Abstract
Innate CD8+ T cells are a heterogeneous population with developmental pathways distinct from conventional CD8+ T cells. However, their biology, classification, and functions remain incompletely understood. We recently demonstrated the existence of a novel population of chemokine (C-X-C motif) receptor 3 (CXCR3)-positive innate CD8+ T cells. Here, we investigated the functional properties of this subset and identified effector molecules and pathways which mediate their function. Adoptive transfer of IL-15 activated CXCR3+ innate CD8+ T cells conferred increased protection against Listeria monocytogenes infection in susceptible IFN-γ−/− mice compared with similarly activated CXCR3− subset. This was associated with enhanced proliferation and IFN-γ production in CXCR3+ cells. Further, CXCR3+ innate cells showed enhanced cytotoxicity against a tumor cell line in vitro. In depth analysis of the CXCR3+ subset showed increased gene expression of Ccl5, Klrc1, CtsW, GP49a, IL-2Rβ, Atp5e, and Ly6c but reduced IFN-γR2 and Art2b. Ingenuity pathway analysis revealed an up-regulation of genes associated with T-cell activation, proliferation, cytotoxicity, and translational initiation in CXCR3+ populations. Our results demonstrate that CXCR3 expression in innate CD8+ T cells defines a subset with enhanced cytotoxic potential and protective antibacterial immune functions. Immunotherapeutic approaches against infectious disease and cancer could utilize CXCR3+ innate CD8+ T-cell populations as novel clinical intervention strategies.—Oghumu, S., Terrazas, C. A., Varikuti, S., Kimble, J., Vadia, S., Yu, L., Seveau, S., Satoskar, A. R. CXCR3 expression defines a novel subset of innate CD8+ T cells that enhance immunity against bacterial infection and cancer upon stimulation with IL-15.
Keywords: granzyme, interferon, Listeria, cytokine, cytotoxicity
The discovery of innate CD8+ T cells has broadened our understanding of T-cell subset classification, development, and function. Phenotypically, these cells are known to have a comparatively restricted T-cell receptor repertoire and express markers for memory T cells, including CD44 and CD122, allowing them to generate rapid effector responses upon cytokine stimulation (1, 2). Moreover, unlike activated CD8+ T cells generated during adaptive immunity, innate CD8+ T cells are present in naive, and even germ-free, mice. A number of studies have identified factors which contribute to the development, maintenance and effector functions of innate CD8+ T cells. Their maturation in the thymus involves nonclassic major histocompatibility complex class I molecules, and unlike conventional CD8+ T cells, they do not require Tec protein tyrosine kinases IL-2 inducible T-cell kinase (Itk) or resting lymphocyte kinase (Rlk) (2). Recently, the signaling lymphocyte activation molecule (SLAM) family receptors coupled with a downstream SLAM-associated protein signaling adaptor molecule have been shown to regulate the development and maintenance of this population, which is dependent on the production of IL-4 (3, 4). Other reports have demonstrated a requirement for IL-15 in the maintenance and mediation of the effector functions of innate CD8+ T cells (5). However, specific mechanisms governing the generation of innate CD8+ T-cell populations, as well as their functions, are still not completely understood.
We have recently demonstrated that a large proportion of innate CD8+ T cells expresses chemokine (C-X-C motif) receptor 3 (CXCR3), and this subset expresses increased levels of activation markers and responds more rapidly to IL-2 and IL-15 stimulation than CXCR3− innate CD8+ T cells (6). This was accompanied by increased expression of IFN-γ as well as the cytotoxic molecule granzyme B. These results present the possibility that these cells could be effective in antitumor immune responses as well as in contributing to immunity against intracellular bacteria. Previous reports have demonstrated a role for class Ib restricted innate CD8+ T-cell populations in early antibacterial immune responses before the onset of adaptive immunity (7–10). CXCR3-expressing subpopulations of innate CD8+ T cells could potentially provide more potent immune responses against a bacterial infectious challenge. Moreover, because activated CD8+ T cells play a vital role in antitumor immunity, strategies aimed at activating CXCR3 expressing innate CD8+ T cells could be a viable approach to cancer immunotherapy.
Given the high importance yet incomplete understanding of the biology and function of the heterogeneous population of innate CD8+ T cells, we have further characterized subsets of this population and identified effector molecules which mediate their function. We have also examined the relative contributions of these populations to antibacterial as well as antitumor cell responses. Our results indicate that CXCR3 expressing innate CD8+ T-cell populations display enhanced cytotoxicity against tumor cells and provide increased protection against primary infection by Listeria monocytogenes in vivo. These studies strongly suggest that CXCR3 expressing innate CD8+ T cells could represent a possible vaccine target in the management of intracellular bacterial infection and neoplastic disease.
MATERIALS AND METHODS
Mouse strains
C57BL/6 wild-type and C57BL/6 IFN-γ knockout mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). CXCR3 IRES Bicistronic EGFP reporter (CIBER) mice (backcrossed to C57BL/6 background for 13 generations) were generated by our group as described previously (6). All mice used were maintained in a pathogen-free animal facility at The Ohio State University in accordance with U.S. National Institutes of Health and institutional guidelines.
Flow cytometry and cell sorting
Single cell suspensions from spleens or lymph nodes were derived from naive CIBER mice, washed with PBS and blocked with normal mouse serum or anti-CD16/CD32 antibodies. In some experiments, T cells were enriched by passing splenocytes through nylon wool column (Polysciences, Warrington, PA, USA) according to the manufacturer’s instructions. Cells were incubated with fluorescently labeled anti-CD8, anti-CD62L, and anti-CD44 antibodies (Biolegend, San Diego, CA, USA). For intracellular staining, stimulated cells were stained for extracellular markers, fixed with 2% para-formaldehyde, permeabilized, and stained with anti–IFN-γ antibodies (Biolegend). Cells were either acquired on a fluorescence activated cell sorter (FACS) Canto flow cytometer or sorted on a FACS Aria cell sorter (BD Biosciences, San Jose, CA, USA) at the flow cytometry core facility at Ohio State University Medical Center. Analysis was performed with CellQuestPro software (BD Biosciences) or FlowJo software (Tree Star Incorporated, Ashland, OR, USA), and sorted populations were used for in vivo and in vitro experiments.
