Abstract
To investigate whether exercise training can reverse age-related impairment of myogenic vasoconstriction in skeletal muscle arterioles, young (4 mo) and old (22 mo) male Fischer 344 rats were randomly assigned to either sedentary or exercise-trained groups. The roles of the endothelium and Kv1 channels in age- and exercise training-induced adaptations of myogenic responses were assessed through evaluation of pressure-induced constriction in endothelium-intact and denuded soleus muscle arterioles in the presence and absence of the Kv1 channel blocker, correolide. Exercise training enhanced myogenic constriction in arterioles from both old and young rats. In arterioles from old rats, exercise training restored myogenic constriction to a level similar to that of arterioles from young sedentary rats. Removal of the endothelium did not alter myogenic constriction of arterioles from young sedentary rats, but reduced myogenic constriction in arterioles from young exercise-trained rats. In contrast, endothelial removal had no effect on myogenic constriction of arterioles from old exercise-trained rats, but increased myogenic vasoconstriction in old sedentary rats. The effect of Kv1 channel blockade was also dependent on age and training status. In arterioles from young sedentary rats, Kv1 blockade had little effect on myogenic constriction, whereas in old sedentary rats Kv1 blockade increased myogenic constriction. After exercise training, Kv1 channel blockade increased myogenic constriction in arterioles from both young and old rats. Thus exercise training restores myogenic constriction of arterioles from old rats and enhances myogenic constriction from young rats through adaptations of the endothelium and smooth muscle Kv1 channels.
Keywords: correolide, myogenic, endothelium
myogenic autoregulation refers to the ability of blood vessels to constrict or dilate in response to intraluminal pressure changes. Disruption of this process in the resistance vasculature, as occurs with aging, can impair regulation of peripheral vascular resistance and tissue-specific blood flow. Aging alters the distribution of rat muscle blood flow during exercise (28), increasing flow to low-oxidative glycolytic muscles and decreasing flow to high-oxidative muscles. This alteration is concomitant with impaired myogenic vasoconstriction in skeletal muscle arterioles from old rats (16, 26). In elderly human subjects, this impairment of myogenic reactivity results in an inability to reduce blood flow velocity within skeletal muscle following an increase in transmural pressure (21). Thus the increased prevalence of orthostatic hypotension and intolerance observed with advancing age (4, 20, 36) may result, in part, from the decline in myogenic reactivity of intramuscular resistance arterioles.
Regular physical activity has been reported to attenuate central and peripheral cardiovascular dysfunction observed with aging. The maldistribution of skeletal muscle blood flow in old rats was rectified following exercise training (1). In young rats, exercise training did not alter myogenic responses in soleus muscle feed arteries (15); however, adaptations to exercise may differ between feed arteries and intramuscular arterioles. The effect of exercise training on myogenic responsiveness has not been examined in skeletal muscle resistance arterioles from either young or old rats. Thus it remains unknown whether exercise training can correct aging-induced impairment of myogenic reactivity in skeletal muscle resistance arterioles.
Development of myogenic tone is determined by the vascular smooth muscle (34), whereas the influence of the vascular endothelium on myogenic responsiveness is less clear. Removal of the endothelium from cremaster arterioles of spontaneously hypertensive rats had no effect on myogenic responses (11). Endothelial denudation did not change the myogenic responsiveness of small femoral arteries (24) or coronary arterioles (18); however, denuded cerebral vessels demonstrated greater myogenic responses compared with intact vessels (14, 17). Denudation also enhanced myogenic constriction of coronary arterioles from old, but not young rats (35). Although it has been established that exercise training reverses age-related endothelial dysfunction in skeletal muscle arterioles (37, 39), the modulatory effects of the endothelium on the myogenic responses in skeletal muscle resistance arterioles in models of aging and exercise training remain unexplored.
Our previous work demonstrated that blockade of voltage-gated potassium (Kv) channels with 4-aminopyridine (4-AP) led to a greater degree of myogenic constriction in soleus muscle arterioles from aged rats compared with young rats (16). Blockade with 4-AP also eliminated age-related differences in myogenic responsiveness. Thus age-related reductions in myogenic responsiveness may be linked to increased Kv channel activity (16). The Kv1 isoform has been reported to be of particular importance in regulation of arteriolar myogenic activity (2). In rat mesenteric arteries, treatment with the Kv1 channel blocker, correolide, resulted in greater myogenic constriction, similar to that seen with 4-AP (31). Although Kv channels are recognized as important regulators of vascular smooth muscle tone and myogenic reactivity of resistance arterioles, their role in exercise training-induced adaptations of myogenic reactivity remains unknown (16).
The primary goal of this study was to determine whether exercise training reverses age-related impairment of myogenic responses in skeletal muscle resistance arterioles. A secondary goal was to determine the contribution of the endothelium and Kv1 channels to observed differences in myogenic responses with advancing age and exercise training. To investigate the mechanisms that underlie potential aging- and exercise training-induced adaptations of the endothelium and vascular smooth muscle, myogenic responses were studied in endothelium-intact and endothelium-denuded arterioles isolated from the soleus muscle, a high-oxidative skeletal muscle (5).
