Abstract
Increasing evidence indicates that both the angiotensin II (ANG II) and γ-aminobutyric acid (GABA) systems play a very important role in the regulation of blood pressure (BP). However, there is little information concerning the interactions between these two systems in the nucleus tractus solitarii (NTS). In the present study, we examined the effects of ANG II on GABAA and GABAB receptor (GAR and GBR) expression in the NTS of Sprague-Dawley rats. The direct effect of ANG II on GBR expression was determined in neurons cultured from NTS. Treatment of neuronal cultures with ANG II (100 nM, 5 h) induced a twofold increase in GBR1 expression, as detected with real-time RT-PCR and Western blots, but had no effect on GBR2 or GAR expression. In electrophysiological experiments, perfusion of neuronal cultures with the GBR agonist baclofen decreased neuronal firing rate by 39% and 63% in neurons treated with either PBS (control) or ANG II, respectively, indicating that chronic ANG II treatment significantly enhanced the neuronal response to GBR activation. In contrast, ANG II had no significant effect on the inhibitory action of the GAR agonist muscimol. In whole animal studies, intracerebroventricular infusion of ANG II induced a sustained increase in mean BP and an elevation of GBR1 mRNA and protein levels in the NTS. These results indicate that ANG II stimulates GBR expression in NTS neurons, and this could contribute to the central nervous system actions of ANG II that result in dampening of baroreflexes and elevated BP in the central actions of ANG II.
Keywords: γ-aminobutyric acid, blood pressure
it is established that the angiotensin system within central cardiovascular control regions exerts regulatory influences in the control of blood pressure (BP) and plays an important role in the development and establishment of hypertension (2, 8, 12, 22). The central nervous system (CNS)-mediated cardiovascular actions of angiotensin II (ANG II) include stimulation of sympathetic nervous system (SNS) outflow, vasopressin release, and dampening of baroreflexes, via stimulation of angiotensin type 1 receptors (AT1R) in the nucleus tractus solitarii (NTS), a brain area that makes an important contribution to baroreflex integration and BP regulation (21, 30). Acute microinjection of ANG II into the medial portion of the NTS produces decreases or increases in BP depending on the volume injected, dose, and region of the ANG II injection (1, 7, 9, 38). The cardiovascular actions of ANG II are mediated not only by acute modulation of neuronal transmission and neuronal activity but also via changes in gene expression that influence long-term BP regulation. However, the identity of the particular genes that are altered by and mediate the chronic actions of ANG II is not established.
It is well known that CNS GABAergic systems play a key role in cardiovascular regulation via stimulation of GABAB receptor (GBR) and GABAA receptor (GAR) in the NTS (6). The ionotropic GAR has an intrinsic Cl− channel, which is responsible for inducing fast inhibitory postsynaptic potentials. The GBR is a G protein-coupled receptor and regulates neuronal activity via activation of K+ channels (31). The activation of K+ channels induces hyperpolarization of the neuronal membrane, which produces chronic inhibition of neuronal activity. A high density of both GAR and GBR and a high density of GABA-containing nerve terminals have been found within the NTS (16). Numerous studies have demonstrated that both GAR and GBR play an important role in the integration of baroreceptor afferent inputs and baroreflex function. Microinjection of the GAR agonist muscimol (24, 37) or the GBR agonist baclofen (5, 16, 25, 29) into the NTS produced a marked pressor response via inhibition of baroreflexes, elevation of sympathetic tone, and vasopressin release. Thus the preponderance of evidence indicates that the actions of GABA and ANG II within the NTS in BP and baroreflex regulation are very similar, suggesting that these factors may act via common cellular and/or intracellular mechanisms when influencing cardiovascular control. However, there is little information concerning the interactions between these two systems in BP regulation in the NTS.
In the present study, we examined whether GAR and/or GBR expression is altered in the NTS of rats infused centrally with ANG II and in cultured NTS neurons treated with ANG II. Our results indicate that GBR1 expression is enhanced in the NTS of normotensive Sprague-Dawley (SD) rats treated with chronic ANG II infusion and that chronic treatment of cultured NTS neurons with ANG II also induces an increase in GBR1 expression. This novel action of ANG II could contribute to ANG II-induced dampening of baroreflexes and the sustained elevation of arterial BP.
METHODS
Animals and materials.
In the experiments described here, we used adult male SD rats (270–320 g) obtained from Charles River Farms (Wilmington, MA). Rats were housed individually and kept on a 12:12-h light-dark cycle in a climate-controlled room. Rat chow (Harlan Tekland, Madison, WI) and water were provided ad libitum. One-day-old SD rat pups were used for the preparation of primary neuronal cultures. All animal protocols were approved by the North Dakota State University Institutional Animal Care and Use Committee (protocol A0741).
Dulbecco's modified Eagle's medium (DMEM) and penicillin-streptomycin mix were obtained from Invitrogen (Grand Island, NY). Crystallized trypsin was from Worthington Biochemicals (Freehold, NJ). Rabbit anti-GBR antibody was purchased from Chemicon International (Temecula, CA). Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 594 goat anti-mouse IgG were purchased from Invitrogen (Carlsbad, CA). Anti-neuron-specific nuclear protein [neuronal nuclei (NeuN)] monoclonal antibody was obtained from Millipore (Billerica, MA). Monoclonal anti-α-internexin antibodies were purchased from Novus Biologicals (Littleton, CO). Plasma-derived horse serum (PDHS), deoxyribonuclease I (DNase I), β-cytosine arabinoside (ARC), ANG II, baclofen, muscimol, ATP, GTP, HEPES, and other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Preparation of neuronal cultures and identification of AT1R-containing neurons.
