Abstract
The way that a motor adaptation is trained, for example, the manner in which it is introduced or the duration of the training period, can influence its internal representation. However, recent studies examining the gradual versus abrupt introduction of a novel environment have produced conflicting results. Here we examined how these effects determine the effector specificity of motor adaptation during visually guided reaching. After adaptation to velocity-dependent dynamics in the right arm, we estimated the amount of adaptation transferred to the left arm, using error-clamp measurement trials to directly measure changes in learned dynamics. We found that a small but significant amount of generalization to the untrained arm occurs under three different training schedules: a short-duration (15 trials) abrupt presentation, a long-duration (160 trials) abrupt presentation, and a long-duration gradual presentation of the novel dynamic environment. Remarkably, we found essentially no difference between the amount of interlimb generalization when comparing these schedules, with 9–12% transfer of the trained adaptation for all three. However, the duration of training had a pronounced effect on the stability of the interlimb transfer: The transfer elicited from short-duration training decayed rapidly, whereas the transfer from both long-duration training schedules was considerably more persistent (<50% vs. >90% retention over the first 20 trials). These results indicate that the amount of interlimb transfer is similar for gradual versus abrupt training and that interlimb transfer of learned dynamics can occur after even a brief training period but longer training is required for an enduring effect.
Keywords: motor adaptation, interlimb generalization, reaching arm movements, training schedule
there is mounting evidence that when training a novel motor skill, the way in which it is introduced can affect future performance (Bock et al. 2005; Savion-Lemieux and Penhune 2005; Yin and Kitazawa 2001). One example is that a novel environment can be introduced suddenly on a single trial or gradually over the course of many trials. Remarkably, the introduction rate for a novel environment has been suggested to affect the retention (Huang and Shadmehr 2009; Kagerer et al. 1997; Kluzik et al. 2008; Wong and Shelhamer 2011), the effector specificity (Malfait and Ostry 2004), and the internal representation of the newly formed motor memory (Berniker and Kording 2008).
One recent study suggested that the rate at which a physical disturbance was introduced during visually guided reaching movements determined whether the adaptation would transfer across arms (Malfait and Ostry 2004). The authors compared two training schedules: short-duration training (15 trials) with abrupt presentation of a velocity-dependent force field and long-duration training (160 trials) with gradual application of the field. They found substantial transfer (∼50%) for the abrupt schedule but no significant transfer for the gradual schedule, suggesting that the latter may lead to increased effector specificity. The substantial transfer Malfait and Ostry observed after abrupt presentation was in line with a previous study (Criscimagna-Hemminger et al. 2003) that showed a large amount of transfer from the right to the left arm. However, the latter experiment did not examine gradual presentation or distinguish the interlimb transfer of the trained adaptation from the ability to improve the learning rate in the untrained arm, because performance in the untrained limb was not assessed until 15 trials into the transfer period. In contrast, two recent studies of visuomotor rotation learning have directly compared the effects of gradual and abrupt presentation on the immediate transfer of motor adaptation from one limb to another (Taylor et al. 2011; Wang et al. 2011a). Intriguingly, both studies found that the effector specificity of motor adaptation is not increased by gradual training when the duration of the training periods is equated. These results appear to be in conflict with those reported for learning physical dynamics. However, two issues complicate the comparison: First, adaptations to physical versus visual perturbations are believed to involve at least partially distinct neural mechanisms (Krakauer et al. 1999; Rabe et al. 2009; Tanaka et al. 2009). Second, the marked difference in training duration between the abrupt and gradual conditions in the Malfait and Ostry (2004) study raises the possibility that effector specificity is determined by the duration rather than the schedule of training.
The conflicting reports described above call the general relationship between the training schedule and the effector specificity of motor adaptation into question. However, understanding the determinants of interlimb transfer is a critical issue as it has implications both for the design of rehabilitation strategies that incorporate interlimb transfer (Raghavan et al. 2006) and for vetting computational models of learning that make predictions about this transfer (Berniker and Kording 2008, 2011). We therefore examined the transfer of learned arm dynamics for gradual versus abrupt presentation schedules, while controlling the duration of training so that the effects of introduction rate and training duration could be independently determined. After training subjects under three different schedules (a short-duration abrupt presentation, a long-duration abrupt presentation, and a long-duration gradual presentation of novel velocity-dependent physical dynamics), we examined interlimb transfer, using error-clamp trials to directly measure the learning-related changes in feedforward motor output (Scheidt et al. 2000; Smith et al. 2006). We find that the training schedule has little effect on effector specificity. However, the transferred adaptation was stable for long-duration training regardless of the introduction rate but decayed rapidly when training was short, indicating that the duration of training determines the stability of interlimb generalization.
METHODS
Participants
Thirty-two healthy subjects without known neurological impairment were recruited from the Harvard University community to participate in the study. Twenty-four subjects completed the main experiment that examined interlimb adaptation transfer measured with error-clamp trials, and eight participated in the control experiment that examined transfer measured with force-field trials. All participants were right handed. The study protocol was approved by the Harvard University Institutional Review Board, and all participants gave informed consent.
Experimental Setup
The experimental paradigm was patterned after the standard force-field adaptation paradigm (Shadmehr and Mussa-Ivaldi 1994). Subjects were trained to move their hands to targets in the horizontal plane while grasping a robot manipulandum (Fig. 1A). The manipulandum measured hand position, velocity, and force, and its motors were used to apply forces to the hand, all at a sampling rate of 200 Hz. The position of the hand was shown as a small round cursor (3 mm) on a vertically oriented computer monitor in front of the participant (refresh rate of 75 Hz). Participants reached to circular targets 1 cm in diameter that were spaced 10 cm apart. We instructed them to “make quick movements to the targets.” Subjects were also told that the reaction time was not important—they could wait as long as they wished after target appearance before starting each movement—but, when ready, they were to move in a rapid motion toward each target. The end point of each movement was used as the starting point for the subsequent movement, and movements were made in two target directions.
Fig. 1.
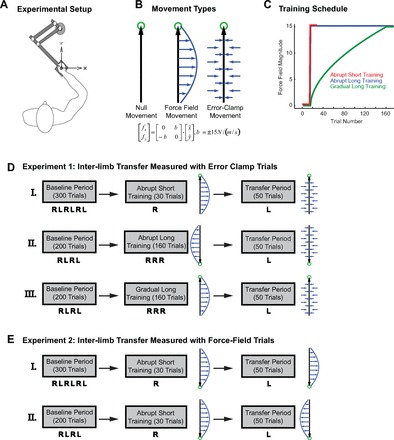
A: experimental setup and protocol. While sitting in front of a computer screen, subjects made reaching movements toward and away from the body (along the y-axis) while holding the handle of the robotic manipulandum. B: 3 trial types employed in the experiment: null trials, force-field trials, and error-clamp trials. During null trials, the motors of the manipulandum were turned off. During force-field trials, the motors of the manipulandum produced forces on the hand (blue arrows) that were proportional in magnitude and perpendicular in direction to the velocity of hand motion (black arrow). Forces were calculated as a function of hand velocity: , with a skew-symmetric viscosity matrix B as shown in the figure. During error-clamp trials, the robot motors constrained the movements in a straight line toward the target by counteracting any motion perpendicular to the target direction. C: time course of force-field amplitude exerted by the robot during the adaptation period for the 3 training schedules. D and E: example experiment protocols showing the sequence, movement direction, and force-field orientation for the different training schedules tested in experiments 1 and 2. The number of trials in each gray rectangle corresponds to the total number of trials for the experimental period. The letters below each experimental period represent the hand used during each block of the period (L, left hand; R, right hand). Thus if the both the left and right hands were used during an experimental period, the total number of trials was divided between the 2 limbs. The direction of the arm movement (vertical black arrow) and force-field direction (blue arrows) were randomized across subjects, and an example protocol is shown on right of each experimental period. Both the right and left hands were used during the baseline period (left). Only the right hand was used during the adaptation period (center), and only the left hand was used during the transfer period (right). Interlimb transfer was measured with error-clamp trials (experiment 1) or force-field trials (experiment 2).
Three trial types were used: null trials, force-field (clockwise or counterclockwise) trials, and error-clamp trials (Fig. 1B). Null trials were used for initial practice and washout between the different epochs of the experiment. During these trials, the motors of the robot manipulandum were turned off. During force-field trials, the motors were used to produce forces on the hand that were proportional in magnitude and perpendicular in direction to the velocity of hand motion. The relationship between force () and velocity () vectors was determined by the 2 × 2 matrix via the relationship . The skew-symmetric shape of B results in a curl force field in which is rotated ±90° from . In other words, the force and velocity vectors are orthogonal. Note that the value of b determines the orientation (clockwise or counterclockwise) and the magnitude of the force field exerted by the manipulandum and has units of N/(m/s). During error-clamp trials, the robot motors were used to constrain movements in a straight line toward the target by counteracting any motion perpendicular to the target direction (Gonzalez Castro et al. 2011a; Joiner et al. 2011; Joiner and Smith 2008; Scheidt et al. 2000; Sing et al. 2013; Smith et al. 2006; Wagner and Smith 2008). This was achieved by applying a stiff one-dimensional spring (6 kN/m) and damper (150 Ns/m) in the axis perpendicular to the target direction. In these trials, perpendicular displacement from a straight line to the target was held to <0.6 mm and averaged ∼0.2 mm in magnitude.