MATERIALS AND METHODS
This study was approved by the Institutional Animal Care and Use Committees at Texas A&M University, West Virginia University, and University of Florida. All methods adhere to the guidelines set forth in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, 8th edition, 2011).
Young (4–6 mo) and old (22–24 mo) male Fischer 344 rats were housed under a 12:12-h light-dark cycle and given food and water ad libitum. Fischer 344 rats were chosen because cardiovascular function decreases with age in these rats, without the development of atherosclerosis or hypertension (19).
Exercise training.
Rats were habituated to treadmill exercise by walking at 5 m/min (0° incline), 5 min/day for 3 days. Following habituation, young and old rats were randomly assigned to either a sedentary group or exercise-trained group. Exercise training consisted of treadmill running at 15 m/min (15° incline), 5 days/wk for 10–12 wk. Exercise duration gradually increased over the first 4 wk from 20 min/day to 60 min/day. Training continued 5 days/wk for 60 min/day for the remainder of the training period.
Microvessel preparation.
Rats were anesthetized with isoflurane (3%/O2 balance) and euthanized by excision of the heart. The soleus-plantaris-gastrocnemius complex was carefully dissected free from both hindlimbs and placed in cold (4°C), filtered physiological saline solution (PSS) containing 145.0 mM NaCl, 4.7 mM KCl, 2.0 mM CaCl2, 1.17 mM MgSO4, 1.2 mM NaH2PO, 5.0 mM glucose, 2.0 mM pyruvate, 0.02 mM EDTA, 3.0 mM MOPS buffer, and 1 g/100 ml BSA, pH 7.4. Sections of soleus muscle were also stored at −80°C for determination of citrate sythase activity to verify the efficacy of the training program. With the aid of a dissecting microscope (Olympus SVH10), first-order (1A) arterioles were isolated from the soleus muscle. The 1A arterioles were defined as the first arterial branch off of the feed artery after the feed artery entered the muscle. The arterioles were transferred to a Lucite chamber containing PSS equilibrated with room air. Each end of the arteriole was cannulated with a micropipette and secured with nylon suture (Alcon 11–0 nylon microfilament). In denuded vessels, removal of the endothelium was achieved by passing 10 ml of air though the lumen of the vessel. Endothelial denudation was verified by lack of vasodilation to acetylcholine (10 μM). After cannulation, the microvessel chamber was transferred to the stage of an inverted microscope (Olympus IX70) equipped with a video camera (Panasonic BP310) and video calipers (Microcirculation Research Institute, Texas A&M) to measure and record intraluminal diameter. Arterioles were pressurized to 50 mmHg with two independent hydrostatic pressure reservoirs. Arterioles exhibiting leaks were discarded. Vessels determined to be free of leaks were warmed to 37°C and allowed to develop spontaneous tone during a 30- to 60-min equilibration period. Vessels that did not develop a minimum of 10% tone were excluded from study. The bathing solution was changed every 20 min during equilibration.
Evaluation of myogenic responses.
Following the equilibration period, active myogenic responses were assessed by increasing intraluminal pressure from 0 mmHg up to 100 mmHg, in 7 mmHg increments. Changes in pressure were maintained for 3 min, and steady-state diameter was recorded. To determine passive responses to pressure, vessels were incubated in calcium-free PSS containing 2.0 mM EDTA for 1 h and allowed to completely relax at an intraluminal pressure of 50 mmHg. Passive diameter changes to increasing intraluminal pressure were then determined.
Role of Kv channels.
Myogenic constriction to incremental pressure changes was also evaluated in the presence of Kv1 channel blocker, correolide (0.1 μM) (Merck Research Laboratories). Vessels were incubated with correolide for 20 min before and during evaluation of myogenic responses.
Data analysis.
Responses were recorded as actual inner diameters. Spontaneous and myogenic tone were expressed as percent constriction relative to maximal diameter, calculated using the following formulas: Spontaneous tone (%) = (IDmax − IDb)/IDmax × 100 and Myogenic constriction (%) = (IDmax − IDs)/IDmax × 100. IDmax is the maximal inner diameter recorded after a 1-h incubation period in calcium-free PSS with 100 μM sodium nitroprusside at an intraluminal pressure of 50 mmHg. IDb is the steady-state baseline diameter. IDs is steady-state diameter measured after each incremental pressure change.
Myogenic responses were compared by repeated measures ANOVA with pairwise comparisons to detect differences within (pressure) and between (experimental groups) factors. Group differences in animal and vessel characteristics were compared by 2-way ANOVA. All data are presented as means ± SE. Statistical significance was set as P ≤ 0.05. In all experiments, “n” indicates the number of animals studied.