Neuronal cultures were prepared from dorsal brain stem including NTS taken from 1-day-old rats as described previously (35). Briefly, trypsin (375 U/ml)- and DNase I (496 U/ml)-dissociated cells were resuspended in DMEM containing 10% PDHS and plated on 35-mm Nunc plastic tissue culture dishes precoated with poly-l-lysine. After the cells were grown for 3 days at 37°C in a humidified incubator with 95% O2 and 5% CO2, they were exposed to 1 μM ARC for 2 days in fresh DMEM containing 10% PDHS. ARC was then removed, and the cells were incubated with DMEM (plus 10% PDHS) for an additional 11–14 days before use. At the time of use, cultures consisted of 90% neurons and 10% astrocyte glia, as determined by immunofluorescent staining with antibodies against neurofilament proteins and glial fibrillary acidic protein (33).
Neurons within these cultures contained AT1R and angiotensin type 2 receptors (AT2R), which are predominantly present in different cell populations (33). The AT1R-positive neurons were identified with a single-cell RT-PCR technique published previously, with minor modifications (14). Glass patch pipettes were washed once in ethanol and three times in distilled water and were then autoclaved for 30 min. After electrophysiological recordings, the neuronal intracellular contents were drawn into the tip of the patch pipette with negative pressure. The procedure was performed carefully under a microscope equipped with a video monitoring system to avoid aspiration of the nucleus. The tip of pipette was then broken off inside an RT-PCR tube containing 5 μl of RNase-free water, and regular RT-PCR was performed with a SuperScript III RT-PCR kit (Invitrogen). The RNA template from whole dishes was used as positive control, and the exclusion of RNA was used as negative control. The following primers were used to identify the AT1R: forward: 5′-CCAAAGTCACCTGCATCAATC-3′, reverse: 5′-CACAATCGCCATAATTATCCTA-3′.
Intracerebroventricular ANG II infusion and BP measurement.
Intracerebroventricular (ICV) ANG II infusion was performed for 2 wk to induce hypertension in male rats. SD rats were fitted with Alzet minipumps (Durect, Cupertino, CA) under isoflurane inhalation as described previously (15). Minipumps were filled with either physiological saline or ANG II, delivering 0.25 μl/h for 2 wk for a final dose of ANG II of 15 ng·kg−1·day−1. Indirect BP was measured by the tail-cuff method as described previously (26). After 2 wk, the animals were killed and the NTS was punched out for expression analysis.
Real-time RT-PCR.
We used real-time PCR to detect changes in the expression of GAR, GBR, AT1R, and AT2R in the NTS of rats and in neuronal cultures. The isolation of total RNA from the NTS tissue and neuronal cultures was performed with RNeasy Mini Kits (Qiagen, Valencia, CA) according to the manufacturer's instructions. RNA purity and concentration were determined spectrophotometrically. For each RT-PCR reaction, 2 μg of total RNA was converted into cDNA with reverse transcriptase (Qiagen). Genomic DNA was eliminated by DNase I. For real-time PCR, the reactions were conducted by placing 10 μl of material into 96-well plates with the SYBR Green PCR kit or TaqMan PCR Master Mix (Applied Biosystems). Oligonucleotide primers specific for GARs were as follows and designed from the GenBank databases with Primer Express (Applied Biosystems): GARα1-forward (F): 5′-aaggacccatgacagtgctc-3′, GARα1-reverse (R): 5′-ggctcccttgtccactcata-3′, GARγ2-F: 5′-gacgatgaccactctcagca-3′, GARγ2-R: 5′-cagtccttgccatccaaac-3.
The specific probes for AT1R, AT2R, GBR1, and GBR2 were obtained from Applied Biosystems, and RT-PCR was performed in an Applied Biosystems PRISM 7000 sequence detection system according to the protocol from the manufacturer. A comparative cycle of threshold fluorescence (CT) method was used with 18S rRNA as an internal control. The CT value for 18S was subtracted from the CT value for the gene of interest to give a ΔCT for each sample. The ΔCT of the control was then subtracted from each sample to give a ΔΔCT value. This was entered into the equation mRNA levels (fold increases of control) = 2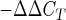 to give the final mRNA levels relative to the control. In each experiment, samples were analyzed in triplicate.
to give the final mRNA levels relative to the control. In each experiment, samples were analyzed in triplicate.
Immunohistochemistry.
Neuronal cultures from neonatal rats were washed briefly with ice-cold Dulbecco's phosphate-buffered saline (PBS) and then fixed for 15 min with PBS containing 0.1% Tween 20 (PBS-Tween) and 4% formaldehyde. Dishes were then washed briefly with PBS-Tween. Goat serum (10%) in PBS-Tween was added to the dish for 30 min at 37°C to reduce nonspecific binding, followed by an additional wash with PBS-Tween. Primary antibodies (rabbit anti-GBR1, 1:500; anti-α-internexin monoclonal antibody, 1:500), diluted in a 0.3-ml total volume of PBS-Tween, were added to the dish and incubated overnight at 4°C. After two 30-min washes with PBS-Tween, the neurons were incubated with secondary antibodies (Alexa Fluor 488 goat anti-rabbit IgG, 1:1,000; Alexa Fluor 594 goat anti-mouse IgG, 1:1,000), washed twice for 30 min each time with PBS-Tween, and mounted with antibleaching medium and a glass coverslip. The staining was detected with a confocal fluorescence microscope, and the fluorescent images were collected and analyzed with computer software as described below.
