001 vs. cells transfected with DN-Src alone.
Stretch-induced fibronectin and TGF-β1 expression is β1-integrin dependent.
Although it had been shown that mechanical stretch induced β1-integrin protein expression in different tissues and that β1-integrin is highly expressed in renal tubular epithelial cells (22, 33, 66, 67, 70), the functions of β1-integrin in renal epithelial cells during mechanical stretch-induced fibrogenesis has never been reported. To address whether the fibrogenic effect of mechanical stretch is β1-integrin dependent, we first examined whether β1-integrin was upregulated by cyclic mechanical stretch in HK-2 cells. As shown in Fig. 7, A and B, respectively, mechanical stretch upregulated β1-integrin protein and mRNA levels in a time-dependent manner. Blockade of β1-integrin, with an anti-integrin β1-blocking antibody, 4B4, abrogated fibronectin and TGF-β1 expression after mechanical stretch (Fig. 8, A, B, and C, respectively). To further confirm the role of β1-integrin in stretch-induced fibronectin and TGF-β1 expression, β1-integrin was silenced by siRNA targeting human β1-integrin (siRNA β1) in HK-2 cells. Compared with scramble siRNA controls, knockdown of β1-integrin significantly inhibited stretch-induced fibronectin and TGF-β1 expression (Fig. 9, A, B, and C, respectively). In addition, both anti-integrin-β1-blocking antibody and siRNA β1 effectively inhibited the mechanical stretch-induced increases in c-Src and STAT3 phosphorylation (Figs. 8, A and B, and 9, A and B, respectively), suggesting that β1-integrin is a critical mediator of fibronectin and TGF-β1 expression, as well as c-Src and STAT3 activation evoked by cyclic mechanical stretch in HK-2 cells.
Fig. 7.
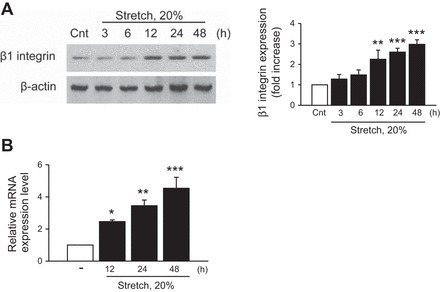
Mechanical stretch upregulates β1-integrin expression in HK-2 cells. HK-2 cells were treated with cycle stretch (20% elongation, 6 cpm) for indicated time periods (A and B). Expression of β1-integrin was determined by Western blotting (A) and quantitative RT-PCR (B). β-Actin was used to verify equivalent loading. Values are means ± SE of 5 independent experiments. Bar graphs depict the quantitative densitometry analysis for Western blot densitometry data. *P < 0.05, **P < 0.01, ***P < 0.001 vs. no stretch (control) cells.
Fig. 8.
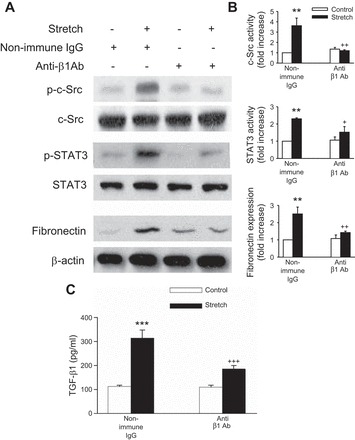
Role of β1-integrin in stretch-induced renal fibrosis. A: HK-2 cells were stimulated with cyclic stretch (20% elongation, 6 cpm) in the presence or absence of blocking antibodies (10 μg/ml) to β1-integrin for 15 min (c-Src and STAT3) or 48 h (fibronectin and TGF-β1) in serum-free medium. Cell lysates were immunoblotted using anti-p-c-Src, anti-p-STAT3, and fibronectin antibodies. Fibronectin protein expression levels were normalized to β-actin. B: bar graphs depict the quantitative densitometry analysis for Western blot densitometry data. C: TGF-β1 was analyzed by ELISA. Values are means ± SE of 5 different experiments. **P < 0.01, ***P < 0.001 vs. no stretch (control) cells. +P < 0.05, ++P < 0.01, +++P < 0.001 vs. anti-β1-integrin antibody-treated cells with stretch.
Fig. 9.
