Abstract
This study demonstrates that the association of mitochondria with vimentin intermediate filaments (VIFs) measurably increases their membrane potential. This increase is detected by quantitatively comparing the fluorescence intensity of mitochondria stained with the membrane potential-sensitive dye tetramethylrhodamine-ethyl ester (TMRE) in murine vimentin-null fibroblasts with that in the same cells expressing human vimentin (∼35% rise). When vimentin expression is silenced by small hairpin RNA (shRNA) to reduce vimentin by 90%, the fluorescence intensity of mitochondria decreases by 20%. The increase in membrane potential is caused by specific interactions between a subdomain of the non-α-helical N terminus (residues 40 to 93) of vimentin and mitochondria. In rho 0 cells lacking mitochondrial DNA (mtDNA) and consequently missing several key proteins in the mitochondrial respiratory chain (ρ0 cells), the membrane potential generated by an alternative anaerobic process is insensitive to the interactions between mitochondria and VIF. The results of our studies show that the close association between mitochondria and VIF is important both for determining their position in cells and their physiologic activity.—Chernoivanenko, I. S., Matveeva, E. A., Gelfand, V. I., Goldman, R. D., Minin, A. A. Mitochondrial membrane potential is regulated by vimentin intermediate filaments.
Keywords: cytoskeleton, mitochondrial respiratory chain, rho 0 cells
Mitochondria are the major source of metabolic energy in cells. They are also responsible for the regulation of intracellular calcium and the sequestration of apoptotic factors (1). Most functions of mitochondria depend on the membrane potential generated and maintained by their inner membrane (2). Thus, alterations in mitochondrial membrane potential are a reliable indicator of their functional state. Dissipation of this membrane potential accompanies the release of proapoptotic factors from the intermembrane space of mitochondria, leading to caspase activation and apoptosis (3, 4).
Compromised mitochondrial functions (e.g., decreased ATP production, elevation of reactive oxygen species) play an important role in a number of pathologic conditions such as age-related diseases, amyotrophic lateral sclerosis, inherited eye disorders, among others (5,8). Interestingly, it has been reported that mitochondrial dysfunctions are frequently associated with diseases caused by mutations in the genes encoding different types of intermediate filaments (IFs). For example, alterations in the morphology, distribution, and function of mitochondria have been reported in patients with a variant of Charcot-Marie-Tooth disease, a neurodegenerative disease caused by mutations in neurofilament proteins (9, 10), and in myopathies and cardiomyopathies caused by desmin IF mutations (11, 12). Dysfunctions of mitochondria have also been demonstrated in patients with epidermolysis bullosa simplex, caused by mutations in genes encoding keratin IFs (13). Similar changes in mitochondria have been reported in animal and cellular models expressing genetically modified IF proteins (14, 15) and in vimentin-null fibroblasts (16).
We recently demonstrated that the expression of vimentin in vimentin-null cells causes an increased accumulation of the membrane potential-dependent dye MitoTracker Red CMXRos in mitochondria (17). Furthermore, we showed that the N-terminal non-α-helical domain of vimentin contains a mitochondrial binding site (18), further supporting a role for vimentin in the regulation of mitochondrial physiology. In this study, we demonstrate that the membrane potential of mitochondria is regulated by their interaction with an N-terminal part of vimentin. Furthermore, we show that these effects of vimentin require interactions of mitochondria that possess intact respiratory chains.
MATERIALS AND METHODS
Cell culture
Cell lines used included rat embryo fibroblast (REF-52), mouse 3T3 (ATCC, Manassas, VA, USA), mouse MFT-6, and vimentin null mouse MFT-16 cells (19). MFT-6 and MFT-16 cells were kindly provided by Prof. R. Evans (University of Colorado, Denver, CO, USA). All cells were maintained in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin, and 100 µg/ml streptomycin. The cell lines ρ0-MFT-6 and ρ0-MFT-16 depleted of mitochondrial DNA were produced by culturing of MFT-6 and MFT-16 cells in 0.1 µg/ml ethidium bromide in culture medium supplemented with 50 µg/ml uridine and 110 µg/ml pyruvate for 6 wk (20). For microscopic observations, cells were plated on coverslips at least 16–20 h before experiments were performed.
Plasmids and transfection
Plasmid enhanced green fluorescent protein (EGFP)-Vim was described previously (21). Cloning of pVim(P56R), pVim(40–93)-Dendra2, and pVim(40–93)(P56R)-Dendra2 was described earlier (18). Transfection was performed using TransIT-LT1 Transfection Reagent (Mirus Bio, Madison, WI, USA). DNA (1 µg) was used for a 40 mm plate of cells containing 2 ml of culture medium. Cells were observed 16–20 h after transfection. To visualize restored VIF in live cells after transfection the EGFP-fused vimentin forms were coexpressed with respective unlabeled variants as described by Yoon et al. (21). Construction of the shRNA expression vectors pG-SHIN2 containing an EGFP reporter for transient transfection is described elsewhere (22, 23). For stable expression of vimentin shRNA (mVIM-T3) was inserted into the pSilencer5.1 H1 (Clontech, Mountain View, CA, USA) retroviral vector (24). Two days after infection, cells were placed under 2 μg/ml puromycin selection. Vimentin silencing was confirmed by immunoblotting and immunofluorescence.
Immunofluorescence
VIFs were stained using the mouse monoclonal antibody V9 (Chemicon International, Hofheim, Germany), or rabbit antibodies directed against human vimentin (25) or mouse vimentin (RVIM-AT) (26). Secondary antibodies conjugated with FITC or tetramethylrhodamine included goat anti-mouse or goat anti-rabbit IgG (The Jackson Laboratory, Bar Harbor, ME, USA). Before staining, cells were either fixed with methanol (−20°C), or 4% formaldehyde in PBS following by permeabilization with 0.1% Triton X-100 as previously described (18, 25).