For GBR staining in the NTS in situ, rats were perfused transcardially with 50 ml of saline followed by 50 ml of 4% paraformaldehyde in PBS. The brain stem was removed, postfixed in 4% paraformaldehyde solution for 1 h, and transferred to PBS containing 20% sucrose. Frozen brain tissues were sectioned in the coronal plane. The NTS sections, identified with a rat brain atlas, were incubated with PBS plus 0.5% Tween 20 (PBS-T) containing 5% goat serum for 60 min. Slices were incubated with primary antibodies (anti-NeuN monoclonal antibody, 1:500; rabbit anti-GBR1, 1:500) overnight at 4°C. After being washed with PBS-T, the sections were incubated with secondary antibodies (Alexa Fluor 488 goat anti-rabbit IgG, 1:1,000; Alexa Fluor 594 goat anti-mouse IgG, 1:1,000) for 2 h. The sections were then washed with PBS-T, and staining was detected with a confocal fluorescence microscope (Olympus, Fluoview FV300), which was connected to a computer to capture and analyze images with Flowview software. The NTS area was focused to scan the image of neurons located on the surface of brain sections with x-y scanning mode. After the current scanning position was set at 0 μm, 50 slides around this level were scanned from −5 μm to +5 μm at steps of 0.20 μm. These 50 images were then stacked and saved in the computer hard drive for each channel, followed by image analysis with Flowview software. In these double-immunolabeling experiments, two different neuronal markers, anti-α-internexin and anti-NeuN antibodies, were used for the cultured neurons and NTS sections, respectively. This selection is based on our past immunocytochemistry experience and published literature, which demonstrates that α-internexin is especially useful for labeling cultured neurons (3) whereas NeuN can be used as a specific neuronal marker for immunostaining neurons in brain sections (20). Each treatment condition was run in triplicate within experiments.
Western blot analysis.
GBR1 protein levels in neuronal cultures and rat brain sections [NTS and paraventricular nucleus (PVN)] were assessed by Western blot analysis as described previously (35). Briefly, neuronal cultures were washed with ice-cold PBS and scraped into a lysing buffer containing 20 mM Tris·HCl (pH 6.8), 150 mM NaCl, 10% glycerol, 1% NP-40, and 8 μl/ml inhibitor cocktail (125 mM PMSF, 2.5 mg/ml aprotinin, 2.5 mg/ml leupeptin, 2.5 mg/ml antipain, and 2.5 mg/ml chymostatin). The samples underwent sonication twice for 5 s each and were centrifuged at 8,000 rpm for 10 min at 4°C. The supernatant was saved for protein assay. The micropunched NTS or PVN tissue from brain sections of saline- or ANG II-treated rats was treated with 1 ml of the same lysis buffer, homogenized for 15 s, boiled for 3 min, ultrasonicated, and centrifuged as above. Supernatants were transferred into new tubes and stored in a −80°C freezer. The protein concentration was determined with a protein assay kit (Bio-Rad Laboratories, Hercules, CA). An aliquot of 20 μg of protein from each sample was separated on a 10% SDS-PAGE gel and was transferred onto nitrocellulose membranes for 2 h at 100 V. After a 10-min wash in PBS-T, membranes were blocked in PBS-T containing 10% milk and 1% BSA for 3 h, followed by an overnight incubation in rabbit anti-GBR1 antibody (dilution 1:500) at 4°C. After a 15-min wash in PBS-T, four 5-min washes in PBS-T were carried out, and membranes were then incubated for 2 h in an anti-rabbit peroxidase-conjugated antibody (dilution 1:15,000). Immunoreactivity was detected by enhanced chemiluminescence autoradiography (ECL Western blotting detection kit, Amersham Pharmacia Biotechnology), and film was analyzed with Quantity One Software (Bio-Rad).
Electrophysiological recordings.
Spontaneous action potentials were recorded with the whole cell patch-clamp technique in current-clamp mode, as described previously (35, 36). Briefly, cultured neurons (11–14 days old) were bathed in a solution containing (in mM) 140 NaCl, 5.4 KCl, 2.0 CaCl2, 2.0 MgCl2, 0.3 NaH2PO4, 10 HEPES, and 10 dextrose, pH 7.4 (NaOH). Experiments were performed with an Axopatch-200B amplifier and Digidata 1200 interface (Axon Instruments, Burlingame, CA) at room temperature. Data acquisition and analysis were performed with the use of pCLAMP 8.0. Neurons in the culture dish (volume 1.5 ml) were superfused at a rate of 2–4 ml/min. The patch pipettes were filled with an internal pipette solution containing (in mM) 140 KCl, 2 MgCl2, 4 ATP, 0.1 guanosine 5′-triphosphate, 10 dextrose, and 10 HEPES, pH 7.2 (KOH) and had resistances of 3–4 MΩ. The whole cell configuration was formed by applying negative pressure to the patch electrode. The resting membrane potential was defined as the potential within a time period of 1 s during which there was no spontaneous action potential firing. Neuronal firing rate was measured as the numbers of fully developed action potentials per second (Hz). In individual experiments, test agents were added sequentially in the superfusate.
Data Analysis.
All data are expressed as means ± SE. Comparisons between experimental groups were performed with ANOVA followed by a Newman-Keuls test. Differences were considered significant at P < 0.05, and individual P values are noted in Figs. 1 and 3–6.
Fig. 1.
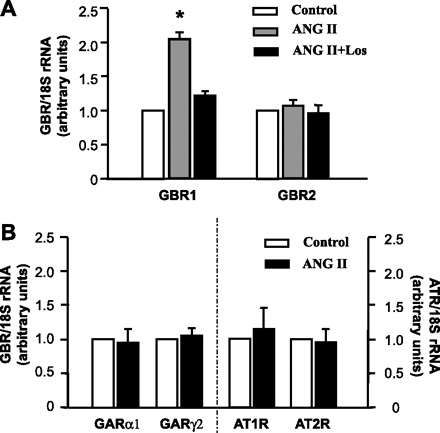
Angiotensin II (ANG II) increases GABAB receptor (GBR)1 mRNA levels in nucleus tractus solitarii (NTS) neuronal cultures. A: GBR mRNA levels in neuronal cultures under the following treatment conditions: PBS (control), ANG II (100 nM), or ANG II (100 nM) + losartan (Los, 1 μM) for 5 h at 37°C. GBR mRNA levels were detected with real-time RT-PCR as described in methods. Data are means ± SE of the fold increase over control (n = 4 experiments). *P < 0.05 vs. control. B: effect of ANG II on the levels of GABAA receptor (GAR) and ANG II type 1 and type 2 receptor (AT1R, AT2R) mRNAs in neuronal cultures treated with PBS control or ANG II (100 nM) for 5 h at 37°C. Data are means ± SE of fold increase over control.