RESULTS
Animals.
Body weight and soleus muscle weight increased with age (Table 1). Exercise training significantly reduced body weight in old rats and increased soleus muscle weight in young rats. Training increased the soleus muscle weight-to-body weight ratio in both young and old rats.
Table 1.
Animal and vessel characteristics
| Characteristic | YSED, n = 25 | YET, n = 28 | OSED, n = 27 | OET, n = 29 |
|---|---|---|---|---|
| Body wt (BW), g | 365 ± 7 | 365 ± 6 | 456 ± 8† | 409 ± 5*† |
| Soleus wt (SW), mg | 140.2 ± 3.4 | 168 ± 3.8* | 177 ± 9.6† | 190 ± 4.0† |
| SW/BW, mg/g | 0.38 ± 0.01 | 0.46 ± 0.01* | 0.39 ± 0.02 | 0.46 ± 0.01*† |
| Citrate synthase activity, μmol·min−1·g wet wt−1 | 21.69 ± 2.52 | 29.67 ± 2.15* | 15.58 ± 1.48† | 19.30 ± 2.91*† |
| Maximal diameter, μm | 119 ± 6 | 137 ± 8 | 122 ± 5 | 131 ± 6 |
| Intact vessel spontaneous tone, % | 28.0 ± 3.0‡ | 42.8 ± 5.2* | 19.4 ± 3.6†‡ | 30.8 ± 4.0†‡ |
| Denuded vessel spontaneous tone, % | 36.6 ± 3.1 | 49.4 ± 2.2* | 28.3 ± 1.7† | 34.4 ± 5.0† |
Values are means ± SE. YSED, young sedentary; YET, young exercise trained; OSED, old sedentary; OET, old exercise trained.
P < 0.05, significantly different from age-matched sedentary group.
P < 0.05, significantly different from respective young group.
P < 0.05, significantly different from denuded vessels.
Citrate synthase activity was greater in soleus muscles from both young and old rats after training (Table 1), indicating the efficacy of the training program.
Isolated vessel characteristics.
Maximal intraluminal diameter of soleus arterioles did not differ between groups. Spontaneous tone development was lower in arterioles from old rats compared with those from young rats. Spontaneous tone development was greater in denuded arterioles compared with intact arterioles in old rats (Table 1).
Aging and exercise training effects.
Myogenic constriction of endothelium-intact soleus muscle arterioles was lower in old rats compared with young rats (Fig. 1), confirming previous findings (16, 26). Exercise training enhanced myogenic constriction in arterioles from both young and old rats. Furthermore, myogenic constriction of arterioles from old, exercise-trained rats was restored to a level similar to that of arterioles from young sedentary rats (Fig. 1). Passive responses to increasing intraluminal pressure were not changed with age or exercise training (data not shown).
Fig. 1.
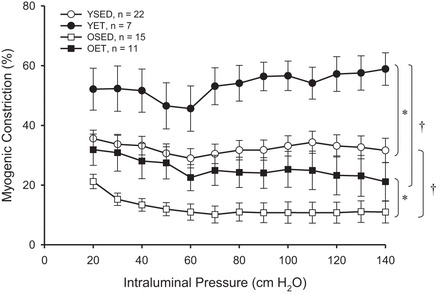
Myogenic constriction in intact soleus muscle arterioles: young sedentary (YSED); young exercise trained (YET); old sedentary (OSED); old exercise trained (OET). Values are means ± SE. †P < 0.05, significantly different from respective young group. *P < 0.05, significantly different from age-matched sedentary group.
Endothelial effects.
Removal of the endothelium eliminated exercise training-induced enhancement of myogenic constriction of arterioles from both young and old rats (Fig. 2). Age-related differences in myogenic constriction of arterioles from sedentary rats were also eliminated by endothelial denudation. In contrast, age-related differences in myogenic constriction persisted in denuded arterioles from exercise-trained rats (Fig. 2).
Fig. 2.
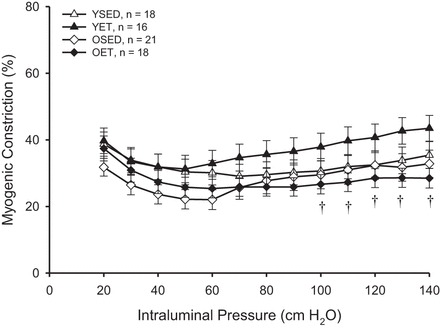
Myogenic constriction in denuded soleus muscle arterioles. Values are means ± SE. †P < 0.05, significantly different from respective young group.
Endothelial denudation did not alter myogenic constriction in arterioles from young sedentary rats (Fig. 3A) or old exercise trained rats (Fig. 3D). Removal of the endothelium significantly decreased myogenic constriction in arterioles from young exercise-trained rats (Fig. 3C), suggesting that production of an endothelial constricting factor was lost with denudation. In contrast, removal of the endothelium significantly increased myogenic constriction in arterioles from old sedentary rats (Fig. 3B), suggesting that denudation eliminated an endothelial vasodilatory factor.