SDS-PAGE and immunoblotting
Electrophoretic separation of proteins was performed using discontinuous 7.5% SDS-PAGE (27). For comparison of the content of vimentin and tubulin in cells treated with shRNAs, the samples were first run on gels that were stained with Coomassie R250. The gels were scanned, and the load for Western blots was normalized according to the scan data. Serial dilutions of cell lysates (1, 1/10, 1/30, 1/100) were carried out to quantitate vimentin and tubulin content. Tubulin was detected using DM1a antibody (Sigma-Aldrich, St. Louis, MO. USA) at a dilution of 1:1000. A vimentin antibody RVIM-AT was used at a dilution of 1:500. To determine the amount of residual vimentin after RNA interference, we compared intensities of bands on blots obtained for serially diluted samples and control cellular extracts. For a quantification of the data, filters were scanned and band intensities were measured using NIH ImageJ software (http://rsbweb.nih.gov/ij).
Analysis of mtDNA depletion in ρ0 cells
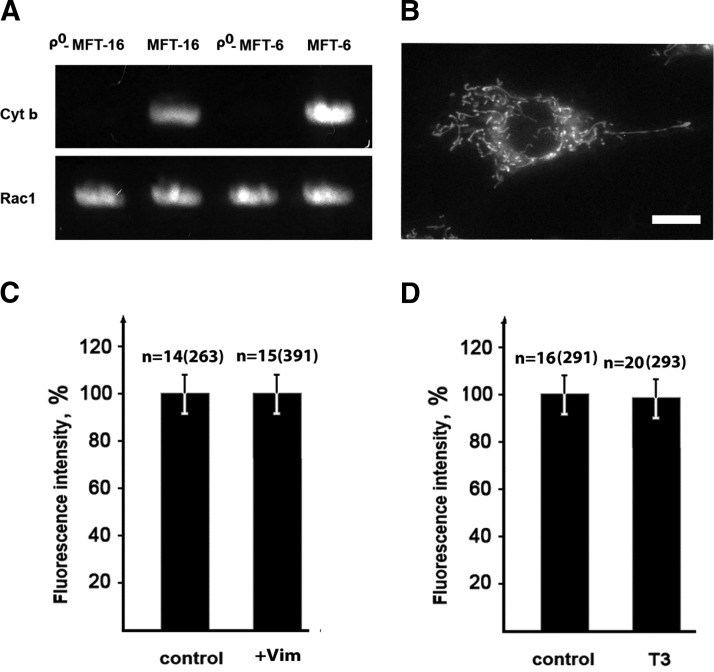
To verify the depletion of mtDNA in cells treated with ethidium bromide (see above), total DNA was isolated and probed for the cyt b gene, which is encoded by mtDNA using PCR and the primers TGATATGAAAAACCATCGTTG and CCCTCAGAATGATATT-TGTCCTCA (28, 29). PCR with primers TGAATTCTATGCAGGCCATCAAGTGT and AGGATCCTTACAACAGCAGGC-ATTTT for amplification of gene rac1 encoded by nuclear DNA was used as a control (Fig. 4A).
Figure 4.
VIFs have no effect on mitochondria membrane potential in ρ0 cells. A) DNA from vimentin-null (ρ0-MFT-16 and MFT-16) and vimentin-containing cells (ρ0-MFT-6 and MFT-6) was used as a template in PCR with primers specific to the cytochrome b gene (encoded by mitochondrial DNA) and primers specific to the Rac1 gene (encoded by nuclear DNA) as a control. PCR products (30 cycles) are shown in agarose gel after electrophoresis. B) Cells ρ0-MFT-16 were stained with the potential-dependent dye TMRE for 40 min. C) Restoration of VIF in ρ0 cells does not increase mitochondrial membrane potential. Vimentin-null cells (ρ0-MFT-16) were transfected with plasmid pIRES2-EGFP-Vim(wt) or pIRES2-EGFP as a control and 16 h later were stained with TMRE for 40 min. D) Depletion of VIF in ρ0 cells does not decrease mitochondrial membrane potential. Cells containing vimentin (ρ0-MFT-6) were transfected with plasmid pG-Shin2-Vim-T3 and 6 d later incubated with TMRE for 40 min. Data represent mean values of TMRE fluorescence as a percentage relative to control levels ± sem. n, number of cells and, in brackets, number of mitochondria. Scale bar, 10 µm.
Live cell imaging
To stain mitochondria, cells were incubated with 25 nM TMRE or 100 nM MitoTracker Red CMXRos for 40 min. To avoid the effects of a dye efflux from cells mediated by P-glycoprotein, a product of the multidrug resistance gene (30), verapamil was added to the incubation mixture to 2.2 μM. Then coverslips were mounted in a chamber filled with DMEM supplemented with 10% FCS. Temperature was maintained at 37 ± 2°C with an Incubator S (PeCon, Erbach, Germany). Epifluorescence microscopy was carried out with an Axiovert 200 M (Carl Zeiss, Jena, Germany) equipped with a Plan-Apochromat 63× 1.4 NA objective. Images were captured with a AxioCam MRm camera (Zeiss, Germany) driven by AxioVision 4.6 (Zeiss, Jena, Germany) software. To minimize phototoxic damage, a 100 W halogen lamp was used as a light source for fluorescent imaging of live cells.
Quantification of mitochondrial fluorescence
The transmembrane potential of mitochondria was analyzed with the potential-dependent fluorescent dye TMRE (Molecular Probes/Life Technologies, Grand Island, NY, USA). To compare the membrane potential of mitochondria under different experimental conditions, the intensity of TMRE fluorescence was measured using ImageJ software (for details see Supplemental Materials and Supplemental Fig. 1). Average pixel intensities inside the contours of individual mitochondria were determined for each organelle. Typically, data from 10–20 cells were collected for each experimental condition and mean values of fluorescence intensity ± sem were calculated.