Fig. 3.
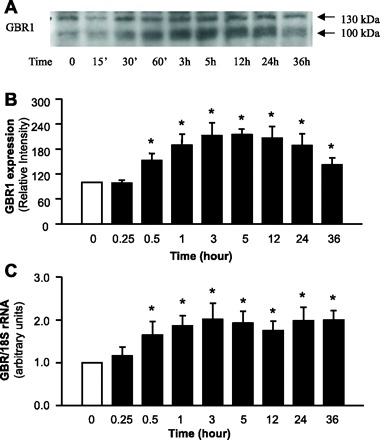
ANG II increases GBR expression in NTS neuronal cultures. Neuronal cultures from rats were incubated with control solution (PBS) or ANG II (100 nM) for 0.25, 0.5, 1, 3, 5, 12, 24, or 36 h. Cells were then lysed. GBR1 protein levels were analyzed by Western blots, and GBR1 mRNA levels were detected with real-time PCR as described in methods. A: representative blot showing GBR1 protein levels under each treatment condition. B: mean ± SE levels of GBR1 protein (n = 4 experiments). C: mean ± SE levels of GBR1 mRNA (n = 3 experiments). *P < 0.05 vs. PBS control.
Fig. 4.
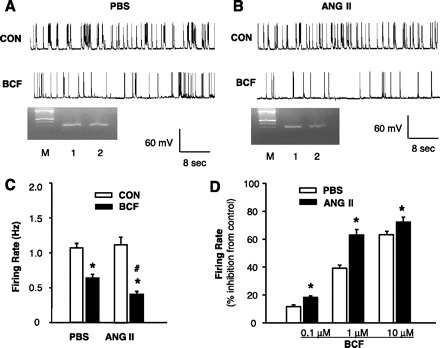
Effect of ANG II on the neuronal responsiveness to GBR agonist baclofen in neuronal cultures. A: representative tracing showing neuronal spontaneous action potentials (APs) recorded from a PBS-treated neuron under basal conditions (CON) and during superfusion of baclofen (BCF, 1 μM). APs were recorded from an AT1R-positive neuron identified by single-cell RT-PCR. B: representative tracing showing APs recorded from an ANG II-treated neuron under basal conditions (CON) and during superfusion of baclofen. APs were recorded from an AT1R-positive neuron by the same methods as in A. Ethidium bromide-stained gels seen in A and B show the PCR DNA products that correspond to AT1R mRNA obtained from the same neuron after the electrophysiological recordings (lanes 2). The RNA from the whole dishes of neurons was used as positive control (lanes 1). M, marker. C: effect of baclofen (1 μM) on neuronal firing in AT1R-positive neurons pretreated with ANG II or PBS control. Data are means ± SE (n = 7). D: dose-dependent inhibition induced by baclofen on neuronal firing in AT1R-positive neurons pretreated with ANG II or PBS control. *P < 0.05 compared with respective control recording; #P < 0.05 compared with PBS treatment.
Fig. 5.
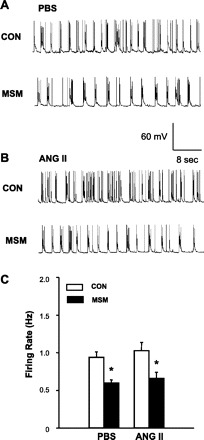
Effect of ANG II on the neuronal responsiveness to GAR agonist muscimol in neuronal cultures. A: representative tracing showing neuronal spontaneous APs recorded from a PBS-treated neuron under basal conditions (CON) and during superfusion of muscimol (MSM, 1 μM). APs were recorded from an AT1R-positive neuron identified by single-cell RT-PCR as described in methods. B: representative tracing showing APs recorded from an ANG II (100 nM, 5 h)-treated neuron under basal conditions (CON) and superfusion of muscimol (1 μM). C: effect of muscimol on neuronal firing in AT1R-positive neurons pretreated with ANG II or PBS control. Data are means ± SE (n = 7). *P < 0.01 compared with respective control recording.
Fig. 6.
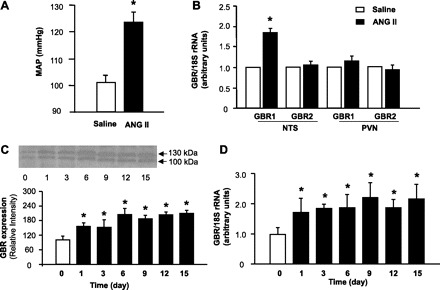
Effect of chronic ANG II infusion on GBR1 and GBR2 mRNA levels in the NTS and paraventricular nucleus (PVN) of rats. A: mean arterial pressure (MAP) measured after intracerebroventricular (ICV) delivery of saline or ANG II (15 ng·kg−1·day−1) for 2 wk via osmotic minipumps. Data are means ± SE (n = 6 rats in each group) of the average MAP measured by the tail-cuff method. *P < 0.05 vs. rats treated with 0.9% saline. B: GBR mRNA levels were detected in the NTS and PVN of the rats from A by real-time RT-PCR as described in methods. Data are mean ± SE GBR mRNA levels (n = 6, triplicate experiments in each rat). *P < 0.05 vs. saline treatment rats. C: GBR protein levels were detected with Western blots in the NTS of rats treated with ANG II (ICV, 15 ng·kg−1·day−1) for 1, 3, 6, 9, 12, or 15 days via osmotic minipumps. Data are means ± SE (n = 4 rats in each group). *P < 0.05 vs. untreated rats. D: GBR mRNA levels were detected with real-time RT-PCR in the NTS of rats treated with the same protocol as in C. *P < 0.05 vs. untreated rats.
RESULTS
Effect of ANG II on GAR, GBR, AT1R, and AT2R mRNA levels in neuronal cultures.
