Abstract
Hypoxia is a common component of many developmental insults and has been studied in early-stage chicken development. However, its impact on cardiac function and arterial-ventricular coupling in late-stage chickens is relatively unknown. To test the hypothesis that hypoxic incubation would reduce baseline cardiac function but protect the heart during acute hypoxia in late-stage chickens, white Leghorn eggs were incubated at 21% O2 or 15% O2. At 90% of incubation (19 days), hypoxic incubation caused growth restriction (−20%) and increased the LV-to-body ratio (+41%). Left ventricular (LV) pressure-volume loops were measured in anesthetized chickens in normoxia and acute hypoxia (10% O2). Hypoxic incubation lowered the maximal rate of pressure generation (ΔP/ΔtMax; −22%) and output (−57%), whereas increasing end-systolic elastance (ELV; +31%) and arterial elastance (EA; +122%) at similar heart rates to normoxic incubation. Both hypoxic incubation and acute hypoxia lengthened the half-time of relaxation (τ; +24%). Acute hypoxia reduced heart rate (−8%) and increased end-diastolic pressure (+35%). Hearts were collected for mRNA analysis. Hypoxic incubation was marked by decreased mRNA expression of sarco(endo)plasmic reticulum Ca2+-ATPase 2, Na+/Ca2+ exchanger 1, phospholamban, and ryanodine receptor. In summary, hypoxic incubation reduces LV function in the late-stage chicken by slowing pressure generation and relaxation, which may be driven by altered intracellular excitation-contraction coupling. Cardiac efficiency is greatly reduced after hypoxic incubation. In both incubation groups acute hypoxia reduced diastolic function.
Keywords: heart development, animal model, embryo, intrauterine growth restriction, prenatal hypoxia
prenatal hypoxia affects about 12% of pregnancies in the United States and often results in intrauterine growth restriction and premature delivery (20, 55). Chronic fetal hypoxia is linked to depressed cardiac function in humans and animal models (15, 18, 32, 37). Some effects of hypoxia in the mammalian fetus are mediated by the placenta or by maternal factors (54), independent of the direct influence of oxygen restriction on the fetal cardiovascular system (16). To understand how cardiac development is directly altered by hypoxia, it is necessary to separate placental and maternal influences from primary fetal responses (3, 23, 47).
The developing chicken is a model that uniquely allows the investigator to isolate the effects of reduced oxygen from maternal and placental mediators. Arterial pressure and heart rate have been successfully measured throughout the majority of chicken ontogeny (9, 13, 14, 43, 44, 53), whereas quantification of cardiac function has been restricted to early stages of development (24, 41, 42, 49, 50) and lung-ventilating chickens before hatching (48). Function of the late-stage fetal chicken heart in which ventilation has not begun (internal pipping occurs at about day 19.5) is less well understood due to technical difficulties such as loss of transparency of embryonic tissues and reduced ease of access to the heart. Thus in vivo cardiac function and arterial-ventricular coupling in the late-stage, nonventilating fetal chicken are not well understood.
Studies of isolated hearts, and of hearts of lung-ventilating fetal chickens, indicate that chronic hypoxia leads to cardiac chamber dilation and reduced cardiac function (38, 48). However, we previously found that hypoxia-incubated fetuses exhibit less arterial pressure and heart rate depression in response to acute hypoxic challenge (21). From this discrepancy, we hypothesized that fetal chicken cardiac adaptation to hypoxic incubation would reduce baseline cardiac function but precondition the animal to maintain cardiac function during acute hypoxia. We further sought to investigate the transcriptional regulation underlying functional changes, focusing on altered mRNA expression of excitation-contraction coupling and growth-controlling genes.
METHODS
Ethical Approval
All protocols were conducted with the approval of the University of North Texas Animal Care and Use Committee under protocol no. 11007.
Animals
White Leghorn chicken (Gallus gallus domesticus) eggs were obtained from the Department of Poultry Science at Texas A&M University. Upon delivery, fertilized eggs were randomly separated into two groups: normoxic incubation (21% O2) or hypoxic incubation (15% O2). The degree of hypoxia was selected to allow comparison with previous studies (12, 21, 27, 38, 39, 48). Both groups were incubated at 38°C with a relative humidity of 60% and hourly rotation in chicken egg incubators (BSS 160 Grumbach, Asslar, Germany). Oxygen concentrations were set using rotameters or a Sechrist Air-Oxygen mixer (model 3500HL, Sechrist Industries, Anaheim, CA), downstream of compressed N2 and air, or air alone, with the mixtures being bubbled through water to ensure appropriate humidity. Gas compositions within both incubation conditions were monitored with an oxygen analyzer (S-3AII, Ametek Applied Electrochemistry, Pittsburgh, PA) via a PowerLab data recording system (ADInstruments, Colorado Springs, CO) connected to a computer running LabChart software (version 7.2, ADInstruments). Fetuses from day 19 of the 21-day incubation period (i.e., 90% of development) were included in the study.
Cardiac Pressure-Volume Recordings
Surgical preparation.
Both incubation groups had cardiac studies performed in room air and in hypoxia. Thus four conditions were compared: 1) normoxic incubation (21% O2) in normoxia, 2) normoxic incubation in acute hypoxia (10% O2), 3) hypoxic incubation (15% O2) in experimental normoxia, and 4) hypoxic incubation in acute hypoxia. Eggs were removed from incubation and candled to locate the fetus. Eggs were then placed in a custom-made, heated (38°C) surgical chamber at 21% O2 under a dissecting microscope (Leica M60, Leica Microsystems, Waukegan, IL) and a small section of eggshell was removed. The underlying shell membrane and chorioallantoic membrane were opened to isolate the fetus. The amniotic membrane was opened and the fetus was located. Temperature was continuously monitored with a probe placed inside the egg (BAT-12, Physitemp Instruments, Clifton, NJ). Pentobarbital (0.75–1.0 mg subcutaneous) was injected to achieve a surgical plane of anesthesia. Movement was monitored to assess anesthesia; additional incremental doses were provided as needed. The chest was opened through a midline incision above the keel. The muscle was blunt dissected away from the keel, and the bone was cut to provide access to the thoracic cavity. The ventral aspect of the pericardium was opened and a pericardial sling was created to support the heart. A loop of silk suture (6-0) was passed around the aorta to intermittently constrict the aorta and increase load on the left ventricle (LV). Heparinized saline (2–2.5 units in 20–25 μl) was given with a fine needle (25–30 gauge) through the LV apex. A calibrated, presoaked, zeroed Scisense 1.2-Fr ADVantage admittance catheter (Transonic Scisense, Toronto, ON, Canada) was introduced into the LV through the 25- to 30-gauge needle hole in the LV apex and oriented with the tip pointed at the aortic valve. The position of the admittance catheter was adjusted to minimize the phase signal (a high phase indicates interference by the myocardium), to maximize the magnitude range, and to obtain a pressure-volume loop free of artifacts. Once a satisfactory signal was obtained, the preparation was allowed to stabilize for about 10 min.