Quantitative analysis of mitochondrial motility
Image analysis was done as described earlier (31) using ImageJ. Because of the variability in mitochondrial morphology, we plotted the coordinates of one end for longer organelles and the center of mass for shorter ones. Mitochondrial motility was assayed by determining displacement distances and velocities. Fast organelle movement was defined as movement with a rate above 200 nm/s. The values of the relative motility of mitochondria were expressed as mean percentages of fast movements in all recorded displacements ± sem. In each experiment, movement of 20–40 individual mitochondria per cell was analyzed in 10–15 cells. The significance of differences was determined statistically by the paired-sample Student’s t test. Variability of the values calculated for different cells in the samples were analyzed by the same method, and this was insignificant.
RESULTS
Mitochondria membrane potential depends on the presence of VIFs
To determine whether the membrane potential of mitochondria depends on their interaction with VIFs, we stained mitochondria with TMRE (32). This probe equilibrates rapidly across membranes, has low toxicity, and shows very little association with other organelles (2, 33). First, we compared the intensity of TMRE fluorescence in mitochondria of vimentin-null cells and in these same cells transfected with vimentin to assemble VIF (see Materials and Methods). The fluorescence intensity of mitochondria increased by ∼35% in the cells expressing VIF compared with the vimentin null cells (Fig. 1A–D). Likewise, when vimentin expression was reduced by ∼90% by the introduction of shRNA into MFT-6 cells, the intensity of mitochondrial fluorescence decreased by ∼20% compared with controls (Fig. 2G). A similar effect on mitochondrial membrane potential was observed in REF-52 and 3T3 cells following vimentin silencing (see Supplemental Fig. 2C). Therefore, the loss of vimentin caused a significant reduction in mitochondrial membrane potential.
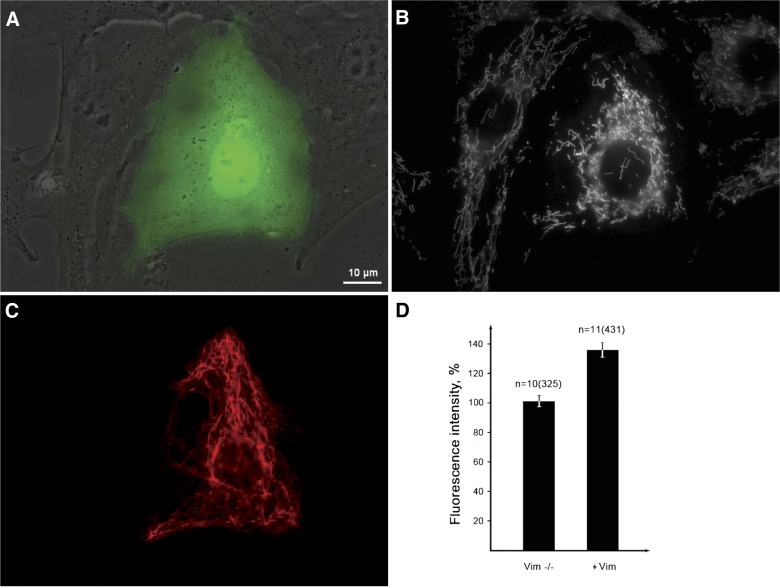
Figure 1.
Vimentin IFs increase the membrane potential of mitochondria. Vimentin-null cells were transfected with plasmid pIRES2-EGFP-Vim(wt) encoding vimentin and EGFP that are translated separately and 16 h later were stained with potential-dependent mitochondria-specific dye TMRE. A) Phase contrast image overlaid with green fluorescence showing transfected cell and (B) the staining with TMRE showing more intensive fluorescence of mitochondria in the transfected cell. C) The same cells fixed and immunostained with antivimentin antibody to show restoration of VIF in a transfected cell. D) Quantitative data expressed as mean values of TMRE fluorescence of mitochondria in nontransfected and transfected cells ± sem. n, number of cells and, in brackets, number of mitochondria. P < 0.01. Scale bar, 10 µm.
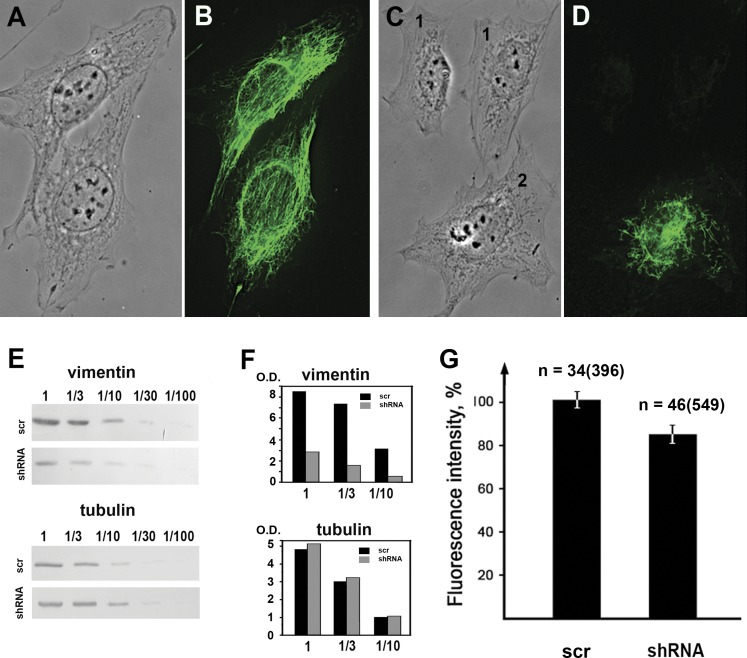
Figure 2.