Fig. 3.
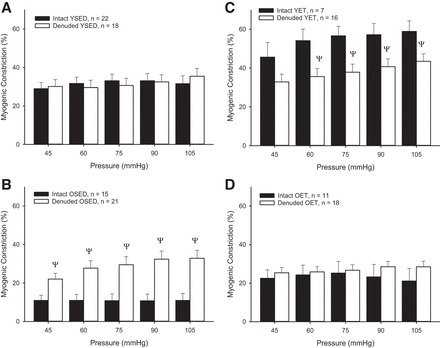
Comparison of myogenic constriction in intact and denuded soleus muscle arterioles from YSED (A), OSED (B), YET (C), and OET (D) rats. Values are means ± SE. ΨP < 0.05, significantly different from intact vessel response.
Kv1 channel activity.
In arterioles from young sedentary rats, Kv1 channel blockade with correolide increased myogenic constriction at low pressures (45 and 60 mmHg) in endothelium-intact arterioles (Fig. 4A), but had no effect in denuded arterioles (Fig. 4B). In intact arterioles from old sedentary rats, Kv1 channel blockade increased myogenic constriction (Fig. 5A), an effect that persisted only at the lowest pressure in denuded vessels (Fig. 5B). Age-related differences in myogenic tone remained following Kv1 channel blockade in endothelium-intact vessels from sedentary rats, but were eliminated following denudation.
Fig. 4.
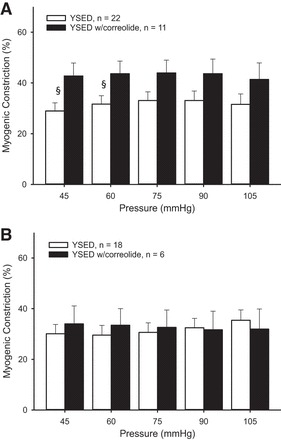
Myogenic constriction in the presence of correolide in intact (A) and denuded (B) soleus muscle arterioles from YSED rats. Values are means ± SE. §P < 0.05, significantly different from treatment.
Fig. 5.
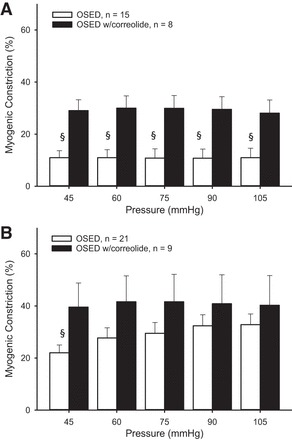
Myogenic constriction in the presence of correolide in intact (A) and denuded (B) soleus muscle arterioles from OSED rats. Values are means ± SE. §P < 0.05, significantly different from treatment.
Treatment with correolide did not alter myogenic constriction in intact vessels from young exercise trained rats (Fig. 6A), whereas incubation with correolide increased myogenic constriction in denuded vessels (Fig. 6B). In intact arterioles from old exercise-trained rats, Kv1 blockade resulted in greater myogenic constriction (Fig. 7A), an effect that persisted at low pressures in denuded vessels (Fig. 7B).
Fig. 6.
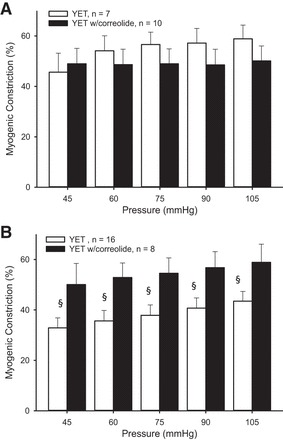
Myogenic constriction in the presence of correolide in intact (A) and denuded (B) soleus muscle arterioles from YET rats. Values are means ± SE. §P < 0.05, significantly different from treatment.
Fig. 7.
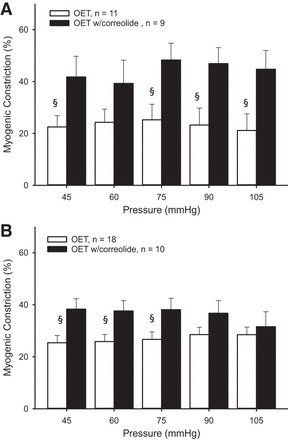
Myogenic constriction in the presence of correolide in intact (A) and denuded (B) soleus muscle arterioles from OET rats. Values are means ± SE. §P < 0.05, significantly different from treatment.
There were no age-related differences in myogenic constriction following Kv1 channel blockade in intact vessels from exercise trained rats. In contrast, age-related differences in myogenic responses were present in denuded vessels from exercise trained rats treated with correolide.
