CLINICAL VIGNETTE
A 38-year-old woman with a history of allergic rhinitis and asthma presented for evaluation of nasal congestion, rhinorrhea, postnasal drip, and sneezing. Although her asthma had remained well controlled with inhaled corticosteroids for many years, her nasal symptoms had been steadily worsening for at least the past 12 months. During her clinical visit, spirometric results were within normal limits, and aeroallergen skin test results were positive for tree, ragweed, grass, dust mite, and cat. On examination, her nasal turbinates were moderately edematous, but no nasal polyps were visualized. A sinus computed tomographic scan revealed only mild sinus disease. She was treated with a short course of oral steroids, antibiotics, and nasal steroids, as well as educated on allergen avoidance. Her nasal symptoms were manageable for 4 years with intranasal steroid therapy alone.
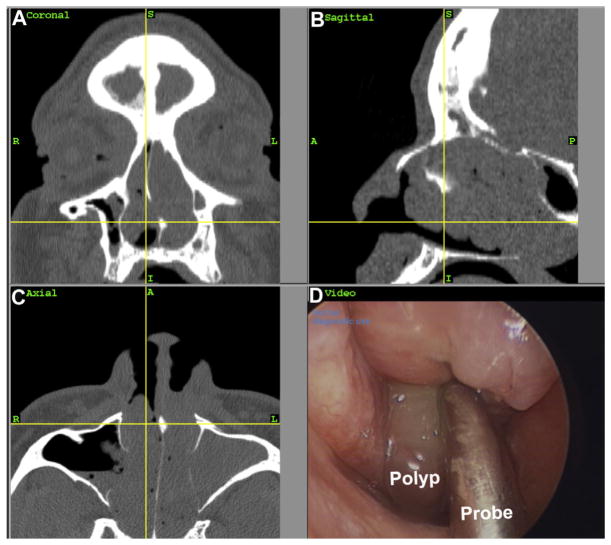
At age 43 years, she began to experience recurrent episodes of worsening nasal congestion, facial pressure, headaches, and hyposmia, each associated with an asthma exacerbation. She was prescribed courses of oral steroids and antibiotics, with only temporary relief of symptoms. A repeat sinus computed tomographic scan at this time showed pansinusitis consistent with chronic rhinosinusitis (CRS), and nasal endoscopy revealed frank polyps (see Fig E1 in this article’s Online Repository at www.jacionline.org). Additionally, the patient recalled recently taking ibuprofen for a headache and experiencing acute shortness of breath and worsening of nasal congestion within 30 minutes of ingestion. Before this episode, she had tolerated aspirin and ibuprofen without problems. Because the patient demonstrated the clinical triad of chronic rhinosinusitis with nasal polyps (CRSwNP), asthma, and sensitivity to a nonsteroidal anti-inflammatory drug, she was given a diagnosis of aspirin-exacerbated respiratory disease (AERD)E1 and instructed to avoid all nonsteroidal anti-inflammatory drugs.
The full review of this article, including a preview of relevant issues to be considered, can be found online at www.jacionline.org. If you wish to receive CME or MOC credit for the article, please see the instructions above.
REVIEW
Nasal polyps are inflammatory outgrowths of the paranasal sinus mucosa and occur in 1% to 4% of the general population.E2 They develop in the setting of chronic mucosal inflammation of the sinuses and can include subgroups of AERD and allergic fungal rhinosinusitis. Nasal polyps most often are benign, occur bilaterally, and typically develop in adulthood. Unilateral nasal polyps should be evaluated for malignancy,E3 and nasal polyps found in children should raise the suspicion for underlying cystic fibrosis.E4
Several theories regarding the development and pathogenesis of nasal polyposis have been proposed. The following is an overview of factors relevant to the biology of nasal polyposis, with a focus on the roles of barrier function, innate immunity, eosinophilic inflammation, and adaptive immunity.