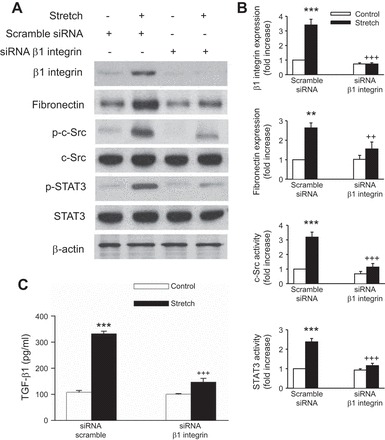
Knockdown of β1-integrin inhibits the expression of stretch-induced c-Src and STAT3 activation and fibronectin and TGF-β1 expression in HK-2 cells. A: HK-2 cells were transfected with control (scramble) siRNA or β1-integrin siRNA followed by cyclic stretch treatment for 15 min (c-Src and STAT3) or 48 h (fibronectin and TGF-β1) in serum-free medium. The phosphorylation of c-Src and STAT3 and expression of fibronectin were determined by Western blotting. Fibronectin protein expression levels were normalized to β-actin. B: bar graphs depict the quantitative densitometry analysis for Western blot densitometry data. Values are means ± SE of 5 different experiments. C: TGF-β1 was analyzed by ELISA. **P < 0.01, ***P < 0.001 vs. no stretch (control) cells. ++P < 0.01, +++P < 0.001 vs. siRNA β1-integrin-treated cells with stretch.
DISCUSSION
Proximal tubular cells secrete a number of profibrotic mediators that may contribute to the pathophysiology of obstructive nephropathy-related disorders. Among these, the expressions of fibronectin and TGF-β1 are upregulated in the UUO model of obstructive nephropathy and may aid the progression to tubulointerstitial fibrosis (12, 25, 31, 34, 37, 55, 66, 68). Understanding the regulatory pathways that control their production may be paramount to developing effective therapeutics to treat these diseases.
Tubular mechanical stretch represents a major insult to proximal tubular cells during obstructive nephropathy. Mechanical stretch represents a unique in vitro model to mimic tubular dilation due to transient increase in intrarenal pressure accompanying obstructive nephropathy and a mechanism to stimulate profibrotic (TGF-β1) and extracellular matrix (fibronectin) gene and protein expression. However, the mechanisms responsible for mechanotransduction of this external strain to TGF-β1 and fibronectin expression are unknown. In this study, cyclic mechanical stretch induces fibronectin and TGF-β1 expression, which was attenuated by pretreatment of proximal tubular cells with a Src family kinase inhibitor, PP2, or by transfection of proximal tubular cells with DN-Src. The involvement of STAT3 in stretch-induced fibronectin and TGF-β1 expression was also confirmed by using a potent and selective STAT3 inhibitor, S3I-201 and by transfection of proximal tubular cells with siRNA of STAT3. Additionally, our results demonstrated for the first time that mechanical stretch is able to induce significant time-dependent increases in β1-integrin mRNA and protein expression in renal proximal tubule cells. Moreover, mechanical stretch-induced fibronectin and TGF-β1 expression was inhibited by blocking antibodies to β1-integrin and by knockdown of β1-integrin by transfection of siRNAs. Thus our results confirmed the notion that the mechanisms underlying the activation of c-Src and STAT3 lead to TGF-β1 synthesis by mechanical stretch and may be essential for the upregulation of fibronectin expression in HK-2 cells. To our knowledge, this is the first direct evidence that the β1-integrin/c-Src/STAT3 signaling pathway mediates fibronectin and TGF-β1 expression induced by mechanical stretch. Thus a novel pathway has been identified for mechanical stretch-initiated cellular events leading to integrin expression, kinase activation, and extracellular matrix synthesis culminating into renal fibrosis.