Microarray analysis
Total RNA was isolated from sorted CXCR3+ and CXCR3− innate CD8+ T-cell as well as naive CD8+ T-cell populations from about 3 to 5 CIBER mice using an RNeasy kit (Qiagen, Valencia, CA, USA). RNA quantity, quality, and integrity were confirmed by Nanodrop and Agilent Bioanalyzer before inclusion in the array. Microarray processing was performed at the Micro Array Shared Resource, The Ohio State University. RNA amplification, fragmentation, and labeling were carried out according to manufacturer’s protocols (Affymetrix, Santa Clara, CA, USA). The arrays (GeneChip Mouse Gene 2.0ST) were hybridized for 16 h at 45°C and 60 rpm. Washing and staining of arrays was performed at the fluidics station 450 according to manufacturer’s protocol (Affymetrix). The microarrays were scanned using an Affymetrix GeneChip Scanner 3000 7G with Affymetrix GeneChip Command Console (AGCC) software. Background correction and quantile normalization was performed to adjust technical bias, and expression levels were summarized over the probe set using the robust multiarray average method (11). A filtering method based on percentage of arrays above noise cutoff was applied to filter out low-expression genes. Affymetrix Expression Console software and R statistical software (http://www.r-project.org/) was used for the analysis. Microarray expression data have been submitted to the Gene Expression Omnibus (GSE accession no. GSE60068).
Ingenuity pathway analysis of gene expression arrays
Molecular interactions among differentially regulated genes between CXCR3+ and CXCR3− innate CD8+ T cells were explored using ingenuity pathway analysis (IPA) (Qiagen). Each mouse gene identifier was mapped to its corresponding gene in the Ingenuity Pathway Knowledge Base. Families of genes that were up- or down-regulated in CXCR3 expressing innate CD8+ T cells compared to CXCR3− innate CD8+ T cells were integrated into predictive network models on the basis of gene interactions within a biologic pathway defined in the literature as contained in the Ingenuity Pathway Knowledge Base.
RT-PCR validation
Total RNA was extracted from naive, CXCR3+ and CXCR3− innate CD8+ T-cell populations using the RNeasy kit (Qiagen). RNA was reverse transcribed to cDNA using the SuperScript Vilo cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA). Primer sequences and cycling conditions for RT-PCR were obtained using the Primer Bank website (http://pga.mgh.harvard.edu/primerbank/index.html), Harvard Medical School. PCR amplification was performed in a CFX 96 RT-PCR cycler (Bio-Rad, Hercules, CA, USA) using SYBR Green (Bio-Rad) for detection. Data were normalized to β-actin and presented as fold induction over naive cells using the ΔΔCT method.
Adoptive transfer and cellular analysis
Spleens were removed aseptically from naive CIBER mice, and single cell suspensions were prepared after lysis of red blood cells. Naive CD8+, CXCR3+ innate CD8+, and CXCR3− innate CD8+ T-cell populations were sorted using FACS Aria (BD Biosciences) as described. Sorted cells were preactivated with IL-15 before adoptive transfer as previously demonstrated (12). One million cells from sorted, preactivated populations were labeled with CellTrace Violet Cell Proliferation Kit (Invitrogen) and injected into IFN-γ knockout mice via tail vein injections. On d 1 after adoptive transfer, recipient mice were infected with L. monocytogenes. On d 4 after adoptive transfer, mice were humanely killed, and spleens were analyzed for the presence of adoptively transferred cells, cell proliferation, and intracellular IFN-γ production (Biolegend) by flow cytometry.
Bacterial infection and enumeration
Listeria monocytogenes strain 10403S (wild type; strain was a gift from Dr. D. Portnoy, University of California, Berkeley, CA, USA) was grown overnight at 37°C in brain–heart infusion. Overnight cultures were diluted 1/20 in brain–heart infusion and grown at 37°C until OD600 = 0.7 to 0.8. Bacteria were washed 3 times and diluted in PBS. Mice were infected by tail vein injection with 104 bacteria. Livers and spleens were collected 72 h after infection. To enumerate colony-forming units, organs were homogenized in PBS, serial dilutions of homogenates were plated on brain–heart infusion agar, and samples were incubated at 37°C for 24 h.
Cytotoxicity assay
Naive CD8+, CXCR3+ innate CD8+, and CXCR3− innate CD8+ T cells were sorted from naive CIBER mice and then stimulated with IL-15 for 48 h. Stimulated CD8+ T-cell subsets were cocultured with P815 mastocytoma cell line (American Type Culture Collection, Manassas, VA, USA) at E:T ratios of 1:1 and 5:1. Cytotoxicity of target cells was measured by lactate dehydrogenase release using the Cytotoxicity Detection Kit (Roche Diagnostics, Indianapolis, IN, USA) with appropriate controls.
Statistical analysis
Statistical analyses were performed by Prism 5 software (GraphPad Software, San Diego, CA, USA). Student’s unpaired t test was used to determine statistical significance of values obtained. P values of <0.05 were considered statistically significant. The R statistical package was used for the analysis of microarray data.
RESULTS
CXCR3+ innate CD8+ cells confer increased protection against L. monocytogenes infection
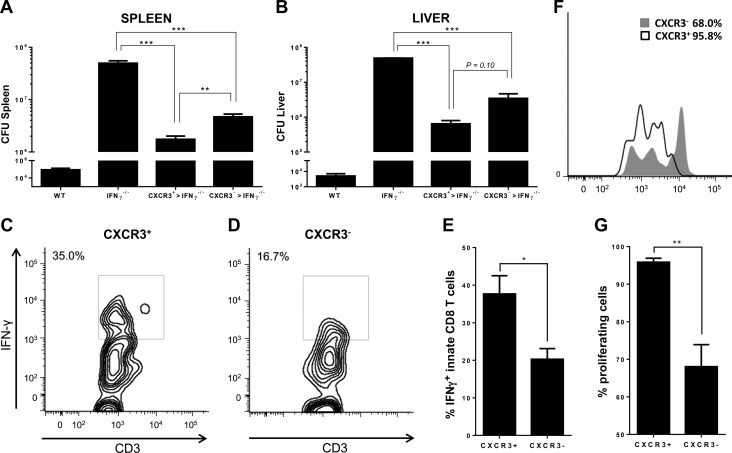
We recently identified a subset of innate CD8+ T cells that express the chemokine receptor CXCR3 (6). These cells were distinct from CXCR3− innate CD8+ T cells in the expression of antiapoptotic factors as well as in the production of granzyme B and IFN-γ after stimulation with IL-2 or IL-15 (6), which suggested an enhanced role for CXCR3+ innate CD8+ T cells in antibacterial immune responses. This led us to test the protective effect of this population against an infectious challenge by an intracellular bacterium. We used an in vivo bacterial infection model using L. monocytogenes, which causes a food-borne disease called listeriosis in animals and humans. Protective immune response against L. monocytogenes is T-cell mediated, and IFN-γ is essential for early control of infection. IFN-γ–deficient mice are highly susceptible to L. monocytogenes infection, and early IFN-γ production primarily by CD8+ T cells is essential for innate immune protection (13). This is therefore a suitable bacterial infection model to test the protective ability of our innate CD8+ T-cell subsets. CXCR3+ and CXCR3− innate CD8+ T cells were sorted from naive CIBER mice and activated in vitro with IL-15. Sorted cells were transferred intravenously into IFN-γ–deficient C57BL/6 mice 1 d before L. monocytogenes infection. Three days after infection, bacterial loads were evaluated in livers and spleens of recipient mice, and the proliferative potential and effector functions of adoptively transferred innate CD8+ T-cell subsets were analyzed. As expected, IFN-γ–deficient mice had much higher bacterial loads in their livers and spleens compared to wild-type controls. IFN-γ–deficient mice adoptively transferred with innate CD8+ T-cell subsets had significantly reduced bacterial burdens in their livers and spleens compared to nontransferred IFN-γ–deficient mice (Fig. 1A, B). Mice that received CXCR3+ innate CD8+ T cells displayed significantly less bacterial loads in the spleens than those that received CXCR3− innate CD8+ T cells (Fig. 1A). Bacterial counts in the liver were also less in CXCR3+ innate CD8+ cell-transferred mice, although the difference was not significant (Fig. 1B).