Role of barrier function and innate immunity
The sinus mucosa forms an important barrier protecting the host from constant exposure to foreign antigens. Compromise of this barrier has been hypothesized to cause dysregulation of local immune homeostasis and lead to a chronic inflammatory state.E5 There have been several studies examining sinus mucosal function and integrity in patients with CRS. Levels of S100A7 and S100A8/9, proteins important in epithelial defense and repair, were reduced in nasal lavage fluid from patients with CRSwNP compared with levels seen in healthy control subjects.E6 Additionally, RNA levels of the serine peptidase inhibitor Kazal type 5 (SPINK5) were reduced in epithelial scrapings from sinonasal tissues from patients with CRSwNP compared with those seen in healthy control subjects.E7 Furthermore, Soyka et alE8 showed that expression of the tight junction proteins occludin and zonula occludens 1 are decreased in nasal polyps also when compared with expression seen in healthy control subjects. Taken together, there appears to be impairment of tight junctions and decreases in various proteins important in epithelial defense, suggesting that the mucosal barrier in patients with CRSwNP is compromised. Similar barrier integrity loss has been reported in patients with atopic dermatitis and asthma.E9,E10 Disruption in barrier integrity generally increases allergic sensitization and could increase epithelial susceptibility to colonization by fungal or bacterial pathogens, allowing access to proteases and allergens that could drive the chronic inflammation characteristic of nasal polyposis.
Eosinophilia and CRS
Studies from the United States and Europe have shown that nasal polyps are characterized by predominantly eosinophilic inflammation.E11 Levels of soluble mediators important in eosinophil activation, proliferation, recruitment, and survival have been shown to be increased in nasal polyp tissue from patients with CRS compared with those in healthy control subjects.E11,E12 Additionally, patients with AERD have even greater levels of nasal polyp eosinophilia than those with CRSwNP alone.E13 The significance of tissue eosinophilia and whether eosinophils have a causative versus bystander role in the development of nasal polyps remain unclear and are subjects of active investigation.E14
As opposed to Western countries, subjects living in Asia are less likely to have a predominant eosinophilic infiltrate in nasal polyp tissue. Instead, a predominantly neutrophilic infiltrate has been more commonly reported in Thai, Chinese, Korean, and Japanese cohorts.E15 It can be hypothesized that differences in CRSwNP among patients living in Eastern versus Western countries might be due to possible genetic factors, environmental factors, or both. Interestingly, a recent study examining cohorts in Korea found a shift from neutrophil- to eosinophil-predominant cases of CRSwNP in a 17-year period, leading the authors to suggest that the specific factors important in CRSwNP pathogenesis might be evolving and are modifiable.E16
Mast cells have also been studied in patients with CRS because of their ability to not only recruit eosinophils and basophils but also produce mediators that can induce vasodilation and tissue edema, findings observed in nasal polyps. Takabayashi et alE17 found increased numbers of mast cells in nasal polyps when compared with nonpolypoid CRS tissue and tissue from healthy control subjects. Additionally, mast cells within nasal polyp epithelium were shown to express tryptase and carboxypeptidase A3, but not chymase, an unexpected finding given mast cells are traditionally classified as either expressing all 3 proteases or just tryptase alone.E17,E18 Taken together, the increased presence and phenotypic differences of mast cells in nasal polyp tissue suggest these cells might play a role in sinus disease pathogenesis.
Numbers of basophils, which are often companion cells to eosinophils, were recently shown to be increased in nasal polyps.E19 Interestingly, however, basophil numbers are lower in nasal polyps of patients with AERD compared with numbers in those with CRSwNP alone, and it has been suggested that this might reflect degranulation of those basophils that appear in nasal polyps from patients with AERD.E19
Role of adaptive immunity
B lymphocytes are integral components of the adaptive immune response, during which they can produce immunoglobulins, secrete various cytokines, and present antigen to T lymphocytes. Studies have shown that activated B cells are more numerous in nasal polyps.E20 Increased levels of B cells in nasal polyps might in part be secondary to increased expression of CXCL12 or CXCL13, which are B cell–attracting chemokines, or caused by increases in a key B-cell survival factor, B cell–activating factor of the TNF family, which is found in polyp tissue.E5 There is evidence to suggest that B cells also differentiate vigorously within nasal tissue to become plasma cells that produce immunoglobulins, and levels of total IgG, IgE, and IgA in nasal polyp tissue are increased compared with those seen in healthy control subjects.E21 Importantly, in this study there were no corresponding differences in circulating levels of total IgG, IgA, or IgE between subjects with CRSwNP and control subjects. It is possible that local immunoglobulin production occurs for the purpose of host defense in patients with CRSwNP because of frequent colonization, infection, or both. It is also being considered that local immunoglobulin production might mediate pathogenesis in nasal polyps, and local synthesis of autoantibodies has been documented and hypothesized to be of potential pathogenic importance.E22 Whether protective or pathogenic, it is becoming clear that there is a robust local B lineage cell response and production of immunoglobulins.