Interlimb Adaptation Transfer Measured Through Error-Clamp Trials
In the first experiment, 24 subjects were trained to move in a velocity-dependent force field with their right hand and were tested for transfer of adaptation to their untrained left hand. A sample of the experimental procedure is depicted in Fig. 1D. In the baseline period of the first testing session (I), subjects completed 3 blocks of 50 trials each for both the right and left hands (6 blocks in total). The letters below each period in Fig. 1D represent the hand used (L, left hand; R, right hand), the sequence used during the period, and the number of blocks within the period. For example, the sequence R L R L R L represents a 6-block sequence beginning with the right hand and switching back and forth to the left hand. Thus subjects switched between the left and right hands between blocks. The first block for each hand during the baseline period contained only null-field trials, whereas the last two blocks in the baseline period for each hand contained a combination of null (80%) and error-clamp (20%) trials. The baseline period was then followed by one of three training sessions (described below). We tested the two training sequences used by Malfait and Ostry (2004) and a training sequence similar to that used by Criscimagna-Hemminger et al. (2003) for interlimb transfer of learned dynamics. The progression of the force-field magnitude, |b|, exerted by the robot for each training sequence is shown in Fig. 1C.
Short abrupt training.
As shown by the red trace in Fig. 1C, after 15 null trials the force field was introduced suddenly and the training period was short (15 force-field trials). As in the Malfait and Ostry (2004) abrupt experiment, the value of |b| changed from 0 to 15 Ns/m between the 15th and the 16th trial and remained at 15 Ns/m for the last 15 movements. Note that the sign of b was randomly varied from one subject to the next. These 30 trials comprised one block. Transfer to the left hand was then tested by one block of 50 consecutive error-clamp trials. The training and transfer periods were thus divided into one block of 30 trials and one block of 50 trials (sequence I in Fig. 1D).
Long abrupt training.
As shown by the blue trace in Fig. 1C, similar to the training sequence used by Criscimagna-Hemminger et al. (2003), after 15 null trials the force field was introduced suddenly and the training period was long (160 force-field trials). As in abrupt short training, the value of |b| switched from 0 to 15 Ns/m between the 15th and the 16th trial but remained at 15 Ns/m for the last 160 movements of training. Transfer to the left hand was again tested by a block of 50 consecutive error-clamp trials. Here the entire training and transfer session was divided into four blocks of ∼50 trials each (sequence II in Fig. 1D).
Long gradual training.
As shown by the green trace in Fig. 1C, after 15 null trials the force field was introduced gradually and the training period was long (160 force-field trials). Following Malfait and Ostry (2004), we changed the value of |b| smoothly from 0 to 15 Ns/m over the first 145 trials in a nonlinear pattern:
| (1) |
where n is the trial number and
| (2) |
Note that this choice of x results in |b| = 1 Ns/m on the first trial after introduction and
| (3) |
on the 145th trial. The amplitude of the field then remained constant at |b| = 15 Ns/m for the final 15 trials. As in the long abrupt training, the entire training and transfer session was divided into four blocks of ∼50 trials each (sequence III in Fig. 1D) and transfer to the left hand was tested during a block of 50 consecutive error-clamp trials.
Subjects completed each of the three sessions spanning a total of 24 blocks in ∼2 h. As depicted in Fig. 1D, subjects completed additional baseline blocks (4 blocks with 200 total trials, 100 trials for each limb) at the start of sessions II and III. These trials served as a washout of the training and transferred adaptation to ensure a return to baseline performance for the start of the next experimental session. As in session I, each baseline block was a combination of null-field (80%) and error-clamp (20%) trials.
To ensure that early training did not influence adaptation later in the experiment, we also switched the direction of the force field and movement direction between consecutive sessions. As depicted in Fig. 1D, the force field and movement direction for first and third training were always in the same direction (in this case clockwise and away from the body). However, the force field and movement direction for the second training session was always in the opposite direction (in this example, counterclockwise and toward the body). The order of training sessions (6 possible combinations of the long abrupt, long gradual, and short abrupt training sessions), force field direction (2 directions, clockwise or counterclockwise), and movement direction (2 directions, toward or away from the body) were counterbalanced between subjects (6 × 2 × 2 = 24 possible combinations). Each of the 24 subjects completed one of the combinations. Subjects were given breaks between blocks outside the testing sessions (i.e., in the baseline periods) but not between blocks within the testing sessions (i.e., the adaptation and transfer periods).
Interlimb Adaptation Transfer Measured Through Force-Field Trials
A second experiment, which contained only null and force-field trials, was conducted as a control on an additional eight subjects to further examine the role of limb stiffness in the interlimb transfer of adaptation to novel dynamics. Critically, in this experiment we compared a kinematic measure of transfer (angular deviation) in conditions for which the effects of limb stiffness and motor adaptation would have contrasting effects. Specifically, we compared transfer into force fields that were the same versus opposite from the trained adaptation, where transfer of the trained adaptation would have opposite effects but increases in stiffness would have identical effects. In particular, transfer of the trained adaptation would result in straighter movements in the trained field but more deviated movements in the opposite field, whereas increased stiffness would result in straighter movements in both fields. An example of the experimental procedure is depicted in Fig. 1E. In the first baseline period, subjects completed three blocks of 50 trials each for both the right and left hands (6 blocks total). As in the main experiment, subjects switched between the left and right hands between blocks. The first two blocks for each hand contained only null trials, and the last block of baseline for each hand contained mostly null trials with four randomly inserted force-field trials (2 clockwise and 2 counterclockwise trials). These force-field trials served as catch trials and provided an estimate of naive performance before training.
After the initial baseline period, subjects completed two short abrupt training and transfer sessions separated by a second baseline period. The force field directions for the two training sessions were the same (clockwise in the example presented in Fig. 1E). Similar to the experiments performed by Criscimagna-Hemminger et al. (2003), transfer of adaptation was assessed by requiring subjects to make movements with their left hand in a force field that was either in the same direction as that during the adaptation or the opposite direction. That is, the force field for one transfer session (sequence I in Fig. 1E) was the same as the adaptation force field, but the force field for the other transfer session (sequence II in Fig. 1E) was the opposite of that experienced during adaptation. Note that an important difference from the study by Criscimagna-Hemminger et al. (2003) is how transfer of adaptation was estimated, as described below. In the second baseline period separating the two training sessions, subjects again completed two blocks of 50 null-field trials for each hand (200 total trials, 100 for each limb). As in the first baseline period, these blocks consisted of null trials with four randomly inserted force-field (catch) trials (2 clockwise and 2 counterclockwise trials).
Subjects completed each of the two training sessions over a total of 14 blocks (∼1 h in duration). The force field direction during training (2 directions, clockwise or counterclockwise), the force field direction during transfer (2 directions, clockwise or counterclockwise), and movement direction (2 directions, toward or away from the body) were counterbalanced between subjects (2 × 2 × 2 = 8 possible combinations). Each subject completed one of the combinations. As in the first experiment, subjects were given breaks between blocks outside of the testing sessions (i.e., the baseline periods) but not between blocks within a testing session.
Quantifying Adaptation and Transfer with Error-Clamp and Force-Field Trials
Since the environmental perturbations applied during this experiment consisted of forces perpendicular to the direction of motion, we focused our analysis of error-clamp trials on the lateral force profiles that participants generated during movement. In general, lateral force could reflect either an adaptive compensation of expected lateral force or an online corrective response to errors detected during the course of movement. Specifically, we looked at the progression of lateral force profiles during error-clamp trials through the baseline, adaptation, and transfer blocks of the experiment. During these error-clamp trials, lateral errors were kept small (<0.6 mm), so lateral force profiles essentially reflected adaptive compensation of the force-field perturbations. Since full compensation of the force-field perturbation on a particular trial required a lateral force profile proportional to the speed profile on that same trial (and this speed profile varied from one trial to another), we assessed the amount of adaptation on each error-clamp trial by computing a force-field compensation factor found by linear regression of the measured lateral force profile on each error-clamp trial onto the ideal force profile required for full force-field compensation on that trial after subtracting out the baseline force pattern (Gonzalez Castro et al. 2011a; Joiner et al. 2011; Joiner and Smith 2008; Sing et al. 2013; Sing and Smith 2010; Smith et al. 2006; Wagner and Smith 2008). Each regression coefficient characterizes the overall amount of force-field compensation in a given trial. If the applied force and the desired force perfectly coincide this learning metric is 1, if they are directly opposed the metric is −1, and if they are unrelated it will be 0.
We also computed the midmovement force based on these force patterns. This measure was computed as the average lateral force relative to baseline in a window extending from 70 ms before to 70 ms after the peak speed point of the movement (the dark gray stripe in Fig. 2A). This midmovement force estimates the force output at the time when the learned change should be maximal.
Fig. 2.
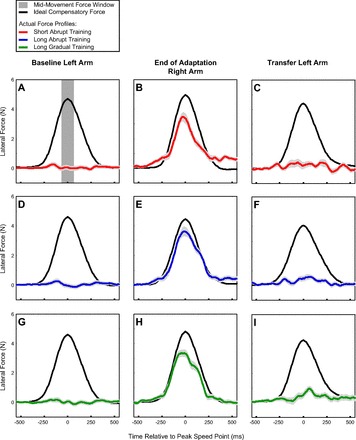
Lateral force profiles associated with training and interlimb transfer in experiment 1. Black trace in each panel shows the ideal compensatory force based on the movement velocity if the full strength force-field perturbation (|b| = 15 Ns/m) were applied. Colored traces show the actual force profiles averaged across subjects measured for the 3 training schedules: abrupt short (red), abrupt long (blue), and gradual long (green). Light gray shaded region represents the SE around the average lateral force profile. A, D, and G: lateral force profiles for the left hand prior to adaptation for the 3 training schedules. Dark gray stripe in A depicts the time window (70 ms before to 70 ms after the peak speed point of the movement) over which the midmovement force was measured. B, E, and H: lateral force profiles for the right hand at the end of adaptation (last 30% of the adaptation period). C, F, and I: lateral force profiles produced by the left hand on the first trial after adaptation of the right hand.