Integrins are heterodimeric cell surface adhesion proteins that link the ECM to the cytoskeleton and consist of different α- and β-subunits. While not possessing kinase domains, integrin receptors can transduce information from the ECM to the cell to activate various intracellular signaling pathways, thereby regulating cellular processes as diverse as cytoskeletal organization, proliferation, differentiation, apoptosis, and cell migration (8, 30, 47, 62, 65). Numerous studies have linked integrins in the pathogenesis of renal fibrosis. For example, deletion of α1-integrin causes the development of severe glomerulosclerosis in a model of glomerular injury by adriamycin (10). Similarly, anti-integrin-α1 antibodies reduced glomerular and tubulointerstitial scarring in rat models of glomerular injury (14). Obstructed kidneys from mice devoid of α5β6-integrin showed significant less tubulointerstitial fibrosis than observed in the wild-type (39). Recently, Amann et al. (3) showed that anti-αvβ3-integrin-blocking antibodies interfered with glomerulosclerosis during experimental Habu glomerulonephritis. In Alport mice, the expression of αvβ6 correlates with renal fibrosis, and blocking this integrin results in reduced deposition of collagen matrix (21). In several models of kidney diseases, ablation of β1-integrin abrogates profibrotic signaling and blocks accumulation of ECM and the development of tubulointerstitial fibrosis (15, 36, 70). However, the molecular mechanisms by which β1-integrin attenuates UUO-induced fibrosis are not well characterized. Although it had been shown that mechanical stretch induced β1-integrin protein expression in different tissues and that β1-integrin is highly expressed in renal tubular epithelial cells (22, 33, 66, 67, 70), the functions of β1-integrin in renal epithelial cells during mechanical stretch-induced fibrogenesis has never been reported.
Our data indicate that knockdown of β1-integrin attenuates fibronectin and TGF-β1 expression in mechanical stress-induced tubular cells. Furthermore, β1-integrin deficiency attenuated stretch-induced phosphorylation of c-Src and STAT3. The fact that both fibronectin and TGF-β1 expression and c-Src and STAT3 phosphorylation can be blocked by treatment with a β1-integrin-blocking antibody and β1-integrin siRNA strongly suggests that β1-integrin represents an important mechanoreceptor in renal tubular cells. Therefore, we present for the first time an alternative paradigm to αvβ6-induced regulation of TGF-β1 synthesis and renal fibrosis and propose the following model of mechanotransduction in HK-2 cells, as shown in Fig. 10: Conceivably, mechanical stretch, exerted by tubular distension, sensed by β1-integrin receptors, leads to increase β1-integrin mRNA and protein levels. β1-Integrin acts as a signaling molecule, activating c-Src. c-Src then phosphorylates STAT3 at Tyr705. This phosphorylation leads to increases synthesis of TGF-β1. Finally, TGF-β1 can modulate the expression of fibronectin, which may play a role in renal fibrosis.
Fig. 10.
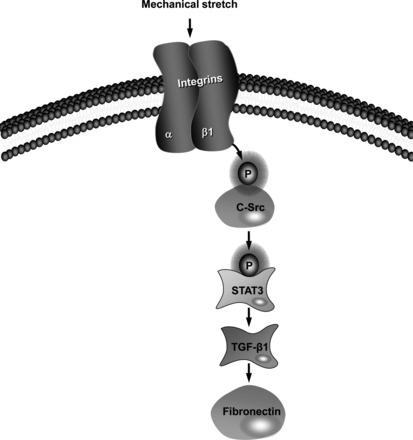
Schematic diagram outlines the proposed pathway of mechanotransduction linking β1-integrin to fibronectin expression in HK-2 cells. Cyclic stretch increases β1-integrin expression, which leads to the phosphorylation and activation c-Src. This results in the increased tyrosine phosphorylation of STAT3, which promotes TGF-β1 production. TGF-β1 increases fibronectin synthesis.
It is well established that STAT3 inhibition plays an important role in protecting the kidney during several types of renal injury. Yang et al. (69) reported that JAK2/STAT1/3 signals were activated in the kidneys after ischemia-reperfusion (I/R) and that blockage of JAK2 by AG490 attenuates I/R-induced renal injury. Others have reported STAT3 activation in renal tubular cells in response to experimental UUO (40, 46) and have demonstrated that S3I-201, a STAT3 inhibitor, decreased the expression of profibrotic markers following obstructive injury (46). In addition, in vitro, S3I-201 attenuated tubular cell profibrotic cellular changes and apoptosis in response to IL-18 stimulation (40). Broadbelt et al. (6) recently demonstrated that pressurization of human proximal tubular (HKC-8) cells leads to phosphorylation of the EGFR with subsequent activation of STAT3. These studies suggest that STAT3 may be an important molecule in the mechanical signaling of renal tubular cells during obstructive nephropathy. In this report, it is demonstrated that STAT3 is required for TGF-β1 and fibronectin expression by immortalized tubular epithelial cells exposed to cyclic mechanical stretch. Indeed, when STAT3 phosphorylation was functionally blocked in HK-2 cells with pharmacological inhibitors or following transient STAT3 siRNA treatment, there was inhibition of expression of TGF-β1 and fibronectin. In contrast, there was no inhibition of c-Src activation. These data offer further evidence for an important role for STAT3 in mediating renal injury.