DISCUSSION
The results of this study provide four main insights into the effects of aging and exercise training on myogenic function of skeletal muscle resistance arterioles. First, confirming our previous observations (16, 26), the myogenic responsiveness of rat skeletal muscle arterioles decreases with advancing age. Second, exercise training reverses the myogenic dysfunction observed with advancing age, and enhances myogenic function in arterioles of healthy, young rats. Third, differences in myogenic constriction in skeletal muscle resistance arterioles observed with aging and exercise training are mediated, in part, by adaptations of the endothelium. Fourth, the modulation of myogenic constriction by Kv1 channels changes with aging in skeletal muscle arterioles, and the effects of exercise training on the modulation of myogenic constriction by Kv1 channels are age specific.
Orthostatic intolerance, observed with aging in both humans (20) and animals (32), may result, in part, from an inability to maintain peripheral vascular resistance due to impaired myogenic reactivity. Previous work has demonstrated that both endothelium-dependent vasodilation and myogenic responsiveness decrease in skeletal muscle arterioles of aged rats (16, 26, 27, 37, 39). The current results confirm that myogenic constriction of soleus muscle arterioles declines with age and demonstrate that this decline in myogenic constriction is due to mechanistic changes in both the endothelium and the vascular smooth muscle.
Exercise training restored myogenic constriction in arterioles from old rats to that of young sedentary rats and enhanced myogenic constriction in arterioles from young rats. Similarly, exercise training has been shown to reverse age-induced vasomotor dysfunction in various vascular beds (3, 7, 8, 25, 37, 39). Impaired endothelium-dependent vasodilation observed with aging was reversed by an exercise training program in both humans (6) and rats (37). Exercise training also attenuated α-adrenergic (8) and angiotensin II-mediated (30) vasoconstriction in rat skeletal muscle arterioles. In rat gastrocnemius muscle, the age-induced redistribution of flow away from the oxidative portion of the muscle toward the glycolytic portion of the muscle during exercise was reversed by exercise training (1). In addition to contributing to improved distribution of skeletal muscle blood flow during exercise, enhancement of myogenic constriction by exercise training may improve dynamic regulation of peripheral vascular resistance with aging, thus contributing to improved blood pressure regulation during postural changes and reduction of orthostatic intolerance in elderly populations.
The current results indicate that endothelial adaptations contribute to alterations in myogenic activity of arterioles that occur with aging and exercise training. Training-induced increases in myogenic activity were eliminated in denuded arterioles. In arterioles from young rats, removal of the endothelium eliminated the enhancement of myogenic constriction produced by exercise training, suggesting that exercise training increased release of an endothelium-derived vasoconstrictor signal that contributes to the enhanced myogenic constriction in intact arterioles. In arterioles from old rats, both exercise training and removal of the endothelium reduce a vasodilatory endothelial signal that contributes to the reduction of myogenic responsiveness in intact vessels. Overall, the current results suggest that, in intact arterioles from young animals, exercise training promotes endothelium-mediated vasoconstriction. In arterioles from young sedentary rats, the current results suggest that a balance exists between endothelium-derived vasodilatory and constrictor mechanisms that modulate myogenic tone. With old age, the balance of endothelial modulation is shifted toward increased vasodilatory modulation that reduces myogenic constriction. Exercise training restores the balance between endothelial vasodilatory and contractile modulation of myogenic responsiveness in arterioles from old rats.
The majority of previous studies have found no effect of endothelial denudation on myogenic responses (11, 18, 23, 43), leading to the conclusion that the endothelium does not play a role in the development of myogenic tone. Of the studies suggesting a role for the endothelium in myogenic responses, most were performed in cerebral arteries (14, 17), indicating the endothelium may be vital to myogenic responsiveness in that vascular bed. Importantly, most studies showing no effect of denudation on myogenic responses were performed in young animals (11, 18, 23). The current results are consistent with these reports; removal of the endothelium did not alter myogenic constriction of arterioles from young sedentary rats. Shipley and colleagues (35) also reported that denudation had no effect on myogenic constriction of coronary arterioles from young rats; however, in old rats, denudation enhanced myogenic constriction in coronary arterioles, similar to our current observations in soleus muscle arterioles. Therefore, although the endothelium may not have a net influence on myogenic constriction in resistance arterioles from healthy, young animals, alterations occurring in the endothelium do appear to contribute to diminished myogenic reactivity in skeletal muscle resistance arterioles with advancing age. Exercise training also appears to induce endothelial adaptations that alter myogenic reactivity in skeletal muscle resistance arterioles, but these endothelial adaptations are age specific.