There is evidence to suggest that many patients with CRSwNP are colonized with Staphylococcus aureus and can have a polyclonal population of IgE antibodies both locallyE23 and systemically.E24 In a subset of colonized patients, specific IgE to S aureus enterotoxins can develop, and these levels directly correlate to the amount of detectable IL-5 and eosinophil cationic protein, as well as to the number of eosinophils present in polyp tissue.E25 The presence of such enterotoxins also influences local T-cell diversity because T lymphocytes in nasal polyp tissue undergo a more significant T-cell receptor V-β expansion compared with those T lymphocytes in circulation.E26 Additionally, polyclonal IgE was shown to activate mast cells ex vivo in patients with allergic rhinitis and nasal polyposis, and it is hypothesized that specific IgE to S aureus enterotoxins functions similarly in vivo.E27
T lymphocytes are very important in the adaptive immune response and have been shown to play an important role in CRS pathogenesis. CD4 T-cell numbers are increased in nasal polyp tissue and appear to be skewed toward a TH2-type phenotype.E28 Thymic stromal lymphopoietin, which is known as the master regulator of TH2 inflammation, is produced by immune cells and epithelial cells and activates dendritic cells to promote a TH2 response.E29 Nagarkar et alE30 have shown that thymic stromal lymphopoietin expression not only is increased in epithelial scrapings from nasal polyps in patients with CRS but also is linked with TH2 inflammation, as evidenced by a positive correlation with IL-5 expression in polyp tissue. Although TH2 cells have been identified in nasal polyps, recent studies indicate that a cell type of potentially more importance is the type 2 innate lymphoid cell.E31 These cells generally drive a TH2-type inflammation that is antigen independent and in which IL-5 and IL-13, but not IL-4, are the predominant cytokines. Finally, CD4+ regulatory T-cell function in patients with CRSwNP might be impaired because studies have shown that forkhead box protein 3 (FOXP3) mRNA expression and TGF-β protein levels are both decreased in nasal polyp tissue compared with those seen in control tissue.E28 This possible imbalance between effector and regulatory T-cell function could thus contribute to the chronicity of the ongoing inflammatory response in nasal polyposis.
Nasal polyps and tissue edema
In addition to an inflammatory cell infiltrate, nasal polyps are also characterized by edematous stromal tissue and the formation of pseudocysts. The retention of plasma proteins, such as albumin, has been suggested to contribute to polyp size and growth,E32 but the precise mechanisms underlying how such proteins are retained remains unclear. However, recent studies have suggested that products of the coagulation cascade might be important in the development of tissue edema observed in patients with CRS.
Levels of fibrin, a key protein involved in clot formation, were found to be increased in nasal polyp tissue, whereas levels of D-dimer, a measure of fibrin breakdown, and tissue plasminogen activator, a protein involved in fibrin degradation, were both reduced.E33 Additionally, levels of an initiator of the coagulation cascade, Factor XIII-A, are also increased in nasal polyp tissue. Factor XIII-A is predominantly produced by M2 macrophages, a subset of macrophages alternatively induced by TH2 cytokines that play a role in tissue repair and fibrosis.E34 Taken together, it is hypothesized that the TH2 environment seen in patients with CRSwNP recruits M2 macrophages, which in turn secrete Factor XIII-A and promote fibrin deposition. The same TH2 environment also is thought to attenuate tissue plasminogen activator and decrease the rate of fibrin degradation. Such imbalance in the generation versus breakdown of fibrin could lead to an enhanced fibrin deposition that could retain plasma proteins and contribute to nasal polyp edema.