Figure 1.
CXCR3+ innate CD8+ cells confer increased protection against L. monocytogenes infection. Bacterial loads in the (A) spleen and (B) liver of L. monocytogenes infected wild-type C57BL/6 mice or IFN-γ knockout mice that received either no cells (IFN-γ−/−), CXCR3+ innate CD8+ T cells (CXCR3+ > IFN-γ−/−), or CXCR3− innate CD8+ T cells (CXCR3− > IFN-γ−/−). C, D) Intracellular IFN-γ production by (C) CXCR3+ or (D) CXCR3− innate CD8+ T cells purified from CIBER mice and adoptively transferred to IFN-γ−/− mice and subsequently infected with L. monocytogenes. E) Percentage of IFN-γ producing, adoptively transferred innate CD8+ T cells in IFN-γ−/− mice infected with L. monocytogenes. F, G) Cellular proliferation of sorted and adoptively transferred CXCR3+ and CXCR3− innate CD8+ T-cell populations in IFN-γ−/− mice infected with L. monocytogenes. Percentages of proliferating cells are depicted in (G). Data are presented as mean ± sem and are representative of 2 separate experiments from 3 to 5 individual mice per group. *P < 0.05, **P < 0.01, ***P < 0.001.
Adoptively transferred CXCR3+ and CXCR3− innate CD8+ T cells were detected in the spleens of infected mice at d 3 after infection. The protective effect of the CXCR3+ innate CD8+ T-cell subset was accompanied by enhanced IFN-γ production compared to CXCR3− innate CD8+ T cells, as demonstrated by intracellular flow cytometric analysis of adoptively transferred cells in the spleens of recipient mice (Fig. 1C–E). Further, we observed that transferred CXCR3+ innate CD8+ T cells proliferated to a greater extent than the CXCR3− innate CD8+ T-cell population after L. monocytogenes infection in vivo (Fig. 1F, G). Taken together, these results demonstrate that CXCR3+ innate CD8+ T cells confer greater protection against infection by L. monocytogenes.
CXCR3+ innate CD8+ T cells show enhanced tumor cytotoxicity in vitro
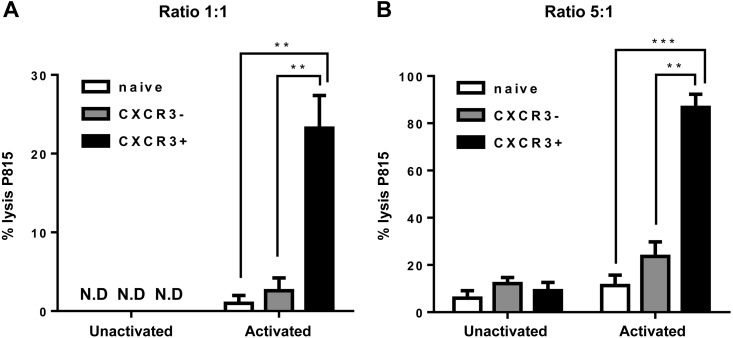
Immune cytotoxic effector cells are major contributors to antitumor immunity (14). Previous work on innate CD8+ T cells and our current detailed transcriptional analysis of this population suggest that CXCR3 expressing innate CD8+ T cells are potent mediators of antitumor immunity (6). We therefore tested the functional ability of CXCR3 expressing and nonexpressing innate CD8+ T cells in mediating cytotoxicity against P815 target cancer cells in vitro. Sorted innate CD8+ cell subsets from naive mice were preactivated with IL-15 in vitro and cocultured with P815 target cells. CXCR3+ innate CD8+ T cells were significantly more potent than CXCR3− innate CD8+ T cells and naive CD8+ T cells at killing target tumor cells in vitro (Fig. 2). Unstimulated cells were not cytotoxic to tumor cells in vitro (Fig. 2). Our data demonstrate that CXCR3+ innate CD8+ cells are potentially more efficient in mediating tumor cell cytotoxicity. These results demonstrate the possibility of exploiting this subset of innate CD8+ T cells in therapeutic approaches to cancer immunosuppression and subsequent tumor regression.
Figure 2.
CXCR3+ innate CD8+ T cells show enhanced tumor cell cytotoxicity in vitro. A, B) Cytotoxic activity of IL-15–stimulated naive, CXCR3+ innate CD8+ T cells, and CXCR3− innate CD8+ T-cell populations against P815 mastocytoma cell line at effector to target ratios of (A) 1:1 and (B) 5:1, as determined by lactate dehydrogenase release assay. Unstimulated cells are also shown. Data are presented as mean ± sem of 3 individual samples and are representative of 2 separate experiments. **P < 0.01, ***P < 0.001. ND, not detectable.