To quantify adaptation and transfer with force-field trials we determined the angular deviation of the movement:
| (4) |
dP and dL represent the perpendicular (lateral) displacement and longitudinal displacements of the hand trajectories at peak tangential speed, which occurs at 230 ± 27 ms into the movement on average in our data set. Note that here the sign of the perpendicular displacement was defined relative to the direction expected in the force field in which it is measured.
Here we quantified the transfer of adaptation in two ways. First, we compared the angular deviation of the hand trajectories during baseline (the force-field catch trials during the baseline period, θbaseline) to the first trial of transfer when the force field was the same as the adaptation force-field, θtransfer,sameFF:
| (5) |
This is a normalized version of the analysis that Malfait and Ostry (2004) employed. However, this measure of transfer is problematic if the limb impedance is not identical in the baseline and transfer trials, because changes in limb impedance would affect the relative values of θbaseline and θtransfer,sameFF even if the true amount of transfer were unchanged. In particular, increased impedance during the transfer block would decrease θtransfer,sameFF and thus increase this measure of transfer independent of the true amount of transfer. We would, in fact, expect this to occur, especially for short-duration training, because recent experience with large motor errors is known to increase impedance (Darainy et al. 2009; Milner and Franklin 2005; Scheidt et al. 2001; Takahashi et al. 2001) and large errors are to be expected near the beginning of the training block that precedes the first transfer trial. We therefore created a second measure in which adaptation was quantified by comparing the angular deviation on the first trial of transfer when the field was the same (θtransfer,sameFF) to when the field was opposite the trained force field, θtransfer,oppositeFF:
| (6) |
Because the conditions that led up to the first-trial transfer test for the trained and opposite fields are identical, the limb impedance would not be expected to differ, and systematic changes in limb impedance between the baseline and transfer blocks should thus not result in biases in this measure of transfer. Note that, because transfer would result in both decreased trajectory deviation for the same field test and increased deviation for the opposite field test, the difference is reduced by a factor of 2 here. Note also that the signs of the perpendicular displacements, and thus the angular deviations computed from them, were defined relative to the direction expected in the force field in which they were measured. Thus θtransfer,sameFF and θtransfer,oppositeFF would be identical and the second transfer metric would be zero if there was no true transfer of adaptation corresponding to equal and opposite trajectory deviations in the same and opposite force fields.
RESULTS
The first experiment examined the interlimb transfer of the adaptation to novel viscous dynamics following three different training schedules: gradual long, abrupt long, and abrupt short training, as illustrated in Fig. 1C. This experiment began with 150 baseline movements with each hand to ensure that subjects were fully comfortable with the task and to establish baseline performance before the onset of training, as diagrammed in Fig. 1D. After this baseline period, subjects were exposed to one of the three training schedules during which a velocity-dependent curl force-field environment was applied to subjects' right hand with a robotic manipulandum (see methods). This training period was followed by a 50-trial generalization block, during which we examined transfer to the untrained limb (the left arm). Error-clamp measurement trials (see methods), in which lateral errors were clamped below 0.6 mm, were randomly interspersed during the baseline and training periods with a frequency of one in six trials to provide estimates of the learning curve for each training schedule based on feedforward changes in the lateral force profiles. The generalization blocks were entirely composed of error-clamp trials so that the lateral force profiles associated with the transfer of the trained dynamics could be directly measured. This also allowed the decay of the transferred adaptation across trials to be distinguished from the error-driven learning or unlearning that would occur if force-field or null-field trials were used, respectively.
Adaptation During Training Period
Participants displayed substantial adaptation to the velocity-dependent force field during the training period for all three schedules that we studied as illustrated in Fig. 2. The rows of this figure show the mean lateral force profiles, F(t), for each schedule before, during, and after training. An adaptation coefficient (see methods) that takes the entire lateral force profile into account based on linear regression of the actual force profile (colored lines in Fig. 2) onto the ideal compensatory force pattern (black lines in Fig. 2) was taken as our primary measure of learning as in previous work (Joiner et al. 2011; Joiner and Smith 2008; Sing et al. 2009; Sing and Smith 2010). This measure was used to compute the learning curve for each training schedule that is plotted in Fig. 3A. The amount of adaptation at the end of training was significantly greater than baseline for all training schedules; however, the amount of adaptation was also significantly different between training schedules (2-way ANOVA, P < 0.001 for the main effects of training and of schedule). On average, subjects learned ∼77 ± 4%, 72 ± 3%, and 56 ± 3% (mean ± SE across subjects) of the force field for the long abrupt, long gradual, and short abrupt training, respectively, as shown in Fig. 3, A and B. This corresponds to decreases of 22% and 27% in final learning when comparing the short abrupt schedule to the long gradual and long abrupt schedules, respectively (2-tailed t-tests, P < 0.001 in both cases), and a decrease of 7% for the long gradual compared with the long abrupt training (2-tailed t-test, P = 0.09). Thus participants displayed clear adaptation to the force-field environment for all three training schedules, and analysis based on adaptation coefficients indicates differences of 20–30% between the adaptation levels achieved for the different training schedules. The existence of differences in the adaptation levels we observed between the three schedules, although relatively small, indicates that analysis of the extent to which each training schedule generalizes should take into account the adaptation levels specifically achieved with that schedule.
Fig. 3.
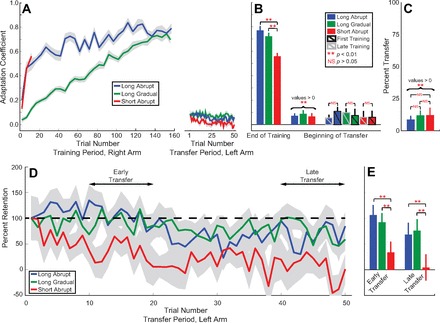
Time course of adaptation and interlimb transfer of adaptation when measured with error-clamp trials. A: the adaptation coefficient for the training of the right hand and transfer to the left hand is plotted against trial number for the 3 training schedules. Light gray background represents SE. Traces on right are the initial adaptation transfer (trial 1 of the transfer period) and the decay of transferred adaptation for the untrained left arm for the 3 training schedules. B, left: comparison of the average amount of adaptation achieved by the right hand at the end of the adaptation period (final 30% of trials) for each training schedule. Center: average amount of adaptation transferred to the left hand on the first trial of transfer after adaptation. Right: summary of the control analysis comparing the amount of adaptation transferred to the left hand when the respective training schedule was in the first session of the experiment (bars with black outline) as opposed to occurring in the second or third training session of the experiment (bars with gray outline). Error bars show SE. **Significant difference, NSno significant difference (α = 0.05). C: % of adaptation transferred to the left hand on the first trial of adaptation transfer: scaled ratio of the adaptation transferred to the amount of adaptation achieved at the end of training. D: decay of the transferred adaptation as % of the initial transferred adaptation. Data were scaled based on the first trial of transfer (i.e., the first trial represented 100% and % on subsequent trials was relative to this initial transfer trial). E: % of initial transferred adaptation early (trials 10–20) and late (trials 40–50) in the transfer period. **Significant differences (2-tailed t-test, P < 0.001).
We used the laterally directed midmovement force measured on error-clamp trials as a secondary measure of adaptation. Analysis of the midmovement force—operationally defined as the mean lateral force in a 140-ms window around the peak speed point as illustrated in Fig. 2A—yields a similar pattern of results. During the baseline period, the mean midmovement force levels applied by the right hand were small in magnitude: long abrupt training, 0.01 ± 0.10 N; long gradual training, 0.04 ± 0.11 N; short abrupt training, −0.15 ± 0.16 N. These force levels were not significantly greater than zero (2-tailed t-test, P > 0.86 in all 3 cases). In contrast, the midmovement force was significantly greater than zero at the end of the training period for all three schedules, as shown in Fig. 2, center: long abrupt training, 3.46 ± 0.24 N; long gradual training, 3.16 ± 0.22 N; short abrupt training, 3.16 ± 0.26 N (1-tailed t-tests, P < 0.001 in all 3 cases). The midmovement force at the end of the training periods was similar for all three schedules, with the average midmovement force within 10% for all three schedules. In line with the adaptation coefficient analysis, the average midmovement force for short abrupt training was nominally lower than for long abrupt training. However, neither this difference nor the difference between the long gradual and long abrupt schedules was significant (2-tailed t-tests, P > 0.34 in both cases).
Generalization of Motor Adaptation to Untrained Limb
We found a small but significantly positive amount of generalization to the untrained limb for all three training schedules as shown in Fig. 3, A–C. Adaptation coefficients during the transfer period were significantly greater than baseline across training schedules, but the amount of transfer was not significantly different between training schedules (2-way ANOVA, P < 0.001 for the main effect of transfer and P = 0.77 for the main effect of schedule; see Fig. 3B). Post hoc tests revealed that all three training schedules resulted in increased adaptation coefficients (1-tailed t-test, P < 0.01 in all cases), indicating transfer that would have been compensatory had the trained force field been applied to the untrained limb for all three schedules. The amount of adaptation generalized to the left hand on the first trial of the generalization period was characterized by baseline-subtracted adaptation coefficients of 0.07 ± 0.02, 0.09 ± 0.03, and 0.07 ± 0.03 for long abrupt, long gradual, and short abrupt training, respectively (summarized in Fig. 3B). As a fraction of the adaptation levels achieved during training (Fig. 3C), these generalization levels corresponded to 9 ± 3%, 12 ± 4%, and 12 ± 6% interlimb transfer, respectively. Like the raw transfer, the percent transfer was significantly greater than zero across training schedules and was not different between schedules (2-way ANOVA, P < 0.001 for the main effect of transfer and P = 0.85 for the main effect of schedule).