Src proteins consist of at least 14 related alternatively spliced gene products which are expressed in a tissue-specific manner (5). Src is activated by dephosphorylation of tyrosine residue at the C terminal to unfold the protein followed by tyrosine residue phosphorylation. The Src family members Fyn, Lyn, and c-Src play a key role in the signaling in response to mechanical stretch (1, 43, 44, 49, 64). For example, in vascular smooth muscle cells (VSMC), mechanical stretch-induced phosphoinositide 3-kinase (PI3-K)/protein kinase B (Akt), p21ras, and ERK1/2 activation has been suggested to be mediated at least in part by Src since pharmacological inhibition or overexpression of a kinase-dead c-Src mutant blocked these effects (26, 56). In vascular endothelial cells deficient of the Src family kinase Fyn, platelet endothelial cell adhesion molecule-1 (PECAM-1) activation by both stretch and flow was blocked, whereas knockdown of c-Src and Yes was ineffective (13). In contrast, treatment with c-Src pharmacological inhibitors or dominant-negative mutants of c-Src blocked stretch-induced focal adhesion kinase (FAK) activation in cardiac myocytes (63). These findings provide compelling evidence that Src family-related proteins play a critical role in cyclic mechanical strain-related signaling. Of the members of the Src kinase family, we have reported that mechanical stretch activates c-Src and enhances the activation of ERK1/2 in renal proximal tubular cells (1). Moreover, a variety of experimental studies implicate c-Src in the pathogenesis of progressive renal fibrosis (27, 29, 45). Additionally, numerous studies indicate that c-Src is critical for TGF-β1-mediated renal fibrosis (7, 23, 48). In line with the previously described role for c-Src in mediating renal fibrosis, we recently demonstrated that blockade of c-Src in proximal tubular cells is associated with an decrease in fibronectin expression induced by angiotensin II and G protein βγ-subunits (2). We now provide evidence that c-Src plays a critical role in regulating renal fibrosis induced by cyclic mechanical stretch. Using both pharmacological inhibitors and dominant-negative mutants of c-Src, we have demonstrated that c-Src mediates the induction of TGF-β1 and fibronectin in response to mechanical stretch. In addition, the activation of c-Src results in an increase in the phosphorylation levels of STAT3, suggesting that it is an upstream regulator of STAT3.
Collectively, the present study provides insight into the expression of β1- integrin receptors in human proximal tubular cells exposed to mechanical stress and sheds light on how the β1-integrin receptor may play a role in renal fibrosis by transducing mechanical stimuli into biochemical signals, including activation of c-Src. Moreover, our studies demonstrates that, in the absence of β1-integrin, human proximal tubular cells fail to activate the signaling cascade that would otherwise lead to the synthesis of profibrotic proteins and ultimately to the development of renal fibrosis, suggesting that β1-integrin inhibition may be a promising novel therapeutic approach in the treatment of a wide variety of fibrotic chronic kidney diseases such as obstructive nephropathy.
GRANTS
This work was supported by University of Michigan-Dearborn and Midwestern University Intramural Awards to L. D. Alexander.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: M.T.H., R.S., and L.D.A. performed experiments; M.T.H., R.S., and L.D.A. analyzed data; M.T.H., R.S., and L.D.A. interpreted results of experiments; M.T.H., R.S., and L.D.A. edited and revised manuscript; M.T.H., R.S., and L.D.A. approved final version of manuscript; L.D.A. provided conception and design of research; L.D.A. prepared figures; L.D.A. drafted manuscript.
ACKNOWLEDGMENTS
Part of this work was presented as an abstract at the Experimental Biology Meeting 2014 in San Diego, CA.