Nasal polyps in patients with AERD
On average, patients with AERD have significantly more extensive sinus inflammation and higher recurrences of nasal polyps after surgery when compared with patients with CRSwNP alone.E35 The biological mechanisms that account for these clinical differences are currently under investigation, with the dysregulation of arachidonic acid metabolism hypothesized to play an important role.E36,E37 Studies have shown reduced levels of prostaglandin E2, an anti-inflammatory lipid mediator, and decreased mRNA levels of COX2, a key enzyme that synthesizes prostaglandin E2, in nasal polyp tissue from patients with AERD compared with patients with CRSwNP alone.E12,E38,E39 In contrast, levels of proinflammatory cysteinyl leukotrienes and mRNA expression of their receptors, cysteinyl leukotriene receptors 1 and 2, were found to be increased in nasal polyps from patients with AERD compared with those from patients with CRSwNP.E12,E40 A recent study by Laidlaw et alE41 reported increased numbers of platelets colocalizing with leukocytes in nasal polyp tissue from patients with AERD compared with those with CRSwNP, and these authors suggest that such adherent platelets might enhance tissue inflammation in patients with AERD in part by contributing to leukocyte adhesion and production of cysteinyl leukotrienes.
THE CASE REVISITED
The patient underwent sinus surgery but had postoperative recurrence of symptoms requiring a second revision surgery 15 months later. Although sinus surgery is the most aggressive treatment modality to address nasal polyps, concomitant medical therapies are also important in the management of this disease. Most commonly, nasal steroids are prescribed based on evidence showing their ability to reduce polyp size, improve sinonasal symptoms, and positively affect quality of life.E42,E43 More recently, a randomized, double-blind, placebo-controlled trial examined the effects of omalizumab, an mAb that binds IgE, in patients with CRSwNP and comorbid asthma.E44 This study found a significant decrease in total nasal endoscopic polyp scores, as well as improvement in nasal symptoms, in the treatment group compared with values in placebo-treated control subjects. The exact mechanisms behind these observations are not known, but one hypothesis is that omalizumab reduces the levels of localized polyclonal IgE in nasal polyp tissue, thus limiting the ability of IgE to activate innate and adaptive immune responses. Interestingly, the treatment was effective in both atopic and nonatopic patients with CRS, suggesting that the mechanism might be more complex. After nasal steroids and surgery, omalizumab was initiated in the present patient, with significant improvement in both her clinical symptoms and endoscopic findings. Aspirin desensitization and subsequent treatment with high doses of aspirin were discussed with the patient because this therapy has been shown to improve sinus and asthma symptoms, as well as reduce the number of revision surgeries.E45 However, the patient declined this treatment.
In conclusion, the biology of nasal polyposis represents a complex interplay between the sinonasal epithelial barrier and the host immune response, which together promote chronic inflammation that is characteristic of CRSwNP. It can be hypothesized that treatments that eliminate the recruitment and/or activation of eosinophils, mast cells, and lymphoid cells producing type 2 cytokines are expected to have beneficial effects in patients with CRSwNP.
FIG E1.
Sinus computed tomography scan demonstrating mucosal thickening in coronal (A), sagittal (B), and axial (C) sections. Nasal endoscopy reveals the presence of a polyp (D).
References
- E1.Samter M, Beers RF., Jr Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann Intern Med. 1968;68:975–83. doi: 10.7326/0003-4819-68-5-975. [DOI] [PubMed] [Google Scholar]
- E2.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23:1–298. 3 p preceding table of contents. [PubMed] [Google Scholar]
- E3.Tritt S, McMains KC, Kountakis SE. Unilateral nasal polyposis: clinical presentation and pathology. Am J Otolaryngol. 2008;29:230–2. doi: 10.1016/j.amjoto.2007.07.001. [DOI] [PubMed] [Google Scholar]
- E4.Mainz JG, Koitschev A. Pathogenesis and management of nasal polyposis in cystic fibrosis. Curr Allergy Asthma Rep. 2012;12:163–74. doi: 10.1007/s11882-012-0250-y. [DOI] [PubMed] [Google Scholar]