Vimentin silencing decreases the membrane potential of mitochondria. Immunofluorescence of control MFT-6 cells (B) and the MFT-6 cells stably expressing vimentin shRNA (D) show that silencing caused the loss of VIF in most cells (1) with a few remaining VIF in the perinuclear region in other cells (2). A, C) Phase contrast of the same cells. E) Western blot analysis of vimentin and α-tubulin in control cells and cells expressing vimentin shRNA. The serial dilutions of cell extracts show the decrease of vimentin expression due to silencing and the α-tubulin content remains unaltered. F) Quantification of Western blot data. Optical densities of vimentin and tubulin bands in control (black) and experimental (gray) samples expressed in arbitrary units. G) Mean values of TMRE fluorescence intensity of mitochondria in control cells and in cells with VIF disruption that express vimentin shRNA. Data represent mean values of TMRE fluorescence as a proportion relative to control levels ± sem. n, number of cells and, in brackets, number of mitochondria; O.D., optical density; scr, scrambled. P < 0.01.
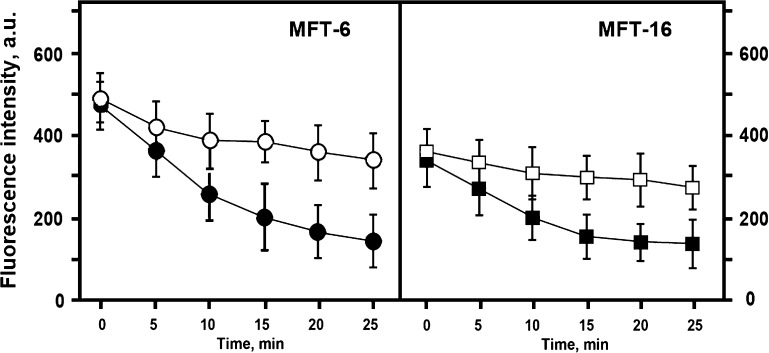
To further confirm that the increased TMRE fluorescence in mitochondria in cells expressing vimentin is attributable to higher membrane potential, we compared the effects of carbonyl cyanide 4-(trifluoromethoxy)-phenylhydrazone (FCCP), a protonophore that inhibits mitochondrial oxidative phosphorylation, in cells with and without VIF networks. Under selected conditions with 0.5 µM FCCP, mitochondria maintained their normal morphology, although their membrane potential was considerably decreased. At higher concentrations of FCCP, the morphology of mitochondria was severely affected, making it virtually impossible to measure their fluorescence intensity. The results show that FCCP decreases TMRE fluorescence to similar levels in both the presence and absence of VIF (Fig. 3). These results further demonstrate that the difference in TMRE fluorescence was due to different mitochondrial membrane potentials in vimentin-null compared with VIF-containing cells.
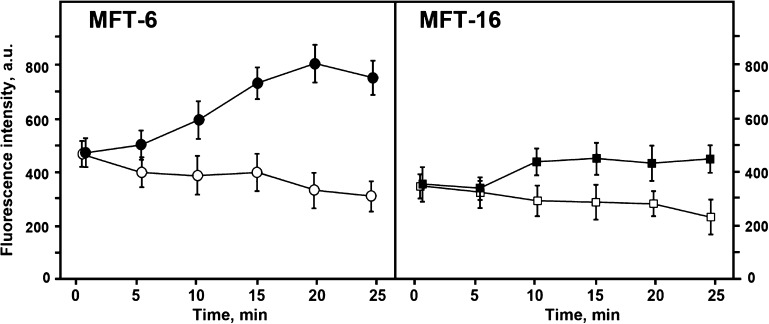
Figure 3.
Effect of FCCP on mitochondrial potential. Mitochondrial membrane potential was monitored based on TMRE fluorescence changes caused by FCCP. Vimentin containing (MFT-6) and vimentin-null cells (MFT-16) were incubated with TMRE for 40 min and then FCCP was added to the final concentration 0.5 µM. Data represent mean values of mitochondria fluorescence measured in 5 min intervals ± sem in arbitrary units. For each time point, mitochondrial fluorescence was analyzed in 10 cells of treated (closed symbols) or control (open symbols) cultures.
There are at least 3 possible explanations for the increased mitochondrial potential in the presence of VIF. These include an increase in the generation of membrane potential by the respiratory chain; an increase in the hydrolysis of ATP produced in the glycolysis in a reverse reaction by ATP-synthase to generate membrane potential; or a decrease in the utilization of the energy of potential by ATP-synthase. To distinguish among these possible scenarios, we first determined if VIFs affect the membrane potential of mitochondria with disrupted respiratory chains. To this end, we prepared ρ0 cell lines lacking mtDNA, which causes the loss of several components of the mitochondrial respiratory chain (20). One cell line (ρ0-MFT-6) contained VIFs and the other (ρ0-MFT-16) was derived from vimentin-null cells. Cell lines were generated by treatment with ethidium bromide (see Materials and Methods) and deletion of mtDNA was confirmed by PCR (see Materials and Methods and Fig. 4A). Despite the lack of a functional respiratory chain in ρ0 cells, the mitochondria membrane potential is retained (34) as shown by the intensity of mitochondrial fluorescence in ρ0-MFT-16 cells stained with TMRE (Fig. 4, B). To determine whether mitochondria in ρ0 cells interacted with microtubules and/or VIF in a fashion similar to controls, we analyzed their relative motility (18). Our data show that in ρ0-MFT-16 cells, mitochondria are transported along microtubules similarly to those in MFT-16 cells (Supplemental Movies 1 and 2). Furthermore, the motility of mitochondria in ρ0-MFT-16 cells decreased upon restoration of VIF (Supplementary Fig. 2D), showing that mitochondria can still interact with vimentin with respect to the modulation of their motile properties, as we have shown previously (18). Therefore, mitochondria with disrupted respiratory chains possess membrane potential, move along microtubules, and can be anchored by VIF (18). Comparison of ρ0-MFT-6 and ρ0-MFT-16 cells shows that elimination of VIF did not alter the mitochondrial potential in ρ0 cells. In agreement with these results, neither restoration of VIF in ρ0-MFT-16 cells (Fig. 4C) nor vimentin knockdown in ρ0-MFT-6 cells (Fig. 4D) has any effect on mitochondria potential. Thus, VIFs increase membrane potential only in mitochondria with fully functional respiratory chains. Therefore, the effect of vimentin can only be explained by either the activation of the electron-transport chain or the inhibition of ATP-synthase. To choose between these 2 possibilities, we treated vimentin-null (MFT-16) and control (MFT-6) containing functional respiratory chains with the ATP-synthase inhibitor oligomycin. Inhibition of this enzyme prevents most of the consumption of the potential energy generated by respiration (35). The resulting data show that TMRE fluorescence intensity increases in the presence of oligomycin in cells containing VIF (MFT-6) to a greater extent than in the null cells (MFT-16) (Fig. 5). In contrast, the treatment of both ρ0 cell lines with oligomycin, which inhibits the F0 complex of ATP synthase (34), had no effects on their mitochondrial potential (data not shown). Therefore, VIF stimulate electron transport chain in mitochondria but do not reduce utilization of the energy of membrane potential by ATP-synthase.