REFERENCES
- 1.Alexander LD, Alagarsamy S, Douglas JG. Cyclic stretch-induced cPLA2 mediates ERK 1/2 signaling in rabbit proximal tubule cells. Kidney Int 65: 551–563, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Alexander LD, Ding Y, Alagarsamy S, Cui X. Angiotensin II stimulates fibronectin protein synthesis via a Gβγ/arachidonic acid-dependent pathway. Am J Physiol Renal Physiol 307: F287–F302, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann K, Haas CS, Schussler J, Daniel C, Hartner A, Schocklmann HO. Beneficial effects of integrin alphavbeta3-blocking RGD peptides in early but not late phase of experimental glomerulonephritis. Nephrol Dial Transplant 27: 1755–1768, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Baraldi A, Zambruno G, Furci L, Ballestri M, Tombesi A, Ottani D, Lucchi L, Lusvarghi E. Beta 1 and beta 3 integrin upregulation in rapidly progressive glomerulonephritis. Nephrol Dial Transplant 10: 1155–1161, 1995. [PubMed] [Google Scholar]
- 5.Bolen JB, Rowley RB, Spana C, Tsygankov AY. The Src family of tyrosine protein kinases in hemopoietic signal transduction. FASEB J 6: 3403–3409, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Broadbelt NV, Chen J, Silver RB, Poppas DP, Felsen D. Pressure activates epidermal growth factor receptor leading to the induction of iNOS via NF-κB and STAT3 in human proximal tubule cells. Am J Physiol Renal Physiol 297: F114–F124, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFbeta-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SY, Lin JS, Yang BC. Modulation of tumor cell stiffness and migration by type IV collagen through direct activation of integrin signaling pathway. Arch Biochem Biophys 555–556: 1–8, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Abair TD, Ibanez MR, Su Y, Frey MR, Dise RS, Polk DB, Singh AB, Harris RC, Zent R, Pozzi A. Integrin alpha1beta1 controls reactive oxygen species synthesis by negatively regulating epidermal growth factor receptor-mediated Rac activation. Mol Cell Biol 27: 3313–3326, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Moeckel G, Morrow JD, Cosgrove D, Harris RC, Fogo AB, Zent R, Pozzi A. Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury. Am J Pathol 165: 617–630, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen-Scarabelli C, Saravolatz IL, McCaukey R, Scarabelli G, Di RJ, Mohanty B, Barry S, Latchman D, Georgiadis V, McCormick J, Saravolatz L, Knight R, Faggian G, Narula J, Stephanou A, Scarabelli TM. The cardioprotective effects of urocortin are mediated via activation of the Src tyrosine kinase-STAT3 pathway. JAKSTAT 2: e24812, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang CK, Sheu ML, Lin YW, Wu CT, Yang CC, Chen MW, Hung KY, Wu KD, Liu SH. Honokiol ameliorates renal fibrosis by inhibiting extracellular matrix and pro-inflammatory factors in vivo and in vitro. Br J Pharmacol 163: 586–597, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiu YJ, McBeath E, Fujiwara K. Mechanotransduction in an extracted cell model: Fyn drives stretch- and flow-elicited PECAM-1 phosphorylation. J Cell Biol 182: 753–763, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook HT, Khan SB, Allen A, Bhangal G, Smith J, Lobb RR, Pusey CD. Treatment with an antibody to VLA-1 integrin reduces glomerular and tubulointerstitial scarring in a rat model of crescentic glomerulonephritis. Am J Pathol 161: 1265–1272, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cybulsky AV, Carbonetto S, Huang Q, McTavish AJ, Cyr MD. Adhesion of rat glomerular epithelial cells to extracellular matrices: role of beta 1 integrins. Kidney Int 42: 1099–1106, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Diamond JR, Kreisberg R, Evans R, Nguyen TA, Ricardo SD. Regulation of proximal tubular osteopontin in experimental hydronephrosis in the rat. Kidney Int 54: 1501–1509, 1998. [DOI] [PubMed] [Google Scholar]