Analysis based on midmovement force yielded comparable results. As illustrated in Fig. 2, the force produced by the left arm after right arm adaptation (Fig. 2, right) was consistently greater than that produced before adaptation (Fig. 2, left). In line with the adaptation coefficient analysis, interlimb transfer measured with midmovement force was significantly greater than baseline across training schedules but not significantly different between training schedules (2-way ANOVA, P < 0.001 for the main effect of transfer and P = 0.40 for the main effect of schedule). The average baseline-subtracted midmovement force for the first trial in the transfer period was 0.47 ± 0.13 N (mean ± SE) for the long abrupt training, 0.59 ± 0.16 N for the long gradual training, and 0.30 ± 0.17 N for the short abrupt training. These values demonstrate that the midmovement force exerted by the left hand after transfer was greater than the force exerted by the left hand prior to adaptation for all three training schedules (1-tailed t-tests, P < 0.03 in all cases). Compared with the midmovement force levels achieved during training, these generalization levels corresponded to 13 ± 4%, 19 ± 5%, and 9 ± 6% interlimb transfer for long abrupt, long gradual, and short abrupt training, respectively. Similar to the results from the adaptation coefficient analysis, the percent transfer of midmovement force was significantly greater than zero across training schedules but was not significantly different between training schedules (2-way ANOVA, P < 0.001 for the main effect of transfer and P = 0.38 for the main effect of schedule).
In experiment 1, all 24 participants were trained and tested for transfer on each of the three training schedules. Although 1) the force-field environments between consecutive training sessions were oppositely directed, 2) there were 100 baseline trials for each hand (200 total baseline trials between the left and right hands) between the training and transfer periods to ensure washout of any previous learning, and 3) the ordering of the three training schedules was fully balanced, the possibility nevertheless existed that earlier training might have some effect on an adaptation session experienced later in the experiment. Therefore, as a control analysis, we compared the amount of adaptation transferred to the untrained limb when each training schedule was experienced first as opposed to second or third in the experiment, effectively reducing the number of participants from 24 to 8 for each schedule. The analysis revealed that the order of the training session had no effect on the amount of adaption transferred to the untrained limb, confirming that the washout we used between training schedules was effective (3-way ANOVA, P = 0.49 for the main effect of session order, P = 0.78 for the effect of schedule, and P < 0.001 for the effect of training). The results of this analysis are graphically presented in Fig. 3B; the bars outlined in black show the transfer measured in the first session alone, whereas the bars outlined in gray show the average transfer measured in the second and third sessions.
Persistence of Generalization to Untrained Limb
In addition to the amount of interlimb transfer measured on the first trial of the transfer period, we also examined the stability of the transferred adaptation from one trial to the next during the transfer period. Since the 50-trial transfer period consisted entirely of error-clamp trials, the decay of interlimb generalization could be examined under conditions in which error signals that could promote or wash out adaptation were essentially absent (Joiner et al. 2011; Scheidt et al. 2000; Sing et al. 2013; Smith et al. 2006). The decay curves shown in Fig. 3D were obtained by normalizing the adaptation coefficient data from the transfer period (Fig. 3A) by the first trial of this period. Although there was no difference in transfer between the training schedules on the first transfer trial, we found significant differences in the subsequent stability of the transferred adaptation. After short abrupt training, the transferred adaptation decays rapidly during the generalization period. We found that generalization decayed to <50% of its initial value by the 10th trial of the transfer period and no clear generalization remained beyond trial 20. In contrast, the long abrupt and long gradual training schedules resulted in generalization that was maintained throughout the entire 50-trial transfer period. Correspondingly, when looking early in the transfer period (between trials 10 and 20), we find that the long abrupt and long gradual schedules retain 106 ± 20% and 92 ± 18% of the initial transfer. These values are significantly greater than what we observe in the short abrupt schedule, where retention is only 34 ± 21% (2-tailed t-tests, P < 0.001 in both cases, as shown in Fig. 3E). Moreover, when looking late in the transfer period (between trials 40 and 50), we find that the long abrupt and long gradual schedules still retain 68 ± 23% and 72 ± 22% of the initial transfer, whereas the short abrupt schedule retains only 4 ± 25% (2-tailed t-tests, P < 0.001 in both cases). Note that the long abrupt and long gradual schedules do not display retention different from one another (2-way ANOVA, P = 0.008 for the effect of trial and P = 0.95 for the effect of schedule between long abrupt and long gradual training). These findings indicate that the duration of the training period is a key determinant of the stability of interlimb generalization, whereas gradual versus sudden introduction of the environment has little effect.
Can Changes in Limb Impedance Explain Previous Reports of Interlimb Transfer?
The present finding of similar, limited interlimb transfer of adaptation under gradual versus abrupt training schedules appears inconsistent with the study of Malfait and Ostry (2004), which reported significant transfer for short abrupt training but no transfer for gradual-onset long-duration training for the same velocity-dependent force-field adaptation. In a qualitative sense, the apparent difference between the present results and Malfait and Ostry's findings is that we find significant generalization to the untrained limb for both gradual and abrupt training, whereas the previous study found significant transfer for abrupt but not gradual training.
However, a quantitative comparison of the results paints a somewhat different picture. Whereas Malfait and Ostry reported ∼50% generalization to the untrained limb for short abrupt training and essentially no generalization for long gradual training, we find ∼12% generalization for both. Given the present results of 12 ± 4% transfer for gradual long training, it is not surprising that a previous study with half the subjects failed to demonstrate significant transfer under this condition given the reduced statistical power that would be expected. A larger discrepancy is the 12 ± 6% transfer we find for the short abrupt training compared with the transfer of ∼50% that Malfait and Ostry found for the same training schedule with the same velocity-dependent dynamic environment [linear viscous curl force fields with a magnitude of 15 N/(m/s) were used in both cases].
However, this previous study measured transfer of force-field adaptation by comparing the movement trajectories made by the untrained (left) arm in the force field before and after adaptation of the right arm. In contrast, we looked at learning-related changes in force profiles during error-clamp trials—measured both by adaptation coefficients and by midmovement force levels. This raises the possibility that the difference between the two sets of results may stem from the way that generalization was measured. Straighter movements within the force field can be achieved by a combination of improved feedforward adaptation, feedback control, and increased stiffness, whereas the amplitude of the force profiles measured during error-clamp trials should depend on the amount of feedforward adaptation independent of limb stiffness and feedback control. Therefore, the larger transfer that Malfait and Ostry observed with short abrupt training relative to long gradual training may have resulted from differences in arm stiffness or short-latency feedback gains. In particular, the observed difference could occur if short abrupt training resulted in increased stiffness or short-latency feedback gains in the untrained limb compared with long gradual training. There is reason to suspect that this may indeed be the case, as the classic findings of Takahashi et al. (2001) and the more recent results of Milner and Franklin (2005) and Franklin et al. (2008) demonstrate that stiffness or short-latency feedback gains increase when large motor errors are experienced. Because motor errors are largest, on average, early in training when there is an abrupt presentation, the short abrupt schedule may result in increased limb stiffness that would reduce motor errors during transfer. However, these studies did not look at interlimb generalization, and so it is unclear whether large motor errors in the right arm would result in increased stiffness in the left.
Note that we mention short-latency rather than long-latency feedback gains because Malfait and Ostry measured transfer at the peak speed of the movement, which generally occurs just 250 ms after movement onset, a point that has been used to measure primarily feedforward effects in several previous studies (Brayanov et al. 2012; Donchin et al. 2003; Joiner et al. 2011; Sing and Smith 2010; Smith et al. 2000; Smith and Shadmehr 2005). Feedback responses can certainly occur within 250 ms. However, because errors at movement onset are generally quite small, only short-latency responses should have substantial effects at this point because of the time that errors take to accumulate as well as the time that the motor commands take to be transduced into force that needs to be integrated into motion. Note also that we have combined limb stiffness and short-latency feedback gains in the preceding paragraph. We fail to distinguish between these mechanisms for two reasons. First, they have very similar effects: Increased stiffness (impedance) reduces the kinematic effects of force perturbations, and increased feedback gains result in motor commands that counteract the kinematic effects of these perturbations. Second, these mechanisms likely act in concert and have not been distinguished in studies showing that effective stiffness is increased after exposure to large motor errors (Franklin et al. 2008; Milner and Franklin 2005; Takahashi et al. 2001). Thus, going forward, we generally lump limb stiffness and short-latency feedback gains together into what we refer to as effective stiffness.
Effect of Limb Impedance on Measuring Interlimb Transfer
In a second experiment, we sought to determine whether differences in effective limb stiffness following training could account for the differences in reported transfer described above. To accomplish this, we conducted an experiment that mirrored Malfait and Ostry's methodology more closely by measuring interlimb generalization with force-field rather than error-clamp trials. Specifically, we examined the trajectory of left arm movements in the force field after training the right arm with the short abrupt schedule. As in Malfait and Ostry (2004), we tested for transfer to clockwise and counterclockwise force fields. Critically, however, we examined transfer not only to the same force field experienced during training but also to the opposite force field. This allowed us to examine changes in effective stiffness while disambiguating stiffness-related effects from specific transfer of the trained adaptation. Transfer of the trained adaptation would lead to reduced motor errors in the same-field condition but increased errors in the opposite-field condition, whereas training-induced increases in effective stiffness would lead to reduced motor errors in both conditions.