Figure 5.
VIFs increase the consumption of potential energy by the ATP-synthase in mitochondria. Vimentin containing (MFT-6) and vimentin-null cells (MFT-16) were incubated with TMRE for 40 min and then oligomycin was added to the final concentration 10 µM and mitochondrial fluorescence was monitored. Data represent mean values of mitochondria fluorescence measured in 5 min intervals ± sem in arbitrary units. For each time point, mitochondrial fluorescence was analyzed in 10 cells of treated (closed symbols) or control (open symbols) cultures.
The N terminus of vimentin is responsible for increasing mitochondrial membrane potential
We have previously demonstrated that the non-α-helical N-terminal domain of vimentin is responsible for modulating the motile properties of mitochondria and that the substitution of Pro-56 to Arg disrupts this interaction (18). To test the possibility that the increase in mitochondrial membrane potential is also mediated by interaction with the vimentin N terminus, we analyzed the effects of vimentin (P56R) expression in null cells (Fig. 6A). The data show that the expression of this mutant vimentin eliminated the effect of VIF on mitochondrial membrane potential (Fig. 6B). Therefore, the effect of VIF on mitochondrial potential depends on interactions between mitochondria and the N terminus of vimentin.
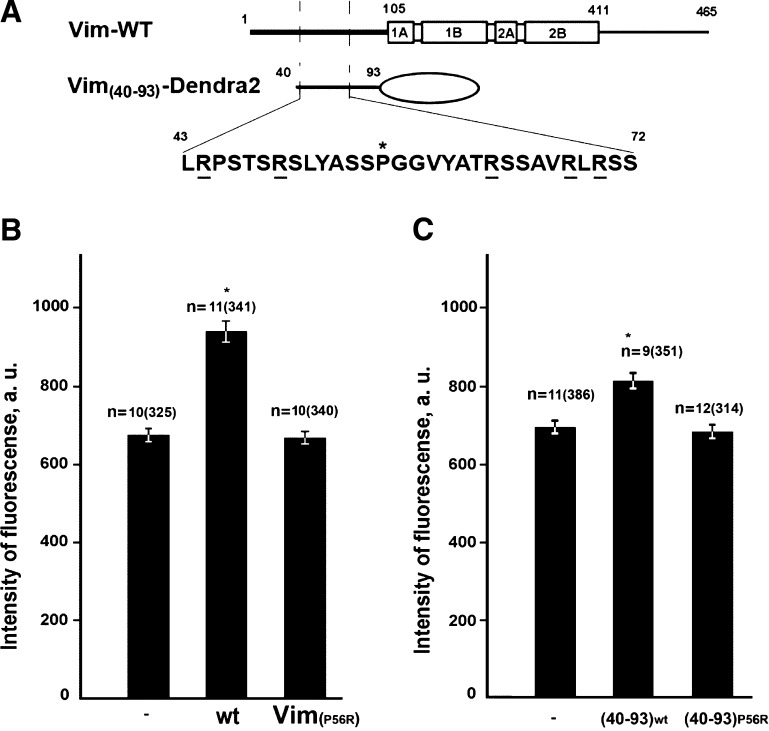
Figure 6.
N-terminal segment of vimentin is responsible for mitochondria potential elevation. A) Schematic representation of constructs used in this assay. Vim-WT–full-length vimentin showing N- and C-terminal domains, and central domain formed by 4 α-helical rich regions and the Vim(40-93)-Dendra-2 fragment of the vimentin N terminus fused to Dendra-2. The purported mitochondria binding site is shown in more detail with positively charged arginines underlined and Pro-56 (asterisk). B) Vimentin-null cells were cotransfected with plasmids encoding either wild-type human vimentin (wt) or its mutant Vim(P56R) together with plasmids encoding their EGFP-labeled variants or (C) transfected with plasmids pVim(40-93)-Dendra-2 or pVim(40-93)-(P56R)-Dendra-2. For controls, vimentin-null cells (-Vim) were transfected with the pEGFP-C1 plasmid. Mitochondria were stained with TMRE for 40 min and the fluorescence intensity of individual mitochondria was measured in mock-transfected cells (-Vim), and cells with restored VIF (see Materials and Methods). Values are mean intensities of fluorescence minus background fluorescence in the vicinity of each analyzed mitochondrion ± sem. n, number of cells and, in brackets, number of mitochondria. *P < 0.001; **P = 0.77.
We have also determined whether the interacting region of the vimentin N terminus containing the binding site is sufficient for increasing the mitochondrial potential by expressing the N-terminal fragment containing amino acids 40–93 fused to Dendra2 (Vim(40–93)-Dendra2) in vimentin-null cells (Fig. 6A). This chimeric protein localized to mitochondria as we determined by live imaging earlier (18) increased their membrane potential (Fig. 6C). However, if Vim(40–93)-(P56R)-Dendra2 unable to localize to mitochondria (18) is expressed, there is no effect on mitochondrial membrane potential (Fig. 6C).