Experimental protocol.
Three recordings of LV pressure and volume were made in normoxia, starting at baseline pressure and during increasing aortic occlusion until maximum LV systolic pressure was achieved. Heart rate and LV pressure were allowed to return to normal between each pressure “ramp.” Ramps were compared to ensure a consistent response, but only one ramp was analyzed from each fetus (Fig. 1).
Fig. 1.
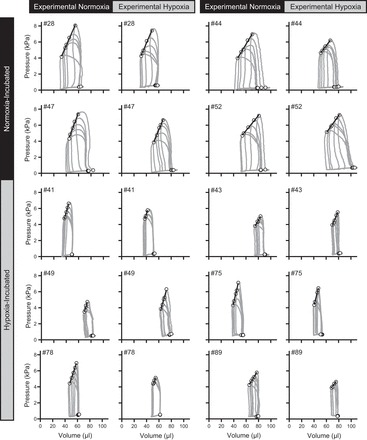
Pressure-volume loop obtained from fetal chickens incubated for 19 days at 21% O2 (black row header) or 15% O2 (grey row header), under the acute influence of either 21% O2 (black column header) or 10% O2 (grey column header). Shown also are end-systolic and end-diastolic points (circles) and the end-systolic pressure-volume relationship (straight line).
Acute experimental hypoxia was achieved by mixing nitrogen gas with room air to achieve 10% O2, warmed to 38°C, and passed into the surgical chamber at a flow rate of 2 l/min. Chamber gas percentage was continuously monitored with an Oxygen analyzer (S-3AII, Ametek Applied Electrochemistry). Recordings of LV pressure and volume starting at baseline and during increasing aortic occlusions were repeated as described beginning 2 min after chamber gas reached 10% O2 and concluded by 5 min after chamber gas reached 10% O2. This level of acute hypoxia was chosen because it was lower than the developmental oxygen concentration for both groups, but all fetuses could maintain normal LV systolic pressure during the data collection period. Catheter data (pressure, phase, magnitude) and oxygen percentage of the chamber were continuously recorded at 100 Hz using a Powerlab and computer (Apple, Cupertino, CA) running LabChart. Heart rate was calculated from LV pulse pressure frequency.
Blood Resistivity
Calculation of chamber volume by the Scisense system requires input of a blood resistivity constant. Chicken fetuses were anesthetized and heparinized as described above. Approximately 200–300 μl of blood were withdrawn into a syringe from the LV, aorta, or main pulmonary artery. Blood was placed within a small plastic container within a small air-tight bag and resistivity was immediately measured with a Scisense reference probe. The gas mixture was changed within the bag to 10% O2, the blood gently but thoroughly mixed, and resistivity measured again. Blood resistivity at 21% O2 was 1.6 ± 0.1 Ωm for normoxic incubation (n = 8) and 2.5 ± 0.1 Ωm for hypoxic incubation (n = 6). In 10% O2, blood resistivity was 1.8 ± 0.1 Ωm (n = 5) for normoxia-incubated and 2.4 ± 0.2 Ωm for hypoxia-incubated (n = 3) fetuses. The oxygen gas concentration at the time of measurement did not have a statistically significant effect on blood resistivity, but incubation oxygen concentration did (P < 0.0001). Rheological changes alter blood resistivity (30, 33) and may explain differences between normoxia- and hypoxia-incubated fetuses (21).
Stroke Volume
Calculation of chamber volume by the Scisense system requires input of a baseline stroke volume constant. Chicken fetuses were anesthetized and heparinized as described, and their thoracic cavities were opened. The heart was exposed and a 1.5-mm flow probe (Transonic, Ithaca, NY) was placed sequentially around each of the brachiocephalic arteries and aorta proximal to the ductus arteriosus, and stable flow was recorded for several minutes in each of the vessels. Because of size and anatomical limitations, simultaneous flow measurements could not be conducted. Blood flows from these vessels were summed to obtain LV stroke volumes used to calibrate the Scisense system.
Cardiac Pressure-Volume Calculations
Regions of interest in the LabChart record were converted to text format with no loss of fidelity and analyzed in the data postprocessor software Advol (Transonic Scisense) using the measured constants, a “heart type” value of 1 (personal communication from A. Kottam of Transonic Scisense), and the phase and magnitude channels (35) to obtain LV volume. Data files were then opened in LabChart v7.3.5 and pressure-volume loops were analyzed using the Pressure-Volume Loop module. End diastole was taken to be inflection between low-pressure ventricular filling and isovolumetric contraction.
Masses and Tissue Collection
Before setting eggs, egg mass was measured. At the completion of the hemodynamic measurements, fetuses were euthanized with an overdose of the anesthetic isoflurane. Yolk-free fetal and heart (including proximal great vessels) masses were measured. A separate set of chicken fetuses incubated as described under normoxic and hypoxic conditions, but that did not undergo surgery, were euthanized, and their hearts were collected for mRNA analysis. After blotting was completed, hearts were dissected into LV freewall, right ventricular (RV) freewall, septum, and atria (combined left and right). Samples were immediately immersed in RNAlater (Life Technologies, Grand Island, NY) and then stored at −20°C.
mRNA Expression Analysis
Tissue processing, RNA isolation, and first-strand cDNA synthesis.
RNA from ventricular tissue was isolated by homogenizing with a TissueLyser LT (Qiagen, Valencia, CA) in TRIzol (Ambion, Grand Island, NY). The RNA samples were cleaned using the RNeasy Mini Kit (Qiagen, Germantown, MD) and analyzed (Synergy H1 Hybrid Multi-Mode Microplate Reader Gen5, data analysis software from BioTek, Winooski, VT) to ensure integrity of RNA before first-strand cDNA synthesis. Total first-strand cDNA was synthesized using the Applied Biosystems High Capacity cDNA Reverse Transcription Kit (Life Technologies) per manufacturer's protocol (10 min at 25°C, 120 min at 37°C, 5 min at 85°C, hold at 4°C), with the addition of 2.5 μM oligo dT (Table 1).
Table 1.