Gene expression of innate CD8+ T-cell subsets
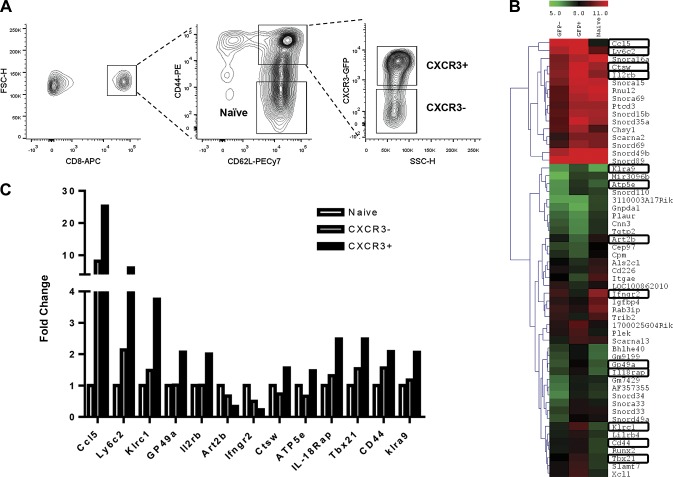
To further characterize potential mechanisms of action behind the potent antibacterial and antitumor immunity characteristic of CXCR3+ innate CD8+ T cells, we performed gene expression profiling of sorted naive, CXCR3+, and CXCR3− innate CD8+ T cells from naive mice (Fig. 3A). When compared to innate CD8+ T cells that do not express CXCR3, CXCR3+ innate CD8+ T cells displayed enhanced expression of the chemokine (C-X-C motif) ligand 5 (CCL5) as well as genes associated with cellular activation and proliferation including killer cell lectin-like receptor subfamily C, member 1 (klrc1), glycoprotein 49A (Gp49a), and IL-2 receptor β (IL-2Rβ). The cysteine protease known to regulate T-cell cytolytic activity, cathepsin W (ctsW), was also up-regulated in CXCR3 expressing innate CD8+ T cells (Fig. 3B, C). This subset also up-regulated genes that have previously been shown to be associated with a T-bet–dependent type 1 CD8 cytotoxic T-cell (Tc1) programming, which leads to enhanced antitumor responses, including T-box 21 (tbx21), klrc1, IL-2 receptor β (il2rb), cytotoxic T lymphocyte (CTL)–associated protein 2 α (ctla2a), CTL-associated protein 2 β (ctla2b), and Fas ligand (faslg), which have been shown to be important in CD8+ T-cell cytotoxicity (15). Other genes significantly up-regulated include ATP synthase, H+ transporting, mitochondrial F1 complex, ε subunit (ATP5e) involved in ATP synthesis and oxidative phosphorylation, and Ly6c important in signal transduction and cytokine production during T-cell activation. In support of this result, previous work in our laboratory demonstrated increased cell surface expression of Ly6c in CXCR3+ innate CD8+ T cells (6). Genes that were significantly down-regulated in CXCR3+ innate CD8+ T cells compared to CXCR3− innate CD8+ T cells include IFN-γ receptor 2 (IFNγR2) and ADP-ribosyl transferase 2b (Art2b), which is involved in NAD+-induced cell death (Fig. 3B, C).
Figure 3.
Gene expression profiling of sorted innate CD8+ T-cell subsets. A) Flow cytometric analysis of lymph node cells from naive CIBER mice showing CXCR3+ and CXCR3− innate CD8+ T-cell populations. Gating strategy used for sorting CXCR3+ and CXCR3− innate CD8+ T-cell populations are also shown. B) Heat map with gene expression patterns of highly dsyregulated genes in naive, CXCR3+, and CXCR3− innate CD8+ T-cell populations as determined by microarray data analysis. Gene expression variations are represented by color. C) Fold induction of significant genes in CXCR3+ and CXCR3− innate CD8+ T-cell populations compared to naive CD8+ T cells, as determined by microarray. Data were obtained from sorted cells from a pool of 5 mice.
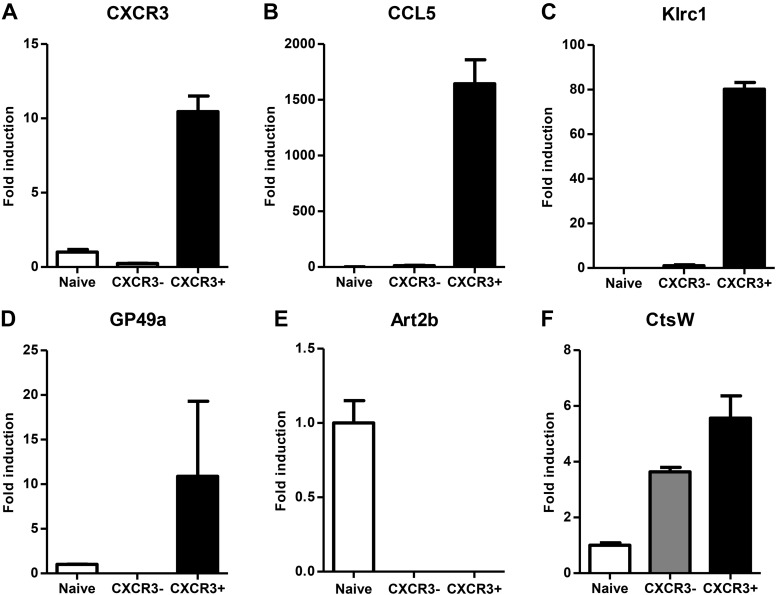
To confirm the results of the gene expression microarray, we performed quantitative RT-PCR (qRT-PCR) validation on RNA obtained from naive, CXCR3+, and CXCR3− innate CD8+ T-cell subsets (Fig. 4). The genes analyzed represent the range of T-cell functions detected by the microarray data. The relative expressions of genes in the T-cell subsets analyzed by qRT-PCR were consistent with the gene expression changes observed by gene microarray. Taken together, our results establish a phenotypic distinction between CXCR3 expressing and nonexpressing subsets of innate CD8+ T cells and define a important functional role for CXCR3+ innate CD8+ T cells in antibacterial and antitumor immune responses.
Figure 4.
qRT-PCR validation of select dysregulated genes among innate CD8+ T-cell subsets. A–F) Relative gene expression of (A) Cxcr3, (B) Ccl5, (C) Klrc1, (D) GP49b, (E) Art2b, and (F) CtsW in sorted CXCR3+ and CXCR3− innate CD8+ T-cell populations from naive CIBER mice as determined by qRT-PCR. Data are presented as fold induction relative to naive CD8+ T cells and as mean ± sem of duplicates obtained by pooling samples from 3 or 4 individual mice.
Ingenuity pathway analysis of differentially regulated genes in CXCR3+ and CXCR3− innate CD8+ T cells
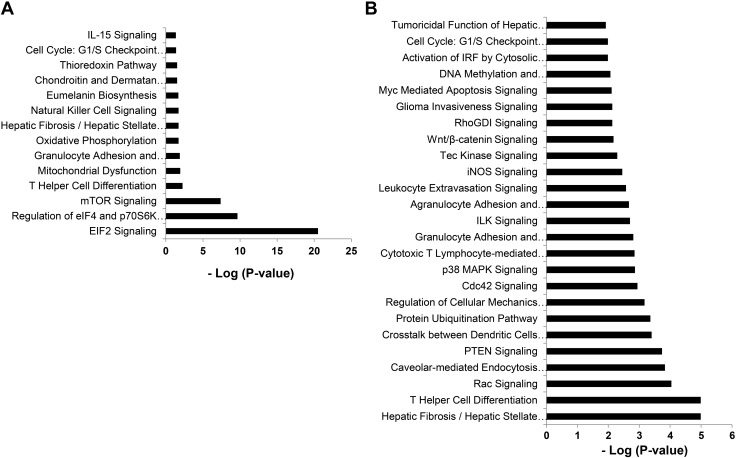
To further characterize potential signaling pathways preferentially utilized by the CXCR3 expressing subset of innate CD8+ T cells which play a role in their function, we performed IPA (Ingenuity Systems, http://www.ingenuity.com/) on genes that were differentially regulated in this subset compared to CXCR3− innate and naive CD8+ T cells. Major canonical pathways that were significantly modulated between CXCR3+ and CXCR3− innate CD8+ T cells are shown in Fig. 5A and include eukaryotic initiation factor (eIF) 2 and eIF4 signaling. eIF2 signaling regulates translation initiation in response to stressors such as bacterial infection. Up-regulated eIF2 signaling has been shown to play a vital role in immunity to intracellular bacterial infection including L. monocytogenes (16). eIF4 and p70S6K also play critical roles in translational regulation. Molecules associated with the significant canonical pathways in CXCR3+ vs. CXCR3− innate CD8+ T cells identified using IPA are listed in Table 1. Significant pathways in the CXCR3+ innate CD8+ vs. naive CD8+ sample set are depicted in Fig. 5B, and molecules associated with these pathways are listed in Table 2.