- E5.Schleimer RP, Kato A, Peters A, Conley D, Kim J, Liu MC, et al. Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proc Am Thorac Soc. 2009;6:288–94. doi: 10.1513/pats.200808-088RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Tieu DD, Peters AT, Carter RG, Suh L, Conley DB, Chandra R, et al. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125:667–75. doi: 10.1016/j.jaci.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Richer SL, Truong-Tran AQ, Conley DB, Carter R, Vermylen D, Grammer LC, et al. Epithelial genes in chronic rhinosinusitis with and without nasal polyps. Am J Rhinol. 2008;22:228–34. doi: 10.2500/ajr.2008.22.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E8.Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol. 2012; 130:1087–96. doi: 10.1016/j.jaci.2012.05.052. [DOI] [PubMed] [Google Scholar]
- E9.Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009;124:R7–12. doi: 10.1016/j.jaci.2009.07.012. [DOI] [PubMed] [Google Scholar]
- E10.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432–46. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- E11.Bachert C, Wagenmann M, Hauser U, Rudack C. IL-5 synthesis is upregulated in human nasal polyp tissue. J Allergy Clin Immunol. 1997;99:837–42. doi: 10.1016/s0091-6749(97)80019-x. [DOI] [PubMed] [Google Scholar]
- E12.Perez-Novo CA, Watelet JB, Claeys C, Van Cauwenberge P, Bachert C. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol. 2005;115:1189–96. doi: 10.1016/j.jaci.2005.02.029. [DOI] [PubMed] [Google Scholar]
- E13.Stevenson DD, Zuraw BL. Pathogenesis of aspirin-exacerbated respiratory disease. Clin Rev Allergy Immunol. 2003;24:169–88. doi: 10.1385/CRIAI:24:2:169. [DOI] [PubMed] [Google Scholar]
- E14.Schleimer RP, Kato A, Kern R. Eosinophils and chronic rhinosinusitis. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. 1. Oxford (UK): Elsevier-Academic Press; 2013. pp. 508–18. [Google Scholar]
- E15.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122:961–8. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- E16.Kim SJ, Lee KH, Kim SW, Cho JS, Park YK, Shin SY. Changes in histological features of nasal polyps in a Korean population over a 17-year period. Otolaryngol Head Neck Surg. 2013;149:431–7. doi: 10.1177/0194599813495363. [DOI] [PubMed] [Google Scholar]
- E17.Takabayashi T, Kato A, Peters AT, Suh LA, Carter R, Norton J, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;130:410–20. doi: 10.1016/j.jaci.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E18.Schwartz LB. Analysis of MC(T) and MC(TC) mast cells in tissue. Methods Mol Biol. 2006;315:53–62. [PubMed] [Google Scholar]
- E19.Mahdavinia M, Carter RG, Ocampo CJ, Stevens W, Kato A, Tan BK, et al. Basophils are elevated in nasal polyps of patients with chronic rhinosinusitis without aspirin sensitivity. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2013.12.1092. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E20.Gevaert P, Holtappels G, Johansson SG, Cuvelier C, Cauwenberge P, Bachert C. Organization of secondary lymphoid tissue and local IgE formation to Staphylococcus aureus enterotoxins in nasal polyp tissue. Allergy. 2005;60:71–9. doi: 10.1111/j.1398-9995.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- E21.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013;131:1075–83. doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E22.Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128:1198–206. doi: 10.1016/j.jaci.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E23.Boase S, Foreman A, Cleland E, Tan L, Melton-Kreft R, Pant H, et al. The microbiome of chronic rhinosinusitis: culture, molecular diagnostics and biofilm detection. BMC Infect Dis. 2013;13:210. doi: 10.1186/1471-2334-13-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E24.Tripathi A, Conley DB, Grammer LC, Ditto AM, Lowery MM, Seiberling KA, et al. Immunoglobulin E to staphylococcal and streptococcal toxins in patients with chronic sinusitis/nasal polyposis. Laryngoscope. 2004;114:1822–6. doi: 10.1097/00005537-200410000-00027. [DOI] [PubMed] [Google Scholar]
- E25.Bachert C, Gevaert P, Holtappels G, Johansson SG, van Cauwenberge P. Total and specific IgE in nasal polyps is related to local eosinophilic inflammation. J Allergy Clin Immunol. 2001;107:607–14. doi: 10.1067/mai.2001.112374. [DOI] [PubMed] [Google Scholar]