First, we examined the direct effect of ANG II on GBR expression in neurons cultured from dorsal brain stem including the NTS. The neuronal cultures were prepared from 1-day-old SD rats as described in methodsand were treated under the following conditions: control solution (PBS), ANG II (100 nM), and ANG II (100 nM) plus losartan (1 μM) for 5 h at 37°C followed by analysis of GBR1 and GBR2 mRNA levels with real-time RT-PCR. Data from four experiments were normalized to 18S RNA and expressed as fold increase compared with control. The results shown in Fig. 1A demonstrate that ANG II treatment resulted in a twofold increase in the mRNA levels of GBR1 but did not alter GBR2 mRNA levels. Coincubation with 1 μM losartan completely attenuated the ANG II-induced increase in GBR1 expression (Fig. 1A). Losartan (1 μM) alone had no significant effect on the basal levels of neuronal GBR1 and GBR2 (data not shown). These data indicate that ANG II enhanced GBR1 expression via stimulation of AT1R. In addition, we also examined the effect of ANG II on GAR, AT1R, and AT2R mRNA levels to test whether the ANG II-induced enhancement of GBR1 expression is secondary to the alteration of GAR, AT1R, or AT2R expression in neurons. These results are shown in Fig. 1B and indicate that ANG II treatment (100 nM, 5 h) did not change GARα1, GARγ2, AT1R, or AT2R mRNA levels in the neuronal cultures.
ANG II increases GBR1 protein levels in cultured NTS neurons.
The effect of ANG II on GBR1 expression in NTS neuronal cultures was examined via immunohistochemistry and Western blotting. Immunohistochemical experiments using a GBR1-specific antibody and an antibody against the neuron-specific marker α-internexin revealed that GBR1 immunoreactivity was primarily localized to neurons (Fig. 2). To determine whether ANG II increases GBR1 expression in these cells, neuronal cultures from rats were incubated with ANG II (100 nM) for 0.25, 0.5, 1, 3, 5, 12, 24, or 36 h, followed by analysis of GBR1 protein expression with Western blots. The results indicate that in NTS neurons ANG II begins to produce an increase in GBR1 expression within 30 min and the effect reaches a maximum by 3–5 h. This action of ANG II persisted through 36 h (Fig. 3, A and B). These observations were also confirmed by examining the effect of ANG II on the mRNA level of GBR1 at each time point with real-time PCR (Fig. 3C).
Fig. 2.
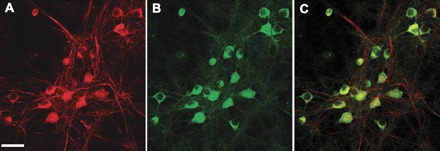
GBR1 immunoreactivity in NTS neurons. A–C: fluorescence images taken from the same field of cultured NTS neurons. A: neurons stained with antibodies to the neuronal marker α-internexin (red). B: the same neurons stained with anti-GBR1 antibodies (green). C: overlap of A and B, showing that GBR1 is neuronally located. Bar, 20 μm.
Effect of ANG II on neuronal responsiveness to GBR agonist baclofen.
To examine the functional interactions between the GABA and angiotensin systems, the effect of the GBR agonist baclofen on neuronal firing rate was examined in AT1R-positive neurons treated with PBS (control) or ANG II. AT1R-positive neurons were identified with single-cell RT-PCR after the electrophysiological recording. The results shown in Fig. 4, A and C, indicate that perfusion of neuronal cultures with baclofen (1 μM) decreased the neuronal firing rate by 39% in PBS-treated neurons; however, in ANG II (100 nM, 5 h)-treated neurons baclofen decreased the neuronal firing rate by 63% (Fig. 4, B and C), indicating that chronic ANG II treatment significantly increased the neuronal responsiveness to baclofen in AT1R-containing neurons (Fig. 4D). By contrast, pretreatment with ANG II (100 nM, 5 h) had no effect on the inhibitory action of baclofen in AT1R-negative neurons (38% inhibition in neurons treated with ANG II, 39% inhibition in neurons treated with PBS; n = 8, P > 0.05).
Effect of ANG II on neuronal responsiveness to GAR agonist muscimol.
We also examined the effect of the GAR agonist muscimol on AT1R-positive neurons treated with control solution (PBS) or ANG II (100 nM, 5 h). AT1R-positive neurons were identified by single-cell RT-PCR after the electrophysiological recordings. The data are shown in Fig. 5, indicating that perfusion of the neurons with muscimol (1 μM) significantly decreased the neuronal activity by 38%, from 0.97 ± 0.7 Hz to 0.60 ± 0.04 Hz in PBS-treated neurons (Fig. 5, A and C). Muscimol (1 μM) produced a similar (36%) decrease in neuronal firing in ANG II (100 nM, 5 h)-treated neurons, from 1.03 ± 0.11 Hz to 0.66 ± 0.08 Hz (Fig. 5, B and C). The results demonstrate that treatment of neurons with ANG II had no effect on the neuronal response to the GAR agonist muscimol.
GBR expression in brains of ANG II infusion-induced hypertensive rats.