- 17.El CM, Attia E, Chen J, Hannafin J, Poppas DP, Felsen D. Cyclooxygenase-2 inhibitor decreases extracellular matrix synthesis in stretched renal fibroblasts. Nephron Exp Nephrol 100: e150–e155, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Elias BC, Mathew S, Srichai MB, Palamuttam R, Bulus N, Mernaugh G, Singh AB, Sanders CR, Harris RC, Pozzi A, Zent R. The integrin beta1 subunit regulates paracellular permeability of kidney proximal tubule cells. J Biol Chem 289: 8532–8544, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev 9: 1896–1908, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Glynne PA, Picot J, Evans TJ. Coexpressed nitric oxide synthase and apical beta(1) integrins influence tubule cell adhesion after cytokine-induced injury. J Am Soc Nephrol 12: 2370–2383, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun WL, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF III, Sheppard D, Gardner HA, Violette SM. Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170: 110–125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han W, Zhao H, Jiao B, Liu F. EPA and DHA increased PPARgamma expression and deceased integrin-linked kinase and integrin beta1 expression in rat glomerular mesangial cells treated with lipopolysaccharide. Biosci Trends 8: 120–125, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Hu M, Che P, Han X, Cai GQ, Liu G, Antony V, Luckhardt T, Siegal GP, Zhou Y, Liu RM, Desai LP, O'Reilly PJ, Thannickal VJ, Ding Q. Therapeutic targeting of SRC kinase in myofibroblast differentiation and pulmonary fibrosis. J Pharmacol Exp Ther 351: 87–95, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang SJ, Hwang YJ, Yun MO, Kim JH, Oh GS, Park JH. Indoxyl 3-sulfate stimulates Th17 differentiation enhancing phosphorylation of c-Src and STAT3 to worsen experimental autoimmune encephalomyelitis. Toxicol Lett 220: 109–117, 2013. [DOI] [PubMed] [Google Scholar]
- 25.Isaka Y, Tsujie M, Ando Y, Nakamura H, Kaneda Y, Imai E, Hori M. Transforming growth factor-beta 1 antisense oligodeoxynucleotides block interstitial fibrosis in unilateral ureteral obstruction. Kidney Int 58: 1885–1892, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki H, Yoshimoto T, Sugiyama T, Hirata Y. Activation of cell adhesion kinase beta by mechanical stretch in vascular smooth muscle cells. Endocrinology 144: 2304–2310, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Jennings BL, Montanez DE, May ME Jr, Estes AM, Fang XR, Yaghini FA, Kanu A, Malik KU. Cytochrome P450 1B1 contributes to increased blood pressure and cardiovascular and renal dysfunction in spontaneously hypertensive rats. Cardiovasc Drugs Ther 28: 145–161, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kagami S, Border WA, Ruoslahti E, Noble NA. Coordinated expression of beta 1 integrins and transforming growth factor-beta-induced matrix proteins in glomerulonephritis. Lab Invest 69: 68–76, 1993. [PubMed] [Google Scholar]
- 29.Kamato D, Burch ML, Piva TJ, Rezaei HB, Rostam MA, Xu S, Zheng W, Little PJ, Osman N. Transforming growth factor-beta signalling: role and consequences of Smad linker region phosphorylation. Cell Signal 25: 2017–2024, 2013. [DOI] [PubMed] [Google Scholar]
- 30.Karamessinis PM, Tzinia AK, Kitsiou PV, Stetler-Stevenson WG, Michael AF, Fan WW, Zhou B, Margaritis LH, Tsilibary EC. Proximal tubular epithelial cell integrins respond to high glucose by altered cell-matrix interactions and differentially regulate matrixin expression. Lab Invest 82: 1081–1093, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Kim D, Lee AS, Jung YJ, Yang KH, Lee S, Park SK, Kim W, Kang KP. Tamoxifen ameliorates renal tubulointerstitial fibrosis by modulation of estrogen receptor alpha-mediated transforming growth factor-beta1/Smad signaling pathway. Nephrol Dial Transplant 29: 2043–2053, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Kreidberg JA, Symons JM. Integrins in kidney development, function, and disease. Am J Physiol Renal Physiol 279: F233–F242, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Lal H, Verma SK, Smith M, Guleria RS, Lu G, Foster DM, Dostal DE. Stretch-induced MAP kinase activation in cardiac myocytes: differential regulation through beta1-integrin and focal adhesion kinase. J Mol Cell Cardiol 43: 137–147, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J, Hwang I, Lee JH, Lee HW, Jeong LS, Ha H. The selective A3AR antagonist LJ-1888 ameliorates UUO-induced tubulointerstitial fibrosis. Am J Pathol 183: 1488–1497, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Lee JS, Lim JY, Kim J. Mechanical stretch induces angiotensinogen expression through PARP1 activation in kidney proximal tubular cells. In Vitro Cell Dev Biol Anim [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 