- E26.Tripathi A, Kern R, Conley DB, Seiberling K, Klemens JC, Harris KE, et al. Staphylococcal exotoxins and nasal polyposis: analysis of systemic and local responses. Am J Rhinol. 2005;19:327–33. [PubMed] [Google Scholar]
- E27.Zhang N, Holtappels G, Gevaert P, Patou J, Dhaliwal B, Gould H, et al. Mucosal tissue polyclonal IgE is functional in response to allergen and SEB. Allergy. 2011;66:141–8. doi: 10.1111/j.1398-9995.2010.02448.x. [DOI] [PubMed] [Google Scholar]
- E28.Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121:1435–41. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- E29.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- E30.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132:593–600.e12. doi: 10.1016/j.jaci.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E31.Kim BS, Wojno ED, Artis D. Innate lymphoid cells and allergic inflammation. Curr Opin Immunol. 2013;6:738–44. doi: 10.1016/j.coi.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E32.Bachert C, Gevaert P, Holtappels G, Cuvelier C, van Cauwenberge P. Nasal polyposis: from cytokines to growth. Am J Rhinol. 2000;14:279–90. doi: 10.2500/105065800781329573. [DOI] [PubMed] [Google Scholar]
- E33.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med. 2013;187:49–57. doi: 10.1164/rccm.201207-1292OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E34.Takabayashi T, Kato A, Peters AT, Hulse KE, Suh LA, Carter R, et al. Increased expression of factor XIII-A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2013;132:584–92. doi: 10.1016/j.jaci.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E35.Awad OG, Lee JH, Fasano MB, Graham SM. Sinonasal outcomes after endoscopic sinus surgery in asthmatic patients with nasal polyps: a difference between aspirin-tolerant and aspirin-induced asthma? Laryngoscope. 2008;118:1282–6. doi: 10.1097/MLG.0b013e318170af1e. [DOI] [PubMed] [Google Scholar]
- E36.Laidlaw TM, Boyce JA. Pathogenesis of aspirin-exacerbated respiratory disease and reactions. Immunol Allergy Clin North Am. 2013;33:195–210. doi: 10.1016/j.iac.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E37.Mullol J, Picado C. Rhinosinusitis and nasal polyps in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2013;33:163–76. doi: 10.1016/j.iac.2012.11.002. [DOI] [PubMed] [Google Scholar]
- E38.Yoshimura T, Yoshikawa M, Otori N, Haruna S, Moriyama H. Correlation between the prostaglandin D(2)/E(2) ratio in nasal polyps and the recalcitrant pathophysiology of chronic rhinosinusitis associated with bronchial asthma. Allergol Int. 2008;57:429–36. doi: 10.2332/allergolint.o-08-545. [DOI] [PubMed] [Google Scholar]
- E39.Picado C, Fernandez-Morata JC, Juan M, Roca-Ferrer J, Fuentes M, Xaubet A, et al. Cyclooxygenase-2 mRNA is downexpressed in nasal polyps from aspirin-sensitive asthmatics. Am J Respir Crit Care Med. 1999;160:291–6. doi: 10.1164/ajrccm.160.1.9808048. [DOI] [PubMed] [Google Scholar]
- E40.Adamusiak AM, Stasikowska-Kanicka O, Lewandowska-Polak A, Danilewicz M, Wagrowska-Danilewicz M, Jankowski A, et al. Expression of arachidonate metabolism enzymes and receptors in nasal polyps of aspirin-hypersensitive asthmatics. Int Arch Allergy Immunol. 2012;157:354–62. doi: 10.1159/000329744. [DOI] [PubMed] [Google Scholar]
- E41.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood. 2012;119:3790–8. doi: 10.1182/blood-2011-10-384826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E42.Lund VJ, Flood J, Sykes AP, Richards DH. Effect of fluticasone in severe polyposis. Arch Otolaryngol Head Neck Surg. 1998;124:513–8. doi: 10.1001/archotol.124.5.513. [DOI] [PubMed] [Google Scholar]
- E43.Aukema AA, Mulder PG, Fokkens WJ. Treatment of nasal polyposis and chronic rhinosinusitis with fluticasone propionate nasal drops reduces need for sinus surgery. J Allergy Clin Immunol. 2005;115:1017–23. doi: 10.1016/j.jaci.2004.12.1144. [DOI] [PubMed] [Google Scholar]
- E44.Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131:110–6. doi: 10.1016/j.jaci.2012.07.047. [DOI] [PubMed] [Google Scholar]
- E45.White AA, Stevenson DD. Aspirin desensitization in aspirin-exacerbated respiratory disease. Immunol Allergy Clin North Am. 2013;33:211–22. doi: 10.1016/j.iac.2012.10.013. [DOI] [PubMed] [Google Scholar]