To validate our in vitro observations, we detected GBR1 and GBR2 mRNA levels with real-time PCR in the NTS of saline- or ANG II-infused rats. The results shown in Fig. 6 demonstrate that ICV ANG II infusion (15 ng·kg−1·day−1) for 2 wk in rats resulted in a significant increase in BP. Mean BP was 104 ± 5 mmHg in saline-infused rats and 123 ± 6 mmHg in ANG II-infused rats (Fig. 6A). The hypertensive state in the ANG II-infused rats was associated with an 80% increase in the GBR1 mRNA levels in the NTS. However, GBR2 mRNA levels were not different in the NTS between ANG II-infused rats and saline-infused rats (Fig. 6B). To examine whether GBR expression is also increased in other brain cardiovascular regulatory regions, we also determined GBR mRNA levels in the PVN, another important central BP regulatory brain region. The results shown in Fig. 6B demonstrate that GBR1 and GBR2 mRNA levels in the PVN were not significantly increased by ANG II infusion and thus were not different between ANG II-infused rats and saline-infused rats. The levels of GBR protein and mRNA were also examined in the NTS of rats infused ICV with ANG II (15 ng·kg−1·day−1 for 1–15 days). The levels of both GBR protein and mRNA were elevated by ANG II infusion. These stimulatory effects of ANG II began at day 1, reached a peak at day 3, and persisted for 15 days after the start of infusion (Fig. 6, C and D). To confirm that GBR1 are expressed on the neurons in the NTS, we localized the GBR1 in NTS brain sections via immunohistochemistry using an anti-GBR1 specific antibody and an antibody against the neuron-specific marker NeuN. The results are shown in Fig. 7, indicating that GBR1 are localized on neurons of NTS.
Fig. 7.
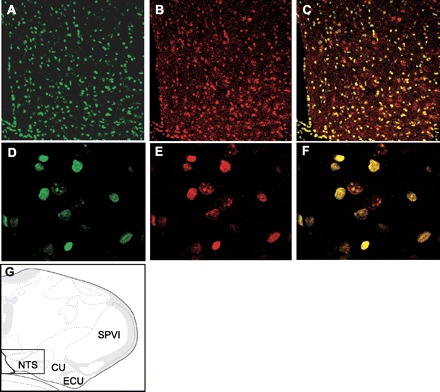
GBR1 localization and expression in neurons of the NTS. A–C: fluorescence confocal images (×10 magnification) demonstrating neurons within the NTS stained with a specific anti-GBR1 antibody (green, A) and a neuron-specific anti-NeuN antibody (red, B). C: overlap of A and B, indicating that GBR is located on neurons. D–F: fluorescence confocal images taken under high magnification (×40) from a section of the NTS brain sections shown in A–C, respectively. G: location of the stained NTS brain sections shown in A–F, based on the rat brain atlas of Swanson (37a). SPVI, spinal tract of the trigeminal interpolar part; ECU, external cuneate nucleus; CU, cuneate nucleus.
DISCUSSION
The present studies present the first evidence that ANG II increases GBR1 expression in NTS neurons. This conclusion is supported by the following observations. 1) GBR1 expression was enhanced by chronic treatment of NTS neuronal cultures with ANG II. 2) The inhibitory response to the GBR agonist baclofen was exaggerated in NTS neurons after chronic treatment with ANG II. 3) High BP resulting from chronic ANG II infusion was associated with elevated GBR1 expression within the NTS of rats. In contrast, GAR expression was not altered by chronic ANG II treatment within the NTS in vivo and in NTS neurons in vitro. The inhibitory effect of the GAR agonist muscimol on neuronal activity observed in NTS neuronal cultures was not altered by chronic ANG II treatment. These results indicate that ANG II may act directly on NTS neurons and enhance functional GBR expression, and that it is associated with elevated GBR sensitivity in NTS neurons.
Alterations in GABAergic function within the NTS are associated with chronic hypertension. An enhanced pressor response to the microinjection of baclofen into the NTS has been observed in several hypertensive animal models including spontaneously hypertensive rats (SHR), deoxycorticosterone acetate salt-induced hypertensive rats, and renal wrap hypertensive rats (11, 39, 40, 23, 43). However, the exact mechanism responsible for the increase in neuronal response to GABA receptor activation is unknown. The observations in the present study demonstrate that GBR1 gene expression in the NTS is elevated by ANG II chronic infusion in vivo and by treatment of NTS neuronal cultures with ANG II in vitro. These results suggest that ANG II may be responsible for the enhanced pressor response to microinjection of GBR agonists into NTS in hypertensive rats, because it has been reported that the actions of ANG II are increased in the central cardiovascular regulatory regions including NTS in these hypertensive animal models (5, 18). The hyperactivity of the angiotensin system would consequently increase GBR1 expression as observed in the present study and enhance inhibitory GABAergic neurotransmission. Exaggerated activity of the GABAergic system within the NTS may elicit an inhibitory action over baroreflex input signals, leading to an increase in sympathetic neural outflow and elevation of BP. This speculation is supported by the observations that microinjection of ANG II into the NTS results in dampening of the baroreflex, increases in sympathetic neural outflow, and elevation of BP (2). However, there are several concerns that require further investigation.
One concern in the present study is the effect on BP of microinjection of ANG II into the NTS. Previous studies indicate that acute microinjection of this peptide into the medial portion of the NTS produces either decreases or increases in BP (1, 7, 9, 38). This controversy has been explained in several ways, for example, differences in the volume injected, dose, and region of the ANG II injection. This controversy may be clarified by the observation that a high dose of ANG II injected into the NTS induced a brief depressor effect, followed by a long-lasting pressor action (28). These data suggest that the pressor effect of ANG II may be the long-term action of this peptide since a high dose of ANG II may take longer to be degraded. The acute depressor and bradycardic actions of ANG II in NTS have been well documented and are thought to be mediated by substance P and nitric oxide (10, 41). However, the chronic actions of ANG II remain to be elucidated. The present observations demonstrate that chronic ANG II treatment-induced BP elevation is associated with elevated GBR expression in NTS. The consequence of enhanced inhibitory GABAergic neurotransmission in NTS could result in baroreflex inhibition and increased SNS outflow. However, this speculation must be confirmed in future in vivo experiments.