The results of experiment 2 are displayed in Fig. 4. Figure 4A shows the trajectory of the first movement with the untrained limb after short abrupt training for each participant. In experiment 2, the direction of the arm movement and the direction of the force field during training and transfer were randomly varied from one subject to the next. We displayed all movements in a standardized (upward) orientation, aligned so that the trained force-field direction was to the right in Fig. 4A to facilitate comparison across the entire data set. The red traces in Fig. 4A show data from sequence I of experiment 2 (Fig. 1E) for which the force fields in the transfer and training blocks were matched. In contrast, the orange traces in Fig. 4A show data from sequence II of experiment 2 (Fig. 1E) for which the force fields in the transfer and training blocks were opposite. For comparison, the thin black traces in Fig. 4A are force-field catch trials (single, unexpected force-field trials) from the baseline period in the untrained limb that can be used to estimate the amount of deviation expected for a naive movement in the force field.
Fig. 4.
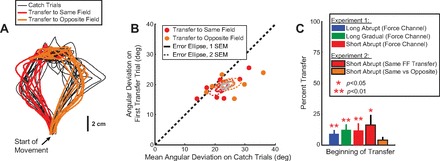
Interlimb transfer of adaptation when measured with force-field trials. A: movement trajectories for the first movement of transfer to the left hand following the short abrupt training schedule. Red traces represent the movements when the adaptation and transfer force fields were the same. Orange traces are the movements when the adaptation and transfer force fields were opposite. Thin black traces are the force-field catch trials for the left hand experienced before training. B: angular deviation of the first transfer trial for the left hand plotted against the mean angular deviation of the 2 force-field (FF) catch trials (unexpected FF trials during baseline). Each circle represents the data for 1 subject; ellipses represent the mean and SE across subjects for each case. Red circles and ellipses represent angular deviations and SE confidence intervals when the training and transfer force fields were the same. Orange circles and ellipses represent angular deviations and SE confidence intervals when the training and transfer force fields were opposite. Gray ellipses are SE confidence intervals over both conditions. In each case, the thick line ellipse represents 1 SE and the thin dashed line ellipse represents 2 SE. (Note that a 1 standard deviation ellipse would be times larger than the 1 SE ellipse shown, as there were 8 participants.) C: comparison of % adaptation transferred to the left hand when measured with error-clamp (experiment 1) and force-field (experiment 2) trials. Symbols above the bars represent % adaptation significantly greater than zero (P < 0.05, 1-tailed t-test). Error bars show SE.
When the movement paths observed for the left hand after same-field versus opposite-field training were directly compared, we found that the trajectory of the first left hand movement in the generalization period after right hand training was significantly straighter than the left hand force-field catch trial trajectories observed at baseline before training. This held true regardless of whether the force field experienced on left hand movements during transfer was the same or the opposite of that experienced by the right hand during adaptation, suggesting increased effective stiffness. To quantify this, we calculated the angular deviation at the peak speed point of the first transfer trial and plotted it against the mean peak speed point angular deviation of the force-field catch trials (Fig. 4B) for each subject. The filled red and orange circles in Fig. 4B represent individual subject data for transfer to the same and opposite fields, respectively. The error ellipses are at one and two SEs around the mean deviation for each condition (thick solid lines and thin dashed lines, respectively, Fig. 4B). Note that 1 standard deviation ellipses would be times larger than the 1 SE ellipses shown, as there were 8 participants. There are two key observations to be made here. First, the majority of the data and the confidence ellipses for both subgroups lie below the unity line (dashed black line, Fig. 4B), indicating that the first transfer trial was generally less deviated (i.e., straighter) than the catch trials. Across subjects, the angular deviation of the first transfer trial was significantly less than the deviation of the force-field catch trials at baseline, as would be expected from an increase in limb impedance and/or low-latency feedback gains. More specifically, when the data from both conditions were combined (gray confidence ellipses, Fig. 4B), the angular deviation on the first trial of transfer was significantly less than the mean of the two force-field catch trials in the baseline period (P = 0.006, 1-tailed paired t-test). When we broke down the data, we found that this reduction was observed both when the training and transfer force fields were the same (P = 0.02, 1-tailed paired t-test) and when they were opposite (P = 0.03, 1-tailed paired t-test). The latter finding is striking because when the training and transfer fields are in opposite directions (orange dots and ellipses, Fig. 4B), the transfer of learning would increase the deviation rather than decrease it. The finding that the reverse occurs suggests not only that there is an increase in the effective impedance of the left arm after training with the right but also that the increased impedance has an effect that outweighs the true transfer of adaptation.
A secondary observation was that when the force field experienced during transfer was the same as that experienced during adaptation (red circles and ellipse, Fig. 4B), we found a slightly greater decrease in the angular deviation compared with when the force fields were opposite in direction (the center of the red ellipse is slightly below the center of the orange ellipse, Fig. 4B). This is in line with the modest transfer of adaptation observed in the first experiment. However, the small improvement in the angular deviation that we observed to be associated with the learned dynamics was not statistically significant in this case (deviations of 19.02 ± 3.71° when the transfer force field was the same vs. 19.98 ± 2.86° when opposite, a nominal 5% decrease; P = 0.30, 1-tailed paired t-test). Correspondingly, there was no significant improvement in the perpendicular displacement at peak speed on the first trial of transfer when the training and transfer force fields were in the same direction compared with opposite (1.95 ± 0.67 cm vs. 2.49 ± 0.96 cm, respectively; P = 0.18, 1-tailed paired t-test). Together, these results suggest that the ability to make straighter movements with the left hand after short abrupt training in the right hand was primarily due to an increase in effective stiffness rather than transfer of the learned dynamics.
Although the order of the sequences in the control experiment (sequences I and II in Fig. 1E) was counterbalanced between subjects, there was a possibility that 1) there was a difference in movement speed between the catch, training, and transfer trials and 2) the order of the experiment had a significant effect on transfer. However, we found no difference in speed between the catch, training, and transfer trials (ANOVA, P = 0.20 for main effect of trial type). When we compared the amount of adaptation transferred to the untrained limb when sequence I was experienced first to when sequence II was experienced first, we found no difference in lateral displacement (2-tailed t-test, P = 0.29), indicating no significant ordering effect. This was supported by a combined analysis of order and the correspondence between the training and transfer force fields that found no effect on the percent change in the angular deviation of the untrained limb (2-way ANOVA, P = 0.78 for main effect of training and transfer force field correspondence and P = 0.15 for the effect of sequence order).
The bar graphs in Fig. 4C summarize the percentage of adaptation transferred to the left hand for the training schedules in the first experiment (error-clamp trials) and the second experiment (force-field trials). In the second experiment we quantified adaptation in two ways. In the first method, we compared the angular deviation of the hand trajectories during baseline (the force-field catch trials during the baseline period) to the first trial of transfer when the force field was the same as the adaptation force field, as in Malfait and Ostry (2004). In this case, we found the transferred adaptation coefficient to be 0.12 ± 0.06, which corresponded to 16 ± 8% interlimb transfer compared with the trained adaptation. However, as noted above, this measure combines the effects of transferred feedforward adaptation and increases in effective limb stiffness due to training. In the second method, we quantified adaptation by comparing the angular deviation on the first trial of transfer when the field was the same versus when the field was opposite to the trained force field. By comparing these two sets of deviations, we can cancel out any general increase in effective limb stiffness due to training and estimate movement deviation due to the transfer of adaptation to the untrained limb. In this case, we found the amount of adaptation transferred to the left hand on the first trial to be 0.04 ± 0.04, which corresponded to 4 ± 5% interlimb transfer. In both cases, the percentage of adaptation transferred (16 ± 8% or 4 ± 5%) was not statistically different from the percentages determined for the three training types by using the error-clamp trials to measure transfer in experiment 1 (ANOVA, P = 0.76). However, comparing the first measure for experiment 2, which includes changes in the movement trajectory due to effective stiffness, to the second, in which the effect of the effective limb stiffness is accounted for, reveals that 75% of the unadjusted estimate of the transferred adaptation (12% of the apparent 16%) is due to increased effective stiffness in the untrained limb. Moreover, the second measure, when effective stiffness is accounted for, is not significantly greater than zero (1-tailed t-test, P = 0.2). Together, the results from experiment 2 show that 1) the amount of adaptation transferred with short abrupt training is modest, in line with the error-clamp based results from experiment 1, 2) transfer to the untrained limb is accompanied by a sizeable increase in effective limb impedance, and 3) carefully designed measurements of adaptation are necessary to control for changes in effective stiffness when estimating true interlimb transfer of learning.
DISCUSSION
We studied the effector specificity of motor adaptation by examining how the manner in which the adaptation is trained influences both the amount of transfer to the untrained limb and the stability of that transfer. We found that abrupt versus gradual training had no effect on either the amount of transfer measured immediately after training or the retention of that transfer thereafter. In contrast, the duration of the training period had no effect on the amount of transfer but a pronounced effect on the stability of the transferred adaptation, with longer training resulting in substantially increased retention. All three training schedules that we studied resulted in statistically significant interlimb transfer, but the amount of transfer was limited to 9–12% of the trained adaptation in all cases. This limited transfer is at odds with a previous report that claimed substantial interlimb transfer for short abrupt training; however, the previous result was obtained by measuring transfer with force-field trials, a method that could be biased by changes in stiffness or short-latency feedback gains. We therefore conducted a second experiment to determine whether short abrupt training would alter the effective stiffness of the untrained limb. In experiment 2, we found large increases in effective stiffness after short abrupt training, providing an explanation for the increased transfer that had previously been reported. Together, our results provide a parsimonious explanation for seemingly discrepant findings from previous studies about the ability of motor adaptation to transfer from one limb to another. We demonstrate that the transfer of learned dynamics to the untrained limb can occur after only a brief exposure, but this transfer is limited in amplitude, independent of training schedule, and stable only after prolonged training.