Figure 5.
Top gene networks generated from ingenuity pathway analysis and significantly modulated between innate and conventional naive CD8 T cells. A) Top networks significantly modulated (log P value) between CXCR3+ and CXCR3− innate CD8+ T-cell populations. B) Top networks significantly modulated (log P value) between CXCR3+ innate CD8+ T cells and naive CD8+ T cells. Molecules associated with these pathways are depicted in Tables 1 and 2.
TABLE 1.
Top canonical pathways and associated molecules modulated between CXCR3+ and CXCR3− innate CD8+ T cells
| Ingenuity canonical pathway | Molecules |
|---|---|
| eIF2 signaling | RPS2, RPS19, RPL30, RPS21, eIF4A2, RPL37A, RPLP0, RPS12, RPS7, RPL14, RPL8, RPL35, RPS16, RPS9, RPL5, RPS3, RPL13, RPL13A, RPSA, RPL38 |
| Regulation of eIF4 and p70S6K signaling | ITGB1, RPS7, RPS16, RPS2, RPS9, RPS19, eIF4A2, RPS21, RPS3, RPS12, RPSA |
| mTOR signaling | RPS7, RPS16, RPS2, RPS9, RPS19, eIF4A2, RPS21, RPS3, RPS12, RPSA |
| T helper cell differentiation | IFNGR2, TBX21, IL18R1 |
| Mitochondrial dysfunction | COX7B, NDUFA3, PSENEN, Atp5e |
| Granulocyte adhesion and diapedesis | ITGB1, XCL1, CCL5, IL18RAP |
| Oxidative phosphorylation | COX7B, NDUFA3, Atp5e |
| Hepatic fibrosis/hepatic stellate cell activation | IFNGR2, CCL5, FASLG, IL18RAP |
| NK cell signaling | Klra4, HCST, KLRC1 |
| Eumelanin biosynthesis | MIF |
| Chondroitin and dermatan biosynthesis | CHSY1 |
| Thioredoxin pathway | TXN |
| Cell cycle: G1/S checkpoint regulation | PA2G4, RPL5 |
| IL-15 signaling | FASLG, IL2RB |
TABLE 2.
Top canonical pathways and associated molecules modulated between CXCR3+ innate CD8+ and naive CD8+ T cells
| Ingenuity canonical pathway | Molecules |
|---|---|
| Hepatic fibrosis / hepatic stellate cell activation | IGFBP4, IGF1R, IFNGR2, CCL5, STAT1, CCR7, FASLG, TIMP2, IL18RAP |
| T helper cell differentiation | IL6ST, IFNGR2, CXCR5, STAT1, TBX21, IL18R1 |
| Rac signaling | ITGB1, JUN, CFL2, CD44, PIP4K2A, ITGA4 |
| Caveolar-mediated endocytosis signaling | ITGB1, ITGAE, HLA-A, ITGB7, ITGA4 |
| PTEN signaling | ITGB1, TGFBR3, IGF1R, CDKN1B, FASLG, ITGA4 |
| Cross-talk between dendritic cells and NK cells | HLA-A, CD226, CCR7, FASLG, IL2RB |
| Protein ubiquitination pathway | USO1, USP28, DNAJC9, PSMD7, HLA-A, PSMB1, ANAPC10, NEDD4L |
| Regulation of cellular mechanics by calpain protease | ITGB1, CDKN1B, ACTN1, ITGA4 |
| Cdc42 signaling | ITGB1, JUN, H2-T10, CFL2, HLA-A, ITGA4 |
| p38 MAPK signaling | ATF1, DUSP10, STAT1, FASLG, IL18RAP |
| CTL-mediated apoptosis of target cells | HLA-A, CASP8, FASLG |
| Granulocyte adhesion and diapedesis | ITGB1, PECAM1, XCL1, CCL5, ITGA4, IL18RAP |
| ILK signaling | ITGB1, JUN, CFL2, LEF1, ITGB7, ACTN1 |
| Agranulocyte adhesion and diapedesis | ITGB1, PECAM1, XCL1, CCL5, ITGB7, ITGA4 |
| Leukocyte extravasation signaling | ITGB1, CD44, PECAM1, ACTN71, TIMP2, ITGA4 |
| iNOS signaling | JUN, IFNGR2, STAT1 |
| Tec kinase signaling | ITGB1, GTF2I, STAT1, FASLG, ITGA4 |
| Wnt/β-catenin signaling | JUN, TGFBR3, HDAC1, CD44, LEF1 |
| RhoGDI signaling | ITGB1, CFL2, CD44, PIP4K2A, ITGA4 |
| Glioma invasiveness signaling | CD44, PLAUR, TIMP2 |
| Myc mediated apoptosis signaling | IGF1R, CASP8, FASLG |
| DNA methylation and transcriptional repression signaling | HDAC1, MBD2 |
| Activation of IRF by cytosolic pattern recognition receptors | JUN, IFNA4, STAT1 |
| Cell cycle: G1/S checkpoint regulation | HDAC1, RPL5, CDKN1B |
| Tumoricidal function of hepatic NK cells | CASP8, FASLG |
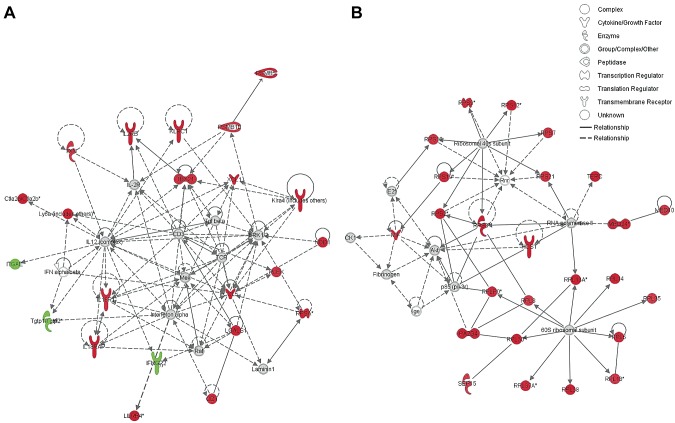
Gene interaction networks analysis of 162 genes differentially expressed in CXCR3+ innate CD8+ T cells relative to CXCR3− innate CD8+ T cells revealed 2 important signaling networks in which most of the genes are up-regulated in CXCR3+ innate CD8+ T cells (Fig. 6). These signaling networks are associated with T-cell activation, proliferation, and cytotoxic effector functions (Fig. 6A), as well as with RNA posttranscriptional modification, translational control, and protein synthesis (Fig. 6B). Interestingly, IFN-γR2 and T-cell–specific GTPase (Tgtp1/2), which is regulated by IFN-γ, are down-regulated in this subset, suggesting a diminished response to IFN-γ activation compared to CXCR3− innate CD8+ T cells.