Another set of questions concerns the intracellular signaling mechanisms underlying ANG II-induced increases in GBR1 expression in NTS neurons. The GBR1 gene promoter region contains several DNA-binding consensus sequences for transcription factors, including the cAMP response element-binding protein (CREB), the depolarization-sensitive upstream stimulatory factor (USF), and the CREB-related factor activating transcription factor-4 (ATF4) (32). Previously, we and others have demonstrated that ANG II increases phosphatidylinositol 3-kinase (PI3-kinase) activity (34, 42) and PI3-kinase is involved in the regulation of expression of several genes via phosphorylation of CREB in neurons (19, 27). Thus it is reasonable to propose that the ANG II-induced increase in GBR1 expression may be mediated by stimulation of PI3-kinase activity. However, the situation may not be that simple. For example, Rho kinase is similar to PI3-kinase inasmuch as Rho kinase is involved in gene expression via phosphorylation of CREB and is involved in the actions of ANG II in neurons (13, 17). Thus it is possible that ANG II-induced increases in GBR1 expression may also involve Rho kinase activation. More interestingly, in the studies on the time course of ANG II-induced GBR1 expression, the stimulatory effect of ANG II on GBR1 protein levels in neuronal cultures peaked at 5 h and started to decline slightly after 12 h (Fig. 3B). The reason for a lessening of the effect of ANG II on GBR1 protein expression in our experiments is not clear, but it is not due to a decline of GBR1 mRNA levels, as shown in Fig. 3C. We speculate that another negative regulatory pathway, which is also triggered by ANG II after 12 h, interferes with protein translation or increases protein degradation in neurons. To summarize, the above evidence suggests that multiple mechanisms and multiple intracellular signaling pathways may be involved in the ANG II-induced increase in GBR1 expression, and these will only be confirmed by additional studies.
In conclusion, ANG II treatment increases GBR1 expression and the inhibitory response to a GBR1 agonist in NTS neurons in culture. GBR1 expression is also increased in the NTS of normal rats made hypertensive via chronic infusion of ANG II. The ANG II-induced elevation of GBR1 expression in NTS could contribute to the damping of baroreflexes by limiting baroreceptor input signals, thus leading to an increase in sympathetic outflow. Interaction between the ANG II and GABA systems may be involved in central BP resetting and the pathogenesis of hypertension.
GRANTS
This work was supported by the American Heart Association, National Center Program (SDG No. 0635050N). The project described was also supported by Grant 2P20-RR-015566 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Averill DB, Diz DI, Barnes KL, Ferrario CM. Pressor responses of angiotensin II microinjected into the dorsomedial medulla of the dog. Brain Res 414: 294–300, 1987. [DOI] [PubMed] [Google Scholar]
- 2.Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull 51: 119–128, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Benson DL, Mandell JW, Shaw G, Benker G. Compartmentation of alpha-internexin and neurofilament triplet proteins in cultured hippocampal neurons. J Neurocytol 25: 181–96, 1996. [DOI] [PubMed] [Google Scholar]
- 4.Brooks PA, Glaum SR. GABAB receptors modulate a tetanus-induced sustained potentiation of monosynaptic inhibitory transmission in the rat nucleus tractus solitarii in vitro. J Auton Nerv Syst 54: 16–26, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Brooks VL, Scrogin KE, McKeogh DF. The interaction of angiotensin II and osmolality in the generation of sympathetic tone during changes in dietary salt intake. Ann NY Acad Sci 940: 380–394, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Callera JC, Bonagamba LG, Nosjean A, Laguzzi R, Machado BH. Activation of GABA receptors in the NTS of awake rats reduces the gain of baroreflex bradycardia. Auton Neurosci 84: 58–67, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Casto R, Phillips MI. Cardiovascular actions of microinjections of angiotensin II in the brain stem of rats. Am J Physiol Regul Integr Comp Physiol 246: R811–R816, 1984. [DOI] [PubMed] [Google Scholar]
- 8.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International Union of Pharmacology, XXIII: the angiotensin II receptors. Pharmacol Rev 52: 415–472, 2000. [PubMed] [Google Scholar]
- 9.Diz DI, Barnes KL, Ferrario CM. Hypotensive actions of microinjections of angiotensin II into the dorsal motor nucleus of the vagus. J Hypertens Suppl 2: S53–S56, 1984. [PubMed] [Google Scholar]
- 10.Diz DI, Fantz DL, Benter IF, Bosch SM. Acute depressor actions of angiotensin II in the nucleus of the solitary tract are mediated by substance P. Am J Physiol Regul Integr Comp Physiol 273: R28–R34, 1997. [DOI] [PubMed] [Google Scholar]
- 11.Durgam VR, Vitela M, Mifflin SW. Enhanced gamma-aminobutyric acid-B receptor agonist responses and mRNA within the nucleus of the solitary tract in hypertension. Hypertension 33: 530–536, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson AV, Washburn DL, Latchford KJ. Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp Biol Med (Maywood) 226: 85–96, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Funakoshi Y, Ichiki T, Shimokawa H, Egashira K, Takeda K, Kaibuchi K, Takeya M, Yoshimura T, Takeshita A. Rho-kinase mediates angiotensin II-induced monocyte chemoattractant protein-1 expression in rat vascular smooth muscle cells. Hypertension 38: 100–104, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Lira G, Lamas M, Romo-Parra H, Gutierrez R. Programmed and induced phenotype of the hippocampal granule cells. J Neurosci 25: 6939–6946, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang BS, Wang H, Leenen FH. Chronic central infusion of aldosterone leads to sympathetic hyperreactivity and hypertension in Dahl S but not Dahl R rats. Am J Physiol Heart Circ Physiol 288: H517–H524, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Hwang BH, Wu JY. Ultrastructural studies on catecholaminergic terminals and GABAergic neurons in nucleus tractus solitarius of the rat medulla oblongata. Brain Res 302: 57–67, 1984. [DOI] [PubMed] [Google Scholar]