DISCUSSION
Our data demonstrate that the association of mitochondria with VIFs increases their membrane potential. This is significant as most mitochondrial functions depend on their membrane potential (1, 36). Indeed, all available data show that in cells with altered IF networks, mitochondrial functions are often diminished (9, 11, 12). Thus, our observation is in good agreement with the fact that cells either null for VIFs or expressing altered arrays of IF contain aberrant mitochondria and are disposed to become apoptotic (16).
How can VIFs interacting with the outer membrane of mitochondria affect the potential on the inner mitochondrial membrane? One possibility resides in the finding that the mitochondrial binding site on vimentin (18) resembles the sequences in proteins known to be responsible for their targeting to the mitochondrial outer membrane (e.g., Bcl-2; Table 1) (37, 38). These amino acid sequences have similar effects on mitochondrial membrane potential (39). The mechanism of action of these targeting domains is still poorly understood, but it has been suggested that they interact with the VDAC (voltage-dependent anion channel). In light of our data showing a positive effect of VIFs on mitochondrial potential only in the presence of a functional respiratory chain, it is interesting to speculate that VIFs may increase the permeability of VDACs for several negatively charged compounds essential for oxidative phosphorylation. These could include such compounds as pyruvate, succinate, ADP, and so on. Such a mechanism could counterbalance the opposing effects of hexokinase (40) and/or tubulin (41), which have been shown to decrease VDAC permeability for these substrates suppressing respiration (42) and thereby reducing the membrane potential. It is also possible that VIFs regulate mitochondrial membrane potential by interacting with other proteins on their surfaces. The finding that vimentin-null fibroblasts undergo apoptosis much more readily than their wild-type counterparts (16) indicates that VIF could potentiate the antiapoptotic effects of other proteins or could also serve as an antiapoptotic factor.
TABLE 1.
Targeting sequences to OMM in some known proteins
| Protein | Sequences | Reference |
|---|---|---|
| Tom 20 H.s. | 1MVGRNSAIA10AGVCGALFIG20YCIYFDRKRR30SDPNFK . . . | Rapaport (38) |
| Tom 20 S.c. | 1MSQSNPILR10GLAITTAIAA20LSATGYAIYF30DYQRRNSPQF40R . . . | Rapaport (38) |
| Tom 70 S.c. | 1MKSFITRNKT10AILATVAATG20TAIGAYYYYN30QLQQQQQRGK40 . . . | Rapaport (38) |
| BCL-2 R.n. | . . . 210FSWLSLKTLSL220ALVGACITLG230AYLGHK | Rapaport (38) |
| Bcl-X R.n. | . . . 210GQERFNRWFL220TGMTVAGVVL230LGSLFSRK | Rapaport (38) |
| Plectin 1b M.m. | 1MEPSGSLFP10SVLVVVGHVV20TLAAVWHWRK30GHRQAKDEQ . . . | Winter et al. (47) |
Positively (in boldface) and negatively (in italics and underlined) charged amino acids. R.n., Rattus norvegicus; H.s., Homo sapiens; M.m., Mus musculus; OMM, outer mitochondria membrane; S.c., Saccharomyces cerevisiae.
Our data demonstrate that the association of vimentin with mitochondria increases their membrane potential and thereby stimulates oxidative phosphorylation. It is also possible that other types of IF proteins affect the membrane potential of mitochondria. Analysis of the amino acid sequences of the N-terminal domains of desmin, keratin 18, neurofilament light chain, and periferin contain sequences that meet the requirements for targeting to the outer mitochondrial membrane (Table 2). These consist of a moderately hydrophobic amino acid sequence containing a proline with 2 flanking clusters of positively charged amino acids (37, 38). However, additional work is required to test the possibility that other IF proteins regulate the membrane potential of mitochondria.
TABLE 2.
Sequences of vimentin and some other IF proteins that could bind to OMM
| Protein | Sequences |
|---|---|
| Vimentin H.s. | . . . 40SALRPSTSRSL50YASSPGGVYA60TRSSAVRLRS70SV . . . |
| Desmin H.s. | . . . 10SSQRVSSYRR20TFGGAPGFPL30GSPLSSPVFP40RAGFGSKGSSSSV . . . |
| Periferin H.s. | . . . 10GLRAGFSSTS20YRRTFGPPPS30LSPGAFSYSS40SSRFSSSRLLGSA . . . |
| NFL H.s. | . . . SSV30RSGYSTARSA40YSSYSAPVSS50SLSVRRSYSS60SSG . . . |
| Keratin 18 H.s. | . . . APSYGAR30PVSSAASVYA40GAGGSGSRIS50VSRSTSFRG . . . |
Positively charged amino acids are in boldface. H.s., Homo sapiens; NFL, neurofilament light chain.
Transport of mitochondria to the sites of increased energy consumption is the function of motor proteins moving along microtubules and actin microfilaments (43, 44). However, in addition to their role in regulating mitochondrial membrane potential, vimentin, desmin, and neurofilament IF, as well as the IF-associated protein, plectin, also control the anchorage of mitochondria in different regions of the cytoplasm. This positioning of mitochondria is crucial to their function (12, 45,47). Our data demonstrating that mitochondria with disrupted respiratory chain in ρ0 cells are motile and can be anchored by VIFs indicate that the positioning function of the VIF cytoskeleton can be independent of the regulation of VIFs’ membrane potential. Thus, the results presented in this study show that VIF not only anchor and localize mitochondria but also play an important role in regulating their membrane potential and their function in controlling the local level of ATP production within different regions of the cytoplasm.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. R. Evans for the cell lines,Natalia Minina for excellent technical assistance, and Dr. P. Chumakov for several useful advices. This work was funded by the Russian Academy of Sciences and Russian Foundation for Basic Research (Grants 06-04-48452-a, 10-04-00414-a, and 13-04-00931-a; to A.A.M.); and the U.S. National Institutes of Health (Grant P01-GM096971; to V.I.G. and R.D.G.).