Our previous work indicated that increased Kv channel activity contributes to age-induced impairment of myogenic activity in skeletal muscle arterioles (16). Because Kv1 channels have been shown to play an important role in regulation of myogenic reactivity in small resistance vessels (31), we hypothesized that Kv1 channel activity increases with aging and decreases with exercise training in soleus muscle arterioles. Since Kv1 channels are thought to be localized to the plasma membrane of the vascular smooth muscle, it seemed unlikely that the myogenic constriction in the presence of Kv1 channel blockade would be significantly altered by the presence of the endothelium. To delineate the role of Kv1 channel activity in the smooth muscle and the endothelium, we assessed myogenic constriction in both intact and denuded arterioles in the presence and absence of correolide, a blocker of Kv1 channel activity. Indeed, our results indicate greater modulation of myogenic constriction by Kv1 channels in arterioles from aged sedentary rats; however, this modulatory effect is exerted both through the endothelium as well as the vascular smooth muscle (Fig. 4, A and B, compared with Fig. 5, A and B). Within the Kv1 family, Kv1.5 channels are abundantly expressed in vascular smooth muscle cells (22, 31). However, Kang et. al. (16) observed that Kv 1.5 channels do not contribute to age-related increases in Kv channel activity in rat soleus arterioles, suggesting that other Kv1 family members are responsible for the age-related Kv1 channel modulation of myogenic tone. In contrast to our hypothesis, blockade of Kv1 channels with correolide increased myogenic constriction in arterioles from both young and old exercise trained rats (Fig. 6B and Fig. 7, A and B), suggesting that exercise training increases Kv1 channel activity in skeletal muscle arterioles. Additionally, the increase in Kv1 channel activity was apparent in both intact and denuded arterioles, suggesting that the increased Kv1 activity alters both endothelial and smooth muscle reactivity to transmural pressure.
Incongruent myogenic constriction between intact and denuded arterioles in the presence of a Kv1 channel blocker may indicate that interactions between the endothelium and vascular smooth muscle of skeletal muscle arterioles are altered by aging and exercise. Myoendothelial gap junctions allow for direct communication between endothelial and vascular smooth muscle cells (10). Yashiro and colleagues (41) demonstrated that selective chelation of endothelial Ca2+ led to prolonged constriction of isolated cremaster arterioles following administration of phenylephrine. Before Ca2+ chelation, phenylephrine led to concurrent spikes in [Ca2+] in endothelial and vascular smooth muscle cells, both of which diminished rapidly. Following chelation, phenylephrine induced a spike in [Ca2+] in the vascular smooth muscle which remained elevated, but no spike in endothelial [Ca2+], paralleling the prolonged constriction observed in the vessels, and suggesting that the phenylephrine-induced rise in [Ca2+] within the vascular smooth muscle diffuses into the endothelium via myoendothelial gap junctions. Connectivity between the endothelium and the vascular smooth muscle may contribute to our findings that blockade of Kv1 channels produced differential modulation of tone in intact vs. denuded arterioles. Further experiments will be needed to determine the mechanisms that underlie the aging- and exercise training-induced adaptations in the endothelium and vascular smooth muscle of skeletal muscle arterioles.
Release of endothelial factors has been reported to activate Kv channels in the smooth muscle of arteries and arterioles from various vascular beds. In pulmonary arteries, nitric oxide-mediated relaxation is inhibited by 4-AP (42), and in middle cerebral arteries relaxation to authentic prostaglandin I2 (PGI2) is partially blocked by 4-AP (9). Activation of eNOS and endothelium-dependent relaxation of aorta induced by apocycnin is prevented by 4-AP, suggesting that Kv channels contribute to both endothelial and smooth muscle responses to this NADPH oxidase inhibitor (12). Hydrogen peroxide elicits relaxation of mesenteric arteries through activation of 4-AP-sensitive channels (29). In the present study, correolide increased myogenic tone of intact arterioles from young sedentary, old sedentary, and old exercise trained rats, but did not alter myogenic tone in arterioles from young exercise trained rats, suggesting that endothelium-derived factors activate Kv1 channels opposing myogenic tone in arterioles from young and old sedentary rats. Exercise training appears to reduce this endothelial modulation of tone by Kv1 channels in arterioles from young, but not old rats. Our previous work indicates an increase in the contribution of hydrogen peroxide to endothelium-dependent vasodilation increases with age (38); thus it is possible that endothelium-derived hydrogen peroxide is contributing to activation of correolide-sensitive Kv1 channels, opposing myogenic tone development in arterioles from young and old sedentary rats. Exercise training appears to reduce this modulation of Kv1 channels in arterioles from young, but not old rats.
In this study, we focused on endothelial mechanisms, and specifically Kv1 channels, in adaptations of myogenic constriction. Other important mechanisms could also contribute to altered myogenic reactivity in skeletal muscle arterioles from aged or exercise trained rats. Indeed, previous work suggests that alterations in calcium-activated channels may also contribute to age-induced impairment of myogenic constriction in skeletal muscle arterioles (16). Although we did not investigate changes to wall structure in this study, remodeling of the vascular wall that occurs with exercise training in both conduit (33, 40) and resistance arteries (13, 39) may contribute to altered myogenic constriction. Exercise training stimulates both local and systemic effects that influence vascular structure and function, and adaptations such as the enhanced myogenic constriction reported here result from these combined effects.