36.Lee K, Boctor S, Barisoni LM, Gusella GL. Inactivation of integrin-beta1 prevents the development of polycystic kidney disease after the loss of polycystin-1. J Am Soc Nephrol [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li S, Mariappan N, Megyesi J, Shank B, Kannan K, Theus S, Price PM, Duffield JS, Portilla D. Proximal tubule PPARα attenuates renal fibrosis and inflammation caused by unilateral ureteral obstruction. Am J Physiol Renal Physiol 305: F618–F627, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lieberthal W, McKenney JB, Kiefer CR, Snyder LM, Kroshian VM, Sjaastad MD. Beta1 integrin-mediated adhesion between renal tubular cells after anoxic injury. J Am Soc Nephrol 8: 175–183, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice. Am J Pathol 163: 1261–1273, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsui F, Rhee A, Hile KL, Zhang H, Meldrum KK. IL-18 induces profibrotic renal tubular cell injury via STAT3 activation. Am J Physiol Renal Physiol 305: F1014–F1021, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyajima A, Chen J, Kirman I, Poppas DP, Darracott Vaughan EJR, Felsen D. Interaction of nitric oxide and transforming growth factor-beta1 induced by angiotensin II and mechanical stretch in rat renal tubular epithelial cells. J Urol 164: 1729–1734, 2000. [PubMed] [Google Scholar]
- 42.Miyajima A, Chen J, Lawrence C, Ledbetter S, Soslow RA, Stern J, Jha S, Pigato J, Lemer ML, Poppas DP, Vaughan ED, Felsen D. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int 58: 2301–2313, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Niediek V, Born S, Hampe N, Kirchgessner N, Merkel R, Hoffmann B. Cyclic stretch induces reorientation of cells in a Src family kinase- and p130Cas-dependent manner. Eur J Cell Biol 91: 118–128, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Niu A, Wen Y, Liu H, Zhan M, Jin B, Li YP. Src mediates the mechanical activation of myogenesis by activating TNFalpha-converting enzyme. J Cell Sci 126: 4349–4357, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oelusarz A, Nichols LA, Grunz-Borgmann EA, Chen G, Akintola AD, Catania JM, Burghardt RC, Trzeciakowski JP, Parrish AR. Overexpression of MMP-7 increases collagen 1A2 in the aging kidney. Physiol Rep 1: e00090, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, Chin YE, Yan H, Dworkin LD, Zhuang S. A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 78: 257–268, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Park SW, Yun JH, Kim JH, Kim KW, Cho CH, Kim JH. Angiopoietin 2 induces pericyte apoptosis via alpha3beta1 integrin signaling in diabetic retinopathy. Diabetes 63: 3057–3068, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Peng F, Zhang B, Wu D, Ingram AJ, Gao B, Krepinsky JC. TGFβ-induced RhoA activation and fibronectin production in mesangial cells require caveolae. Am J Physiol Renal Physiol 295: F153–F164, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plotkin LI, Mathov I, Aguirre JI, Parfitt AM, Manolagas SC, Bellido T. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol 289: C633–C643, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Proietti C, Salatino M, Rosemblit C, Carnevale R, Pecci A, Kornblihtt AR, Molinolo AA, Frahm I, Charreau EH, Schillaci R, Elizalde PV. Progestins induce transcriptional activation of signal transducer and activator of transcription 3 (Stat3) via a Jak- and Src-dependent mechanism in breast cancer cells. Mol Cell Biol 25: 4826–4840, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ricardo SD, Ding G, Eufemio M, Diamond JR. Antioxidant expression in experimental hydronephrosis: role of mechanical stretch and growth factors. Am J Physiol Renal Physiol 272: F789–F798, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Ricardo SD, Franzoni DF, Roesener CD, Crisman JM, Diamond JR. Angiotensinogen and AT1 antisense inhibition of osteopontin translation in rat proximal tubular cells. Am J Physiol Renal Physiol 278: F708–F716, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Roy-Chaudhury P, Hillis G, McDonald S, Simpson JG, Power DA. Importance of the tubulointerstitium in human glomerulonephritis. II. Distribution of integrin chains beta 1, alpha 1 to 6 and alpha V. Kidney Int 52: 103–110, 1997. [DOI] [PubMed] [Google Scholar]