Glossary
- ρ0 cells or rho 0 cells
cells depleted of mtDNA
- EGFP
enhanced green fluorescent protein
- IF
intermediate filament
- FBS
fetal bovine serum
- FCCP
carbonyl cyanide 4-(trifluoromethoxy)-phenylhydrazone
- MFT
transformed murine fibroblast
- pEGFP
plasmid EGFP
- RVIM-AT
rabbit anti-Vim antibody
- shRNA
small hairpin RNA
- TMRE
tetramethylrhodamine-ethyl ester
- VIF
vimentin intermediate filament
- mtDNA
mitochondrial DNA
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Nicholls D. G., Budd S. L. (2000) Mitochondria and neuronal survival. Physiol. Rev. 80, 315–360 [DOI] [PubMed] [Google Scholar]
- 2.Nicholls D. G., Ward M. W. (2000) Mitochondrial membrane potential and neuronal glutamate excitotoxicity: mortality and millivolts. Trends Neurosci. 23, 166–174 [DOI] [PubMed] [Google Scholar]
- 3.Green D. R., Reed J. C. (1998) Mitochondria and apoptosis. Science 281, 1309–1312 [DOI] [PubMed] [Google Scholar]
- 4.Kroemer G., Dallaporta B., Resche-Rigon M. (1998) The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 60, 619–642 [DOI] [PubMed] [Google Scholar]
- 5.Jarrett S. G., Lewin A. S., Boulton M. E. (2010) The importance of mitochondria in age-related and inherited eye disorders. Ophthalmic Res. 44, 179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamata H., Manfredi G. (2010) Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mech. Ageing Dev. 131, 517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocha M., Apostolova N., Hernandez-Mijares A., Herance R., Victor V. M. (2010) Oxidative stress and endothelial dysfunction in cardiovascular disease: mitochondria-targeted therapeutics. Curr. Med. Chem. 17, 3827–3841 [DOI] [PubMed] [Google Scholar]
- 8.Scaglia F. (2010) The role of mitochondrial dysfunction in psychiatric disease. Dev. Disabil. Res. Rev. 16, 136–143 [DOI] [PubMed] [Google Scholar]
- 9.Brownlees J., Ackerley S., Grierson A. J., Jacobsen N. J., Shea K., Anderton B. H., Leigh P. N., Shaw C. E., Miller C. C. (2002) Charcot-Marie-Tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum. Mol. Genet. 11, 2837–2844 [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Ollé R., López-Toledano M. A., Goryunov D., Cabrera-Poch N., Stefanis L., Brown K., Liem R. K. (2005) Mutations in the neurofilament light gene linked to Charcot-Marie-Tooth disease cause defects in transport. J. Neurochem. 93, 861–874 [DOI] [PubMed] [Google Scholar]
- 11.Milner D. J., Mavroidis M., Weisleder N., Capetanaki Y. (2000) Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J. Cell Biol. 150, 1283–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capetanaki Y. (2002) Desmin cytoskeleton: a potential regulator of muscle mitochondrial behavior and function. Trends Cardiovasc. Med. 12, 339–348 [DOI] [PubMed] [Google Scholar]
- 13.Uttam J., Hutton E., Coulombe P. A., Anton-Lamprecht I., Yu Q. C., Gedde-Dahl T. Jr., Fine J. D., Fuchs E. (1996) The genetic basis of epidermolysis bullosa simplex with mottled pigmentation. Proc. Natl. Acad. Sci. USA 93, 9079–9084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumemura H., Harada M., Yanagimoto C., Koga H., Kawaguchi T., Hanada S., Taniguchi E., Ueno T., Sata M. (2008) Mutation in keratin 18 induces mitochondrial fragmentation in liver-derived epithelial cells. Biochem. Biophys. Res. Commun. 367, 33–40 [DOI] [PubMed] [Google Scholar]
- 15.Maloyan A., Osinska H., Lammerding J., Lee R. T., Cingolani O. H., Kass D. A., Lorenz J. N., Robbins J. (2009) Biochemical and mechanical dysfunction in a mouse model of desmin-related myopathy. Circ. Res. 104, 1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tolstonog G. V., Shoeman R. L., Traub U., Traub P. (2001) Role of the intermediate filament protein vimentin in delaying senescence and in the spontaneous immortalization of mouse embryo fibroblasts. DNA Cell Biol. 20, 509–529 [DOI] [PubMed] [Google Scholar]
- 17.Chernoivanenko I. S., Matveeva E. A., Minin A. A. (2010) Vimentin intermediate filaments increase mitochondrial membrane potential. (2011) Membr. Cell Biol. Biochemistry (Moscow) (Supplement A)5, 21–28 [Google Scholar]
- 18.Nekrasova O. E., Mendez M. G., Chernoivanenko I. S., Tyurin-Kuzmin P. A., Kuczmarski E. R., Gelfand V. I., Goldman R. D., Minin A. A. (2011) Vimentin intermediate filaments modulate the motility of mitochondria. Mol. Biol. Cell 22, 2282–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holwell T. A., Schweitzer S. C., Evans R. M. (1997) Tetracycline regulated expression of vimentin in fibroblasts derived from vimentin null mice. J. Cell Sci. 110, 1947–1956 [DOI] [PubMed] [Google Scholar]
- 20.King M. P., Attardi G. (1989) Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science 246, 500–503 [DOI] [PubMed] [Google Scholar]
- 21.Yoon M., Moir R. D., Prahlad V., Goldman R. D. (1998) Motile properties of vimentin intermediate filament networks in living cells. J. Cell Biol. 143, 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kojima S., Vignjevic D., Borisy G. G. (2004) Improved silencing vector co-expressing GFP and small hairpin RNA. Biotechniques 36, 74–79 [DOI] [PubMed] [Google Scholar]