Figure 6.
Ingenuity pathway analysis of differentially regulated genes between CXCR3+ innate, CXCR3− innate, and naive CD8+ T cells subsets. A) Functional pathway analysis of immune related gene interactions associated with cellular activation, differentiation, and cytotoxicity between innate CD8+ T-cell subsets. B) Functional pathway analysis of genes associated with 40S and 60S ribosomal subunits as well as others involved in translation initiation between innate CD8+ T-cell subsets. Genes in red are significantly up-regulated and genes in green are significantly down-regulated in CXCR3+ compared to CXCR3− innate CD8+ T cells.
DISCUSSION
Our results provide compelling evidence for the phenotypic and functional heterogeneity of innate CD8+ T-cell subsets and a role for CXCR3 expression in predicting distinct effector functions within this diverse population. CXCR3 has canonically been associated with type 1 immune responses, driven by the master regulator, T-bet, and defined by IFN-γ production, resulting in host protective responses against intracellular pathogens and during a tumor challenge (14, 15, 17–19). Our present work, coupled with previous data from our laboratory (6), strongly suggests comparable phenotypic characteristics between the CXCR3 expressing subset of innate CD8+ T cells and Tc1 effector cells (15, 20, 21), such as the enhanced expression of Ly6c, granzyme B, T-bet, and IFN-γ. Added supporting evidence for the role of CXCR3+ innate CD8+ T cells in type 1 effector responses was the observed significant down-regulation of IFN-γR2 in this population. It has been shown that Th1 cells do not respond to IFN-γ stimulation (22, 23), and reduced IFN-γR2 expression is essential for mediating the effector functions of Th1 cells (24). It is therefore not surprising that CXCR3+ innate CD8+ T cells contribute to type 1 immunity during the early stages of an infectious challenge. However, it is noteworthy that the expression of CD62L in CXCR3+ innate CD8+ T cells, as well as the relative abundance of this population in naive (and even germ-free) mice (6), distinguish them from Tc1 effector cells, which usually arise after an infectious challenge. Functionally, this subset of innate CD8+ T cells seems to be involved in antibacterial and antitumor effector functions during the innate phase of the immune response.
Our rationale for using IL-15 to preactivate innate CD8+ T-cell subsets before our assays was to demonstrate the behavior of these cells in a naturally occurring inflammatory environment, such as during microbial infection or neoplastic disease. IL-15 is significantly produced in microbial or tumor microenvironments, which is necessary for the optimal activity of innate lymphocytes including innate CD8+ T cells, NK cells, and NKT cells (12, 25, 26). Interestingly, IL-15 has been shown to be required for the maintenance of the total innate CD8+ T-cell population (1, 5). In previous studies using innate CD8+ T cells in an in vivo infection model with L. monocytogenes, authors preactivated these cells with IL-15 (12). Further, these studies showed that unstimulated and IL-2 stimulated innate CD8+ T cells provided minimal protection against Listeria infection. In keeping with these studies, we preactivated our cells with IL-15. The novelty of our study is that, unlike previous work that adoptively transferred total innate CD8+ T cells after preactivation with IL-15, we reveal a specific subset that is more responsive to this cytokine, which has biologic relevance in tumor killing and antibacterial activity.
CD8+ T cells play an important role in the control and elimination of L. monocytogenes and other intracellular pathogens (8, 9, 13, 27–30). Our results point out that among the subsets of innate CD8+ cells, those expressing CXCR3 played a major role in controlling the early growth of L. monocytogenes. Enhanced IFN-γ production by these cells correlated with reduced bacterial burden, suggesting that the protective ability of CXCR3+ innate CD8+ T cells is largely mediated by IFN-γ production, which has been shown to be important for protection against L. monocytogenes (13). Although other cells, such as NK cells, have been shown to produce IFN-γ early after L. monocytogenes infection, they are less efficient than CD8+ T cells at restoring protective innate responses in IFN-γ knockout mice (13). This was shown to be due to NK cell localization at the red pulp of infected mice, whereas CD8+ cells were found to be in the T-cell area of the spleen, colocalized with bacteria and macrophages (13). As a chemokine receptor, CXCR3 does play a role in the migration of effector lymphocytes to infected sites (19), and this may further facilitate protection by CXCR3+ innate CD8+ T cells. However, the ability to produce larger amounts of IFN-γ appears to be major reason behind the enhanced protection by CXCR3+ innate CD8+ T cells compared to the CXCR3− subset, as observed in our study. It is therefore apparent that CXCR3 defines an innate CD8+ T-cell phenotype that mediates a potent antibacterial immune response.
Although the lineage development of CXCR3+ and CXCR3− innate CD8+ T cells are parallel but separate from the pathway of conventional T-cell development (1, 31, 32), the transcriptional profiles of these subsets of innate CD8+ cells are suggestive of distinct pathways of differentiation and effector activity. Evidence for enhanced cytolytic activity of CXCR3+ innate CD8+ T-cell population is seen in enhanced CatW expression, a cysteine protease expressed by NK cells and CTLs that has been shown to be associated with cytotoxicity of target cells (33, 34). GP49a expression was also enhanced in CXCR3+ innate CD8+ T cells. Although related to the inhibitory receptor GP49b, which down-regulates activation signals that lead to cytotoxicity in T cells and NK cells (35), GP49a lacks an intracellular immunoreceptor tyrosine-based inhibitory motif (ITIM) and appears to exert an opposite effect by eliciting the activation of these cells (36), evidently contributing to their cytotoxicity. Other genes observed to be highly expressed in CXCR3+ innate CD8+ T cells include klra4 and klrc1, generally expressed by NK cells, and are associated with cellular cytotoxicity as well as the chemokine ccl5, which is highly expressed by cytotoxic NK and CD8+ T cells in response to IL-15 signaling (37, 38).