- 54.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112: 1486–1494, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sawashima K, Mizuno S, Mizuno-Horikawa Y, Kudo T, Kurosawa T. Protein restriction ameliorates renal tubulointerstitial nephritis and reduces renal transforming growth factor-beta expression in unilateral ureteral obstruction. Exp Nephrol 10: 7–18, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Sedding DG, Hermsen J, Seay U, Eickelberg O, Kummer W, Schwencke C, Strasser RH, Tillmanns H, Braun-Dullaeus RC. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res 96: 635–642, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Sonomura K, Okigaki M, Kimura T, Matsuoka E, Shiotsu Y, Adachi T, Kado H, Ishida R, Kusaba T, Matsubara H, Mori Y. The kinase Pyk2 is involved in renal fibrosis by means of mechanical stretch-induced growth factor expression in renal tubules. Kidney Int 81: 449–457, 2012. [DOI] [PubMed] [Google Scholar]
- 58.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev 9: 1883–1895, 1995. [DOI] [PubMed] [Google Scholar]
- 59.Tang CH, Yang RS, Chen YF, Fu WM. Basic fibroblast growth factor stimulates fibronectin expression through phospholipase C gamma, protein kinase C alpha, c-Src, NF-kappaB, and p300 pathway in osteoblasts. J Cell Physiol 211: 45–55, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Tang CH, Yang RS, Fu WM. Prostaglandin E2 stimulates fibronectin expression through EP1 receptor, phospholipase C, protein kinase Calpha, and c-Src pathway in primary cultured rat osteoblasts. J Biol Chem 280: 22907–22916, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Tang XH, Huang SM, Tan SQ, Ma YL. [Effect of rosiglitazone on integrin beta1 expression and apoptosis of proximal tubular cell exposed to high glucose]. Sichuan Da Xue Xue Bao Yi Xue Ban 38: 291–294, 2007. [PubMed] [Google Scholar]
- 62.Tian B, Lessan K, Kahm J, Kleidon J, Henke C. Beta 1 integrin regulates fibroblast viability during collagen matrix contraction through a phosphatidylinositol 3-kinase/Akt/protein kinase B signaling pathway. J Biol Chem 277: 24667–24675, 2002. [DOI] [PubMed] [Google Scholar]
- 63.Torsoni AS, Constancio SS, Nadruz W Jr, Hanks SK, Franchini KG. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ Res 93: 140–147, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Wang S, Gong H, Jiang G, Ye Y, Wu J, You J, Zhang G, Sun A, Komuro I, Ge J, Zou Y. Src is required for mechanical stretch-induced cardiomyocyte hypertrophy through angiotensin II type 1 receptor-dependent beta-arrestin2 pathways. PLoS One 9: e92926, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z, Symons JM, Goldstein SL, McDonald A, Miner JH, Kreidberg JA. (Alpha)3(beta)1 integrin regulates epithelial cytoskeletal organization. J Cell Sci 112: 2925–2935, 1999. [DOI] [PubMed] [Google Scholar]
- 66.Wei X, Xia Y, Li F, Tang Y, Nie J, Liu Y, Zhou Z, Zhang H, Hou FF. Kindlin-2 mediates activation of TGF-beta/Smad signaling and renal fibrosis. J Am Soc Nephrol 24: 1387–1398, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wernig F, Mayr M, Xu Q. Mechanical stretch-induced apoptosis in smooth muscle cells is mediated by beta1-integrin signaling pathways. Hypertension 41: 903–911, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Xu W, Shao X, Tian L, Gu L, Zhang M, Wang Q, Wu B, Wang L, Yao J, Xu X, Mou S, Ni Z. Astragaloside IV ameliorates renal fibrosis via the inhibition of mitogen-activated protein kinases and antiapoptosis in vivo and in vitro. J Pharmacol Exp Ther 350: 552–562, 2014. [DOI] [PubMed] [Google Scholar]
- 69.Yang N, Luo M, Li R, Huang Y, Zhang R, Wu Q, Wang F, Li Y, Yu X. Blockage of JAK/STAT signalling attenuates renal ischaemia-reperfusion injury in rat. Nephrol Dial Transplant 23: 91–100, 2008. [DOI] [PubMed] [Google Scholar]
- 70.Yeh YC, Wei WC, Wang YK, Lin SC, Sung JM, Tang MJ. Transforming growth factor-β1 induces Smad3-dependent β1 integrin gene expression in epithelial-to-mesenchymal transition during chronic tubulointerstitial fibrosis. Am J Pathol 177: 1743–1754, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zent R, Yan X, Su Y, Hudson BG, Borza DB, Moeckel GW, Qi Z, Sado Y, Breyer MD, Voziyan P, Pozzi A. Glomerular injury is exacerbated in diabetic integrin alpha1-null mice. Kidney Int 70: 460–470, 2006. [DOI] [PubMed] [Google Scholar]