Primers for quantitative PCR studies in ventricular freewalls of chickens at 90% of development (19 day) after incubation in 21% O2 or 15% O2
| Gene | Sequence | GenBank Accession Number |
|---|---|---|
| 18S ribosomal RNA | Sense: TGTCCCAGCCCCTGTCTCTC | AF173612.1 (Gallus gallus) |
| Antisense: CGCCGGTCCAAGAATTTCAC | ||
| Na+/Ca2+ exchanger 1 | Sense: GTTGACTTCCGGACAGAGGA | NM_001079473.1 (Gallus gallus) |
| Antisense: ACTGGCCTCGAGAATACCCT | ||
| Cardiac ryanodine receptor | Sense: CAGTCCTTGGCCATTACAAC | XM_419553.4 (Gallus gallus) |
| Antisense: CCTGCTGGATCCTCTATTTC | ||
| Sarco(endo)plasmic reticulum Ca2+-ATPase 2 | Sense: CCGATCCTCGTGCTGTAAAT | M66385.1 (Gallus gallus) |
| Antisense: TGTTGGAGTGGGGTTCTCTC | ||
| Phospholamban | Sense: AGGATCGGGTACAGGATCAG | NM_205410.1 (Gallus gallus) |
| Antisense: GGCAGTTGGAGACAAGGTTC | ||
| Collagen α-2(I) chain | Sense: TGGTAGGGTTGGGCCAATCG | NM_001079714.2 (Gallus gallus) |
| Antisense: GCACCTTGGTTGCCAGTGAC | ||
| Chicken cardiac natriuretic peptide B | Sense: CCTCTTGCTGCTTCTCATCC | NM_204925.1 (Gallus gallus) |
| Antisense: GCTCATCGCTGTCATCTGTG | ||
| Notch1 | Sense: CTCCAACTGCGATACCAAC | XM_004945971.1 (Gallus gallus) |
| Antisense: CAGACACTGGCATTGGAAG | ||
| Kinase insert domain receptor (VEGFR-2) | Sense: GAAGAGGAGGACGCTGGTTC | NM_001004368.1 (Gallus gallus) |
| Antisense: GGTGCCATCCATTTTAGCGG | ||
| Vascular endothelial growth factor A | Sense: ATGAGATGTGCGGGTTGCTG | NM_001110355.1 (Gallus gallus) |
| Antisense: TGCGCTATGTGCTGACTCTG |
Quantitative PCR.
Candidate genes from genomic databases were identified by analysis of sequence alignments of chicken (Gallus gallus) target sequences. Genes assessed in the excitation-contraction coupling pathway include Na+/Ca2+ exchanger 1 (NCX1), sarco(endo)plasmic reticulum Ca2+-ATPase 2 (SERCA2), phospholamban (PLN), and cardiac ryanodine receptor (RYR). The growth-associated genes assessed include collagen α-2(I) chain (COL1A2), kinase insert domain receptor (KDR, also known as VEGFR-2), vascular endothelial growth factor A (VEGF-A), Notch1, and Cyclin D1. B-type natriuretic peptide (BNP) family gene chicken cardiac natriuretic peptide B (chNP) was also assessed. qPCR primer design was accomplished using Primer 3 software program (San Diego Biology Workbench 3.2, http://workbench.sdsc.edu/). All primers (Eurofins MWG Operon, Huntsville, AL) were tested to optimize annealing temperatures and ensure single product formation. Each primer pair produced a single PCR product as evidenced by melt curve analysis and gel electrophoresis (Table 1). Amplification products were sequenced at Eurofins MWG Operon, and the sequences were compared with other DNA sequences using BLAST in The Gene Index database (TGI, http://compbio.dfci.harvard.edu/tgi/ncbi/blast/blast.html) to confirm amplification of the target gene. The BLAST queries resulted in correct gene hits with BLAST e-values <10-15. As part of our validation strategy, we also compared similar sequences with lesser e-values before assigning identities to the target genes.
After PCR product validation and assay optimization, qPCR assays were conducted in a 96-well format under the optimal PCR conditions with Applied Biosystems Power SYBR Green Master Mix (Life Technologies) using the relative standard curve method on a Stratagene Mx3005P QPCR System (Agilent Technologies, Santa Clara, CA). Reactions for each cDNA sample were performed in triplicate, including the standard curve, which was generated from pooled samples and quantified before serial dilution. PCR amplifications were performed for 1 cycle at 95°C for 10 min, 42 cycles with denaturation at 94°C for 20 s, annealing at optimum temperature for primers (55–58°C) for 30 s, and extension at 72°C for 30 s followed by melt curve analysis. All samples were normalized against 18S ribosomal RNA (18S) quantities.
Statistics
Physiological data from chronic and acute hypoxia were analyzed by repeated measures analysis of variance (ANOVA; Prism 6.0d, Graphpad Software, San Diego, CA). If the F-statistic justified further comparisons based on either incubation condition or oxygen at the time of measurement, Bonferroni-corrected comparisons between groups were performed within Prism and the multiplicity-adjusted P value is reported (57). If the F-statistic justified further comparisons based on both incubation condition and oxygen at the time of measurement, comparisons were carried out in the GraphPad QuickCalcs Post test calculator using the Bonferroni method (31). Heart mass, body mass, and gene expression values were compared by unpaired two-tailed t-test. Physiological data, mass, and gene expression are shown as means ± SE. Intercepts and slopes relating LV pressure to volume were compared between incubation groups and between experimental conditions using mixed models with unstructured autoregressive covariance matrices (SAS version 9.3, SAS, Cary, NC). To facilitate these comparisons, we incorporated interaction between LV volume and incubation condition and between LV volume and experimental condition. These regression data are presented as estimate ± SE. A P value of <0.05 was taken to be significant.
RESULTS
Fetal and Heart Mass
Hypoxia-incubated chicken fetuses were 20% smaller than normoxia-incubated fetuses at 19 days of incubation, indicating developmental growth restriction (P < 0.0001; Table 2). Heart mass was similar between the groups; consequently the heart to body mass was 17% greater in hypoxia-incubated fetuses (P < 0.02; Table 2). LV mass relative to body mass was 41% greater in hypoxia-incubated fetuses (P < 0.002; Table 2). Absolute LV, RV, and septal masses were similar in both groups (Table 2).
Table 2.
Chicken egg, body, and heart masses at 90% of development (day 19) after incubation in 21% O2 or 15% O2
| Control Incubation | Hypoxic Incubation | |
|---|---|---|
| Egg, g | 51.2 ± 0.9 (12) | 52.9 ± 1.0 (14) |
| Body, g | 26.5 ± 0.5 (13) | 21.2 ± 0.4 (14) * |
| Heart, μg | 149.2 ± 4.0 (13) | 138.1 ± 5.4 (14) |
| Relative to body, μg/g | 5.63 ± 0.16 (13) | 6.60 ± 0.34 (14) * |
| Left ventricle, μg | 33.7 ± 1.8 (9) | 35.9 ± 1.9 (8) |
| Relative to body, μg/g | 1.27 ± 0.07 (9) | 1.78 ± 0.12 (8) * |
| Septum, μg | 32.9 ± 1.6 (9) | 29.7 ± 2.8 (8) |
| Relative to body, μg/g | 1.24 ± 0.07 (9) | 1.46 ± 0.13 (8) |
| Right ventricle, μg | 32.0 ± 1.8 (9) | 28.8 ± 1.8 (8) |
| Relative to body, μg/g | 1.21 ± 0.07 (9) | 1.42 ± 0.08 (8) |
Data are presented as means ± SE; (n = number of chickens). Egg mass was measured when eggs were set.
P < 0.05 compared with control by unpaired t-test.