In conclusion, myogenic constriction in skeletal muscle resistance arterioles is diminished with advancing age. Exercise training restores myogenic constriction in arterioles from aged rats and further enhances reactivity in arterioles from young rats. These changes in myogenic constriction with aging and exercise training appear to be mediated by both endothelial and vascular smooth muscle mechanisms.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01 HL-077224 and R01 HL-090937 and National Aeronautics and Space Administration (NASA) Space Biology (NNX12AL41G and NNX14AQ57G).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
P.G., F.R.M.S., J.M.D., S.A.S., and A.J.D. performed experiments; P.G., F.R.M.S., J.M.D., S.A.S., A.J.D., and J.M.M.-D. analyzed data; P.G., F.R.M.S., S.A.S., A.J.D., M.D.D., and J.M.M.-D. interpreted results of experiments; P.G., F.R.M.S., and J.M.M.-D. prepared figures; P.G. and J.M.M.-D. drafted manuscript; P.G., F.R.M.S., J.M.D., S.A.S., A.J.D., M.D.D., and J.M.M.-D. edited and revised manuscript; P.G., F.R.M.S., J.M.D., S.A.S., A.J.D., M.D.D., and J.M.M.-D. approved final version of manuscript; M.D.D. and J.M.M.-D. conception and design of research.
ACKNOWLEDGMENTS
The authors thank MERCK for the gift of correolide and Xueling Tseng, Catherine Dominguez, and Zachary Grimm for their technical assistance.
REFERENCES
- 1.Behnke BJ, Ramsey MW, Stabley JN, Dominguez JM, Davis RT, McCullough DJ, Muller-Delp JM, Delp MD. Effects of aging and exercise training on skeletal muscle blood flow and resistance artery morphology. J Appl Physiol 113: 1699–1708, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole WC, Chen TT, Clément-Chomienne O. Myogenic regulation of arterial diameter: role of potassium channels with a focus on delayed rectifier potassium current. Can J Physiol Pharmacol 83: 755–765, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Davis RT, Stabley JN, Dominguez JM, Ramsey MW, McCullough DJ, Lesniewski LA, Delp MD, Behnke BJ. Differential effects of aging and exercise on intra-abdominal adipose arteriolar function and blood flow regulation. J Appl Physiol 114: 808–815, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension 32: 298–304, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996. [DOI] [PubMed] [Google Scholar]
- 6.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation 102: 1351–1357, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez JM, Prisby RD, Muller-Delp JM, Allen MR, Delp MD. Increased nitric oxide-mediated vasodilation of bone resistance arteries is associated with increased trabecular bone volume after endurance training in rats. Bone 46: 813–819, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donato AJ, Lesniewski LA, Delp MD. Ageing and exercise training alter adrenergic vasomotor responses of rat skeletal muscle arterioles. J Physiol 579: 115–125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong H, Waldron GJ, Cole WC, Triggle CR. Roles of calcium-activated and voltage-gated delayed rectifier potassium channels in endothelium-dependent vasorelaxation of the rabbit middle cerebral artery. Br J Pharmacol 123: 821–832, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dora KA. Coordination of vasomotor responses by the endothelium. Circ J 74: 226–232, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Falcone JC, Davis MJ, Meininger GA. Endothelial independence of myogenic response in isolated skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 260: H130–H135, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Han WQ, Wong WT, Tian XY, Huang Y, Wu LY, Zhu DL, Gao PJ. Contributory role of endothelium and voltage-gated potassium channels in apocynin-induced vasorelaxations. J Hypertens 28: 2102–2110, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Hanna MA, Taylor CR, Chen B, La HS, Maraj JJ, Kilar CR, Behnke BJ, Delp MD, Muller-Delp JM. Structural remodeling of coronary resistance arteries: effects of age and exercise training. J Appl Physiol 117: 616–623, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harder DR. Pressure-induced myogenic activation of cat cerebral arteries is dependent on intact endothelium. Circ Res 60: 102–107, 1987. [DOI] [PubMed] [Google Scholar]
- 15.Jasperse JL, Laughlin MH. Vasomotor responses of soleus feed arteries from sedentary and exercise-trained rats. J Appl Physiol 86: 441–449, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Kang LS, Kim SJ, Dominguez JM, Sindler AL, Dick GM, Muller-Delp JM. Aging and muscle fiber type alter K channel contributions to the myogenic response in skeletal muscle arterioles. J Appl Physiol 107: 389–398, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katusic ZS, Shepherd JT, Vanhoutte PM. Endothelium-dependent contraction to stretch in canine basilar arteries. Am J Physiol Heart Circ Physiol 252: H671–H673, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Kuo L, Chilian WM, Davis MJ. Coronary arteriolar myogenic response is independent of endothelium. Circ Res 66: 860–866, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Lakatta EG. Cardiovascular system. In: Handbook of Physiology, edited by Masoro EJ. New York: Oxford Univ. Press, 1995, p. 413–474. [Google Scholar]