Gene expression analyses of sorted innate CD8+ T-cell populations show that the CXCR3 expressing subset is more responsive to cytokine stimulation by IL-15 and IL-18, which further enhances their proliferative and cytotoxic potential. These cells display enhanced expression of IL-2Rβ, IL-18R1, and IL-18R accessory protein (IL-18RAP), indicating enhanced responsiveness to IL-2, IL-15, and IL-18, cytokines known to potentiate the cytotoxic effects of NK cells and CTLs (5, 29). Previous work by our group showed increased IFN-γ production and granzyme B expression upon stimulation by IL-2, IL-15, or IL-12/IL-18 (6), which confirms that CXCR3+ innate CD8+ T cells are preferentially activated by these cytokines. Moreover, the greater cytolytic activity of IL-15 stimulated CXCR3+ innate CD8+ T cells against P815 target cells, as revealed in this study, corroborates the enhanced sensitivity of this population to cytokine stimulation and the potential for antitumor immune responses.
A significant finding in our study using IPA was the increased expression of genes involved in translational initiation. A significant number of ribosomal proteins that make up the 40S and 60S ribosomal subunit as well as eIF4A were up-regulated in the CXCR3+ subset of innate CD8+ T cells. In response to various stimuli or stressors such as oxidative stress or viral infection, translation initiation occurs through a coordinated process involving ribosomal proteins, modification enzymes, and ribosome-associated translation factors (16). This process appears to be enhanced in CXCR3+ innate CD8+ T cells, which is consistent with their observed preactivated state. Biologic triggers that induce this activated state in the absence of infectious stimuli and factors responsible for the maintenance of this population of homeostatically active cells are still not clearly understood.
In conclusion, we demonstrated that CXCR3 expressing innate CD8+ T cells display enhanced tumor cytotoxicity and confer protection against L. monocytogenes infection. Our group has previously shown that CXCR3 is essential for immunity against the intracellular parasite Leishmania major (19). Furthermore, the importance of CXCR3 in antitumor responses was well characterized in a recent breast cancer tumor model (18). Consistent with these findings, our results suggest that CXCR3 expression in innate CD8+ T cells significantly amplifies its cytotoxic potential and protective immune ability characterized by the production of IFN-γ. Immunotherapeutic approaches to infectious disease and cancer management that use adoptive transfer of effector cells (14) could utilize CXCR3+ innate CD8+ T-cell populations as novel clinical intervention strategies.
Acknowledgments
This work was supported by the U.S. National Institutes of Health (NIH) (Grants R03-AI090231, RC4-AI092624, R34-AI100789, R21-AT004160, and R03-CA164399) (to A.R.S.), NIH National Institute of Dental and Craniofacial Research (Training Grant T32DE014320) (to S.O.), and National Council of Science and Technology, Mexico (CONACYT) (to C.T.).
Glossary
- CCL5
chemokine (C-X-C motif) ligand 5
- CIBER
CXCR3 IRES Bb-cistronic EGFP reporter mouse
- CTL
cytotoxic T lymphocyte
- CXCR3
chemokine (C-X-C motif) receptor 3
- eIF
eukaryotic initiation factor
- FACS
fluorescence activated cell sorter
- IPA
ingenuity pathway analysis
- qRT-PCR
quantitative RT-PCR
REFERENCES
- 1.Dubois S., Waldmann T. A., Müller J. R. (2006) ITK and IL-15 support two distinct subsets of CD8+ T cells. Proc. Natl. Acad. Sci. USA 103, 12075–12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg L. J. (2007) Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat. Rev. Immunol. 7, 479–485 [DOI] [PubMed] [Google Scholar]
- 3.Sintes J., Cuenca M., Romero X., Bastos R., Terhorst C., Angulo A., Engel P. (2013) Cutting edge: Ly9 (CD229), a SLAM family receptor, negatively regulates the development of thymic innate memory-like CD8+ T and invariant NKT cells. J. Immunol. 190, 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verykokakis M., Boos M. D., Bendelac A., Kee B. L. (2010) SAP protein-dependent natural killer T-like cells regulate the development of CD8(+) T cells with innate lymphocyte characteristics. Immunity 33, 203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itsumi M., Yoshikai Y., Yamada H. (2009) IL-15 is critical for the maintenance and innate functions of self-specific CD8(+) T cells. Eur. J. Immunol. 39, 1784–1793 [DOI] [PubMed] [Google Scholar]
- 6.Oghumu S., Dong R., Varikuti S., Shawler T., Kampfrath T., Terrazas C. A., Lezama-Davila C., Ahmer B. M., Whitacre C. C., Rajagopalan S., Locksley R., Sharpe A. H., Satoskar A. R. (2013) Distinct populations of innate CD8+ T cells revealed in a CXCR3 reporter mouse. J. Immunol. 190, 2229–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho H., Choi H.-J., Xu H., Felio K., Wang C.-R. (2011) Nonconventional CD8+ T cell responses to Listeria infection in mice lacking MHC class Ia and H2-M3. J. Immunol. 186, 489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Orazio S. E., Halme D. G., Ploegh H. L., Starnbach M. N. (2003) Class Ia MHC-deficient BALB/c mice generate CD8+ T cell-mediated protective immunity against Listeria monocytogenes infection. J. Immunol. 171, 291–298 [DOI] [PubMed] [Google Scholar]
- 9.Seaman M. S., Wang C. R., Forman J. (2000) MHC class Ib-restricted CTL provide protection against primary and secondary Listeria monocytogenes infection. J. Immunol. 165, 5192–5201 [DOI] [PubMed] [Google Scholar]
- 10.Xu H., Chun T., Choi H. J., Wang B., Wang C. R. (2006) Impaired response to Listeria in H2-M3-deficient mice reveals a nonredundant role of MHC class Ib–specific T cells in host defense. J. Exp. Med. 203, 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irizarry R. A., Hobbs B., Collin F., Beazer-Barclay Y. D., Antonellis K. J., Scherf U., Speed T. P. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4, 249–264 [DOI] [PubMed] [Google Scholar]
- 12.Dhanji S., Chow M. T., Teh H. S. (2006) Self-antigen maintains the innate antibacterial function of self-specific CD8 T cells in vivo. J. Immunol. 177, 138–146 [DOI] [PubMed] [Google Scholar]