Measured Cardiac Function
LV output, as determined by stroke volume and heart rate, was affected both by incubation group (P < 0.0001) and by acute hypoxia (P < 0.02; Table 3). LV output of hypoxia-incubated fetal chickens was 58% lower than the normoxic incubation group in room air (P < 0.0001; Table 3) and 55% lower in acute hypoxia (P < 0.001). We could not detect a difference in cardiac output within incubation groups between normoxia and acute hypoxia. LV stroke volume in the hypoxia-incubated group was less than half that of the normoxia-incubated group (P < 0.001; Table 3). In room air, LV output normalized to body mass in room air was 0.25 ± 0.01 ml·min−1·g−1 in the normoxia-incubated group and 0.12 ± 0.01 ml·min−1·g−1 in the hypoxia-incubated group (P < 0.0001). In acute hypoxia, normalized LV output was 0.20 ± 0.02 ml·min−1·g−1 in the normoxia-incubated group and 0.11 ± 0.01 ml·min−1·g−11 in the hypoxia-incubated group (P < 0.001).
Table 3.
Left ventricular hemodynamic and end-systolic pressure-volume parameters for chickens at 90% of development (day 19) after incubation in 21% O2 or 15% O2
| Normoxic Incubation (n = 4) |
Hypoxic Incubation (n = 6) |
|||
|---|---|---|---|---|
| Normoxia | Acute hypoxia | Normoxia | Acute hypoxia | |
| Heart rate, beats/min | 216 ± 6 | 193 ± 12 | 211 ± 9 | 199 ± 8 |
| End-systolic pressure, kPa | 4.29 ± 0.15 | 4.48 ± 0.28 | 4.18 ± 0.18 | 4.22 ± 0.15 |
| End-diastolic pressure, kPa | 0.34 ± 0.03 | 0.51 ± 0.07† | 0.39 ± 0.06 | 0.47 ± 0.05† |
| Output, ml/min | 6.5 ± 0.2 | 5.3 ± 0.6 | 2.7 ± 0.2* | 2.4 ± 0.2* |
| Stroke volume, μl | 30.0 ± 0.5 | 28.2 ± 4.5 | 12.9 ± 0.6* | 11.9 ± 0.9* |
| Stroke work, kPa·μl | 118 ± 4 | 113 ± 24 | 49 ± 3* | 45 ± 4* |
| EA, kPa/μl | 0.1433 ± 0.0057 | 0.1681 ± 0.0216 | 0.3281 ± 0.0195* | 0.3610 ± 0.0251* |
| ELV, kPa/μl‡ | 0.1414 ± 0.009 | 0.1431 ± 0.009 | 0.1819 ± 0.0148 | 0.1906 ± 0.0160 |
| EA/ELV | 1.01 ± 0.04 | 1.17 ± 0.15 | 1.80 ± 0.11* | 1.89 ± 0.13* |
| V0, μl | 14.11 | 15.48 | 33.63 | 33.63 |
| ΔP/ΔtMax, kPa/s | 132 ± 10 | 122 ± 9 | 101 ± 5* | 98 ± 5* |
| ΔP/ΔtMin, kPa/s | −123 ± 4 | −126 ± 4 | −111 ± 7 | −108 ± 7 |
| τ, ms | 17.3 ± 0.8 | 21.2 ± 1.6† | 21.4 ± 0.9* | 25.5 ± 1.2*† |
Data are presented as means ± SE; n = number of chickens. EA, effective arterial elastance; ELV, left ventricular end-systolic elastance; V0, end-systolic pressure-volume relationship intercept at 0 kPa pressure; ΔP/ΔtMax, maximal pressure generation rate; ΔP/ΔtMin, minimal pressure generation rate; τ, time constant of relaxation.
P < 0.05 hypoxic incubation different from normoxic incubation in same acute oxygen concentration;
P < 0.05 acute hypoxia different from normoxia within incubation group.
Hypoxic incubation different from normoxic incubation by mixed models with unstructured autoregressive covariance matrices.
Acute hypoxia reduced heart rate by 8% (P < 0.02), but differences were not detectable between incubation groups. Incubation oxygen concentration did not affect heart rate at 19 days of incubation. Neither hypoxic incubation nor acute hypoxia significantly altered the period spent in systole (120 ± 2 ms). Hypoxic incubation did not significantly affect diastolic period, but it was significantly lengthened by acute hypoxia (164 ± 5 ms in room air, 191 ± 9 ms in 10% O2).
End-systolic pressure was not different between incubation groups and did not differ with acute hypoxia (4.28 ± 0.09 kPa, equivalent to 32.1 ± 0.1 mmHg; Table 3). Maximum end-systolic pressure generated during aortic occlusions also did not differ by incubation or acute hypoxia (normoxic incubation and experimental normoxia: 7.8 ± 0.4 kPa, normoxic incubation and acute hypoxia: 7.9 ± 0.4 kPa; hypoxic incubation and experimental normoxia: 7.4 ± 0.5 kPa, hypoxic incubation and acute hypoxia: 6.9 ± 0.4 kPa; equivalent to 51–59 mmHg). End-diastolic pressure was affected by acute hypoxia (P < 0.0001) but not by incubation oxygen concentration. No significant differences were found in end-diastolic volume (normoxic incubation and experimental normoxia: 74.6 ± 4.8 μl, normoxic incubation and acute hypoxia: 77.2 ± 9.9 μl; hypoxic incubation and experimental normoxia: 68.4 ± 6.8 μl, hypoxic incubation and acute hypoxia: 67.2 ± 5.3 μl). During acute hypoxia, end-diastolic pressure increased by 21% in the hypoxia-incubated group (P < 0.02) and 50% in the normoxia-incubated group (P < 0.0005).
Calculated Cardiac Function
Stroke work, the product of change in ventricular pressure and stroke volume, was affected by incubation oxygen concentration (P = 0.0003) but not acute hypoxia. In both room air and during acute hypoxia, stroke work in the hypoxia-incubation group was less than 50% of that in normoxia-incubation group (P < 0.0004; Table 3). The maximum derivative of change in systolic pressure over time (ΔP/ΔtMax) was affected by incubation oxygen concentration (P < 0.02). ΔP/ΔtMax was about 22% lower in hypoxia-incubated chicken hearts in both room air (P < 0.0001) and in acute hypoxia (P < 0.001; Table 3 and Fig. 3). ΔP/ΔtMax was 6% lower during acute hypoxia compared with ΔP/ΔtMax in room air (P < 0.04), but there were no significant differences between specific groups by posttest. LV relaxation was also affected by both incubation oxygen (P < 0.03) and acute hypoxia (P = 0.0003). The minimal derivative of change in systolic pressure over time (ΔP/ΔtMin) was not affected by incubation or acute hypoxia (Table 3). The time constant of relaxation (τ) in hypoxia-incubated fetuses was 22% greater than in the normoxia-incubated group, indicating a slower rate of relaxation (P < 0.01 for both experimental oxygen concentrations; Fig. 3). Acute hypoxia increased τ by 20% over control values for hypoxia-incubated chicken fetuses (P < 0.01) and for normoxia-incubated fetuses (P < 0.05).
Fig. 3.