- 20.Lipsitz LA. Abnormalities in blood pressure homeostasis that contribute to falls in the elderly. Clin Geriatr Med 1: 637–648, 1985. [PubMed] [Google Scholar]
- 21.Lott MEJ, Herr MD, Sinoway LI. Effects of age on brachial artery myogenic responses in humans. Am J Physiol Regul Integr Comp Physiol 287: R586–R591, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Zhang J, Tang G, Wang R. Modulation of voltage-dependent K channel current in vascular smooth muscle cells from rat mesenteric arteries. J Membr Biol 180: 163–175, 2001. [DOI] [PubMed] [Google Scholar]
- 23.MacPherson RD, McLeod LJ, Rasiah RL. Myogenic response of isolated pressurized rabbit ear artery is independent of endothelium. Am J Physiol Heart Circ Physiol 260: H779–H784, 1991. [DOI] [PubMed] [Google Scholar]
- 24.Matchkov VV, Tarasova OS, Mulvany MJ, Nilsson H. Myogenic response of rat femoral small arteries in relation to wall structure and [Ca2 ] i. Am J Physiol Heart Circ Physiol 283: H118–H125, 2002. [DOI] [PubMed] [Google Scholar]
- 25.McCullough DJ, Davis RT, Dominguez JM, Stabley JN, Bruells CS, Behnke BJ. Effects of aging and exercise training on spinotrapezius muscle microvascular Po2 dynamics and vasomotor control. J Appl Physiol 110: 695–704, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller-Delp J, Spier SA, Ramsey MW, Lesniewski LA, Papadopoulos A, Humphrey JD, Delp MD. Effects of aging on vasoconstrictor and mechanical properties of rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 282: H1843–H1854, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol 96: 81–88, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu SY, Kang K, Kim B, Bae YM, Cho H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflugers Archiv 467: 285–297, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park Y, Prisby RD, Behnke BJ, Dominguez JM, Lesniewski LA, Donato AJ, Muller-Delp J, Delp MD. Effects of aging, TNF-α, and exercise training on angiotensin II-induced vasoconstriction of rat skeletal muscle arterioles. J Appl Physiol 113: 1091–1100, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plane F, Johnson R, Kerr P, Wiehler W, Thorneloe K, Ishii K, Chen T, Cole W. Heteromultimeric Kv1 channels contribute to myogenic control of arterial diameter. Circ Res 96: 216–224, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Ramsey MW, Behnke BJ, Prisby RD, Delp MD. Effects of aging on adipose resistance artery vasoconstriction: possible implications for orthostatic blood pressure regulation. J Appl Physiol 103: 1636–1643, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Rowley NJ, Dawson EA, Birk GK, Cable NT, George K, Whyte G, Thijssen DH, Green DJ. Exercise and arterial adaptation in humans: uncoupling localized and systemic effects. J Appl Physiol 110: 1190–1195, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci 96: 313–326, 1999. [PubMed] [Google Scholar]
- 35.Shipley RD, Muller-Delp JM. Aging decreases vasoconstrictor responses of coronary resistance arterioles through endothelium-dependent mechanisms. Cardiovasc Res 66: 374–383, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Shiraki K, Sagawa S, Yousef MK, Konda N, Miki K. Physiological responses of aged men to head-up tilt during heat exposure. J Appl Physiol 63: 576–581, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sindler AL, Reyes R, Chen B, Ghosh P, Gurovich AN, Kang LS, Cardounel AJ, Delp MD, Muller-Delp JM. Age and exercise training alter signaling through reactive oxygen species in the endothelium of skeletal muscle arterioles. J Appl Physiol 114: 681–693, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spier SA, Delp MD, Meininger CJ, Donato AJ, Ramsey MW, Muller-Delp JM. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol 556: 947–958, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thijssen DH, Dawson EA, van den Munckhof IC, Birk GK, Timothy Cable N, Green DJ. Local and systemic effects of leg cycling training on arterial wall thickness in healthy humans. Atherosclerosis 229: 282–286, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Yashiro Y, Duling BR. Integrated Ca2 signaling between smooth muscle and endothelium of resistance vessels. Circ Res 87: 1048–1054, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Zhao YJ, Wang J, Rubin LJ, Yuan XJ. Inhibition of K(V) and K(Ca) channels antagonizes NO-induced relaxation in pulmonary artery. Am J Physiol Heart Circ Physiol 272: H904–H912, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann PA, Knot HJ, Stevenson AS, Nelson MT. Increased myogenic tone and diminished responsiveness to ATP-sensitive K channel openers in cerebral arteries from diabetic rats. Circ Res 81: 996–1004, 1997. [DOI] [PubMed] [Google Scholar]