Quantitative Comparison to Previous Work on Effector Specificity
Our findings are in line with recent studies examining the effector specificity of visuomotor adaptation (Taylor et al. 2011; Wang et al. 2011a). In agreement with the present findings, these studies demonstrate 1) no effect of introduction rate on the transfer of motor adaptation and 2) incomplete transfer of learning to the untrained limb: 15–25% transfer of visuomotor learning when assessed in a blocked paradigm compared with the 9–12% transfer of force field learning that we observe (Taylor et al. 2011; Wang et al. 2011a; Wang and Sainburg 2003). Despite the nominally higher levels of interlimb transfer, the general agreement between the findings for visuomotor learning and the present results suggests similar mechanisms for the interlimb transfer of these motor adaptations, even though different neural mechanisms are involved in the adaptation to physical versus visual perturbations (Krakauer et al. 1999; Rabe et al. 2009; Tanaka et al. 2009).
In contrast, the amount of transfer that we observe (9–12%) is considerably less than the 50% transfer reported in previous work examining effector specificity in the adaptation to novel dynamics (Malfait and Ostry 2004). However, as argued above, that study may have overestimated the amount of transfer by examining the trajectories of hand paths within the force field, because the effects of increases in effective limb stiffness and interlimb transfer may have been conflated—both result in straighter movements. In line with this idea, several previous studies have shown that the large errors observed in the early adaptation to novel dynamics produce an increase in limb impedance or short-latency feedback gains (Darainy et al. 2009; Milner and Franklin 2005; Scheidt et al. 2001; Takahashi et al. 2001) in conjunction with the formation of an unrefined internal model of the dynamics (Sing et al. 2009). A key advantage of the error-clamp measurement trials we employed in experiment 1 is that the robot motors counteract any motion perpendicular to the target direction, effectively eliminating lateral errors and thus largely removing the effects of limb impedance or online error feedback responses that determine the effective impedance of the limb. This allows us to directly measure changes in learned dynamics uncontaminated by changes in effective impedance. To determine the significance of limb impedance modulation in interlimb transfer, we conducted a second experiment that compared transfer into force-field environments that were the same as or opposite to the trained field. We tested for transfer in opposing force fields because the transfer of adaptation itself should differentially affect the movement trajectories: When the force field is the same as that experienced during training, the first trial of the transfer should be straighter after the training period. When the force field is opposite to that experienced during training, the first trial of the transfer should be more deviated after the training period. Critically, we found that even when the force field was opposite to that experienced during training, the first trial of the transfer tended to be significantly straighter after the training period. This was evidenced by angular deviations on the first trial of the opposite-field transfer period that were significantly less than the deviations of force-field catch trials experienced before training. This finding suggests that there is an increase in the impedance of the left limb after the training of the right that reduces errors independent of the environment experienced during the transfer period. In fact, we found that changes in effective limb impedance or short-latency feedback gains accounted for ∼75% of the apparent transfer of learning following short abrupt training (see the final paragraph in results). Applying this finding to the Malfait and Ostry (2004) results suggests that the 50% reduction in lateral errors they observed might correspond to ∼12% true transfer of adaptation, which is squarely in line with the present findings. Thus the differences in the reported transfer for force-field adaptation are likely explained by how adaptation was assessed.
The Criscimagna-Hemminger et al. (2003) study also claimed substantial transfer of adaptation to the untrained limb by using a kinematic measure, perpendicular displacement, to assess transfer. However, unlike the Malfait and Ostry (2004) study, the authors normalized this displacement in an attempt to account for stiffness. Unfortunately, the normalization procedure used by Criscimagna-Hemminger et al. (2003), although generally reasonable, is of questionable reliability at the beginning of the transfer period—the crucial time point for estimating transfer. This is the case because, for the normalized measure they used, the raw transfer (which, as discussed in detail above, is largely conflated with stiffness) is effectively estimated on different trials than the stiffness estimate used to normalize it. Moreover, the stiffness estimate for this measure critically depends on the displacement observed on infrequent catch trials (frequency of 1 in 6 movements). Thus it is highly likely that the stiffness estimate effectively misses the high, but rapidly decreasing stiffness that occurs at the onset of the transfer period, and as a result the decrease in perpendicular displacement due to stiffness is unlikely to be properly accounted for.
A second key, but related, issue is that the transfer period in the Criscimagna-Hemminger study consisted of force-field training trials and transfer was not analyzed earlier than 15 trials into the testing period, allowing subjects to learn with the “untrained” limb for at least 15 trials before transfer was measured. Thus the Criscimagna-Hemminger study effectively tested for a combination of 1) direct interlimb transfer of learning and 2) any increase in the rate of learning with the untrained limb (i.e., an interlimb version of savings; Kojima et al. 2004; Sing and Smith 2010; Smith et al. 2006; Zarahn et al. 2008) compared with the condition in which the untrained limb was tested before prior training with the opposite limb. Both the present study and the Malfait and Ostry (2004) study were carefully designed so that interlimb savings could not contribute to the primary results because the very first transfer trial was analyzed before any learning could occur in the untrained limb. Therefore, the Criscimagna-Hemminger study could not distinguish the direct interlimb transfer of the trained adaptation that we have examined from the ability to improve the rate of learning in the untrained arm.
Contributions of Interlimb Transfer to Motor Control
Including the studies described above (Taylor et al. 2011; Wang et al. 2011a; Wang and Sainburg 2003), there has been extensive research examining the intermanual transfer of visuomotor rotation learning (Sainburg and Wang 2002; Wang et al. 2011b; Wang and Sainburg 2004a, 2004b, 2006a, 2006b). Despite some heterogeneity in training methods, assessment, and data, these previous studies generally point toward an overall picture in which there is incomplete transfer of learning to the untrained limb. Results for prism adaptation have been substantially more varied. In particular, prism adaptation studies with real-time continuous feedback of movement have generally shown limited transfer, whereas studies in which only terminal end point feedback was available to the participants have often shown substantially greater transfer exceeding 50% (Choe and Welch 1974; Cohen 1967, 1973; Craske 1967; Hamilton 1964; Harris 1963; Kitazawa et al. 1997; Michel et al. 2007; Redding and Wallace 2008, 2009; Taub and Goldberg 1973; Tuan and Jones 1997; Uhlarik and Canon 1971). The nearly complete transfer observed with end point-only feedback has been attributed to static sensory realignment rather than movement-related motor learning, as changes in sensory perception about the target location can explain the majority of transfer (Redding and Wallace 2002, 2008, 2009, 2011; Uhlarik and Canon 1971). This finding provides an intriguing explanation for why the transfer of 9–12% we observed in the present study, where we examined the adaptation to movement-specific velocity-dependent dynamical perturbation, is even more modest than that seen with visuomotor rotation learning. In contrast to the movement-specific perturbation we studied, visuomotor rotation learning is based on a perturbation present during movement as well as at the end point, with visual feedback generally available at both stages, suggesting that the adaptation may include a contribution from changes in sensory perception. This point of view is in line with findings of small but highly significant perceptual changes associated with visuomotor rotation and visuomotor shift adaptation (Cressman and Henriques 2009, 2010, 2012; Simani et al. 2007) and further supported 1) by the findings that continuous and end point feedback during reaching as well as movement-related and end point-related tasks are somewhat promiscuous in their effects (Ghez et al. 2007; Redding and Wallace 2008, 2009; Scheidt and Ghez 2007) and 2) by the finding that visuomotor rotation learning with end point-only feedback can result in substantial interlimb transfer (Taylor et al. 2011). In summary, the present results contribute to an emerging picture that shows relatively little interlimb transfer of movement-related motor learning, suggesting that motor adaptation is largely specific to the trained arm.
Understanding the manner in which learning generalizes is one of the most powerful methods for understanding the internal representations used for motor learning (Shadmehr 2004). Although there is some controversy about how widely force-field learning generalizes within a single limb, there is converging evidence that the generalization of motor adaptation is mostly local across movement directions within the trained arm (Gandolfo et al. 1996; Gonzalez-Castro et al. 2011b; Krakauer et al. 2000; Malfait et al. 2002, 2005; Wu and Smith 2013), across movement directions for both limbs during bimanual movement (Yokoi et al. 2011), and across different postures (Baraduc and Wolpert 2002; Brayanov et al. 2012; Gandolfo et al. 1996), simultaneously in different coordinate frame representations (Brayanov et al. 2012). Thus the present findings about the generalization of motor adaptation across limbs contributes to the emerging picture that motor adaptation is specific to a number of different movement features and thus generalizes in a fashion that is simultaneously local to the trained adaptation across this feature space. A notable exception is the generalization of motor adaptation across different movement speeds, and in particular to faster speeds. Although there is some disagreement here as well (Mattar and Ostry 2010), current evidence appears to suggest that motor adaptation can extrapolate across movement speeds rather than showing local decay around the trained speed range (Goodbody and Wolpert 1998; Joiner et al. 2011). This is similar to the extrapolation of saccade adaptation to larger-amplitude saccades (Noto et al. 1999). An exciting direction for future work would be to meaningfully connect the extent to which learning is local versus extrapolable across different features.