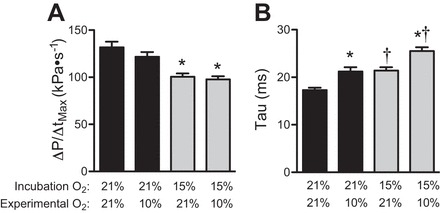
Cardiac function in the 90% of incubation (day 19) chicken fetus is altered by chronic and acute hypoxia. A: the maximal rate of LV contraction (ΔP/ΔtMax) is reduced by hypoxic incubation but not acute hypoxia. B: the half-time of relaxation (τ) is lengthened both by hypoxic incubation and by acute hypoxia. Data are presented as means ± SE. *P < 0.05 different from normoxic incubation in same experimental oxygen concentration; †P < 0.05 different from experimental normoxia of same incubation group.
End-systolic elastance (ELV), which is the slope of LV pressure versus LV volume, was significantly steeper in the hypoxia-incubated group compared with normoxia-incubated group (P = 0.003; Table 3). The regression intercepts at 0 μl LV volume were significantly less in the hypoxic incubation fetuses (P = 0.03). The end-systolic pressure-volume relationships (ESPVR) intercept at 0 kPa pressure (V0) was calculated from the regression intercept at 0 μl LV volume and ELV. V0 was right shifted by hypoxic incubation (Table 3). The ESPVR was not affected by acute experimental oxygen conditions. Effective arterial elastance (EA), defined as end-systolic pressure divided by stroke volume, was affected by incubation oxygen concentration (P < 0.0001) but not acute hypoxia (Table 3). EA was ∼121% greater in the hypoxia-incubated compared with normoxia-incubated group (P < 0.05 in both experimental oxygen conditions). The ratio EA/ELV was higher in hypoxia-incubated than normoxia-incubated chicken hearts in room air (44%; P = 0.0007) and in acute hypoxia (38%; P < 0.002; Table 3).
Excitation-Contraction Coupling Gene Expression
Cardiac RYR mRNA levels were more than 50% lower in hypoxia-incubated fetal chickens compared with the normoxia-incubated group in both LV (P < 0.02) and RV (P < 0.02; Fig. 2). Hypoxic incubation also decreased NCX1 mRNA by 40% in the RV (P < 0.05), and although there was a similar tendency in the LV, it was not significant (P < 0.07; Fig. 2). SERCA2 mRNA levels were 37% lower in the hypoxia-incubated LV (P < 0.02; Fig. 2). PLN mRNA levels were 44% lower in both ventricles in the hypoxia-incubated fetus compared with the normoxia-incubated control (LV: P < 0.02; RV: P < 0.03; Fig. 2).
Fig. 2.
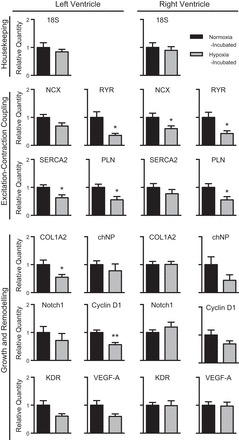
mRNA expression of select genes in left and right ventricles of normoxia-incubated (n = 9; black bars) and hypoxia-incubated (n = 8; shaded bars) chicken fetuses at 90% of incubation (day 19). 18S, 18S ribosomal RNA; NCX1,Na+/Ca2+ exchanger 1; RYR, cardiac ryanodine receptor; SERCA2, sarco(endo)plasmic reticulum Ca2+-ATPase 2; PLN, phospholamban; COL1A2, collagen α-2(I) chain; chNP, chicken cardiac natriuretic peptide B; KDR, kinase insert domain receptor (or VEGFR-2); VEGF-A, vascular endothelial growth factor A. Data are presented as means ± SE. Different from normoxia: *P < 0.05, **P < 0.005.
Expression of Growth-Associated Genes
Expression of growth-regulating genes KDR (also known as VEGFR-2) and VEGF-A were unchanged, although they tended to be lower in the LV of hypoxia-incubated fetuses (both P < 0.06; Fig. 2). Expression of cyclin D1 was 40% lower in the hypoxia-incubated LV (P < 0.003), although proliferation-associated Notch1 expression was not different (Fig. 2). Neither gene was different in the RV (Fig. 2). The LV (but not RV) mRNA relative concentrations of COL1A2 chain, which encodes one of the chains for type 1 collagen, was 45% lower in hypoxic incubation group (P < 0.04; Fig. 2). Expression of the chicken chNP was similar between incubation conditions (Fig. 2).
DISCUSSION
This study represents the first direct measurements of cardiac pressure-volume relationships in the intact late-stage chicken before ventilation. Furthermore, although ELV (41, 42), and EA (59) have been described separately in the stage 21–24 chicken embryo, this is the first description of simultaneous in ovo cardiac pressure-volume measurements in a nonmammalian embryonic or fetal organism. The primary findings of this study were that at 90% of incubation (day 19) hypoxic incubation led to greatly increased EA (an index of afterload; 58), slower LV contraction, and slower LV relaxation (Table 3, Fig. 3). During acute hypoxia (10% O2), the τ, a diastolic parameter of function, was increased in both normoxic and hypoxic incubation groups. These functional changes were concurrent with decreased expression of genes involved in cardiac calcium handling. Together, the data suggest that chronic developmental hypoxia reduces cardiac function and decreases cardiac energy efficiency.
Arterial-Cardiac Coupling
The EA/ELV ratio captures the interaction between LV performance and arterial properties that affect energetic efficiency (7). Just before lung ventilation (90% of incubation, or day 19) the EA/ELV ratio is 1 in the normoxia-incubated chicken fetus (Table 3), which is similar to normal values in healthy adult mammals at rest and in fetal lambs (7, 25). This suggests that mechanical efficacy and energetic efficiency are balanced similarly in fetal chickens as in adult and fetal mammals.
Hypoxia-incubated fetal chickens had a greater EA than control fetuses. End-systolic and end-diastolic pressures were similar between incubation groups at similar end-diastolic pressures; EA is different because stroke volume in the hypoxic incubation group is less than half that in the normoxic incubation group. Although EA was greater in hypoxia-incubated fetuses, ELV did not increase proportionally. The ESPVR relationship is steeper in hypoxia-incubated fetuses, but ELV and V0 must be interpreted together, and the picture they provide is one of reduced contractility (4). Supporting this conclusion is our finding that the maximal LV rate of pressure generation was 22% lower in hypoxic-incubated chicken fetuses. Furthermore, LV stroke work in the hypoxic-incubated group was less than half the value in the control group, despite similar LV masses.
Increased EA may be due to thicker, stiffer arteries (7, 40), resulting from differences in arterial anatomy and resting vascular tone (38). These vascular changes may be specific to certain vascular beds. For instance, aortic wall thickness is increased, lumen diameter is reduced, and peripheral vascular sympathetic innervation increased in chronically hypoxic fetal chickens (38). In contrast, hindlimb arterial resistance is not different between fetal chickens from hypoxic and normoxic incubation (21). Furthermore, our prior studies indicate that vascular adrenergic pathways in the chicken fetus are stimulated mainly by catecholamines of adrenomedullary rather than sympathetic origin (9, 10, 28). The impact of a higher EA may affect posthatching physiology given that it has been linked to exercise intolerance and systolic pressure sensitivity (8, 19), Indeed, rats subjected to intrauterine growth restriction have impaired cardiovascular performance in response to dobutamine challenge as adults (60). Furthermore, adult chickens that were incubated under hypoxic conditions have enlarged hearts and systolic dysfunction (27).