- 23.Mendez M. G., Kojima S., Goldman R. D. (2010) Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 24, 1838–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helfand B. T., Mendez M. G., Murthy S. N., Shumaker D. K., Grin B., Mahammad S., Aebi U., Wedig T., Wu Y. I., Hahn K. M., Inagaki M., Herrmann H., Goldman R. D. (2011) Vimentin organization modulates the formation of lamellipodia. Mol. Biol. Cell 22, 1274–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helfand B. T., Mendez M. G., Pugh J., Delsert C., Goldman R. D. (2003) A role for intermediate filaments in determining and maintaining the shape of nerve cells. Mol. Biol. Cell 14, 5069–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avsyuk A. Y., Khodyakov A. L., Baibikova E. M., Solovyanova O. B., Nadezhdina E. S. (1997) Stability of vimentin intermediate filaments in interphasic cells. Dokl. Rus. Acad. Nauk 357, 130–133 [PubMed] [Google Scholar]
- 27.Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 28.Kocher T. D., Thomas W. K., Meyer A., Edwards S. V., Pääbo S., Villablanca F. X., Wilson A. C. (1989) Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 86, 6196–6200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sorokin P. A., Medvedev D. A., Vasil’ev V. P., Vasil’eva E. D. (2011) Further studies of mitochondrial genome variability in Ponto-Caspian Proterorhinus species (Actinopterygii: Perciformes: Gobiidae) and their taxonomic implications. Acta Ichthyol. Piscat. 41, 95–104 [Google Scholar]
- 30.De Flora S., Camoirano A., Cartiglia C., Ferguson L. (1997) Modulation of the potency of promutagens and direct acting mutagens in bacteria by inhibitors of the multidrug resistance mechanism. Mutagenesis 12, 431–435 [DOI] [PubMed] [Google Scholar]
- 31.Minin A. A., Kulik A. V., Gyoeva F. K., Li Y., Goshima G., Gelfand V. I. (2006) Regulation of mitochondria distribution by RhoA and formins. J. Cell Sci. 119, 659–670 [DOI] [PubMed] [Google Scholar]
- 32.Koopman W. J. H., Distelmaier F., Esseling J. J., Smeitink J. A. M., Willems P. H. G. M. (2008) Computer-assisted live cell analysis of mitochondrial membrane potential, morphology and calcium handling. Methods 46, 304–311 [DOI] [PubMed] [Google Scholar]
- 33.Scaduto R. C. Jr., Grotyohann L. W. (1999) Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys. J. 76, 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buchet K., Godinot C. (1998) Functional F1-ATPase essential in maintaining growth and membrane potential of human mitochondrial DNA-depleted rho degrees cells. J. Biol. Chem. 273, 22983–22989 [DOI] [PubMed] [Google Scholar]
- 35.Brand M. D., Nicholls D. G. (2011) Assessing mitochondrial dysfunction in cells. Biochem. J. 435, 297–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saraste M. (1999) Oxidative phosphorylation at the fin de siècle. Science 283, 1488–1493 [DOI] [PubMed] [Google Scholar]
- 37.Allen R., Egan B., Gabriel K., Beilharz T., Lithgow T. (2002) A conserved proline residue is present in the transmembrane-spanning domain of Tom7 and other tail-anchored protein subunits of the TOM translocase. FEBS Lett. 514, 347–350 [DOI] [PubMed] [Google Scholar]
- 38.Rapaport D. (2003) Finding the right organelle. Targeting signals in mitochondrial outer-membrane proteins. EMBO Rep. 4, 948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimizu S., Eguchi Y., Kamiike W., Funahashi Y., Mignon A., Lacronique V., Matsuda H., Tsujimoto Y. (1998) Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc. Natl. Acad. Sci. USA 95, 1455–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azoulay-Zohar H., Israelson A., Abu-Hamad S., Shoshan-Barmatz V. (2004) In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem. J. 377, 347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rostovtseva T. K., Sheldon K. L., Hassanzadeh E., Monge C., Saks V., Bezrukov S. M., Sackett D. L. (2008) Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA 105, 18746–18751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monge C., Beraud N., Kuznetsov A. V., Rostovtseva T., Sackett D., Schlattner U., Vendelin M., Saks V. A. (2008) Regulation of respiration in brain mitochondria and synaptosomes: restrictions of ADP diffusion in situ, roles of tubulin, and mitochondrial creatine kinase. Mol. Cell. Biochem. 318, 147–165 [DOI] [PubMed] [Google Scholar]
- 43.Hollenbeck P. J., Saxton W. M. (2005) The axonal transport of mitochondria. J. Cell Sci. 118, 5411–5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller K. E., Sheetz M. P. (2006) Direct evidence for coherent low velocity axonal transport of mitochondria. J. Cell Biol. 173, 373–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leterrier J. F., Rusakov D. A., Nelson B. D., Linden M. (1994) Interactions between brain mitochondria and cytoskeleton: evidence for specialized outer membrane domains involved in the association of cytoskeleton-associated proteins to mitochondria in situ and in vitro. Microsc. Res. Tech. 27, 233–261 [DOI] [PubMed] [Google Scholar]
- 46.Chada S. R., Hollenbeck P. J. (2003) Mitochondrial movement and positioning in axons: the role of growth factor signaling. J. Exp. Biol. 206, 1985–1992 [DOI] [PubMed] [Google Scholar]
- 47.Winter L., Abrahamsberg C., Wiche G. (2008) Plectin isoform 1b mediates mitochondrion-intermediate filament network linkage and controls organelle shape. J. Cell Biol. 181, 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.