Stability of Interlimb Transfer Compared with Intralimb Retention
Our results are also comparable to a recent study by Huang and Shadmehr (2009) that examined how intralimb retention was influenced by how the perturbation was introduced and the duration of training when adapting to novel physical dynamics. However, in contrast to the present results, the authors argued that the stability of a learned adaptation depends on the rate of introduction rather than the duration of training. As in our study, they presented a velocity-dependent curl force-field perturbation either abruptly or gradually and with a short (20 trials) or long (50 trials) duration for the abrupt presentation. The data showed that retention of the adaptation within the trained limb was influenced by the duration of training. Retention rapidly decreased after short abrupt training but decreased at slower rates after long gradual and long abrupt training. Although the decay curves were extremely similar for their long gradual and long abrupt training, the authors suggested that introduction rate rather than training duration determined the stability of adaptation. They argued that the abrupt training resulted in a faster decay rate because of a significant interaction between training schedule (abrupt vs. gradual) and epoch (training vs. decay period). However, a significant interaction is exactly what would be expected when comparing unnormalized data if the rate constant for decay is identical between schedules but the amount of learning achieved during the training period is different. Indeed, the authors found significantly increased training period adaptation for the abrupt long condition, which would lead to a greater absolute rate of decay if the exponential rate constant was exactly matched between conditions. This issue could have been avoided if normalized data were analyzed so that decay rates relative to the level of adaption achieved could be compared for long gradual and long abrupt training. Thus, although the authors argued for a greater decay rate in the abrupt long condition, their experimental results are in line with what would be predicted if there were identical rate constants for decay in the abrupt long and gradual long conditions. This suggests that a reexamination of this issue may be warranted for intralimb transfer, but as it stands the Huang and Shadmehr (2009) data on intralimb decay appear in agreement with the present findings demonstrating that the decay rate for interlimb transfer depends on the amount of training rather than on the rate at which the perturbation is introduced (abrupt vs. gradual).
The similarity in the decay of motor memory for intralimb retention and interlimb transfer could be explained by models of interlimb transfer that posit that during training dual internal models are simultaneously updated for each limb (Parlow and Kinsbourne 1989; Sainburg and Wang 2002). Although two models are formed during the training, the hemisphere of the untrained limb is hypothesized to maintain a weaker representation of the required dynamics. Under this framework, the idea would be that the transfer period for the untrained limb is in fact a retention test of this weaker internal model. Therefore, we suggest that after the short training session there is a relatively unrefined internal model of the dynamics that quickly decays during the retention period for the trained and untrained limbs. However, with longer training the internal model becomes more stable, resulting in a slower decay of the adaptation.
Neural Correlates of Force-Field Adaptation and Their Relation to Interlimb Transfer
Imaging and patient studies have attempted to identify the neural substrates for the interlimb transfer of motor learning. For example, a recent imaging study distinguished the neural correlates of learning and transfer in order to test the validity of different models of interlimb transfer for visuomotor rotation (Anguera et al. 2007). Although no imaging study has examined the interlimb transfer of learning force-field dynamics, Criscimagna-Hemminger et al. (2003) examined a split-brain patient to test whether the transfer depended on the interhemispheric communication via the corpus callosum. The authors found that the patient was able to transfer learning between the limbs, indicating that interlimb generalization of learned dynamics does not require interhemispheric connections. However, the issue of assessing transfer with force-field trials outlined above may preclude this conclusion.
Imaging studies that examined force-field adaptation have shown that there is bilateral activation of primary and secondary somatosensory cortex during early learning (Diedrichsen et al. 2005) and bilateral activation of prefrontal cortex and the deep nuclei of the cerebellum late in learning (Nezafat et al. 2001), suggesting that both hemispheres, and perhaps dual internal models, may be involved and updated during training. In addition, some cells in primary motor cortex and dorsal premotor cortex demonstrate effector-independent activity; the cells discharge for both contralateral and ipsilateral arm movements, supporting the view that both contralateral and ipsilateral training may affect the same neural substrates (Cisek et al. 2003; Donchin et al. 1998; Steinberg et al. 2002). Presently it is difficult to distinguish whether interlimb transfer of novel dynamics is the result of two separate internal models formed by a common error or the transfer of the learning from the trained to the untrained hemisphere. Future imaging investigations directly comparing different training durations and the interlimb generalization of the adaptation would clarify how these motor memories are stored and accessed within the brain by different effectors.
GRANTS
This work was supported by the Sloan Research Fellowship from the Alfred P. Sloan Foundation and a Scholar Award from the McKnight Endowment for Neuroscience to M. A. Smith.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.M.J., J.B.B., and M.A.S. conception and design of research; W.M.J. performed experiments; W.M.J. and M.A.S. analyzed data; W.M.J., J.B.B., and M.A.S. interpreted results of experiments; W.M.J. and M.A.S. prepared figures; W.M.J., J.B.B., and M.A.S. drafted manuscript; W.M.J., J.B.B., and M.A.S. edited and revised manuscript; W.M.J., J.B.B., and M.A.S. approved final version of manuscript.
REFERENCES
- Anguera JA, Russell CA, Noll DC, Seidler RD. Neural correlates associated with intermanual transfer of sensorimotor adaptation. Brain Res 1185: 136–151, 2007. [DOI] [PubMed] [Google Scholar]
- Baraduc P, Wolpert DM. Adaptation to a visuomotor shift depends on the starting posture. J Neurophysiol 88: 973–981, 2002. [DOI] [PubMed] [Google Scholar]
- Berniker M, Kording K. Estimating the sources of motor errors for adaptation and generalization. Nat Neurosci 11: 1454–1461, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berniker M, Kording K. Estimating the relevance of world disturbances to explain savings, interference and long-term motor adaptation effects. PLoS Comput Biol 7: e1002210, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock O, Thomas M, Grigorova V. The effect of rest breaks on human sensorimotor adaptation. Exp Brain Res 163: 258–260, 2005. [DOI] [PubMed] [Google Scholar]
- Brayanov JB, Press DZ, Smith MA. Motor memory is encoded as a gain-field combination of intrinsic and extrinsic action representations. J Neurosci 32: 14951–14965, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe CS, Welch RB. Variables affecting the intermanual transfer and decay of prism adaptation. J Exp Psychol 102: 1076–1084, 1974. [DOI] [PubMed] [Google Scholar]
- Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol 89: 922–942, 2003. [DOI] [PubMed] [Google Scholar]
- Cohen MM. Continuous versus terminal visual feedback in prism aftereffects. Percept Mot Skills 24: 1295–1302, 1967. [DOI] [PubMed] [Google Scholar]
- Cohen MM. Visual feedback, distribution of practice, and intermanual transfer of prism aftereffects. Percept Mot Skills 37: 599–609, 1973. [DOI] [PubMed] [Google Scholar]
- Craske B. Adaptation to prisms: change in internally registered eye-position. Br J Psychol 58: 329–335, 1967. [DOI] [PubMed] [Google Scholar]
- Cressman EK, Henriques DY. Sensory recalibration of hand position following visuomotor adaptation. J Neurophysiol 102: 3505–3518, 2009. [DOI] [PubMed] [Google Scholar]
- Cressman EK, Henriques DY. Reach adaptation and proprioceptive recalibration following exposure to misaligned sensory input. J Neurophysiol 103: 1888–1895, 2010. [DOI] [PubMed] [Google Scholar]
- Cressman EK, Henriques DY. Visuomotor adaptation and proprioceptive recalibration. J Mot Behav 44: 435–444, 2012. [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, Shadmehr R. Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol 89: 168–176, 2003. [DOI] [PubMed] [Google Scholar]
- Darainy M, Mattar AA, Ostry DJ. Effects of human arm impedance on dynamics learning and generalization. J Neurophysiol 101: 3158–3168, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R. Neural correlates of reach errors. J Neurosci 25: 9919–9931, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin O, Francis JT, Shadmehr R. Quantifying generalization from trial-by-trial behavior of adaptive systems that learn with basis functions: theory and experiments in human motor control. J Neurosci 23: 9032–9045, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin O, Gribova A, Steinberg O, Bergman H, Vaadia E. Primary motor cortex is involved in bimanual coordination. Nature 395: 274–278, 1998. [DOI] [PubMed] [Google Scholar]
- Franklin DW, Burdet E, Tee KP, Osu R, Chew CM, Milner TE, Kawato M. CNS learns stable, accurate, and efficient movements using a simple algorithm. J Neurosci 28: 11165–11173, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo F, Mussa-Ivaldi FA, Bizzi E. Motor learning by field approximation. Proc Natl Acad Sci USA 93: 3843–3846, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C, Scheidt R, Heijink H. Different learned coordinate frames for planning trajectories and final positions in reaching. J Neurophysiol 98: 3614–3626, 2007. [DOI] [PubMed] [Google Scholar]
- Gonzalez Castro LN, Monsen CB, Smith MA. The binding of learning to action in motor adaptation. PLoS Comput Biol 7: e1002052, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez Castro LN, Wu H, Smith M. Adaptation to dynamic environments displays local generalization for voluntary reaching movements. Conf Proc IEEE Eng Med Biol Soc 2011: 4050–4052, 2011b. [DOI] [PubMed] [Google Scholar]