Cardiac Function
Indices of depressed cardiac function in this study parallel previously reported consequences of developmental hypoxia or intrauterine growth restriction in the sheep and human heart (6, 22, 29), suggesting that placental and maternal factors are not primarily responsible for cardiovascular adaptations to this stress. We found that developmental hypoxia decreased ΔP/ΔtMax and increased τ, similar to findings from prior studies of the isolated, Langendorff-perfused fetal chicken heart at 90% of incubation (day 19; 38), and isolated muscle bundles from the 95% incubation (day 20) hypoxia-incubated chicken heart (48). RYR is responsible for calcium-induced calcium release from the sarcoplasmic reticulum, and decreased ΔP/ΔtMax may be a result of the more than 50% reduction in expression of this gene (Fig. 2). Similarly, slow relaxation in this study is likely linked to the substantial reduction in expression of NCX, which moves cytosolic calcium to the extracellular space during diastole, and SERCA2, which concentrates cytosolic calcium in the sarcoplasmic reticulum during diastole. These genes, together with the SERCA2-regulating PLN, are critically important for cardiac function and were profoundly regulated by developmental hypoxia. Although mRNA expression levels do not directly predict functional protein levels, the mRNA expression levels of genes involved in excitation-contraction coupling parallel the functional changes.
Cardiac Growth
Although stroke volume after hypoxic incubation was halved, heart mass and end-diastolic volume were similar between incubation conditions. Hypoxia-incubated chickens had low yolk-free body mass for their developmental stage, but their heart masses were similar to the normoxia-incubated group. Similar mass does not necessarily indicate normal cardiac morphology, and altered cardiac geometry resulting from altered growth may affect cardiac performance. Differences in wall stress between the LV and RV freewalls, at the equal arterial pressures of the fetal circulation (34), may account for differences in gene expression patterns between the two ventricles. Interestingly, there was a large reduction in LV (but not RV) Col1A2 mRNA expression in hypoxia-incubated hearts (Fig. 2). The product of Col1A2 is a component of type 1 collagen, and mutations in this gene are associated with Ehlers-Danlos syndrome. If this indicates abnormal development of the fibrous myocardial scaffolding, hypoxic incubation may sensitize the heart to diastolic load. Alternatively, as heart weights were similar between incubation groups, translational and posttranslational regulation may support growth at normal levels despite reduced mRNA expression.
Heart Failure or Adaptation?
It has been suggested that the cardiac changes seen in the chronically hypoxic fetal chicken are due to failure (38, 48). This contradicts our own findings that hypoxic incubation may protect the fetal chicken during acute hypoxia by limiting arterial pressure and heart rate reductions (21). In this study, we found τ was longer in hypoxia-incubated fetuses as well as lengthened by acute hypoxia in both groups. However, we also found that cardiac output was similarly preserved during acute hypoxia in both hypoxia-incubated and normoxia-incubated fetuses. Furthermore, expression for the BNP-family gene chNP, which is analogous to mammalian BNP (1, 2, 36), did not increase with hypoxic incubation. It is important to note that, whereas increased cardiac natriuretic peptide expression is a hallmark of failure in mammals (56), birds lack the atrial natriuretic peptide gene (51), and the correlation between increased chNP expression during cardiac failure in birds has not been investigated. Therefore, further investigations regarding the correlation between cardiac failure and chNP gene expression need to be completed to establish the predictive value of the level chNP reported here in chicken hearts. Expression changes for the excitation-contraction coupling and growth genes assessed also only partially resembles the pathophysiology described in adult heart failure (5, 52). While heart failure certainly can occur in the fetus (45), it is clear that the fetal chicken response to chronic hypoxia cannot be characterized as classic failure.
Limitations of The Study
While fetal chickens are sensitive to halogenated ether anesthetics such as isoflurane, which are preferred for pressure-volume studies in rodents, it is not practical to use inhalation anesthetics before lung ventilation. In this study chicken fetuses were studied under a barbiturate anesthetic and consequently heart rates were lower than previously reported (11, 21). Furthermore, the heart type parameter, which contributes to calculation of volume for the Scisense system, could not be directly measured in chicken fetuses due to their small heart size and rapid heart rate. Consequently, the function of the admittance catheter was essentially equivalent to that of a conductance catheter. To compensate, cardiac output and stroke volume were measured in a separate set of fetuses, allowing calculation of LV volume. While these shortcomings are acknowledged, both experimental groups used the same experimental equipment, and were subjected to the same experimental manipulations and surgical procedures. Therefore, comparisons of functional differences between the groups in this study reflect how developmental hypoxia affects function of the heart.
In conclusion, these findings support our hypothesis that late-stage fetal chickens develop systolic and diastolic dysfunction during chronic hypoxia. We found hypoxic incubation led to less energetically favorable coupling of the heart to the systemic circulation. However, these hypoxia-incubated fetal chickens had a similar response to normoxia-incubated fetuses to severe acute hypoxic challenge as measured by change in LV output. This study demonstrates that late-stage chicken fetuses can be used to study in vivo cardiac function, allowing developmental studies isolating hypoxic and nutritional restriction from placental and maternal effects.
Perspectives and Significance
Hypoxic stress during development has been investigated in a number of vertebrate models (6, 18, 32, 46). Chronic fetal hypoxia has been linked to depressed fetal cardiac function in mammals (15, 18, 32, 37) and is predictive of cardiovascular disease states later in life (16, 26, 27). However, the role of maternal and placental mediating factors on the fetal cardiac response to hypoxia is not well understood. In this study, we found depressed cardiac function in chronically hypoxic fetal chickens lacking placental and maternal mediators, suggesting the primary importance of low oxygen in the fetus for that cardiac response. Gene expression changes and preservation of LV output during acute hypoxia in this study, though, suggest that the depressed function in chronically hypoxic fetal chickens may not be best characterized as cardiac failure. This new finding may be due to the lack of maternal and placental factors contributing to the fetal response to hypoxia, as they are absent in the fetal chicken. Alternatively the difference between the mammalian and the chicken fetus may be attributable to evolutionary divergence. Phenotypic plasticity of the developing heart may increase the ability of birds to maintain function during hypoxic bouts in perihatching (or perinatal) life, suggesting a fetal adaptive response to environmental stress in chickens as has been suggested in humans and other mammals (17).