- 17.Ito K, Hirooka Y, Sakai K, Kishi T, Kaibuchi K, Shimokawa H, Takeshita A. Rho/Rho-kinase pathway in brain stem contributes to blood pressure regulation via sympathetic nervous system: possible involvement in neural mechanisms of hypertension. Circ Res 92: 1337–1343, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Katsunuma N, Tsukamoto K, Ito S, Kanmatsuse K. Enhanced angiotensin-mediated responses in the nucleus tractus solitarii of spontaneously hypertensive rats. Brain Res Bull 60: 209–214, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Leinninger GM, Backus C, Uhler MD, Lentz SI, Feldman EL. Phosphatidylinositol 3-kinase and Akt effectors mediate insulin-like growth factor-I neuroprotection in dorsal root ganglia neurons. FASEB J 18: 1544–1546, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Gao Y, Freire CD, Raizada MK, Toney GM, Sumners C. Microphage migration inhibitory factor in the PVN attenuates the central pressor and dipsogenic actions of angiotensin II. FASEB J 20: 1748–1750, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Matsumura K, Averill DB, Ferrario CM. Angiotensin II acts at AT1 receptors in the nucleus of the solitary tract to attenuate the baroreceptor reflex. Am J Physiol Regul Integr Comp Physiol 275: R1611–R1619, 1998. [DOI] [PubMed] [Google Scholar]
- 22.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35: 901–918, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Mei L, Zhang J, Mifflin S. Hypertension alters GABA receptor-mediated inhibition of neurons in the nucleus of the solitary tract. Am J Physiol Regul Integr Comp Physiol 285: R1276–R1286, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Mohler H, Benke D, Fritschy JM. GABA(B)-receptor isoforms molecular architecture and distribution. Life Sci 68: 2297–300, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Mueller PJ, Hasser EM. Putative role of the NTS in alterations in neural control of the circulation following exercise training in rats. Am J Physiol Regul Integr Comp Physiol 290: R383–R392, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Pachori AS, Wang H, Gelband CH, Ferrario CM, Katovich MJ, Raizada MK. Inability to induce hypertension in normotensive rat expressing AT1 receptor antisense. Circ Res 86: 1167–1172, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Perkinton MS, Sihra TS, Williams RJ. Ca2+-permeable AMPA receptors induce phosphorylation of cAMP response element-binding protein through a phosphatidylinositol 3-kinase-dependent stimulation of the mitogen-activated protein kinase signaling cascade in neurons. J Neurosci 19: 5861–5874, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rettig R, Healy DP, Printz MP. Cardiovascular effects of microinjections of angiotensin II into the nucleus tractus solitarii. Brain Res 364: 233–40, 1986. [DOI] [PubMed] [Google Scholar]
- 29.Ruggeri P, Cogo CE, Picchio V, Molinari C, Ermirio R, Calaresu FR. Influence of GABAergic mechanisms on baroreceptor inputs to nucleus tractus solitarii of rats. Am J Physiol Heart Circ Physiol 271: H931–H936, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clin Exp Pharmacol Physiol 29: 514–21, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Slugg RM, Zheng SX, Fang Y, Kelly MJ, Ronnekleiv OK. Baclofen inhibits guinea pig magnocellular neurones via activation of an inwardly rectifying K+ conductance. J Physiol 551: 295–308, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiger JL, Bandyopadhyay S, Farb DH, Russek SJ. cAMP response element-binding protein, activating transcription factor-4, and upstream stimulatory factor differentially control hippocampal GABABR1a and GABABR1b subunit gene expression through alternative promoters. J Neurosci 24: 6115–6126, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sumners C, Myers LM, Kalberg CJ, Raizada MK. Physiological and pharmacological comparisons of angiotensin II receptors in neuronal and astrocyte glial cultures. Prog Neurobiol 34: 355–385, 1990. [DOI] [PubMed] [Google Scholar]
- 34.Sun C, Du J, Sumners C, Raizada MK. PI3-kinase inhibitors abolish the enhanced chronotropic effects of angiotensin II in spontaneously hypertensive rat brain neurons. J Neurophysiol 90: 3155–3160, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Sun C, Li H, Leng L, Raizada MK, Bucala R, Sumners C. Macrophage migration inhibitory factor: an intracellular inhibitor of angiotensin II-induced increases in neuronal activity. J Neurosci 24: 9944–9952, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun C, Sellers KW, Sumners C, Raizada MK. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res 96: 659–666, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki M, Kuramochi T, Suga T. GABA receptor subtypes involved in the neuronal mechanisms of baroreceptor reflex in the nucleus tractus solitarii of rabbits. J Auton Nerv Syst 43: 27–35, 1993. [DOI] [PubMed] [Google Scholar]
- 37a.Swanson LW. Brain Maps: Structure of the Rat Brain (3rd ed.). San Diego, CA: Elsevier, 2004, P. 153.
- 38.Tan PS, Potas JR, Killinger S, Horiuchi J, Goodchild AK, Pilowsky PM, Dampney RA. Angiotensin II evokes hypotension and renal sympathoinhibition from a highly restricted region in the nucleus tractus solitarii. Brain Res 1036: 70–76, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Tsukamoto K, Sved AF. Enhanced gamma-aminobutyric acid-mediated responses in nucleus tractus solitarius of hypertensive rats. Hypertension 22: 819–825, 1993. [DOI] [PubMed] [Google Scholar]
- 40.Vitela M, Mifflin SW. γ-Aminobutyric acidB receptor-mediated responses in the nucleus tractus solitarius are altered in acute and chronic hypertension. Hypertension 37: 619–622, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Wong LF, Polson JW, Murphy D, Paton JF, Kasparov S. Genetic and pharmacological dissection of pathways involved in the angiotensin II-mediated depression of baroreflex function. FASEB J 16: 1595–1601, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Yang H, Raizada MK. Role of phosphatidylinositol 3-kinase in angiotensin II regulation of norepinephrine neuromodulation in brain neurons of the spontaneously hypertensive rat. J Neurosci 19: 2413–2423, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang W, Herrera-Rosales M, Mifflin S. Chronic hypertension enhances the postsynaptic effect of baclofen in the nucleus tractus solitarius. Hypertension 49: 1–5, 2007. [DOI] [PubMed] [Google Scholar]