- Goodbody SJ, Wolpert DM. Temporal and amplitude generalization in motor learning. J Neurophysiol 79: 1825–1838, 1998. [DOI] [PubMed] [Google Scholar]
- Hamilton CR. Intermanual transfer of adaptation to prisms. Am J Psychol 77: 457–462, 1964. [PubMed] [Google Scholar]
- Harris CS. Adaptation to displaced vision: visual, motor, or proprioceptive change? Science 140: 812–813, 1963. [DOI] [PubMed] [Google Scholar]
- Huang VS, Shadmehr R. Persistence of motor memories reflects statistics of the learning event. J Neurophysiol 102: 931–940, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, Ajayi O, Sing GC, Smith MA. Linear hypergeneralization of learned dynamics across movement speeds reveals anisotropic, gain-encoding primitives for motor adaptation. J Neurophysiol 105: 45–59, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, Smith MA. Long-term retention explained by a model of short-term learning in the adaptive control of reaching. J Neurophysiol 100: 2948–2955, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res 115: 557–561, 1997. [DOI] [PubMed] [Google Scholar]
- Kitazawa S, Kimura T, Uka T. Prism adaptation of reaching movements: specificity for the velocity of reaching. J Neurosci 17: 1481–1492, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluzik J, Diedrichsen J, Shadmehr R, Bastian AJ. Reach adaptation: what determines whether we learn an internal model of the tool or adapt the model of our arm? J Neurophysiol 100: 1455–1464, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y, Iwamoto Y, Yoshida K. Memory of learning facilitates saccadic adaptation in the monkey. J Neurosci 24: 7531–7539, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghilardi MF, Ghez C. Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci 2: 1026–1031, 1999. [DOI] [PubMed] [Google Scholar]
- Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20: 8916–8924, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait N, Gribble PL, Ostry DJ. Generalization of motor learning based on multiple field exposures and local adaptation. J Neurophysiol 93: 3327–3338, 2005. [DOI] [PubMed] [Google Scholar]
- Malfait N, Ostry DJ. Is interlimb transfer of force-field adaptation a cognitive response to the sudden introduction of load? J Neurosci 24: 8084–8089, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait N, Shiller DM, Ostry DJ. Transfer of motor learning across arm configurations. J Neurosci 22: 9656–9660, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar AA, Ostry DJ. Generalization of dynamics learning across changes in movement amplitude. J Neurophysiol 104: 426–438, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C, Pisella L, Prablanc C, Rode G, Rossetti Y. Enhancing visuomotor adaptation by reducing error signals: single-step (aware) versus multiple-step (unaware) exposure to wedge prisms. J Cogn Neurosci 19: 341–50, 2007. [DOI] [PubMed] [Google Scholar]
- Milner TE, Franklin DW. Impedance control and internal model use during the initial stage of adaptation to novel dynamics in humans. J Physiol 567: 651–664, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezafat R, Shadmehr R, Holcomb HH. Long-term adaptation to dynamics of reaching movements: a PET study. Exp Brain Res 140: 66–76, 2001. [DOI] [PubMed] [Google Scholar]
- Noto CT, Watanabe S, Fuchs AF. Characteristics of simian adaptation fields produced by behavioral changes in saccade size and direction. J Neurophysiol 81: 2798–2813, 1999. [DOI] [PubMed] [Google Scholar]
- Parlow SE, Kinsbourne M. Asymmetrical transfer of training between hands: implications for interhemispheric communication in normal brain. Brain Cogn 11: 98–113, 1989. [DOI] [PubMed] [Google Scholar]
- Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, Donchin O. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol 101: 1961–1971, 2009. [DOI] [PubMed] [Google Scholar]
- Raghavan P, Krakauer JW, Gordon AM. Impaired anticipatory control of fingertip forces in patients with a pure motor or sensorimotor lacunar syndrome. Brain 129: 1415–1425, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Strategic calibration and spatial alignment: a model from prism adaptation. J Mot Behav 34: 126–138, 2002. [DOI] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Intermanual transfer of prism adaptation. J Mot Behav 40: 246–262, 2008. [DOI] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Asymmetric visual prism adaptation and intermanual transfer. J Mot Behav 41: 83–94, 2009. [DOI] [PubMed] [Google Scholar]
- Redding GM, Wallace B. Prism adaptation in left-handers. Atten Percept Psychophys 73: 1871–1885, 2011. [DOI] [PubMed] [Google Scholar]
- Sainburg RL, Wang J. Interlimb transfer of visuomotor rotations: independence of direction and final position information. Exp Brain Res 145: 437–447, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savion-Lemieux T, Penhune VB. The effects of practice and delay on motor skill learning and retention. Exp Brain Res 161: 423–431, 2005. [DOI] [PubMed] [Google Scholar]
- Scheidt RA, Dingwell JB, Mussa-Ivaldi FA. Learning to move amid uncertainty. J Neurophysiol 86: 971–985, 2001. [DOI] [PubMed] [Google Scholar]
- Scheidt RA, Ghez C. Separate adaptive mechanisms for controlling trajectory and final position in reaching. J Neurophysiol 98: 3600–3613, 2007. [DOI] [PubMed] [Google Scholar]
- Scheidt RA, Reinkensmeyer DJ, Conditt MA, Rymer WZ, Mussa-Ivaldi FA. Persistence of motor adaptation during constrained, multi-joint, arm movements. J Neurophysiol 84: 853–862, 2000. [DOI] [PubMed] [Google Scholar]
- Shadmehr R. Generalization as a behavioral window to the neural mechanisms of learning internal models. Hum Mov Sci 23: 543–568, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simani MC, McGuire LM, Sabes PN. Visual-shift adaptation is composed of separable sensory and task-dependent effects. J Neurophysiol 98: 2827–2841, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing GC, Joiner WM, Nanayakkara T, Brayanov JB, Smith MA. Primitives for motor adaptation reflect correlated neural tuning to position and velocity. Neuron 64: 575–589, 2009. [DOI] [PubMed] [Google Scholar]
- Sing GC, Orozco SP, Smith MA. Limb motion dictates how motor learning arises from arbitrary environmental dynamics. J Neurophysiol 109: 2466–2482, 2013. [DOI] [PubMed] [Google Scholar]
- Sing GC, Smith MA. Reduction in learning rates associated with anterograde interference results from interactions between different timescales in motor adaptation. PLoS Comput Biol 6: e1000893, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Brandt J, Shadmehr R. Motor disorder in Huntington's disease begins as a dysfunction in error feedback control. Nature 403: 544–549, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol 4: 1035–1043, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol 93: 2809–2821, 2005. [DOI] [PubMed] [Google Scholar]
- Steinberg O, Donchin O, Gribova A, Cardosa de Oliveira S, Bergman H, Vaadia E. Neuronal populations in primary motor cortex encode bimanual arm movements. Eur J Neurosci 15: 1371–1380, 2002. [DOI] [PubMed] [Google Scholar]
- Takahashi CD, Scheidt RA, Reinkensmeyer DJ. Impedance control and internal model formation when reaching in a randomly varying dynamical environment. J Neurophysiol 86: 1047–1051, 2001. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Sejnowski TJ, Krakauer JW. Adaptation to visuomotor rotation through interaction between posterior parietal and motor cortical areas. J Neurophysiol 102: 2921–2932, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub E, Goldberg LA. Prism adaptation: control of intermanual transfer by distribution of practice. Science 180: 755–757, 1973. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Wojaczynski GJ, Ivry RB. Trial-by-trial analysis of intermanual transfer during visuomotor adaptation. J Neurophysiol 106: 3157–3172, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan KM, Jones R. Adaptation to the prismatic effects of refractive lenses. Vision Res 37: 1851–1857, 1997. [DOI] [PubMed] [Google Scholar]
- Uhlarik JJ, Canon LK. Influence of concurrent and terminal exposure conditions on the nature of perceptual adaptation. J Exp Psychol 91: 233–239, 1971. [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Smith MA. Shared internal models for feedforward and feedback control. J Neurosci 28: 10663–10673, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Joshi M, Lei Y. The extent of interlimb transfer following adaptation to a novel visuomotor condition does not depend on awareness of the condition. J Neurophysiol 106: 259–264, 2011a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Przybyla A, Wuebbenhorst K, Haaland KY, Sainburg RL. Aging reduces asymmetries in interlimb transfer of visuomotor adaptation. Exp Brain Res 210: 283–290, 2011b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Mechanisms underlying interlimb transfer of visuomotor rotations. Exp Brain Res 149: 520–526, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Interlimb transfer of novel inertial dynamics is asymmetrical. J Neurophysiol 92: 349–360, 2004a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Limitations in interlimb transfer of visuomotor rotations. Exp Brain Res 155: 1–8, 2004b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. The symmetry of interlimb transfer depends on workspace locations. Exp Brain Res 170: 464–471, 2006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Interlimb transfer of visuomotor rotations depends on handedness. Exp Brain Res 175: 223–230, 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AL, Shelhamer M. Saccade adaptation improves in response to a gradually introduced stimulus perturbation. Neurosci Lett 500: 207–211, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HG, Smith MA. The generalization of visuomotor learning to untrained movements and movement sequences based on movement vector and goal location remapping. J Neurosci 33: 10772–10789, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin PB, Kitazawa S. Long-lasting aftereffects of prism adaptation in the monkey. Exp Brain Res 141: 250–253, 2001. [DOI] [PubMed] [Google Scholar]
- Yokoi A, Hirashima M, Nozaki D. Gain field encoding of the kinematics of both arms in the internal model enables flexible bimanual action. J Neurosci 31: 17058–17068, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarahn E, Weston GD, Liang J, Mazzoni P, Krakauer JW. Explaining savings for visuomotor adaptation: linear time-invariant state-space models are not sufficient. J Neurophysiol 100: 2537–2548, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