GRANTS
D. A. Crossley 2nd was supported by the National Science Foundation under award number IOS-0845741. S. S. Jonker was supported by the Office of Research on Women's Health and Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (NIH) under awards numbered K12HD043488 and NIH 1R011HD071068. S. S. Jonker and G. D. Giraud were supported by the National Institute of Child Health and Human Development under award number P01HD034430. E. N. Davis was supported by the M. J. Murdock Charitable Trust. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.S.J. and D.A.C. conception and design of research; S.S.J., H.M.E., and E.N.D. performed experiments; S.S.J. analyzed data; S.S.J., G.D.G., and D.A.C. interpreted results of experiments; S.S.J. prepared figures; S.S.J. drafted manuscript; S.S.J., G.D.G., H.M.E., E.N.D., and D.A.C. edited and revised manuscript; S.S.J., G.D.G., H.M.E., E.N.D., and D.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank A. Kottam for technical guidance in optimizing the use of the Scisense catheter in chicken fetuses and D. Peters for statistical assistance.
REFERENCES
- 1.Akizuki N, Kangawa K, Minamino N, Matsuo H. Cloning and sequence analysis of complementary DNA encoding a precursor for chicken natriuretic peptide. FEBS Lett 280: 357–362, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Bezie Y, Mesnard L, Longrois D, Samson F, Perret C, Mercadier JJ, Laurent S. Interactions between endothelin-1 and atrial natriuretic peptide influence cultured chick cardiac myocyte contractility. Eur J Pharmacol 311: 241–248, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Brown LD, Green AS, Limesand SW, Rozance PJ. Maternal amino acid supplementation for intrauterine growth restriction. Front Biosci 3: 428–444, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkhoff D, Mirsky I, Suga H. Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289: H501–H512, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Busk PK, Hinrichsen R. Cyclin D in left ventricle hypertrophy. Cell Cycle 2: 91–95, 2003. [PubMed] [Google Scholar]
- 6.Cetin I, Kantar A, Unal S, Cakar N. The assessment of time-dependent myocardial changes in infants with perinatal hypoxia. J Matern Fetal Neonatal Med 25: 1564–1568, 2012. [DOI] [PubMed] [Google Scholar]
- 7.Chantler PD, Lakatta EG, Najjar SS. Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol (1985) 105: 1342–1351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CH, Nakayama M, Nevo E, Fetics BJ, Maughan WL, Kass DA. Coupled systolic-ventricular and vascular stiffening with age: implications for pressure regulation and cardiac reserve in the elderly. J Am Coll Cardiol 32: 1221–1227, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Crossley D 2nd, Altimiras J. Ontogeny of cholinergic and adrenergic cardiovascular regulation in the domestic chicken (Gallus gallus). Am J Physiol Regul Integr Comp Physiol 279: R1091–R1098, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Crossley DA 2nd, Burggren WW, Altimiras J. Cardiovascular regulation during hypoxia in embryos of the domestic chicken Gallus gallus. Am J Physiol Regul Integr Comp Physiol 284: R219–R226, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Crossley DA 2nd, Jonker SS, Hicks JW, Thornburg KL. Maturation of the angiotensin II cardiovascular response in the embryonic White Leghorn chicken (Gallus gallus). J Comp Physiol B 180: 1057–1065, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dzialowski EM, von Plettenberg D, Elmonoufy NA, Burggren WW. Chronic hypoxia alters the physiological and morphological trajectories of developing chicken embryos. Comp Biochem Physiol A Mol Integr Physiol 131: 713–724, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Faber JJ. Mechanical function of the septating embryonic heart. Am J Physiol 214: 475–481, 1968. [DOI] [PubMed] [Google Scholar]
- 14.Faber JJ, Green TJ, Hornburg KL. Embryonic stroke volume and cardiac output in the chick. Dev Biol 41: 14–21, 1974. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert RD. Fetal myocardial responses to long-term hypoxemia. Comp Biochem Physiol A Mol Integr Physiol 119: 669–674, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Giussani DA, Niu Y, Herrera EA, Richter HG, Camm EJ, Thakor AS, Kane AD, Hansell JA, Brain KL, Skeffington KL, Itani N, Wooding FB, Cross CM, Allison BJ. Heart disease link to fetal hypoxia and oxidative stress. Adv Exp Med Biol 814: 77–87, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Gluckman PD, Hanson MA, Bateson P, Beedle AS, Law CM, Bhutta ZA, Anokhin KV, Bougneres P, Chandak GR, Dasgupta P, Smith GD, Ellison PT, Forrester TE, Gilbert SF, Jablonka E, Kaplan H, Prentice AM, Simpson SJ, Uauy R, West-Eberhard MJ. Towards a new developmental synthesis: adaptive developmental plasticity and human disease. Lancet 373: 1654–1657, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Hassan WA, Brockelsby J, Alberry M, Fanelli T, Wladimiroff J, Lees CC. Cardiac function in early onset small for gestational age and growth restricted fetuses. Eur J Obstet Gynecol Reprod Biol 171: 262–265, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol 38: 796–802, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Hutter D, Kingdom J, Jaeggi E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: a review. Int J Pediatr 2010: 401323, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iversen NK, Wang T, Baatrup E, Crossley DA 2nd. The role of nitric oxide in the cardiovascular response to chronic and acute hypoxia in White Leghorn chicken (Gallus domesticus). Acta Physiol (Oxf) 211: 346–357, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Kamitomo M, Longo LD, Gilbert RD. Right and left ventricular function in fetal sheep exposed to long-term high-altitude hypoxemia. Am J Physiol Heart Circ Physiol 262: H399–H405, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy 2012: 179827, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leatherbury L, Connuck DM, Gauldin HE, Kirby ML. Hemodynamic changes and compensatory mechanisms during early cardiogenesis after neural crest ablation in chick embryos. Pediatr Res 30: 509–512, 1991. [DOI] [PubMed] [Google Scholar]
- 25.Lewinsky RM, Szwarc RS, Benson LN, Ritchie JW. Determinants of increased left ventricular output during in utero ventilation in fetal sheep. Pediatr Res 36: 373–379, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig 10: 265–274, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Lindgren I, Altimiras J. Prenatal hypoxia programs changes in β-adrenergic signaling and postnatal cardiac contractile dysfunction. Am J Physiol Regul Integr Comp Physiol 305: R1093–R1101, 2013. [DOI] [PubMed] [Google Scholar]
- 28.Lindgren I, Crossley D 2nd, Villamor E, Altimiras J. Hypotension in the chronically hypoxic chicken embryo is related to the β-adrenergic response of chorioallantoic and femoral arteries and not to bradycardia. Am J Physiol Regul Integr Comp Physiol 301: R1161–R1168, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Miyague NI, Ghidini A, Fromberg R, Miyague LL. Alterations in ventricular filling in small-for-gestational-age fetuses. Fetal Diagn Ther 12: 332–335, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Mohapatra SN, Hill DW. The changes in blood resistivity with haematocrit and temperature. Eur J Intensive Care Med 1: 153–162, 1975. [DOI] [PubMed] [Google Scholar]