- 13.Berg R. E., Crossley E., Murray S., Forman J. (2005) Relative contributions of NK and CD8 T cells to IFN-gamma mediated innate immune protection against Listeria monocytogenes. J. Immunol. 175, 1751–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grange M., Buferne M., Verdeil G., Leserman L., Schmitt-Verhulst A.-M., Auphan-Anezin N. (2012) Activated STAT5 promotes long-lived cytotoxic CD8+ T cells that induce regression of autochthonous melanoma. Cancer Res. 72, 76–87 [DOI] [PubMed] [Google Scholar]
- 15.Grange M., Verdeil G., Arnoux F., Griffon A., Spicuglia S., Maurizio J., Buferne M., Schmitt-Verhulst A.-M., Auphan-Anezin N. (2013) Active STAT5 regulates T-bet and eomesodermin expression in CD8 T cells and imprints a T-bet-dependent Tc1 program with repressed IL-6/TGF-β1 signaling. J. Immunol. 191, 3712–3724 [DOI] [PubMed] [Google Scholar]
- 16.Shrestha N., Bahnan W., Wiley D. J., Barber G., Fields K. A., Schesser K. (2012) Eukaryotic initiation factor 2 (eIF2) signaling regulates proinflammatory cytokine expression and bacterial invasion. J. Biol. Chem. 287, 28738–28744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shrikant P. A., Rao R., Li Q., Kesterson J., Eppolito C., Mischo A., Singhal P. (2010) Regulating functional cell fates in CD8 T cells. Immunol. Res. 46, 12–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oghumu S., Varikuti S., Terrazas C., Kotov D., Nasser M. W., Powell C. A., Ganju R. K., Satoskar A. R. (2014) CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunology 143, 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosas L. E., Barbi J., Lu B., Fujiwara Y., Gerard C., Sanders V. M., Satoskar A. R. (2005) CXCR3−/− mice mount an efficient Th1 response but fail to control Leishmania major infection. Eur. J. Immunol. 35, 515–523 [DOI] [PubMed] [Google Scholar]
- 20.Cerwenka A., Morgan T. M., Harmsen A. G., Dutton R. W. (1999) Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection. J. Exp. Med. 189, 423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodland D. L., Dutton R. W. (2003) Heterogeneity of CD4(+) and CD8(+) T cells. Curr. Opin. Immunol. 15, 336–342 [DOI] [PubMed] [Google Scholar]
- 22.Bach E. A., Szabo S. J., Dighe A. S., Ashkenazi A., Aguet M., Murphy K. M., Schreiber R. D. (1995) Ligand-induced autoregulation of IFN-gamma receptor beta chain expression in T helper cell subsets. Science 270, 1215–1218 [DOI] [PubMed] [Google Scholar]
- 23.Pernis A., Gupta S., Gollob K. J., Garfein E., Coffman R. L., Schindler C., Rothman P. (1995) Lack of interferon gamma receptor beta chain and the prevention of interferon gamma signaling in TH1 cells. Science 269, 245–247 [DOI] [PubMed] [Google Scholar]
- 24.Tau G. Z., von der Weid T., Lu B., Cowan S., Kvatyuk M., Pernis A., Cattoretti G., Braunstein N. S., Coffman R. L., Rothman P. B. (2000) Interferon γ signaling alters the function of T helper type 1 cells. J. Exp. Med. 192, 977–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitani A., Nishimura H., Hirose K., Washizu J., Kimura Y., Tanaka S., Yamamoto G., Noguchi T., Yoshikai Y. (1999) Interleukin-15 production at the early stage after oral infection with Listeria monocytogenes in mice. Immunology 97, 92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennedy M. K., Glaccum M., Brown S. N., Butz E. A., Viney J. L., Embers M., Matsuki N., Charrier K., Sedger L., Willis C. R., Brasel K., Morrissey P. J., Stocking K., Schuh J. C., Joyce S., Peschon J. J. (2000) Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg R. E., Crossley E., Murray S., Forman J. (2003) Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J. Exp. Med. 198, 1583–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D’Orazio S. E., Troese M. J., Starnbach M. N. (2006) Cytosolic localization of Listeria monocytogenes triggers an early IFN-gamma response by CD8+ T cells that correlates with innate resistance to infection. J. Immunol. 177, 7146–7154 [DOI] [PubMed] [Google Scholar]
- 29.Hu J., Sahu N., Walsh E., August A. (2007) Memory phenotype CD8+ T cells with innate function selectively develop in the absence of active Itk. Eur. J. Immunol. 37, 2892–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson L. J., Kolumam G. A., Thomas S., Murali-Krishna K. (2006) Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J. Immunol. 177, 1746–1754 [DOI] [PubMed] [Google Scholar]
- 31.Broussard C., Fleischacker C., Horai R., Chetana M., Venegas A. M., Sharp L. L., Hedrick S. M., Fowlkes B. J., Schwartzberg P. L. (2006) Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity 25, 93–104 [DOI] [PubMed] [Google Scholar]
- 32.Huang W., Hu J., August A. (2013) Cutting edge: innate memory CD8+ T cells are distinct from homeostatic expanded CD8+ T cells and rapidly respond to primary antigenic stimuli. J. Immunol. 190, 2490–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoeckle C., Gouttefangeas C., Hammer M., Weber E., Melms A., Tolosa E. (2009) Cathepsin W expressed exclusively in CD8+ T cells and NK cells, is secreted during target cell killing but is not essential for cytotoxicity in human CTLs. Exp. Hematol. 37, 266–275 [DOI] [PubMed] [Google Scholar]
- 34.Wex T., Wex H., Hartig R., Wilhelmsen S., Malfertheiner P. (2003) Functional involvement of cathepsin W in the cytotoxic activity of NK-92 cells. FEBS Lett. 552, 115–119 [DOI] [PubMed] [Google Scholar]
- 35.Katz H. R., Vivier E., Castells M. C., McCormick M. J., Chambers J. M., Austen K. F. (1996) Mouse mast cell gp49B1 contains two immunoreceptor tyrosine-based inhibition motifs and suppresses mast cell activation when coligated with the high-affinity Fc receptor for IgE. Proc. Natl. Acad. Sci. USA 93, 10809–10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee K. H., Ono M., Inui M., Yuasa T., Takai T. (2000) Stimulatory function of gp49A, a murine Ig-like receptor, in rat basophilic leukemia cells. J. Immunol. 165, 4970–4977 [DOI] [PubMed] [Google Scholar]
- 37.Chenoweth M. J., Mian M. F., Barra N. G., Alain T., Sonenberg N., Bramson J., Lichty B. D., Richards C. D., Ma A., Ashkar A. A. (2012) IL-15 can signal via IL-15Rα, JNK, and NF-κB to drive RANTES production by myeloid cells. J. Immunol. 188, 4149–4157 [DOI] [PubMed] [Google Scholar]
- 38.Perera L. P., Goldman C. K., Waldmann T. A. (1999) IL-15 induces the expression of chemokines and their receptors in T lymphocytes. J. Immunol. 162, 2606–2612 [PubMed] [Google Scholar]