- 31.Motulsky H. (2015) GraphPad QuickCalcs: Post test calculator. (Online) GraphPad Software. http://graphpad.com/quickcalcs/posttest1.cfm (27January 2015). [Google Scholar]
- 32.Ostadalova I, Ostadal B, Kolar F. Effect of prenatal hypoxia on contractile performance and responsiveness to Ca2+ in the isolated perinatal rat heart. Physiol Res 44: 135–137, 1995. [PubMed] [Google Scholar]
- 33.Pilwat G, Zimmermann U. Determination of intracellular conductivity from electrical breakdown measurements. Biochim Biophys Acta 820: 305–314, 1985. [DOI] [PubMed] [Google Scholar]
- 34.Pinson CW, Morton MJ, Thornburg KL. An anatomic basis for fetal right ventricular dominance and arterial pressure sensitivity. J Dev Physiol 9: 253–269, 1987. [PubMed] [Google Scholar]
- 35.Porterfield JE, Kottam AT, Raghavan K, Escobedo D, Jenkins JT, Larson ER, Trevino RJ, Valvano JW, Pearce JA, Feldman MD. Dynamic correction for parallel conductance, GP, and gain factor, alpha, in invasive murine left ventricular volume measurements. J Appl Physiol (1985) 107: 1693–1703, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reilly T, Gregg CM, Wideman RF Jr, Jarrett-Zaczek D. A potent hypotensive factor in chicken left ventricle. Proc Soc Exp Biol Med 186: 288–293, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Rizzo G, Capponi A, Rinaldo D, Arduini D, Romanini C. Ventricular ejection force in growth-retarded fetuses. Ultrasound Obstet Gynecol 5: 247–255, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Rouwet EV, Tintu AN, Schellings MW, van Bilsen M, Lutgens E, Hofstra L, Slaaf DW, Ramsay G, Le Noble FA. Hypoxia induces aortic hypertrophic growth, left ventricular dysfunction, and sympathetic hyperinnervation of peripheral arteries in the chick embryo. Circulation 105: 2791–2796, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Ruijtenbeek K, le Noble FA, Janssen GM, Kessels CG, Fazzi GE, Blanco CE, De Mey JG. Chronic hypoxia stimulates periarterial sympathetic nerve development in chicken embryo. Circulation 102: 2892–2897, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Sharma SK, Lucitti JL, Nordman C, Tinney JP, Tobita K, Keller BB. Impact of hypoxia on early chick embryo growth and cardiovascular function. Pediatr Res 59: 116–120, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Stekelenburg-de Vos S, Steendijk P, Ursem NT, Wladimiroff JW, Delfos R, Poelmann RE. Systolic and diastolic ventricular function assessed by pressure-volume loops in the stage 21 venous clipped chick embryo. Pediatr Res 57: 16–21, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Stekelenburg-de Vos S, Steendijk P, Ursem NT, Wladimiroff JW, Poelmann RE. Systolic and diastolic ventricular function in the normal and extra-embryonic venous clipped chicken embryo of stage 24: a pressure-volume loop assessment. Ultrasound Obstet Gynecol 30: 325–331, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Tazawa H. Measurement of blood pressure of chick embryo with an implanted needle catheter. J Appl Physiol 51: 1023–1026, 1981. [DOI] [PubMed] [Google Scholar]
- 44.Tazawa H, Lomholt JP, Johansen K. Direct measurement of allantoic blood flow in the chicken, Gallus domesticus. Responses to alteration in ambient temperature and PO2. Comp Biochem Physiol A Comp Physiol 81: 641–642, 1985. [DOI] [PubMed] [Google Scholar]
- 45.Thakur V, Fouron JC, Mertens L, Jaeggi ET. Diagnosis and management of fetal heart failure. Can J Cardiol 29: 759–767, 2013. [DOI] [PubMed] [Google Scholar]
- 46.Thompson JA, Piorkowska K, Gagnon R, Richardson BS, Regnault TR. Increased collagen deposition in the heart of chronically hypoxic ovine fetuses. J Dev Orig Health Dis 4: 470–478, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Thorn SR, Rozance PJ, Brown LD, Hay WW Jr. The intrauterine growth restriction phenotype: fetal adaptations and potential implications for later life insulin resistance and diabetes. Semin Reprod Med 29: 225–236, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tintu A, Rouwet E, Verlohren S, Brinkmann J, Ahmad S, Crispi F, van Bilsen M, Carmeliet P, Staff AC, Tjwa M, Cetin I, Gratacos E, Hernandez-Andrade E, Hofstra L, Jacobs M, Lamers WH, Morano I, Safak E, Ahmed A, le Noble F. Hypoxia induces dilated cardiomyopathy in the chick embryo: mechanism, intervention, and long-term consequences. PLos One 4: e5155, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tobita K, Keller BB. End-systolic myocardial stiffness is a load-independent index of contractility in stage 24 chick embryonic heart. Am J Physiol Heart Circ Physiol 276: H2102–H2108, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Tobita K, Keller BB. Maturation of end-systolic stress-strain relations in chick embryonic myocardium. Am J Physiol Heart Circ Physiol 279: H216–H224, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Trajanovska S, Inoue K, Takei Y, Donald JA. Genomic analyses and cloning of novel chicken natriuretic peptide genes reveal new insights into natriuretic peptide evolution. Peptides 28: 2155–2163, 2007. [DOI] [PubMed] [Google Scholar]
- 52.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. J Clin Invest 123: 37–45, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Mierop LH, Bertuch CJ Jr. Development of arterial blood pressure in the chick embryo. Am J Physiol 212: 43–48, 1967. [DOI] [PubMed] [Google Scholar]
- 54.van Patot MC, Ebensperger G, Gassmann M, Llanos AJ. The hypoxic placenta. High Alt Med Biol 13: 176–184, 2012. [DOI] [PubMed] [Google Scholar]
- 55.Vuguin PM. Animal models for small for gestational age and fetal programming of adult disease. Horm Res 68: 113–123, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang D, Gladysheva IP, Fan TH, Sullivan R, Houng AK, Reed GL. Atrial natriuretic peptide affects cardiac remodeling, function, heart failure, and survival in a mouse model of dilated cardiomyopathy. Hypertension 63: 514–519, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wright SP. Adjusted P-values for simultaneous inference. Biometrics 48: 1005–1013, 1992. [Google Scholar]
- 58.Yang B, Larson DF, Watson R. Age-related left ventricular function in the mouse: analysis based on in vivo pressure-volume relationships. Am J Physiol Heart Circ Physiol 277: H1906–H1913, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Yoshigi M, Hu N, Keller BB. Dorsal aortic impedance in stage 24 chick embryo following acute changes in circulating blood volume. Am J Physiol Heart Circ Physiol 270: H1597–H1606, 1996. [DOI] [PubMed] [Google Scholar]
- 60.Zohdi V, Jane Black M, Pearson JT. Elevated vascular resistance and afterload reduce the cardiac output response to dobutamine in early growth-restricted rats in adulthood. Br J Nutr 106: 1374–1382, 2011. [DOI] [PubMed] [Google Scholar]


